Abstract
Objectives
Relaxation of vascular smooth muscle (VSM) requires re-uptake of cytosolic Ca2+ into the sarcoplasmic reticulum (SR) via the Sarco/Endoplasmic Reticulum Ca2+ ATPase (SERCA), or extrusion via the Plasma Membrane Ca2+ ATPase (PMCA) or sodium Ca2+ exchanger (NCX). Peroxynitrite, a reactive species formed in vascular inflammatory diseases, upregulates SERCA activity to induce relaxation but, chronically, can contribute to atherogenesis and altered vascular function by escalating endoplasmic reticulum stress. Our objectives were to determine if peroxynitrite-induced relaxation and Ca2+ handling processes within vascular smooth muscle cells were altered as atherosclerosis develops.
Methods
Aortae from control and ApoE−/− mice were studied histologically, functionally and for protein expression levels of SERCA and PMCA. Ca2+ responses were assessed in dissociated aortic smooth muscle cells in the presence and absence of extracellular Ca2+.
Results
Relaxation to peroxynitrite was concentration-dependent and endothelium-independent. The abilities of the SERCA blocker thapsigargin and the PMCA inhibitor carboxyeosin to block this relaxation were altered during fat feeding and plaque progression. SERCA levels were progressively reduced, while PMCA expression was upregulated. In ApoE−/− VSM cells, increases in cytosolic Ca2+ [Ca2+]c in response to SERCA blockade were reduced, while SERCA-independent Ca2+ clearance was faster compared to control.
Conclusion
As atherosclerosis develops in the ApoE−/− mouse, expression and function of Ca2+ handling proteins are altered. Up-regulation of Ca2+ removal via PMCA may offer a potential compensatory mechanism to help normalise the dysfunctional relaxation observed during disease progression.
Keywords: Atherosclerosis, Ca2+, SERCA, Peroxynitrite, PMCA
Highlights
-
•
Expression and function of SERCA and PMCA are temporally altered in ApoE−/− VSM.
-
•
TG-induced increases in [Ca2+]c were reduced in ApoE−/− aortic SM cells.
-
•
Ca2+ extrusion is upregulated in isolated ApoE−/− aortic SM cells.
Abbreviations
- Ca2+
calcium ion
- [Ca2+]c
cytosolic calcium concentration
- CE
carboxyeosin
- MLCK
myosin light chain kinase
- NCX
sodium/Ca2+ exchanger
- NO
nitric oxide
- ONOO−
peroxynitrite
- PLB
phospholamban
- PMCA
plasma membrane Ca2+ ATPase
- RNS
reactive nitrogen species
- RyR
ryanodine receptor
- SERCA
sarco/endoplasmic reticulum ATPase
- SOCE
store operated Ca2+ entry
- SOCC
store operated Ca2+ channel
- SR
sarcoplasmic reticulum
- TG
thapsigargin
- VLDL
very low density lipoprotein
- hfd
high fat diet
- VSM
vascular smooth muscle
1. Introduction
Increased oxidant stress is implicated in the progression of several diseases affecting the vasculature, including hypertension, diabetes and atherosclerosis [1–4]. In vascular smooth muscle (VSM), reactive oxygen (ROS) and nitrogen species (RNS) can alter core functions [5], including migration, cell growth, vascular reactivity and inflammatory processes [6–8]; all critical factors in the development and progression of atherosclerosis and cardiovascular disease. One such RNS, peroxynitrite (ONOO−), is the reaction product of nitric oxide (NO) and superoxide (O2.) and has direct and indirect effects on the relaxation of blood vessels [9–12]. However, the precise mechanism by which ONOO− induces relaxation remains unclear.
Ca2+ is the fundamental second messenger controlling VSM contraction and relaxation, and can enter the cytoplasm via membrane depolarisation, non-selective cation channels, store operated Ca2+ entry (SOCE) or from the sarcoplasmic reticulum (SR) [13]. Ca2+ clearance occurs either via re-uptake into the SR via sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pumps [14], which utilise energy from ATP hydrolysis to maintain a 10000-fold Ca2+ gradient between the SR lumen and cell cytoplasm, or extrusion into the extracellular space via the plasma membrane Ca2+ ATPase (PMCA) [15] or sodium Ca2+ exchanger (NCX) [16]. In healthy vessels, ONOO− has been shown to induce relaxation of rat aorta following constriction to phenylephrine [12]. This relaxation was not reduced by inhibitors of nitric oxide synthase or endothelial denudation, and was thought to occur via elevation of cGMP levels, membrane hyperpolarisation and direct activation of myosin phosphatase activity in the smooth muscle. A further potential mechanism for ONOO−-induced vascular relaxation is via increased uptake of Ca2+ into the internal SR store via SERCA or extrusion across the plasma membrane. Lower levels of ONOO− (10–50 μM) have been shown, in purified cardiac fractions and aorta homogenates, to increase the activity of SERCA via reversible S-glutathiolation [9]. Conversely, higher ONOO− concentrations (>100 μM) were associated with tyrosine nitration and SERCA inhibition. ONOO− has also been determined to contribute to atherogenesis [17], and chronically elevated levels of RNS in atherosclerotic rabbit arteries impair SERCA activity by irreversible oxidation of relevant cysteine thiols [9]. Oxidative stress has also been shown to target the plasma membrane pump PMCA in disease states; altering Ca2+ extrusion in platelets [18] and neuronal tissue [19], but its role in PMCA activity in smooth muscle has not yet been investigated. A role for NCX in VSM remains controversial [20–22], although what is clear is that NCX is expressed in this cell type and is also sensitive to ROS which may disrupt Ca2+ homoeostasis under oxidative stress [23]. Therefore, significant evidence exists for the interplay of reactive species and Ca2+ signalling mechanisms [24]. In addition, defects in smooth muscle Ca2+ handling has been shown to affect responses to vasodilators [25].
In this study, we have utilised pharmacological Ca2+ pump inhibitors to examine the effect of up to 4 months high fat feeding of atherosclerosis-prone ApoE−/− mice on ONOO−-mediated relaxation. Mice on a high fat diet are reported to have approximately 5% lesion coverage of the aorta [39] but, even prior to the development of lesions, changes in smooth muscle function are apparent [26]. This indicates the importance of circulating factors and perhaps the influence of other cell types present in the lesion on smooth muscle function. We have determined the expression levels of SERCA and PMCA during disease progression and have explored how alterations in these proteins impacts upon Ca2+ handling by examining single cell Ca2+ responses in the presence and absence of extracellular Ca2+. Changes in ONOO− responses have been correlated with high fat feeding and plaque development.
2. Materials and methods
2.1. Animal model and aorta preparation
Adult male C57/BL-6 or apolipoprotein E deficient mice (ApoE−/−) [27–29] were used. ApoE−/− mice were placed on high fat diet (hfd) for 2 or 4 months to accelerate plaque progression. Age-matched chow fed ApoE−/− mice were also assessed. Procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and Directive 2010/63/EU of the European Parliament. Mice were terminally anaesthetised via intraperitoneal injection of sodium pentobarbital (200 mg/ml). Thoracic aortae were removed to ice cold oxygenated (95% O2:5% CO2) Krebs' solution and cleaned of adherent tissue.
2.2. Histological analysis
Cleaned thoracic aortae were fixed in neutral-buffered formalin, embedded in paraffin and 4 μm sections cut on a rotary microtome. Morphological analysis was performed on haematoxylin and eosin stained sections. Nitrotyrosine (Millipore) and α-actin (Abcam) were detected using rabbit primary antibodies (diluted 1 in 100) and visualised using biotin labelled secondary antibody-streptavidin-HRP complexes and DAB (3,3′ diaminobenzidine) chromogenic substrate (Vector Laboratories). Images were analysed using ImmunoRatio analysis software (IBT, University of Tampere), which calculates the percentage of DAB staining over total nuclear area.
2.3. Small vessel wire myography
Descending thoracic aorta was systematically divided into rings of 2 mm, and endothelium was removed. Rings were mounted on a small vessel wire myograph (Danish Myotech), placed under a resting tension of 1 g and allowed to equilibrate. Reproducible responses were obtained to 40 mM KCl and 30 nM 9,11-Dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U46619, Tocris) and endothelial function was assessed by the addition of 10 μM acetylcholine before commencing experiments. Rings were pre-contracted to U46619 (30 nM) and then cumulative doses of ONOO− (Calbiochem, 1 × 10−6 – 5x10−4 M) added at 10 min intervals. ONOO− was diluted in argon-purged dH2O and kept in the dark at 4 °C in order to protect activity. 3 μM thapsigargin (TG, Sigma) or 10 μM carboxyeosin (CE, Marker Gene Technologies) were added 30 min prior to ONOO−.
2.4. Protein expression/immunoblotting
Denuded aortae were pulverised in liquid nitrogen and incubated in lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.25% (w/v) Na-deoxycholate, 1% (v/v) TX-100, 1× protease inhibitor cocktail (Roche)) for 30 min at 4 °C. Lysates were spun to remove debris, and protein concentrations determined using Coomassie Plus Protein Assay Reagent (Perbio, USA). Denatured samples were subjected to electrophoresis (NuPAGE, 3–8% tris–acetate gels (Invitrogen)) and western blotting. SERCA was detected using rabbit polyclonal anti-SERCA2b (a kind gift from Prof. F Wuytack, Leuven). SERCA2b was identified as a dimer, with a fainter monomer band detected at 110 kDa. Protein abundance was quantified from a range of protein loads using Quantity One software (BioRad). PMCA (Affinity Bioreagents) was detected and quantified similarly. Expression was normalised to GAPDH (Cell Signaling Technology).
2.5. Single cell isolation and Ca2+ measurement in the presence and absence of extracellular Ca2+
Single aortic smooth muscle cells were enzymatically isolated [21] and changes in Ca2+ levels measured as fluorescence using the membrane permeable dye Fluo-3 AM (10 μM, Molecular Probes/Invitrogen). Cells were loaded with Fluo-3 for 30 min prior to the beginning of the experiment. Cells were allowed to settle prior to perfusion with bathing solution containing (in mM): 80 Na glutamate, 40 NaCl, 20 tetraethylammonium chloride, 1.1 MgCl2, 3 CaCl2, 10 HEPES, and 30 glucose; adjusted to pH 7.4 with NaOH. The Ca2+−free extracellular solution additionally contained (mM): MgCl2, 3 (substituted for Ca2+); and EGTA, 1. Fluorescence was quantified using a microfluorimeter, which consisted of an inverted microscope (Olympus IX81) and a photomultiplier tube with a bi-alkali photocathode. Fluo-3 was excited at 488 nm (bandpass 9 nm) from a PTI Delta Scan (Photon Technology International Inc., London, UK) through the epi-illumination port of the microscope. Excitation light was passed through a field stop diaphragm to reduce background fluorescence and reflected off a 505 nm long-pass dichroic mirror. Emitted light was guided through a 535 nm barrier filter (bandpass 35 nm) to a photomultiplier in photon counting mode. Caffeine (10 mM) was applied by hydrostatic pressure ejection using a pneumatic pump (PicoPump PV 830, World Precision Instruments). TG (1 μM) was perfused into the solution bathing the cells.
2.6. Statistical analysis
Myography data was analysed via Graphpad Prism software and significance determined using 2-way ANOVA. EC50 and Emax values were determined using non-linear fit curve analysis of individual data sets followed by ANOVA. Student's t-tests (unpaired) were performed on protein expression data. Changes in [Ca2+]c were expressed as ratios (F/F0 or ΔF/F0) of fluorescence counts (F) relative to baseline (control) values before stimulation (F0), and were analysed via Origin (OriginLab) and WinFluor (University of Strathclyde) data analysis software. Data are expressed as means ± SEM and p < 0.05 was taken to be indicative of statistical significance. Confidence intervals are also shown where appropriate.
3. Results
3.1. Protein nitrosylation is increased in atherosclerotic ApoE−/− mouse aortae
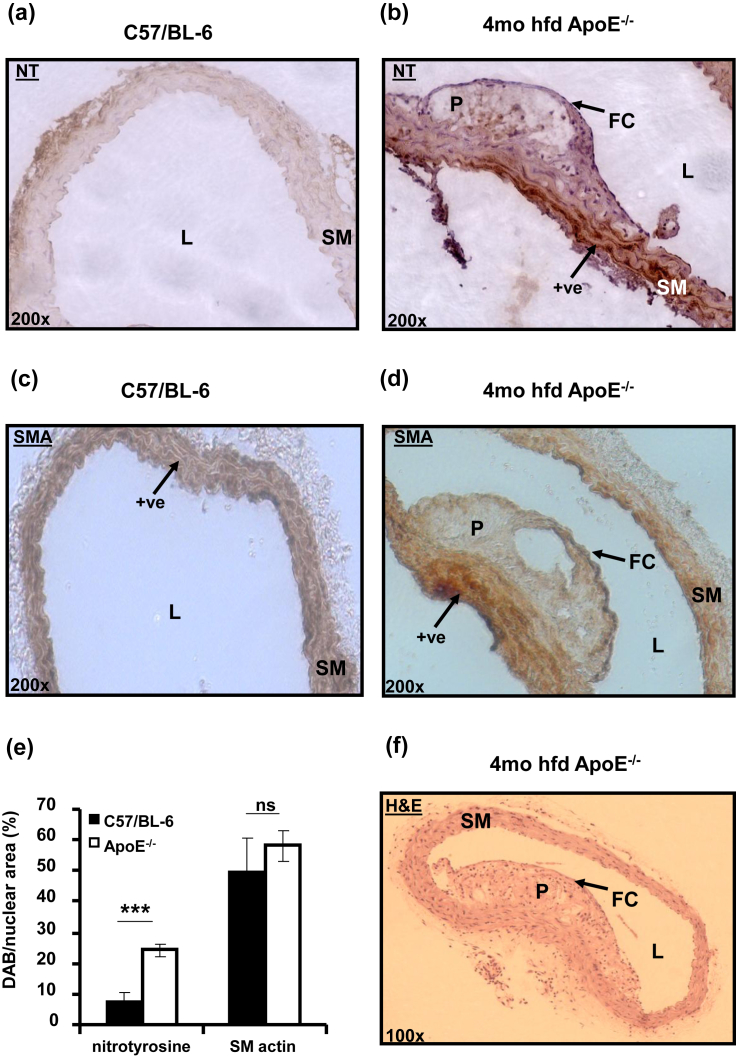
Nitrotyrosine expression was higher in ApoE−/− thoracic aorta in comparison to C57/BL-6 and particularly marked in medial and adventitial areas around atherosclerotic plaques (Fig. 1a and b). Some diffuse staining also was found within the plaque. There was significantly more nitrotyrosine detected (Fig. 1b) in the 4 month high fat fed ApoE−/− aorta (7.50 ± 3.01% vs. 24.3 ± 2.15% expressed as percentage of nuclear area, Fig. 1e ***p < 0.001). The increased ONOO− generation during atherosclerosis could lead to nitrosylation and modified function of vascular Ca2+ handling proteins. Spleen size in atherosclerotic mice was also significantly and progressively increased with time spent on high fat diet (0.27 ± 0.03, 0.40 ± 0.04* and 0.52 ± 0.07** % of total body weight for C57/BL-6, 2 month and 4 month high fat fed ApoE−/− respectively, *p < 0.05 and **p < 0.01 vs. C57/BL-6); an indicator of progressive inflammatory responses in these mice which is known to be correlated to peroxynitrite bioavailability. Smooth muscle α-actin staining revealed similar numbers of smooth muscle cells in C57/BL-6 and ApoE−/− aortic sections (Fig. 1 c, d and e, 49.83 ± 10.71% vs. 58.14 ± 5.03%), with smooth muscle staining also being observed in the fibrous cap of plaques in ApoE−/− aortae (Fig. 1d). Haematoxylin and eosin staining revealed typical plaque morphology in ApoE−/− aortic cross-sections from mice which have been fed a high fat diet for 4 months (Fig. 1f).
Fig. 1.
Protein nitrosylation is increased in atherosclerotic ApoE−/− mouse thoracic aortae. (a, b) Representative cross-sectional images of nitrotyrosine stained C57/BL-6 and 4 month hfd ApoE−/− mouse thoracic aortae (200× magnification). (c, d) Smooth muscle actin staining. (e) Mean DAB staining/nuclear area (%) for nitrotyrosine and actin. (f) H&E stained aorta from 4 month hfd ApoE−/−. L = lumen, SM = smooth muscle, P = plaque, FC = fibrous cap, +ve = areas of positive staining ***p < 0.001 vs. C57/BL-6, n = 5 mice per group.
3.2. Mouse thoracic aorta relaxes to ONOO− in a dose-dependent manner
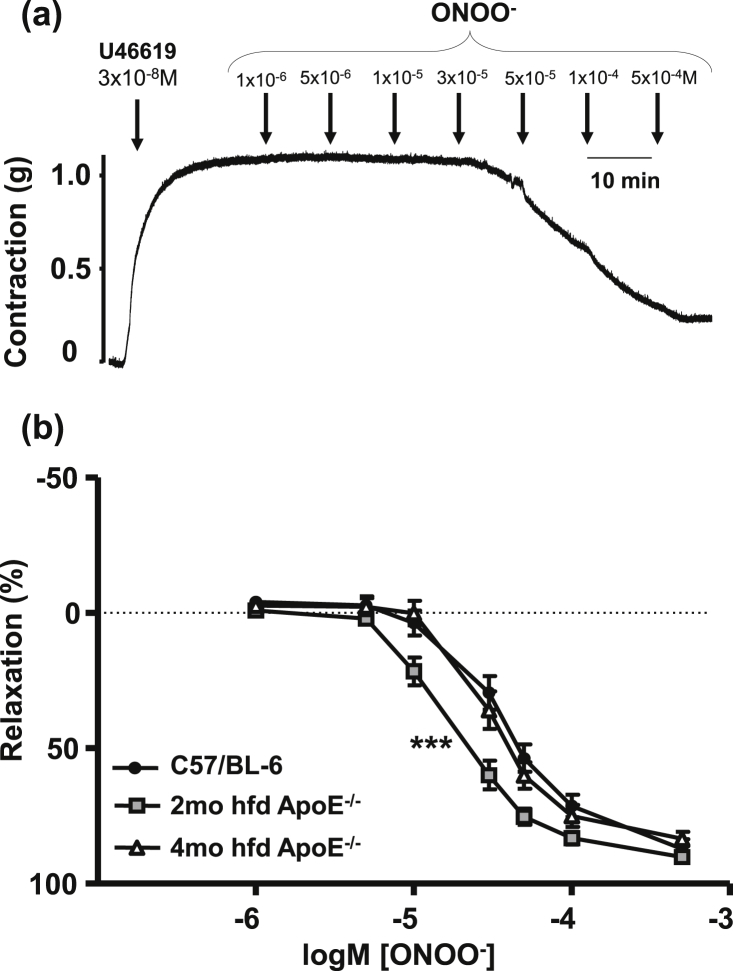
Endothelium denuded aortic rings relaxed to ONOO− in a concentration dependent manner (Fig. 2a). In 2 month ApoE−/− mice sensitivity to ONOO− was increased (Fig. 2b, ***p < 0.001 vs. C57/BL-6) but this had normalised by four months on high fat diet. EC50 values were 3.9, 1.9, 3.3 × 10−5 M (95% confidence intervals of 3.2–4.8, 1.6 to 2.3 and 2.7 to 3.9 × 10−5 M) and Emax values were 87.8 ± 4.7, 89.6 ± 2.9, 82.5 ± 3.7% for C57/BL-6, 2 month and 4 month high fat diet ApoE−/−, respectively. An overview of EC50 and Emax values is given on Table 1. No significant difference was observed between endothelial denuded or intact vessels (data not shown).
Fig. 2.
Mouse thoracic aorta relaxes to ONOO− in a dose-dependent manner. (a) Representative trace of 3x10-8 M U46619-induced contraction of mouse aorta and subsequent relaxation to increasing doses of ONOO− (1 × 10−6 – 5x10−4 M). (b) ONOO−-induced relaxation in C57/BL-6, 2 and 4 month hfd ApoE−/− aortae. All data presented are from endothelial denuded vessels. ***p < 0.001 for 2 month hfd ApoE−/− vs. C57/BL-6, minimum of 7 mice per group.
Table 1.
EC50 and Emax values for ONOO−-induced relaxations.
| EC50 (M) | Change in EC50 (M) (compared to control) | Emax(%) | Change in Emax( %) (compared to control) | ||
|---|---|---|---|---|---|
| C57/BL-6 | Control | 3.9 × 10−5 (CI: 3.2–4.8 × 10−5) | N/A | 87.8 (SEM: 4.7) | N/A |
| 3 μM TG | 4.9 × 10−5 (CI: 3.5–6.9 × 10−5) | +1.0 × 10−5 | 22.3 (SEM: 4.3) | −65.6 | |
| 10 μM CE | 3.4 × 10−5 (CI: 2.1–5.5 × 10−5) | −0.5 × 10−5 | 97.5 (SEM: 9.1) | +9.6 | |
| 2mo hfd ApoE−/− | Control | 1.9 × 10−5 (CI: 1.6–2.3 × 10−5) | N/A | 89.6 (SEM: 2.9) | N/A |
| 3 μM TG | 8.5 × 10−5 (CI: 6.8–10.6 × 10−5) | +6.6 × 10−5 | 83.6 (SEM: 4.9) | −6.6 | |
| 10 μM CE | 4.6 × 10−5 (CI: 2.8–7.8 × 10−5) | +2.7 × 10−5 | 82.6 (SEM: 9.5) | −7.0 | |
| 4mo hfd ApoE−/− | Control | 3.3 × 10−5 (CI: 2.7–3.9 × 10−5) | N/A | 82.5 (SEM: 3.7) | N/A |
| 3 μM TG | No relaxation | No relaxation | No relaxation | No relaxation | |
| 10 μM CE | 2.5 × 10−5 (CI: 1.9–3.3 × 10−5) | −0.8 × 10−5 | 82.2 (SEM: 4.0) | −0.2 |
3.3. Expression of SERCA2b is progressively reduced while PMCA levels are increased in aortic smooth muscle from ApoE−/− mice
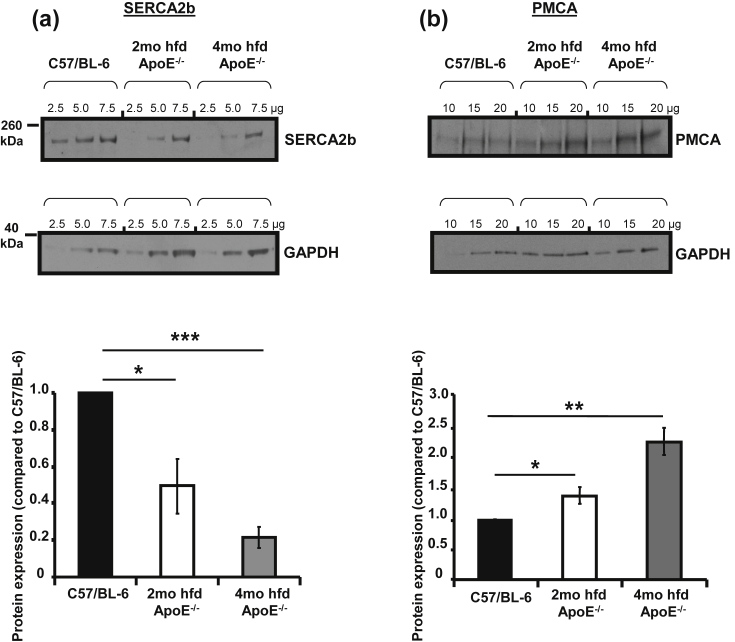
Expression of SERCA2b (Fig. 3a) was significantly down-regulated in aortic smooth muscle from ApoE−/− (45% reduction, *p < 0.05 vs. C57/BL-6 after 2 months fat feeding and 75%, ***p < 0.001 vs. C57/BL-6 after 4 months). In contrast, expression of PMCA (Fig. 3b) was significantly upregulated (by 45%, *p < 0.05 vs. C57/BL-6 following 2 months high fat feeding and by **137%, p < 0.01 vs. C57/BL-6 following 4 months). We found no significant changes in NCX expression at 2 months and a significant reduction at 4 months (data not shown).
Fig. 3.
Expression levels of SERCA2b are progressively reduced while PMCA levels are increased in aortic smooth muscle from ApoE−/− mice. (a, b) Representative immunoblots and averaged protein expression data for SERCA2b, pan-PMCA and GAPDH. Protein loads were 2.5, 5, 7.5 μg for SERCA and 10, 15, 20 μg for PMCA. Slopes of plotted band densities were standardised to GAPDH. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. C57/BL-6, minimum of 5 independent experiments.
3.4. Efficacy of Ca2+ pump inhibitors is temporally altered as atherosclerosis progresses
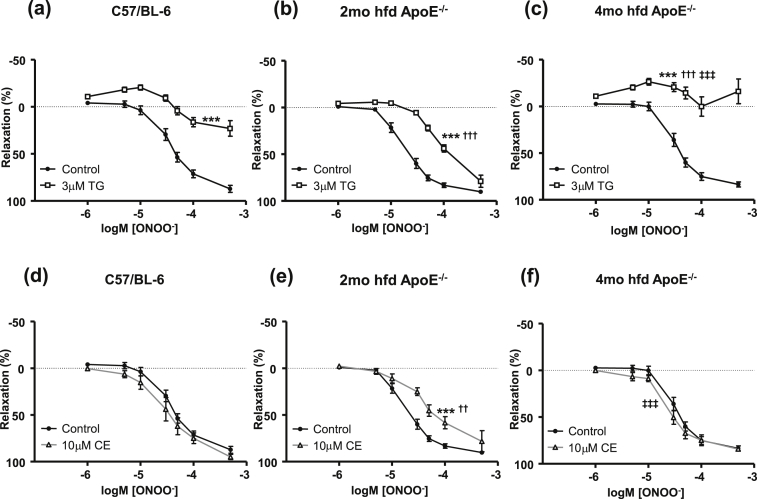
As ONOO− is known to activate SERCA [9], the expression of which is progressively reduced in atherosclerotic VSM (Fig. 3a), we next evaluated the function of SERCA using the irreversible SERCA inhibitor thapsigargin (TG). Contraction of endothelium denuded aortic rings to U46619 did not differ among groups or between controls and TG pre-treated tissues. TG (3 μM) was found to inhibit ONOO−-induced relaxation in control and atherosclerotic aorta [Fig. 4a–c]. A 65.5% reduction in Emax was observed in TG treated C57/BL-6 preparations, while a complete abolition of relaxation was observed in 4 month high fat diet ApoE−/− preparations (98.5% reduction in Emax). Increased SOCE may influence the function of TG at this latter time-point, and this will be discussed in detail later. 2 month high fat diet ApoE−/− aortae were less sensitive to TG (only 6% reduction in Emax), which implies that other Ca2+ removal mechanisms can compensate to mediate the relaxation to ONOO− at this stage of disease progression.
Fig. 4.
Efficacy of TG and CE is temporally altered as atherosclerosis progresses. (a–c) ONOO−-induced relaxation following 3 μM TG pre-incubation. ***p < 0.001 vs. control, †††p < 0.001 vs. TG treated C57/BL-6, ‡‡‡p < 0.001 vs. TG treated 2mo hfd ApoE−/−. (d–f) ONOO−-relaxation following pre-incubation with 10 μM CE. ***p < 0.001 vs. control, ††p < 0.01 vs. CE treated C57/BL-6, ‡‡‡p < 0.001 vs. CE treated 2mo hfd ApoE−/−. All experiments performed following endothelial denudation. Minimum of 5 mice per group.
To investigate the contribution of PMCA, vessels were pre-treated with 10 μM of the PMCA inhibitor CE (Fig. 4d–f). Contraction to U46619 did not differ between non-CE and CE treated tissues. Significant inhibition of ONOO−-induced relaxation was only found in 2 month fat fed aortae (***p < 0.001 vs. no CE control). A higher concentration of CE (30 μM) was ineffective in blocking relaxation in 4 month high fat diet ApoE−/− aortae. An overview of EC50 and Emax values following preincubation with TG or CE is also shown on Table 1
We hypothesised that PMCA activity may be responsible for increased relaxation to ONOO− in 2 months fat fed ApoE−/− and so attempted to study intracellular calcium regulation in isolated aortic smooth muscle cells.
3.5. Ca2+ extrusion is upregulated in atherosclerotic mouse aortae
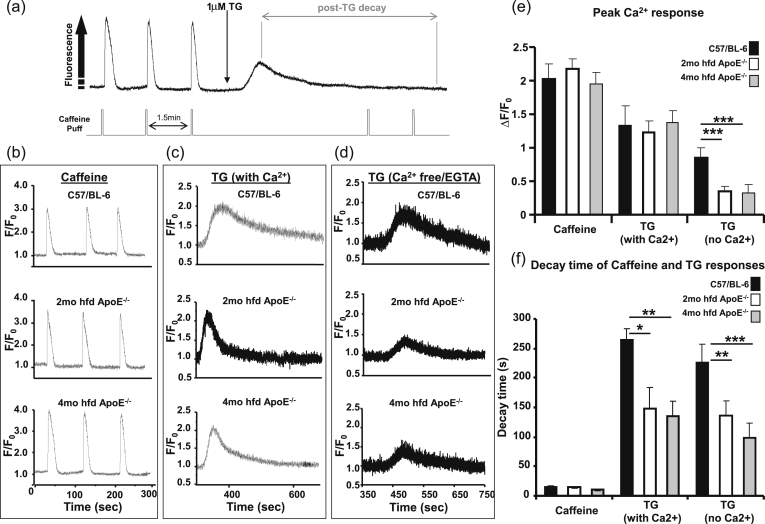
Changes in [Ca2+]c in response to the ryanodine receptor (RyR) agonist caffeine and the SERCA inhibitor TG were measured in freshly isolated VSM cells. TG induced a transient [Ca2+]c increase in all sample groups and completely inhibited any further caffeine induced responses, confirming store emptying following SERCA inhibition (Fig. 5a). Caffeine responses were similar across control and atherosclerotic groups (Fig. 5b and e), but the decay times of the TG-induced Ca2+ transients (after SERCA blockade) were found to be significantly faster in cells from ApoE−/− mice (Fig. 5c and f, decay times (s): 264 ± 25, 149 ± 29* and 135 ± 23** for C57/BL-6, 2 month and 4 month high fat fed ApoE−/− respectively, *p < 0.05 vs. C57/BL-6). This effect was also observed in the absence of extracellular Ca2+ (Fig. 5 d and f, decay times (s): 228 ± 31, 138 ± 26** and 100 ± 26*** for C57/BL-6, 2 month and 4 month high fat fed ApoE−/− respectively) *p < 0.05, **P < 0.01, ***p < 0.001 vs. C57/BL-6. The peak Ca2+ response to TG was reduced in the absence of extracellular Ca2+ and there was a significantly greater reduction in cells from ApoE−/− mice (Fig. 5d and e, ΔF/F0: 0.85 ± 0.16, 0.36 ± 0.07*** and 0.33 ± 0.12*** for C57/BL-6, 2 month and 4 month high fat fed ApoE−/− respectively, ***p < 0.0001 vs. C57/BL-6). This suggests a difference in contribution of SERCA, as well as SOCE, between cells from C57/BL-6 and ApoE−/− mice. Since SERCA was blocked with TG in these experiments, the increased expression of PMCA in atherosclerotic VSMs (Fig. 3c), may be responsible for the more rapid sarcolemmal Ca2+ extrusion observed in ApoE−/− cells in Fig. 5c and f.
Fig. 5.
Ca2+ extrusion is upregulated in atherosclerotic mouse aortae. (a) Typical experimental protocol. (b–d) Representative responses to 10 mM caffeine and 1 μM TG (in the presence and absence extracellular Ca2+). (e) Mean peak Ca2+ responses (ΔF/F0). (f) Decay times (s) of Ca2+ transients. *p < 0.05, **p < 0.01 and ***p < 0.001 vs C57/BL-6. The average of multiple cells from a single mouse was used as 1 data point, n = 5–6 mice per group.
4. Discussion
In this study, we have presented evidence showing novel time-dependent and progressive changes in Ca2+ handling within VSM cells during high fat feeding in ApoE−/− mice. In agreement with previous investigations in canine coronary and cerebral arteries and rabbit and rat aortae [12,30–33], the pro-oxidant ONOO− relaxed pre-contracted C57/BL-6 and ApoE−/− mouse aorta. However, we additionally report for the first time that this vasodilator response (Fig. 2b), and the expression and function of VSM SERCA and PMCA (Figs. 3 and 4), are altered with time on high fat diet and reveal modifications and compensatory mechanisms of Ca2+ handling in the disease state (Fig. 5).
Facilitation of cellular Ca2+ removal requires a combination of SR Ca2+ uptake via SERCA and plasma membrane extrusion [34] via PMCA and NCX, although the extent of the involvement of the latter protein is controversial in aortic smooth muscle [20–22,35]. Importantly though, the majority of Ca2+ clearance in mouse aortic VSM (60–70%) has been attributed to the combined function of PMCA and SERCA and not NCX and SERCA [36]. We therefore focussed this study on the impact of SERCA and PMCA. The changes we observed in relaxation to ONOO− over time in vessels from fat fed animals will be influenced not only by our observed changes in SERCA function and SOCE, but also by the compensatory changes we have identified in the expression and function of PMCA. We suggest the altered balance and influence of these processes accounts for the altered sensitivity to ONOO− observed in this study.
Surprisingly, ONOO−-induced relaxation was enhanced in ApoE−/− mice which had been high fat fed for 2 months while, after 4 months on diet, this enhancement was lost (Fig. 2b). Improved smooth muscle cell function in ApoE deficient mice has not been reported previously, however a recent study by Beleznai et al. [40] did reveal increased endothelial cell function and sensitivity to dilation in the mesenteric arteries of ApoE−/− of 9–14 weeks of age. This increased sensitivity was reduced in older ApoE−/− [40], which correlates with our findings presented here on aortic smooth muscle. Paradoxically, although we observed greater relaxation of aortae from 2 month fat fed ApoE−/− mice, SERCA expression was significantly reduced in comparison to controls. We would suggest that a compensatory upregulation of calcium extrusion occurs in the smooth muscle of ApoE−/− mice, which allows for augmented relaxation at the earlier 2 month time-point but is “over-ridden” by smooth muscle dysfunction as age and time on high fat diet increases. Our data suggests that PMCA is likely to be responsible for the increased calcium extrusion and enhanced relaxation to ONOO− at 2 months high fat diet since expression was increased (Fig. 3b) and the PMCA inhibitor carboxyeosin normalised the enhanced relaxation to ONOO− (Fig. 4e).
Release of Ca2+ from the SR will also activate SOCE, which is upregulated in ApoE knockout mice [26]. We have confirmed this finding in our single cell experiments through the addition of the SERCA blocker (and, consequently, SOCC activator) TG in extracellular Ca2+ -containing and Ca2+- free/EGTA conditions (Fig. 5). We also report here for the first time that the TG-induced increase in [Ca2+]c (in extracellular Ca2+-free conditions) is reduced in aortic smooth muscle from ApoE−/− (Fig. 5e) and this is in line with our finding that SERCA protein expression is reduced in these samples (Fig. 3a). SERCA plays an important role in ONOO−-induced relaxation, since pre-treatment with TG caused significant reduction in relaxation in aortae from C57/BL-6 and ApoE−/− mice (Fig. 4a–c). SERCA is highly sensitive to oxidative damage and, in ApoE−/− mice with demonstrable atherosclerotic plaques, we found nitrotyrosine staining around the plaque correlated with a progressive reduction in SERCA protein expression and altered effectiveness of TG in inhibiting ONOO-induced relaxation. TG reduced ONOO−-induced relaxation most notably in C57/BL-6 and 4 month ApoE−/− preparations while, in 2 month ApoE−/− aortae, inhibition by TG was much less marked. The lack of sensitivity to SERCA blockade at 2 months is likely due to reduced SERCA expression (Fig. 3a) but this does not correlate with data at 4 months where SERCA expression was further reduced but the relaxation to ONOO− was more sensitive to TG. The observed increase in SOCC function in VSM from ApoE−/− mice goes some way to explaining the increased efficacy of TG in blocking ONOO−-induced relaxation in aorta from 4 month high fat fed ApoE−/− mice (Fig. 4c), as TG will cause indirect SOCC activation as a consequence of SR Ca2+ store depletion. Therefore, increased Ca2+ entry via SOCCs potentially precludes relaxation following incubation with TG at this later time point.
Interestingly, a recent paper by Barriga et al. [41] reported that SERCA2 protein levels are reduced in cultured atrial myocytes by the addition of LDL. Data from our laboratory has indicated there is no significant decrease in SERCA2b expression between age-matched chow-fed C57/BL-6 and ApoE−/− aortae (data not shown). Other studies have shown raised LDL levels with time spent on high fat diet [42–44], with approximately a two-fold increase in comparison to chow-fed animals. Since we have shown reduced SERCA expression following 2 and 4 months high fat feeding, it is probable that in addition to the impact of atherosclerosis, increased LDL levels may play a role in altered SERCA expression and function.
No other investigators to date have specifically investigated the temporal relationship between atherosclerotic development, high fat feeding and the relationship between smooth muscle cell SERCA and PMCA, although other studies have observed a compensatory relationship between the two in other cell types. Over-expression of PMCA has been shown to lead to down-regulation of the SERCA pump in rat aortic endothelial cells by Liu et al. [37] and work by Wang et al. [38] indicates that PMCA functions co-operatively with SERCA and NCX in rabbit aortic endothelium. We therefore believe that this study represents an important step forward in the understanding of the temporal and compensatory alterations observed in VSM function between healthy and hypercholesterolaemic ApoE−/− mice.
5. Conclusion
For the first time we have demonstrated time-dependent changes in the expression and activity of Ca2+ regulatory mechanisms in VSM during progressive high fat feeding in atherosclerosis-prone mice. Compensatory changes, comprising a rise in PMCA expression, coupled with a fall in SERCA expression and function act as important mechanisms to sustain ‘normal’ relaxation to some vasodilator agents as a pathological condition is developing. Data from single cells suggest enhanced Ca2+ extrusion, likely via PMCA may be responsible for maintenance of close to normal relaxation responses for a period of time and perhaps augmented vasodilator response at early stages of fat feeding. This study highlights the importance of examining multiple time points during disease development and the interdependence and complexity of SERCA, SOCE and PMCA function during atherosclerosis.
Source of funding
This work was supported by project grants from the British Heart Foundation (BHF-PG/07/012/22342), (BHF-PG/10/79/28603), The Royal Society (RG110356) and Tenovus Scotland (S12/4).
Disclosures
No conflict of interest declared.
Acknowledgements
Many thanks to: Professor Frank Wuytack, Laboratory for Ca2+ transport ATPases, KU Leuven, Belgium for kindly supplying the antibody to SERCA2b, Professor Gail McConnell, Centre for Biophotonics, University of Strathclyde for technical expertise/microscope use and Dr John Dempster, SIPBS, University of Strathclyde for Winfluor electrophysiology software.
References
- 1.White C.R., Brock T.A., Chang L.Y. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci USA. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touyz R.M., Briones A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 3.Touyz R.M., Schiffrin E.L. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 4.Park J.G., Oh G.T. The role of peroxidases in the pathogenesis of atherosclerosis. BMB Rep. 2011;44:497–505. doi: 10.5483/bmbrep.2011.44.8.497. [DOI] [PubMed] [Google Scholar]
- 5.Taniyama Y., Griendling K.K. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 6.De Keulenaer G.W., Ushio-Fukai M., Yin Q. Convergence of redox-sensitive and mitogen-activated protein kinase signaling pathways in tumor necrosis factor-alpha-mediated monocyte chemoattractant protein-1 induction in vascular smooth muscle cells. Arterioscler Throm Vasc Biol. 2000;20:385–391. doi: 10.1161/01.atv.20.2.385. [DOI] [PubMed] [Google Scholar]
- 7.Lyle A.N., Griendling K.K. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiol (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 8.Yung L.M., Leung F.P., Yao X., Chen Z.Y., Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 9.Adachi T., Weisbrod R.M., Pimentel D.R. S-glutathiolation by peroxynitrite activates serca during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi M., Faraci F., Heistad D. Peroxynitrite hyperpolarizes smooth muscle and relaxes internal carotid artery in rabbit via atp-sensitiveK+ channels. Am J Physiol Heart Circ Physiol. 2005;289:H2244–H2250. doi: 10.1152/ajpheart.00254.2005. [DOI] [PubMed] [Google Scholar]
- 11.Tiefenbacher C.P., Kreuzer J. Nitric oxide-mediated endothelial dysfunction–is there need to treat? Curr Vasc Pharmacol. 2003;1:123–133. doi: 10.2174/1570161033476718. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Li W., Altura B.T., Altura B.M. Peroxynitrite-induced relaxation in isolated rat aortic rings and mechanisms of action. Toxicol Appl Pharmacol. 2005;209:269–276. doi: 10.1016/j.taap.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Sanders K.M. Signal transduction in smooth muscle: invited review: mechanisms of calcium handling in smooth muscles. J Appl Physiol. 2001;91:1438–1449. doi: 10.1152/jappl.2001.91.3.1438. [DOI] [PubMed] [Google Scholar]
- 14.Periasamy M., Kalyanasundaram A. Serca pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 15.Di Leva F., Domi T., Fedrizzi L., Lim D., Carafoli E. The plasma membrane Ca2+ atpase of animal cells: structure, function and regulation. Arch Biochem Biophys. 2008;476:65–74. doi: 10.1016/j.abb.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Floyd R., Wray S. Calcium transporters and signalling in smooth muscles. Cell Calcium. 2007;42:467–476. doi: 10.1016/j.ceca.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Dickhout J.G., Hossain G.S., Pozza L.M., Zhou J., Lhotak S., Austin R.C. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: Implications in atherogenesis. Arterioscler Throm Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 18.Jardin I., Redondo P.C., Salido G.M., Pariente J.A., Rosado J.A. Endogenously generated reactive oxygen species reduce PMCA activity in platelets from patients with non-insulin-dependent diabetes mellitus. Platelets. 2006;17:283–288. doi: 10.1080/09537100600745187. [DOI] [PubMed] [Google Scholar]
- 19.Hettiarachchi N.T., Boyle J.P., Bauer C.C. Peroxynitrite mediates disruption of ca2+ homeostasis by carbon monoxide via Ca2+ atpase degradation. Antioxid Redox Signal. 2012;17:744–755. doi: 10.1089/ars.2011.4398. [DOI] [PubMed] [Google Scholar]
- 20.Kamishima T., McCarron J.G. Ca2+ removal mechanisms in rat cerebral resistance size arteries. Biophys J. 1998;75:1767–1773. doi: 10.1016/S0006-3495(98)77618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainbow R.D., Macmillan D., McCarron J.G. The sarcoplasmic reticulum Ca2+ store arrangement in vascular smooth muscle. Cell Calcium. 2009;46:313–322. doi: 10.1016/j.ceca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura J. Topics on the Na+/Ca2+ exchanger: involvement of Na+/Ca2+ exchanger in the vasodilator-induced vasorelaxation. J Pharmacol Sci. 2006;102:27–31. doi: 10.1254/jphs.fmj06002x5. [DOI] [PubMed] [Google Scholar]
- 23.Huschenbett J., Zaidi A., Michaelis M.L. Sensitivity of the synaptic membrane Na+/Ca2+ exchanger and the expressed ncx1 isoform to reactive oxygen species. Biochem Biophys Acta. 1998;1374:34–46. doi: 10.1016/s0005-2736(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 24.Trebak M., Ginnan R., Singer H.A., Jourd'heuil D. Interplay between calcium and reactive oxygen/nitrogen species: an essential paradigm for vascular smooth muscle signaling. Antioxid Redox Signal. 2010;12:657–674. doi: 10.1089/ars.2009.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levitsky D.O., Clergue M., Lambert F. Sarcoplasmic reticulum calcium transport and Ca(2+)-atpase gene expression in thoracic and abdominal aortas of normotensive and spontaneously hypertensive rats. J Biol Chem. 1993;268:8325–8331. [PubMed] [Google Scholar]
- 26.Van Assche T., Fransen P., Guns P.J., Herman A.G., Bult H. Altered Ca2+ handling of smooth muscle cells in aorta of apolipoprotein e-deficient mice before development of atherosclerotic lesions. Cell Calcium. 2007;41:295–302. doi: 10.1016/j.ceca.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Piedrahita J.A., Zhang S.H., Hagaman J.R., Oliver P.M., Maeda N. Generation of mice carrying a mutant apolipoprotein e gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plump A.S., Smith J.D., Hayek T. Severe hypercholesterolemia and atherosclerosis in apolipoprotein-e-deficient mice created by homologous recombination in es cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 29.Reddick R., Zhang S., Maeda N. Atherosclerosis in mice lacking apo e. Evaluation of lesional development and progression. Arterioscler Throm Vasc Biol. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 30.Dowell F.J., Martin W. The effects of peroxynitrite on rat aorta: Interaction with glucose and related substances. Eur J Pharmacol. 1997;338:43–53. doi: 10.1016/s0014-2999(97)01320-4. [DOI] [PubMed] [Google Scholar]
- 31.Inesi G., Hua S., Xu C. Studies of Ca2+ atpase (serca) inhibition. J Bioenerg Biomembr. 2005;37:365–368. doi: 10.1007/s10863-005-9472-1. [DOI] [PubMed] [Google Scholar]
- 32.Liu S., Beckman J.S., Ku D.D. Peroxynitrite, a product of superoxide and nitric oxide, produces coronary vasorelaxation in dogs. J Pharmacol Exp Ther. 1994;268:1114–1121. [PubMed] [Google Scholar]
- 33.Moro M.A., Darley-Usmar V.M., Lizasoain I. The formation of nitric oxide donors from peroxynitrite. Br J Pharmacol. 1995;116:1999–2004. doi: 10.1111/j.1476-5381.1995.tb16404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida Y., Paul R.J. Ca2+ clearance in smooth muscle: lessons from gene-altered mice. J Smooth Muscle Res. 2005;41:235–245. doi: 10.1540/jsmr.41.235. [DOI] [PubMed] [Google Scholar]
- 35.Liu B., Peel S.E., Fox J., Hall I.P. Reverse modeNa+/Ca2+ exchange mediated by stim1 contributes to Ca2+ influx in airway smooth muscle following agonist stimulation. Respir Res. 2010;11:168. doi: 10.1186/1465-9921-11-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pritchard T.J., Bowman P.S., Jefferson A., Tosun M., Lynch R.M., Paul R.J. Na(+)-K(+)-atpase and Ca(2+) clearance proteins in smooth muscle: a functional unit. Am J Physiol Heart Circ Physiol. 2010;299:H548–H556. doi: 10.1152/ajpheart.00527.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B.-F., Xu X., Fridman R., Muallem S., Kuo T.H. Consequences of functional expression of the plasma membrane ca pump isoform 1a. J Biol Chem. 1996;271:5536–5544. doi: 10.1074/jbc.271.10.5536. [DOI] [PubMed] [Google Scholar]
- 38.Wang X., Reznick S., Li P., Liang W., van Breemen C. Ca(2+) removal mechanisms in freshly isolated rabbit aortic endothelial cells. Cell Calcium. 2002;31:265–277. doi: 10.1016/s0143-4160(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 39.Vittone F., Liberman A., Vasic D. Sitagliptin reduces plaque macrophage content and stabilises arteriosclerotic lesions in ApoE (−/−) mice. Diabetologia. 2012;55:2267–2275. doi: 10.1007/s00125-012-2582-5. [DOI] [PubMed] [Google Scholar]
- 40.Beleznai T., Takano H., Hamill C. Enhanced K+-channel-mediated endothelium-dependent local and conducted dilationof small mesenteric arteries from ApoE−/− mice. Cardiovasc Res. 2011;92:199–208. doi: 10.1093/cvr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barriga M., Cal R., Cabello N. Low density lipoproteins promote unstablecalcium handling accompanied by reduced SERCA2 and connexin-40 expression incardiomyocytes. PLoS One. 2013;8:e58128. doi: 10.1371/journal.pone.0058128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali Z.A., Alp N.J., Lupton H. Increased in-stent stenosis in ApoE knockoutmice: insights from a novel mouse model of balloon angioplasty and stenting. Arterioscler Thromb Vasc Biol. 2007;27:833–840. doi: 10.1161/01.ATV.0000257135.39571.5b. [DOI] [PubMed] [Google Scholar]
- 43.Ma K.L., Liu J., Ni J. Inflammatory stress exacerbates the progression of cardiac fibrosis inhigh-fat-fed apolipoprotein E knockout mice via endothelial-mesenchymaltransition. Int J Med Sci. 2013;10:420–426. doi: 10.7150/ijms.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upmacis R.K., Crabtree M.J., Deeb R.S. Profound biopterin oxidation and protein tyrosine nitration in tissues of ApoE-null mice on an atherogenic diet: contribution of inducible nitricoxide synthase. Am J Physiol Heart Circ Physiol. 2007;293:H2878–H2887. doi: 10.1152/ajpheart.01144.2006. [DOI] [PubMed] [Google Scholar]