Abstract
Macrophages are among the first cellular actors facing the invasion of microorganisms. These cells are able to internalize pathogens and destroy them by means of toxic mediators, many of which are produced enzymatically and have strong oxidizing capacity. Indeed, macrophages count on the NADPH oxidase complex activity, which is triggered during pathogen invasion and leads to the production of superoxide radical inside the phagosome. At the same time, the induction of nitric oxide synthase results in the production of nitric oxide in the cytosol which is able to readily diffuse to the phagocytic vacuole. Superoxide radical and nitric oxide react at diffusion controlled rates with each other inside the phagosome to yield peroxynitrite, a powerful oxidant capable to kill microorganisms. Peroxynitrite toxicity resides on oxidations and nitrations of biomolecules in the target cell. The central role of peroxynitrite as a key effector molecule in the control of infections has been proven in a wide number of models. However, some microorganisms and virulent strains adapt to survive inside the potentially hostile oxidizing microenvironment of the phagosome by either impeding peroxynitrite formation or rapidly detoxifying it once formed. In this context, the outcome of the infection process is a result of the interplay between the macrophage-derived oxidizing cytotoxins such as peroxynitrite and the antioxidant defense machinery of the invading pathogens.
1. Macrophage activation, superoxide and nitric oxide generation
It is well known that macrophages play a main role fighting against invading pathogens in the first stages of an infection. They are able to mount a strong response aimed to create a hostile environment for pathogens. Nonetheless, its function is not limited to host defense against foreign organisms, they have a wide range of action regarding important aspects of homeostasis, e.g. wound healing, clearance of senescent cells, tumoricidal activity, adipose tissue metabolism, among others (1). All of these varied functions are not performed by a homogeneous cell population but by sets of cells distinctly stimulated depending on the tissue context. Two main types of macrophages have been described with antagonistic actions: M1 or classically activated macrophages are responsible for host defense from microorganisms and show a pro-inflammatory phenotype; whereas M2 or alternatively activated macrophages have an immunosuppressive profile regulating re-establishment of homeostasis after inflammation and wound healing (1). It is beyond the aim of this work discussing all the aspects of macrophage roles on physiology and pathology, but rather we will focus on microbicidal mechanisms of classically activated macrophages (M1) to deal with potentially hazardous organisms.
Macrophages are equipped with specialized receptors that bind to particular motifs on pathogens, the so called pattern recognition receptors (PRRs), including the Toll-like receptors, C-type-lectin receptors and NOD-like receptors. Union between these receptors and their ligands leads to macrophage activation and production of chemical mediators which contribute to inter-cell communications. For instance, activated macrophages secrete IL-12, promoting interferon-γ (IFNγ) production by T-helper lymphocytes; which in turn further stimulates macrophages (2). One of the more powerful microbicidal tools proven to be activated by IFNγ and other proinflammatory cytokines (IL-1β, IL-16 and TNF-α) is the inducible nitric oxide synthase or iNOS, which synthesizes nitric oxide (•NO) from arginine and NADPH (3). Induction of iNOS upon IFN-γ signaling depends on the activation of STAT1 (part of the JAK/STAT pathway), responsible of enhancing the transcription rate of this gene. Conversely, cytokines like IL-4 and IL-13 produced by T-helper cells 2 (regulatory phenotype) activate STAT6, which blocks iNOS gene expression (4–6).
Not only IFNγ, but also other pro-inflammatory stimuli such as IL-1β, TNF-α or the pathogen itself augment iNOS expression, but it occurs through a different mechanism involving NF-κB. It is the case of Trypanosoma cruzi triggered iNOS induction in macrophages, which depends on the interaction through the Toll-like receptor 4 (7–8). The fact that two or more different signals lead to the same result using separate pathways means that they can synergize and promote a greater effect. Indeed, it is well studied in the murine model, where expression of iNOS is the maximum in the presence of both IFN-γ, and a pathogen stimulus such as lipopolisacharide (LPS), see Figure 1. However regulation of the amount of protein is achieved not only adjusting the transcription rate, but also increasing the stability of mRNA and the protein itself (3). Experimental induction of iNOS takes 4 to 5 hours of cytokine exposure (or other stimulus), after which, production of •NO remains active for up to 16 hours (9–10). Once synthesized, part of iNOS total protein is incorporated in vesicles of 50 to 80 nm that are translocated to phagosome when the macrophage is activated promoting a local increase in •NO, while the rest of the enzyme is distributed throughout the cytosol of the cell (11). Nonetheless, •NO is a small and hydrophobic species and therefore it is able to diffuse and to go across membranes, even enter the phagosome (12).
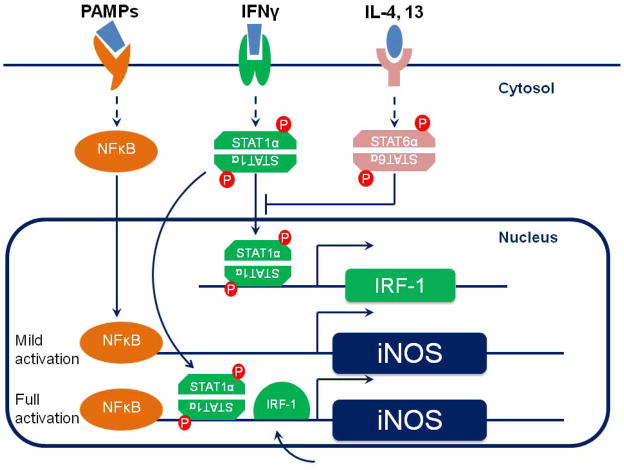
Figure 1. Regulation of iNOS expression.
PAMPs recognition through TLR-4 leads to the activation of NFκB, which is translocated to the nucleus and moderately enhances the iNOS promoter activity. IFNγ signaling depends on the JAK/STAT pathway. Phosphorylated STAT1α binds to the regulatory region of the iNOS gene and also to that of the IFNγ regulatory factor (IRF-1), which in turn promotes iNOS transcription as well. When both IFNγ and a pathogen motif are present, iNOS expression is maximal.
Biological effects of •NO are the most varied and are reviewed elsewhere (13–15), but in large concentrations like it is formed in the context of inflammation, this radical becomes part of the macrophage artillery to eliminate the threat. The actual responsible for most of the cytotoxic effect of nitric oxide are derivatives that arise from its reaction with active redox centers or other radicals (i.e. superoxide).
In a similar way to neutrophils but to a lesser extent, macrophages undergo pronounced oxygen consumption after pathogen recognition and phagocytosis. This phenomenon is known as the “respiratory burst” and it is the result of the recruitment of an enzymatic complex in the phagosome membrane, NADPH oxidase. Cytosolic subunits of the oxidase are phosphorylated and migrate to the membrane to form a functional enzyme with membrane-bound subunits (16). Some stimulus, such as LPS or TNFα, has a “priming” effect, increasing the phosphorylation state of cytosolic subunits (mainly p47phox), resulting in an enhanced activity of the NADPH oxidase upon activation (17–18). After NADPH oxidase assembling, the formation of superoxide (O2•−) takes place inside the phagosome for up two hours approximately (Figure 2) (10). Being O2•− a short lived radical with limited diffusion within the cell, most of its importance as cytotoxic agent comes from derivative species (hydrogen peroxide, hydroxyl radical, peroxynitrite). The fate of superoxide in biological systems depends on the concentration of other actors, such as superoxide dismutase, metal ion centers and •NO. As aforementioned this latter is formed in the cytosol of macrophages and readily diffuses across phagosome membrane due to its hydrofobic nature. Whenever •NO and superoxide meet each other, they react at diffusion-controlled rate (~1.0 × 1010 M−1s−1, (19)) forming peroxynitrite (ONOO-), a strong oxidant capable of causing oxidation and nitration of both proteins and lipids. Thus, macrophage manages itself to form peroxynitrite inside the phagosome where the pathogen is to be eliminated.
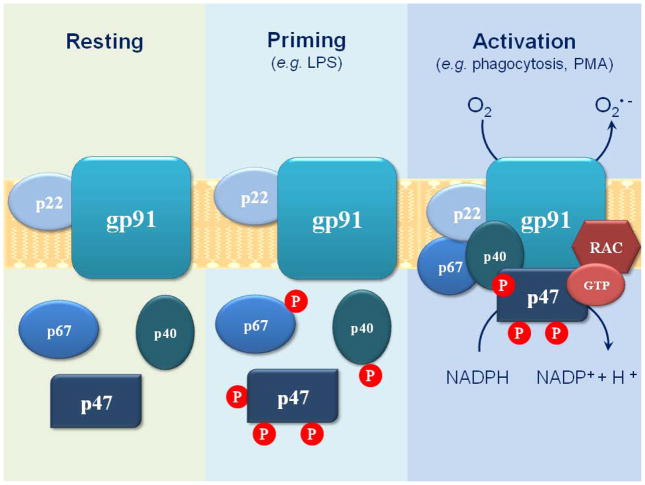
Figure 2. NADPH oxidase assembling.
The NADPH oxidase is composed of three cytosolic (p67phox, p47phox, and p40phox) and two membrane-bound subunits (gp91phox and p22phox). Activation during phagocytosis leads to phosphorylation of cytosolic components and their migration to the membrane to form an active complex. Low doses of LPS or other stimuli, such as TNFα, are not sufficient to promote the oxidase activation, but lead to the primed state, increasing the phosphorylation level of cytosolic subunits.
2. Peroxynitrite formation and some relevant reactions: oxidations and nitrations
Under physiological conditions peroxynitrite anion, ONOO−, is in equilibrium with peroxynitrous acid, ONOOH (pka=6.8), and given their different diffusional and chemical properties, local pH affects peroxynitrite reactivity. Both can promote one or two electron oxidation on biomolecules, particularly with transition metal centers and thiols (20–22). In fact, the reactions with metalloproteins, thiols and carbon dioxide (CO2) are responsible for most of its consumption in vivo (23).
There are many examples of protein thiols that are oxidized by peroxynitrite. Reaction of peroxynitrite towards one specific sulfhydryl on glyceraldehide-3-phosphate dehydrogenase leads to inactivation of the enzyme (24). Likewise, oxidation of critical thiols by peroxynitrite on complex I and II inhibits the electron transport in the mitochondrial respiration chain (25). The low molecular weight thiol, glutathione, reaches millimolar concentrations inside cells and for some time it was considered a direct cellular scavenger of peroxynitrite. However, the reaction with glutathione is not as fast to prevail over others in vivo. Rather, it is a target for secondary products of peroxynitrite, (analyzed recently in Ref (23)).
On the other hand, peroxynitrite is able to oxidize a number of proteins bearing transition metal centers, such as aconitase, whose reaction with peroxynitrite leads to disruption of the 4Fe-4S center and its inactivation (22). Hemoproteins (e.g. myeloperoxydase, oxyhemoglobin, citocrome c2+) as well as Cu-Zn-, Mn-, and Fe-containing superoxide dismutases (SODs) are also targets for peroxynitrite.
However, many of the reactions of peroxynitrite with molecular targets depend on the formation of secondary radicals. At 37ºC and pH 7.4, ONOOH spontaneously homolyses to hydroxyl (•OH) and nitrogen dioxide (•NO2) radicals with yields of about 30% (26–27). Being this a relatively slow reaction (rate constant = 0.9s−1) and given the abundance of other targets for peroxynitrite this turns out to be a minor route in biological systems (28). In the absence of such targets the rest of peroxynitrite would eventually isomerize to nitrate (NO3−).
The other important target in biological milieu is CO2, present at high concentration both intra-and extracellularly (1–2 mM). Its reaction with ONOO− produces an unstable intermediate, nitroso-peroxocarboxylate (ONOOCO2), which decomposes generating carbonate radical (CO3•−) and •NO2 in approximately 35% yields (29). Carbonate radical reacts fast with some metal centers and aminoacidic residues in proteins, mainly tryptophan, methionine, and tyrosine. Its reaction with the latter gives rise to tyrosyl radical, which combines with •NO2 at diffusion-controlled rate to result in 3-nitrotyrosine formation. Carbonate radical is highly effective in the one-electron abstraction needed to form tyrosyl radical, but it can also be formed by reaction of tyrosine with other oxidants, e.g. oxo-metal complexes, lipid peroxyl radicals and less relevant, hydroxyl radical.
Other mechanism of nitration independent of peroxynitrite formation is more relevant in neutrophils and eosinophils, where myeloperoxidase (MPO) and eosinophil peroxidase (EPO) and •NO end products can lead to tyrosine nitration trough •NO2 formation in a hemeperoxidase-dependent manner (30–32). For example, MPO -null mice exhibit a decrease in 3-nitrotyrosine formation in various models of inflammation, though still considerable level of nitration is usually detected (30, 33–34). In fact, it is possible to detect nitrated bacteria inside immunostimulated neutrophils (35) and some proposed this phenomenon to be dependent on MPO in macrophages (36–37). However, current evidence in macrophages indicates that themain nitrating agent must be peroxynitrite, since MPO expression normally negligible in these cells (38).
Massive protein oxidation has been shown to be fatal to the cell. Peroxynitrite formation in macrophages during phagocytosis leads to protein oxidation and nitration in the engulfed pathogen. Detection of 3-nitrotyrosine in a number of invading parasites and bacteria is in accordance to peroxynitrite dependent cytotoxicity (38–40). For example, resistant (C57Bl/6) mice infected with L. amazoniensis parasites showed extensive protein nitration associated with phagolysosomes at early infection stages, while in susceptible mice (BALB/c) poor iNOS expression and tyrosine nitration was detected (41). Importantly, invoking peroxynitrite as the main nitrating species implies that the “timing” of nitration must parallel the time range when the simultaneous formation of O2•− and •NO occurs. In this regard, as the O2•− production is exhausted more rapidly than that of NO in immunostimulated macrophages (i.e. in 2 hours), significant protein tyrosine nitration should be observed at early times, as reported recently (38). While in murine macrophages under optimal immunostimulation conditions O2•− and •NO formation rates are similar resulting in almost stoichiometric peroxynitrite formation rates, conditions that lead to variable flux ratios of O2•−/•NO do not preclude peroxynitrite formation, although the levels will be restricted to the limiting precursor radical. Moreover, and in spite of initial belief, peroxynitrite-dependent tyrosine nitration is not significantly inhibited under in vivo conditions by excess O2•− or •NO (due to interference in the nitration steps) since SOD-catalyzed dismutation or alternative •NO reactions and diffusion, respectively, minimize their secondary reactions with, for example, tyrosyl radical (42).
Peroxynitrite- dependent nitration is not restricted to proteins, fatty acid present in membranes and lipoproteins are also susceptible to this modification. Fatty acid nitration mechanism is not fully understood yet, but peroxynitrite is one of the proposed agents. There is evidence that nitro-fatty acids are formed in vivo in the picomolar range and its concentration increases during inflammatory processes. Nitrated lipids are found in plasma and other components present in the vascular compartment, and given their anti-inflammatory properties; they are thought to have a protective role against oxidative damage associated with inflammatory diseases (43–44). In fact, nitroarachidonic acid has been recently shown to have anti-inflammatory activities during macrophage activation. Specifically it inhibits the NADPH oxidase activation modulating the superoxide formation, through mechanisms that are not fully understood yet (45). All of the aforementioned oxidation processes take place in a “battle” between the macrophage and the antioxidants systems developed by pathogens. They are equipped with enzymatic (and non-enzymatic) tools to detoxify peroxynitrite and it derivatives. Most notably, peroxirredoxins are a group of enzymes capable of reducing hydrogen peroxide, peroxynitrite (and other peroxides) at high rates and specificity. Overexpression of these enzymes is often related to pathogen virulence and this aspect is discussed latter in this review.
3. Peroxynitrite as a cytotoxic effector: evidence in vitro and in vivo
When dealing with oxidants chemistry, special care must be taken in order to identify the real effector in a particular process. Regarding cytotoxic properties of peroxynitrite we must be sure the effect comes from itself, and not because of its precursors, i.e. superoxide (as its dismutation product, hydrogen peroxide) or nitric oxide. Microbicidal activity of peroxynitrite has been thoroughly demonstrated in vitro, by exposing cells to either fluxes or bolus addition of ONOO−; or co-culturing them with •NO y O2•− producing cells; and in vivo, pharmacologically modulating peroxynitrite formation or even working with knockout mice for one or more of the enzymes related to oxidant production.
There is growing evidence in literature identifying peroxynitrite as a potent cytotoxic agent in macrophages when counter-attacking a pathogen invasion (see Table I). The most common strategies chosen to study the role of peroxynitrite in the host-pathogen interactions are either based in the use of inhibitors of iNOS and NADPH oxidase or knockout mice models for one of these proteins with the aim of modulating peroxynitrite formation and evaluating the outcome from protein oxidation/nitration on cell (or animal) viability. As we see in table I mice lacking some of the subunits of NADPH oxidase are more prone to infection of a number of pathogens, as well as iNOS deficiency leads to poorer responses.
Table I.
Evidence of the formation and cytotoxicity of macrophage-derived peroxynitrite towards pathogenic organisms
| Pathogen | Model | Results | Ref. | |
|---|---|---|---|---|
| Bacteria | Escherichia coli | Exposure to •NO and peroxynitrite. | More toxicity of peroxynitrite. Identification of nitrated proteins. | (78–80) |
| Salmonella thiphimurium | Infection in vitro and in vivo with a Cu-Zn SOD deficient strain of S. thiphimurium. | Cu-Zn SOD deficient S. thiphimurium showed less virulence in vivo and in vivo. Infection in macrophages unable to form •NO or O2•− restores it infectivity. | (81–82) | |
| Brucella abortus | Infection in macrophages and treatment with inhibitors. Infection in knockout mice for iNOS and gp91phox. | IFNγ-activated macrophages present enhanced B.abortus killing capacity, and inhibition of iNOS resulted in poor control of pathogen replication. iNOS and gp91phox KO mice are less effective controlling infection. | (83–84) | |
| Mycobacterium tuberculosis | Exposure to peroxynitrite. Infection in vitro (macrophages) and in vivo in knockout mice for iNOS. | Peroxynitrite effectively killed M. tuberculosis while •NO or O2•− alone did not. Peroxynitrite-forming macrophages controlled the infection. iNOS -/- mice are more susceptible to infection. | (85) | |
| Rhodococus equi | Exposure to oxidants in vitro. Infection in iNOS and NADPH oxidase deficient mice. | R. equi is susceptible to peroxynitrite exposure. IFNγ is involved in control of infection in vivo. Knockout mice for iNOS and NADPH oxidase succumb to infection. | (86) | |
| Parasites | Cryptosporidium parvum | Infection in piglets. Treatment with iNOS inhibitors and peroxynitrite scavengers. | Animals treated with iNOS inhibitors or peroxynitrite scavengers are more susceptible to infection. Co-localization of iNOS and nitro-tyrosine. | (39) |
| Leishmania amazoniensis | Exposure of amastigotes to •NO and peroxynitrite. Infection in mice. | •NO is cytostatic and peroxynitrite is cytotoxic. NO2-Tyr detection on phagocytic vacuole and in the parasite. | (40–41) | |
| Leishmania major | Infection in macrophages and mice with L.major strain deficient in a pseudoperoxidase capable of detoxifying peroxynitrite. | Deletion of a pseudoperoxidase in L. major renders the cell more susceptible to SIN-1, and less infective in macrophages and mice. | (87) | |
| Leishmania donovani | Infection in mice deficient it iNOS and NADPH oxidase. | KO mice present higher susceptibility to L. donovani infection. | (88) | |
| Leishmania chagasi | Overexpression of 2 L. chagasi peroxirredoxins and infection in macrophages. | Enhanced expression of peroxirredoxins resulted in higher resistance to oxidants and promoted parasite survival in macrophages. | (69) | |
| Trypanosoma cruzi | Infection in macrophages and mice with peroxirredoxins overexpressers and wt strains. Use of both iNOS and NADPH oxidase inhibitors. | Control of infection dependent of peroxynitrite formation. Detection of NO2-Tyr on internalized parasites. Mice showed higher parasite loads when inoculated with strains overexpressing peroxirredoxins. | (38) | |
| Virus | Coxsackievirus | Exposure to peroxynitrite. Infection in mice, using iNOS inhibitors and peroxynitrite scavengers. | Peorxynitrite inhibits viral replication in vitro. iNOS inhibitors and peroxynitrite scavengers favor viral replication. NO2-Tyr associated with viral proteins. | (89) |
| Fungus | Candida albicans | Exposure to oxidant in vitro. Infection in macrophages; treatment with iNOS inhibitors and O2•− scavengers. | Peroxynitrite showed candidacidal activity in vitro. | (90) |
| Nematodes | Brugia malayi | Co-cultures with macrophages and use of iNOS inhibitors. Exposure to •NO and ONOO− in vitro. Characterization of resistant and susceptible rodents derived macrophages. | Parasites are susceptible to macrophages-derived peroxynitrite. Resistant rodents showed higher of •NO and O2•− production. | (91–92) |
This kind of experiments has brought light into many questions regarding peroxynitrite importance in microorganisms killing. Nevertheless, we must be careful when interpreting the data they provide. We have to bear in mind antimicrobial mechanisms triggered by each pathogen may be different. Redundancy and synergy among immune system cytotoxic tools are crucial properties that assure success in controlling the infection, but also make it difficult to interpret correctly data obtained in knockout models.
At the moment, there is no effective and specific NADPH oxidase inhibitor. More advances have been achieved in the development of iNOS inhibitors, but specificity it is dramatically important in order not to affect physiological •NO production by other isoforms in animal models. A central aspect regarding the importance of •NO and peroxynitrite toxicity in vivo in human pathology, is the difficulty of detecting iNOS protein and activity in human macrophages. There is a strong argument in the literature about the relevance of macrophage-derived •NO in controlling invasion of microorganisms given that nobody has found experimental conditions in vitro to induce iNOS in human cells (unlike what is observed in murine macrophages). However, data obtained in different inflammatory pathologies in patients, ranging from inflamed mediastinal lymph nodes (46) to tissue material from lungs with tuberculosis show higher iNOS expression in macrophages in relation to control individuals (47–48). In the case of tuberculosis, it has been found that •NO exhalation is increased in patients (49). Moreover, lung tissue of patients exhibit augmented nitro-tyrosine immunostaining, unequivocally indicative of enhanced •NO formation (48). Thus, the data support the existence of iNOS activity during inflammatory processes in vivo in humans. Perhaps the negative results in the attempt of reproduce this activity in human cultured macrophages reflect only the fact that no proper conditions and stimuli have been found for its induction (50).
4. Redox Biochemistry in the phagosome
The phagosome is a vesicle formed after a microorganism is engulfed by the plasmatic membrane of the macrophage. Its dimensions depend on the phagocytosed particle, but it varies in the μm range. Later on, this phagocytic vacuole is fused with lysosomes forming the phagolysosome, with a decrease in the pH and an increase in proteolytic activity (51). Concomitantly with pathogen internalization the respiratory burst is triggered and superoxide is formed towards the interior of the phagocytic vacuole. If nitric oxide is present, peroxynitrite will be produced as long as the respiratory burst lasts (from 90 to 120 min), see Figure 3 (10). Both ONOO− and ONOOH are able to cross biological membranes either through the 4,49- diisothiocyanatostilbene-2,29-disulfonic acid (DIDS) sensitive Cl−/HCl3− exchanger or passive diffusion, respectively (52). Nevertheless, in biological milieu peroxynitrite diffusion is limited by the presence of other abundant targets, most notably CO2. Thus, for extracellular microorganisms peroxynitrite toxicity depends on the distances between the source and the target. The scenario changes in the phagosome, because during parasite engulfment it is surrounded leaving a very narrow space between the two membranes. Here, distance between the source of peroxynitrite and the target is minimal (10–20 nm) so the reaction with carbon dioxide or other targets is minimized and peroxynitrite reaches the target cell causing the oxidative damage discussed in section 2 (53).
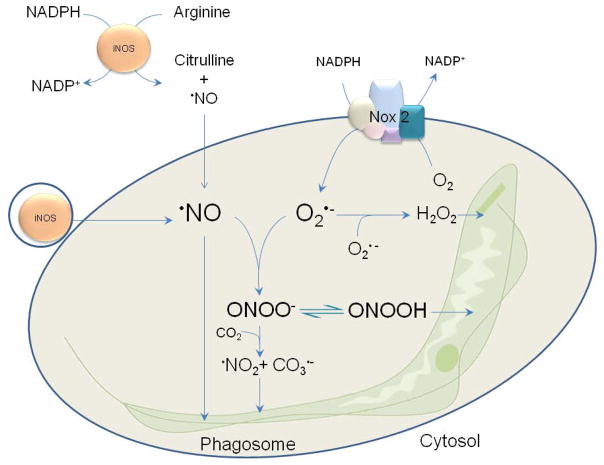
Figure 3. Redox biochemistry in the phagosome.
The scheme shows how a parasite (e.g Trypanosoma cruzi) is engulfed and attacked with powerful oxidants in a very narrow space. NADPH oxidase is activated immediately after phagocytosis directing superoxide production towards the interior of the phagosome. Superoxide dismutation leads to the formation of hydrogen peroxide, which has a cytotoxic effect at high doses. If iNOS is active in the cytosol or in small vesicles, nitric oxide will diffuse across membranes exerting cytostatic or cytotoxic effects on the microorganism. When both superoxide and nitric oxide co-exist in the phagosome they result in peroxynitrite formation, with lethal consequences for the pathogen.
The directional activity of NOX 2 in the phagosome membrane towards the vacuole’s interior determines the site of formation of peroxynitrite, since superoxide has limited diffusion while nitric oxide can readily cross membranes. This is extremely important to assure that powerful oxidants are produced in the same compartment that contains the target cell in order to prevent its consumption by other reactions. Intraphagosomal formation of peroxynitrite has been demonstrated by means of fluorescents probes (dihydrorhodamine) and detection of 3-nitro-tyrosine inside this compartment (Figure 4) (38).
Figure 4. Intraphagosomal peroxynitrite formation.
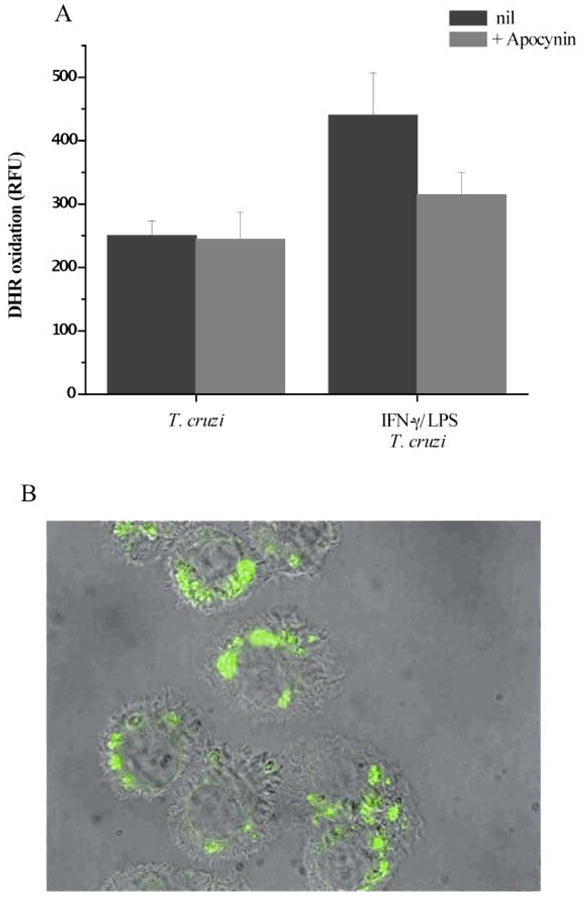
T. cruzi trypomastigotes preloaded with DHR were exposed to macrophages, and cells were washed 30 min after incubation. A, RH 123 accumulation after 2 h of infection in unstimulated (T. cruzi) or preactivated macrophages (IFNγ /LPS + T. cruzi) was determined in a fluorescence plate reader. The effect of apocynin (NADPH oxidase inhibitor) was evaluated under both conditions. RFU, relative fluorescence units. B, activated macrophages plated in slides were infected with DHR-loaded trypomastigotes (5:1, parasite:macrophage ratio). Merged DIC and fluorescence images were obtained 2 h after infection; the green fluorescence corresponds to oxidized DHR (magnification, X400). This research was originally published in J Biol Chem, Álvarez MN et al, “Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: consequences for oxidative killing and role of microbial peroxiredoxins in infectivity”, J Biol Chem 2011, 286: 6627–6640. © the American Society for Biochemistry and Molecular Biology.
Once we know it is formed, a more difficult question to answer is how much peroxynitrite is formed, and how much is needed to kill pathogens? Many efforts have been made to determine the rates of formation of these oxidants in the course of infection in phagocytic cells. Different cell types showed distinct levels of superoxide and nitric oxide production, and it also varies with the stimulus, i.e. pathogen and/or cytokines (54). Moreover, some techniques used to measure oxidants production may lead to under- or over- estimated values of concentrations and rates of formation. Most of the published data regarding cellular production of nitric oxide, superoxide and peroxynitrite are based in addressing oxidant concentrations in a particular volume (e.g. well or plate containing culture medium). Perhaps these numbers are not the most relevant to understand events happening in the phagosome, a compartment in which the space where oxidants are being formed is extremely scarce. The number of oxidant molecules, and even more interesting, number of hits of these species upon the phagocytosed pathogen are much more informative data.
Previous works of our group performed exposing Trypanosoma cruzi epimastigotes to peroxynitrite in absence or in presence of CO2 allowed to establish an LD50 (lethal dosis 50) of <0.3 nmol/106 cells. Taking this “average” cytotoxicity the estimated amount of ONOO− needed to kill an individual parasite would be <0.3 fmol/T. cruzi, (~108 molecules) which is line with the estimated range of its formation inside the phagosome, 0.25–0.35 fmol in 120 min (53). Hurst et al had also determined a LD50 for E.coli of 106–107molecules of ONOO− per cell.
5. Evasion from the peroxynitrite-dependent pathogen killing
In spite of the diverse defensive mechanisms mounted by macrophages, many pathogens manage to survive, sometimes for long periods inside macrophages. They have developed evasion mechanisms usually intended to detoxify or inhibit •NO or O2•− (and therefore, ONOO−) production in macrophages.
One way to prevent peroxynitrite formation is to inhibit NADPH oxidase as a source for O2•−. In this sense Leishmania donovani lipophosphoglycan (LPG) prevents NADPH oxidase assembly in the phagosome (55). Salmonella typhimurium secrets a protein (SPI2) that also blocks activation of this complex and strains lacking this gene are highly attenuated for virulence in mice, except in those unable to express NADPH oxidase (56).
Other evasion mechanisms consist in inhibition of •NO synthesis. For instance, adenoviruses impair •NO production by macrophages by interfering activation of iNOS transcription (57). Toxoplasma gondii exposes phosphatidylserine and trigger TGF-b1secretion by macrophages, antagonizing IFNγ mediated iNOS induction (58). Leishmania amazonensis decreases iNOS activity as well, although without affecting its transcription rate (59).
One common feature found in several pathogens is the ability to induce host arginase or even their own. This enzyme is part of the urea cycle and it catalyzes hydrolysis of L-arginine into urea and ornithine. Enhancement of arginase activity reduces •NO production by host cell due to substrate depletion for iNOS and therefore, contributes to invading pathogen survival (60). Helicobacter pilori, and Salmonella 13eroxid (serovar Typhimurium) are two of examples of bacteria where induction of arginase represents a virulence factor. The first two up-regulate arginase II expression in macrophages, and deleting or inhibiting this enzyme favors •NO mediated killing (61–62). There is also evidence regarding other pathogens which uses arginase for its own benefit, like Schistosoma S. mansoni, Candida albicans and Hepatitis C virus; for a review see reference (60).
Instead of inhibition its formation, others have developed enzymes or molecules to detoxify oxidants once formed. One interesting example are truncated hemoglobins expressed by Mycobacterium tuberculosis (63) Mycobacterium bovis (64) and Mycobacterium leprae (65), that serve as •NO scavengers (66).
Trypanosomatids and some bacteria (Mycobacterium spp) have developed complex antioxidant systems that protect them against the oxidative assault within phagocytes. Trypanosoma cruzi has five peroxidases located in different subcellular compartments capable of detoxifying hydrogen peroxide, hydroperoxides and ONOO−. Two of them are 2-cystein peroxirredoxins, one found in the mitochondria (MPX) and the other in the cytosol (MPX). Both reduce ONOO−, H2O2, and short-chain organic hydroperoxides at expenses of reducing power of trypanothione. Overexpression of MPX and CPX results in higher resistance of T.cruzi to ONOO− challenging in vitro and enhanced survival when infecting macrophages (38). Interestingly, it has been show that these enzymes are augmented during differentiation to the infective form, which is the one that faces the oxidative attack of phagocytes. Moreover, content of these enzymes correlates with virulence of natural strains (67–68). Similarly, Leishmania spp resistance to peroxides and virulence is also related with peroxirredoxins expression (69–71).
Another important group of enzymes related to immune response evasion are the superoxide dismutases (SODs), present in all of the aerobic organisms. These enzymes catalyze the O2•−dismutation to H2O2 and protect cells from endogenous generated O2•− (e.g. in the mitochondria). Interestingly, some microorganisms have taken advantage of superoxide dismutases to defend themselves from the oxidative damage when phagocytized by macrophages, expressing one or more isoform of this enzyme in the periplasm. In this sense, E.coli and Salmonella typhimurium lower the damage caused by superoxide and secondary products (ONOO− and H2O2) by having one and two periplasmic CuZn-SODs, respectively (72–73). The presence of the gene encoding the periplasmic SOD has been shown to correlate with virulence in different strains of both E.coli and Salmonella typhimurium (74–75). In the same way, Trypanosoma cruzi apparently also excrete a Fe-SOD as a defense mechanism (76–77).
6. Concluding Remarks and perspectives
The role of macrophage-derived peroxynitrite as a potent cytotoxic mediator has been thoroughly addressed in a wide spectrum of models involving different microorganisms and hosts. The chemistry of peroxynitrite is complex and its formation and effects are sometimes difficult to prove, but with the combination of the use of proper inhibitors, specific probes and the study of footprints on biomolecules it is possible to get insights in the mechanisms mediating peroxynitrite cytotoxicity. The success or fail in pathogen killing is a result of the interplay between the macrophage oxidant “artillery” and the antioxidant defense mechanisms developed by the invading microorganism. Future efforts should be directed to exploit these biochemical insights for a better resolution of microbial infections.
Acknowledgments
CP was partially supported by a fellowship from Universidad de la República. This work received financial support from Programa de Desarrollo de Ciencias Básicas (PEDECIBA), Universidad de la República and the National Institutes of Health (RO1 AI095173).
References
- 1.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–550. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 3.Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Coccia EM, Stellacci E, Marziali G, Weiss G, Battistini A. IFN-gamma and IL-4 differently regulate inducible NO synthase gene expression through IRF-1 modulation. Int Immunol. 2000;12:977–985. doi: 10.1093/intimm/12.7.977. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan C, Thuring H, Dlaska M, Rollinghoff M, Weiss G. Mechanism of suppression of macrophage nitric oxide release by IL-13: influence of the macrophage population. J Immunol. 1997;159:4506–4513. [PubMed] [Google Scholar]
- 6.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 7.Rottenberg ME, Castanos-Velez E, de Mesquita R, Laguardia OG, Biberfeld P, Orn A. Intracellular co-localization of Trypanosoma cruzi and inducible nitric oxide synthase (iNOS): evidence for dual pathway of iNOS induction. Eur J Immunol. 1996;26:3203–3213. doi: 10.1002/eji.1830261254. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira AC, de Alencar BC, Tzelepis F, Klezewsky W, da Silva RN, Neves FS, Cavalcanti GS, Boscardin S, Nunes MP, Santiago MF, Nobrega A, Rodrigues MM, Bellio M. Impaired innate immunity in Tlr4(-/-) mice but preserved CD8+ T cell responses against Trypanosoma cruzi in Tlr4-, Tlr2-, Tlr9- or Myd88-deficient mice. PLoS Pathog. 2010;6:e1000870. doi: 10.1371/journal.ppat.1000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez MN, Trujillo M, Radi R. Peroxynitrite formation from biochemical and cellular fluxes of nitric oxide and superoxide. Methods Enzymol. 2002;359:353–366. doi: 10.1016/s0076-6879(02)59198-9. [DOI] [PubMed] [Google Scholar]
- 11.Vodovotz Y, Russell D, Xie QW, Bogdan C, Nathan C. Vesicle membrane association of nitric oxide synthase in primary mouse macrophages. J Immunol. 1995;154:2914–2925. [PubMed] [Google Scholar]
- 12.Moller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem. 2005;280:8850–8854. doi: 10.1074/jbc.M413699200. [DOI] [PubMed] [Google Scholar]
- 13.Stuehr DJ, Gross SS, Sakuma I, Levi R, Nathan CF. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989;169:1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 15.Hill BG, Dranka BP, Bailey SM, Lancaster JR, Jr, Darley-Usmar VM. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J Biol Chem. 2010;285:19699–19704. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babior BM. The respiratory burst oxidase. Curr Opin Hematol. 1995;2:55–60. doi: 10.1097/00062752-199502010-00008. [DOI] [PubMed] [Google Scholar]
- 17.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Zarember KA, Kuhns DB, Gallin JI. Impaired priming and activation of the neutrophil NADPH oxidase in patients with IRAK4 or NEMO deficiency. J Immunol. 2009;182:6410–6417. doi: 10.4049/jimmunol.0802512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 20.Quijano C, Alvarez B, Gatti RM, Augusto O, Radi R. Pathways of peroxynitrite oxidation of thiol groups. Biochem J. 1997;322(Pt 1):167–173. doi: 10.1042/bj3220167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 22.Castro L, Rodriguez M, Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994;269:29409–29415. [PubMed] [Google Scholar]
- 23.Carballal S, Bartesaghi S, Radi R. Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbagen.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souza JM, Radi R. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch Biochem Biophys. 1998;360:187–194. doi: 10.1006/abbi.1998.0932. [DOI] [PubMed] [Google Scholar]
- 25.Rubbo H, Denicola A, Radi R. Peroxynitrite inactivates thiol-containing enzymes of Trypanosoma cruzi energetic metabolism and inhibits cell respiration. Arch Biochem Biophys. 1994;308:96–102. doi: 10.1006/abbi.1994.1014. [DOI] [PubMed] [Google Scholar]
- 26.Augusto O, Gatti RM, Radi R. Spin-trapping studies of peroxynitrite decomposition and of 3-morpholinosydnonimine N-ethylcarbamide autooxidation: direct evidence for metal-independent formation of free radical intermediates. Arch Biochem Biophys. 1994;310:118–125. doi: 10.1006/abbi.1994.1147. [DOI] [PubMed] [Google Scholar]
- 27.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radi R. Peroxynitrite reactions and diffusion in biology. Chem Res Toxicol. 1998;11:720–721. doi: 10.1021/tx980096z. [DOI] [PubMed] [Google Scholar]
- 29.Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 30.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 31.Hurst JK. What really happens in the neutrophil phagosome? Free Radic Biol Med. 2012;53:508–520. doi: 10.1016/j.freeradbiomed.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 33.Brovkovych V, Gao XP, Ong E, Brovkovych S, Brennan ML, Su X, Hazen SL, Malik AB, Skidgel RA. Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L96–103. doi: 10.1152/ajplung.00450.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277:46116–46122. doi: 10.1074/jbc.M209124200. [DOI] [PubMed] [Google Scholar]
- 35.Evans TJ, Buttery LD, Carpenter A, Springall DR, Polak JM, Cohen J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc Natl Acad Sci U S A. 1996;93:9553–9558. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in cytokine-activated murine macrophages. Involvement of a peroxidase/nitrite pathway rather than peroxynitrite. J Biol Chem. 2001;276:34051–34058. doi: 10.1074/jbc.M100585200. [DOI] [PubMed] [Google Scholar]
- 37.Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in mouse peritoneal macrophages activated in vitro and in vivo: evidence against an essential role of peroxynitrite. FASEB J. 2001;15:355–2364. doi: 10.1096/fj.01-0295com. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J Biol Chem. 2011;286:6627–6640. doi: 10.1074/jbc.M110.167247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gookin JL, Allen J, Chiang S, Duckett L, Armstrong MU. Local peroxynitrite formation contributes to early control of Cryptosporidium parvum infection. Infect Immun. 2005;73:3929–3936. doi: 10.1128/IAI.73.7.3929-3936.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giorgio S, Linares E, Ischiropoulos H, Von Zuben FJ, Yamada A, Augusto O. In vivo formation of electron paramagnetic resonance-detectable nitric oxide and of nitrotyrosine is not impaired during murine leishmaniasis. Infect Immun. 1998;66:807–814. doi: 10.1128/iai.66.2.807-814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linares E, Giorgio S, Mortara RA, Santos CX, Yamada AT, Augusto O. Role of peroxynitrite in macrophage microbicidal mechanisms in vivo revealed by protein nitration and hydroxylation. Free Radic Biol Med. 2001;30:1234–1242. doi: 10.1016/s0891-5849(01)00516-0. [DOI] [PubMed] [Google Scholar]
- 42.Quijano C, Romero N, Radi R. Tyrosine nitration by superoxide and nitric oxide fluxes in biological systems: modeling the impact of superoxide dismutase and nitric oxide diffusion. Free Radic Biol Med. 2005;39:728–741. doi: 10.1016/j.freeradbiomed.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Trostchansky A, Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic Biol Med. 2008;44:1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Trostchansky A, Bonilla L, Gonzalez-Perilli L, Rubbo H. Nitro-Fatty Acids: Formation, Redox Signaling, and Therapeutic Potential. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.5023. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Perilli L, Alvarez MN, Prolo C, Radi R, Rubbo H, Trostchansky A. Nitroarachidonic acid prevents NADPH oxidase assembly and superoxide radical production in activated macrophages. Free Radic Biol Med. 2013;58:126–133. doi: 10.1016/j.freeradbiomed.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, Radi R, Cayota AM. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–3366. [PubMed] [Google Scholar]
- 47.Nicholson S, da Bonecini-Almeida MG, Lapa e Silva JR, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho JL. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. [Google Scholar]
- 48.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med. 2002;166:178–186. doi: 10.1164/rccm.2201023. [DOI] [PubMed] [Google Scholar]
- 49.Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998;11:809–815. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 50.Nathan C. Inducible nitric oxide synthase in the tuberculous human lung. Am J Respir Crit Care Med. 2002;166:130–131. doi: 10.1164/rccm.2205016. [DOI] [PubMed] [Google Scholar]
- 51.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci U S A. 1998;95:3566–3571. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez MN, Piacenza L, Irigoin F, Peluffo G, Radi R. Macrophage-derived peroxynitrite diffusion and toxicity to Trypanosoma cruzi. Arch Biochem Biophys. 2004;432:222–232. doi: 10.1016/j.abb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Cape JL, Hurst JK. The role of nitrite ion in phagocyte function--perspectives and puzzles. Arch Biochem Biophys. 2009;484:190–196. doi: 10.1016/j.abb.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lodge R, Diallo TO, Descoteaux A. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell Microbiol. 2006;8:1922–1931. doi: 10.1111/j.1462-5822.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 56.Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 57.Cao W, Bao C, Lowenstein CJ. Inducible nitric oxide synthase expression inhibition by adenovirus E1A. Proc Natl Acad Sci U S A. 2003;100:7773–7778. doi: 10.1073/pnas.1337185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seabra SH, de Souza W, Damatta RA. Toxoplasma gondii exposes phosphatidylserine inducing a TGF-beta1 autocrine effect orchestrating macrophage evasion. Biochem Biophys Res Commun. 2004;324:744–752. doi: 10.1016/j.bbrc.2004.09.114. [DOI] [PubMed] [Google Scholar]
- 59.Balestieri FM, Queiroz AR, Scavone C, Costa VM, Barral-Netto M, de Abrahamsohn IA. Leishmania (L) amazonensis-induced inhibition of nitric oxide synthesis in host macrophages. Microbes Infect. 2002;4:23–29. doi: 10.1016/s1286-4579(01)01505-2. [DOI] [PubMed] [Google Scholar]
- 60.Das P, Lahiri A, Chakravortty D. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 2010;6:e1000899. doi: 10.1371/journal.ppat.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis ND, Asim M, Barry DP, de Sablet T, Singh K, Piazuelo MB, Gobert AP, Chaturvedi R, Wilson KT. Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J Immunol. 2011;186:3632–3641. doi: 10.4049/jimmunol.1003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lahiri A, Das P, Chakravortty D. Arginase modulates Salmonella induced nitric oxide production in RAW264.7 macrophages and is required for Salmonella pathogenesis in mice model of infection. Microbes Infect. 2008;10:1166–1174. doi: 10.1016/j.micinf.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Couture M, Yeh SR, Wittenberg BA, Wittenberg JB, Ouellet Y, Rousseau DL, Guertin M. A cooperative oxygen-binding hemoglobin from Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1999;96:11223–11228. doi: 10.1073/pnas.96.20.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ouellet H, Ouellet Y, Richard C, Labarre M, Wittenberg B, Wittenberg J, Guertin M. Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc Natl Acad Sci U S A. 2002;99:5902–5907. doi: 10.1073/pnas.092017799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fabozzi G, Ascenzi P, Renzi SD, Visca P. Truncated hemoglobin GlbO from Mycobacterium leprae alleviates nitric oxide toxicity. Microb Pathog. 2006;40:211–220. doi: 10.1016/j.micpath.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Gardner PR. Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases. J Inorg Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Piacenza L, Peluffo G, Alvarez MN, Martinez A, Radi R. Trypanosoma cruzi Antioxidant Enzymes As Virulence Factors in Chagas Disease. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piacenza L, Zago MP, Peluffo G, Alvarez MN, Basombrio MA, Radi R. Enzymes of the antioxidant network as novel determiners of Trypanosoma cruzi virulence. Int J Parasitol. 2009;39:1455–1464. doi: 10.1016/j.ijpara.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barr SD, Gedamu L. Role of peroxidoxins in Leishmania chagasi survival. Evidence of an enzymatic defense against nitrosative stress. J Biol Chem. 2003;278:10816–10823. doi: 10.1074/jbc.M212990200. [DOI] [PubMed] [Google Scholar]
- 70.Daneshvar H, Wyllie S, Phillips S, Hagan P, Burchmore R. Comparative proteomics profiling of a gentamicin-attenuated Leishmania infantum cell line identifies key changes in parasite thiol-redox metabolism. J Proteomics. 2012;75:1463–1471. doi: 10.1016/j.jprot.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 71.Harder S, Bente M, Isermann K, Bruchhaus I. Expression of a mitochondrial peroxiredoxin prevents programmed cell death in Leishmania donovani. Eukaryot Cell. 2006;5:861–870. doi: 10.1128/EC.5.5.861-870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 73.Korshunov SS, Imlay JA. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of Gram-negative bacteria. Mol Microbiol. 2002;43:95–106. doi: 10.1046/j.1365-2958.2002.02719.x. [DOI] [PubMed] [Google Scholar]
- 74.Sanjay MK, Srideshikan SM, Vanishree VL, Usha MS, Raj AP, Gaddad SM, Shivannavar CT. Copper, Zinc-Superoxide Dismutase from Clinically Isolated Escherichia coli: Cloning, Analysis of sodC and Its Possible Role in Pathogenicity. Indian J Microbiol. 2011;51:326–331. doi: 10.1007/s12088-011-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fang FC, DeGroote MA, Foster JW, Baumler AJ, Ochsner U, Testerman T, Bearson S, Giard JC, Xu Y, Campbell G, Laessig T. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc Natl Acad Sci U S A. 1999;96:7502–7507. doi: 10.1073/pnas.96.13.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mateo H, Sanchez-Moreno M, Marin C. Enzyme-linked immunosorbent assay with purified Trypanosoma cruzi excreted superoxide dismutase. Clin Biochem. 2010;43:1257–1264. doi: 10.1016/j.clinbiochem.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Cespedes A, Longoni SS, Sauri-Arceo CH, Rodriguez-Vivas RI, Villegas N, Escobedo-Ortegon J, Barrera-Perez MA, Sanchez-Moreno M, Bolio Gonzalez ME, Marin C. Seroprevalence of antibodies against the excreted antigen superoxide dismutase by Trypanosoma cruzi in dogs from the Yucatan Peninsula (Mexico) Zoonoses Public Health. 2013;60:277–283. doi: 10.1111/j.1863-2378.2012.01520.x. [DOI] [PubMed] [Google Scholar]
- 78.Brunelli L, Crow JP, Beckman JS. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch Biochem Biophys. 1995;316:327–334. doi: 10.1006/abbi.1995.1044. [DOI] [PubMed] [Google Scholar]
- 79.Zhu L, Gunn C, Beckman JS. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- 80.McLean S, Bowman LA, Sanguinetti G, Read RC, Poole RK. Peroxynitrite toxicity in Escherichia coli K12 elicits expression of oxidative stress responses and protein nitration and nitrosylation. J Biol Chem. 2010;285:20724–20731. doi: 10.1074/jbc.M109.085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Groote MA, Ochsner UA, Shiloh MU, Nathan C, McCord JM, Dinauer MC, Libby SJ, Vazquez-Torres A, Xu Y, Fang FC. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci U S A. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 83.Jiang X, Leonard B, Benson R, Baldwin CL. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 1993;151:309–319. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- 84.Ko J, Gendron-Fitzpatrick A, Splitter GA. Susceptibility of IFN regulatory factor-1 and IFN consensus sequence binding protein-deficient mice to brucellosis. J Immunol. 2002;168:2433–2440. doi: 10.4049/jimmunol.168.5.2433. [DOI] [PubMed] [Google Scholar]
- 85.Hickman-Davis J, Gibbs-Erwin J, Lindsey JR, Matalon S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc Natl Acad Sci U S A. 1999;96:4953–4958. doi: 10.1073/pnas.96.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Darrah PA, Hondalus MK, Chen Q, Ischiropoulos H, Mosser DM. Cooperation between reactive oxygen and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages. Infect Immun. 2000;68:3587–3593. doi: 10.1128/iai.68.6.3587-3593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bose M, Saha R, Sen Santara S, Mukherjee S, Roy J, Adak S. Protection against peroxynitrite by pseudoperoxidase from Leishmania major. Free Radic Biol Med. 2012;53:1819–1828. doi: 10.1016/j.freeradbiomed.2012.08.583. [DOI] [PubMed] [Google Scholar]
- 88.Murray HW, Nathan CF. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Padalko E, Ohnishi T, Matsushita K, Sun H, Fox-Talbot K, Bao C, Baldwin WM, 3rd, Lowenstein CJ. Peroxynitrite inhibition of Coxsackievirus infection by prevention of viral RNA entry. Proc Natl Acad Sci U S A. 2004;101:11731–11736. doi: 10.1073/pnas.0400518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vazquez-Torres A, Jones-Carson J, Balish E. Peroxynitrite contributes to the candidacidal activity of nitric oxide-producing macrophages. Infect Immun. 1996;64:3127–3133. doi: 10.1128/iai.64.8.3127-3133.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas GR, McCrossan M, Selkirk ME. Cytostatic and cytotoxic effects of activated macrophages and nitric oxide donors on Brugia malayi. Infect Immun. 1997;65:2732–2739. doi: 10.1128/iai.65.7.2732-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gupta R, Bajpai P, Tripathi LM, Srivastava VM, Jain SK, Misra-Bhattacharya S. Macrophages in the development of protective immunity against experimental Brugia malayi infection. Parasitology. 2004;129:311–323. doi: 10.1017/s0031182004005682. [DOI] [PubMed] [Google Scholar]