Abstract
The sudden appearance of calcified skeletons among many different invertebrate taxa at the Precambrian-Cambrian transition may have required minor reorganization of preexisting secretory functions. In particular, features of the skeletal organic matrix responsible for regulating crystal growth by inhibition may be derived from mucous epithelial excretions. The latter would have prevented spontaneous calcium carbonate overcrusting of soft tissues exposed to the highly supersaturated Late Proterozoic ocean [Knoll, A. H., Fairchild, I. J. & Swett, K. (1993) Palaios 8, 512-525], a putative function for which we propose the term "anticalcification." We tested this hypothesis by comparing the serological properties of skeletal water-soluble matrices and mucous excretions of three invertebrates--the scleractinian coral Galaxea fascicularis and the bivalve molluscs Mytilus edulis and Mercenaria mercenaria. Crossreactivities recorded between muci and skeletal water-soluble matrices suggest that these different secretory products have a high degree of homology. Furthermore, freshly extracted muci of Mytilus were found to inhibit calcium carbonate precipitation in solution.
Full text
PDF
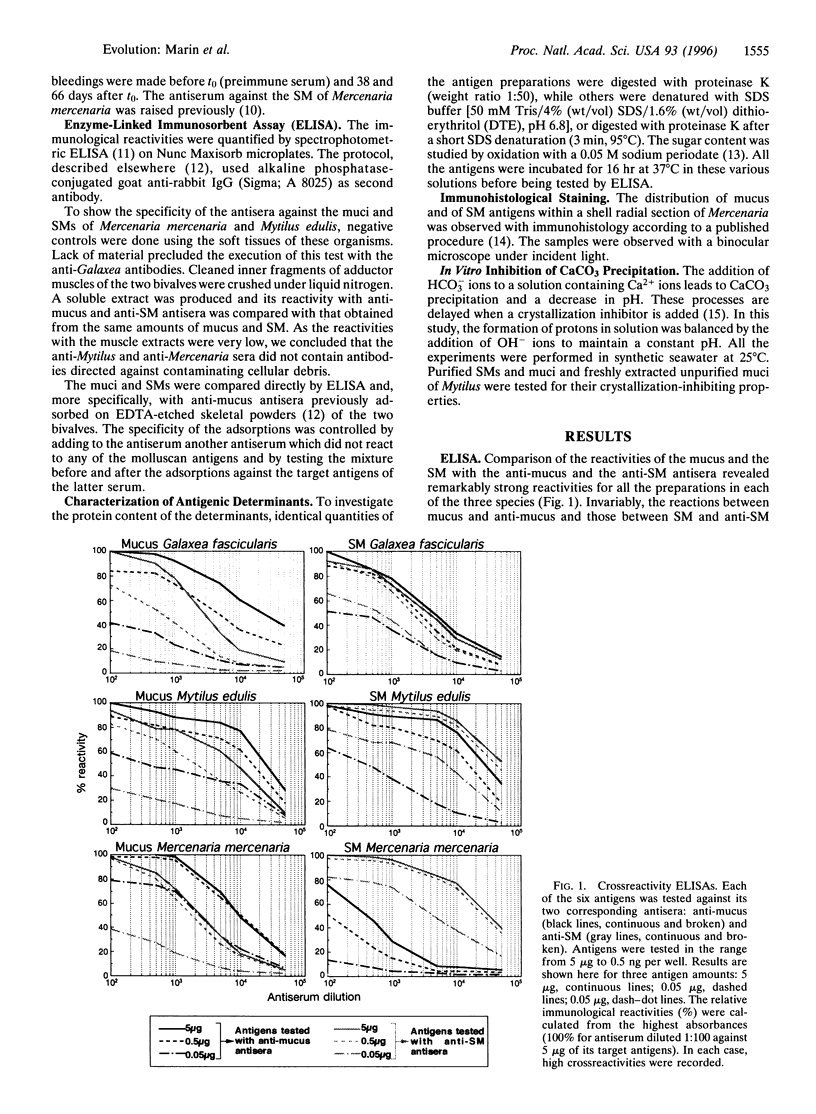
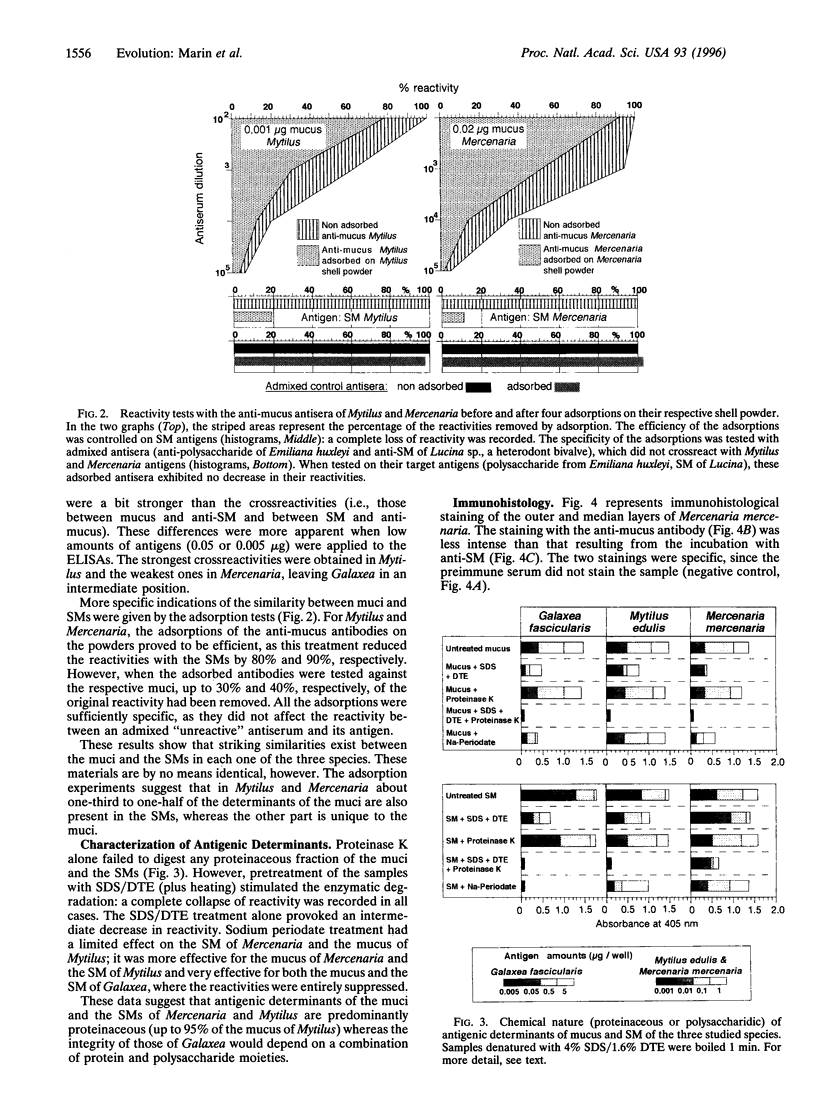
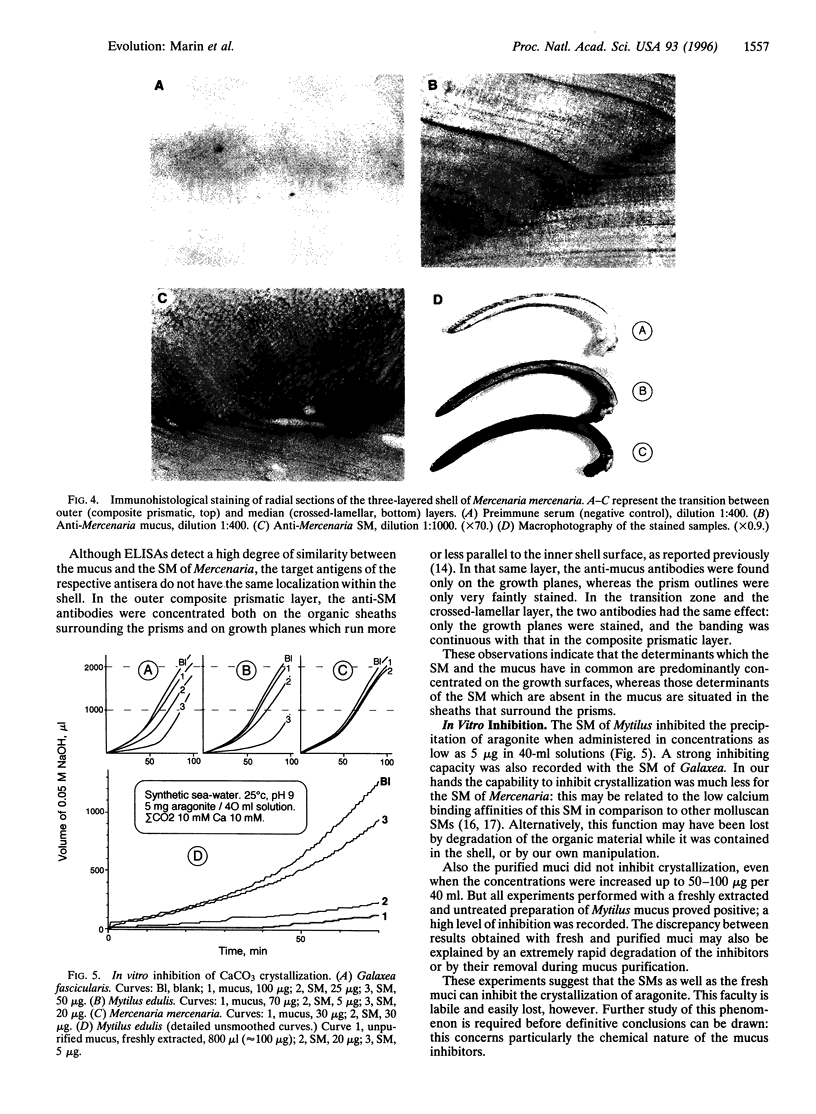


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn H. Y., Sue L. F., Ma J. K., Pinkstaff C. A., Pore R. S., Overman D. O., Malanga C. J. Synthesis and secretion of mucous glycoprotein by the gill of Mytilus edulis. I. Histochemical and chromatographic analysis of [14C]glucosamine bioincorporation. Biochim Biophys Acta. 1988 Jul 14;966(1):122–132. doi: 10.1016/0304-4165(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Clark M. F., Adams A. N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977 Mar;34(3):475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- Humbert W., Kirsch R., Simonneaux V. Is mucus involved in biocrystallization? Study of the intestinal mucus of the sea-water eel Anguilla anguilla L. Cell Tissue Res. 1986;245(3):599–604. doi: 10.1007/BF00218561. [DOI] [PubMed] [Google Scholar]
- Knoll A. H., Fairchild I. J., Swett K. Calcified microbes in Neoproterozoic carbonates: implications for our understanding of the Proterozoic/Cambrian transition. Palaios. 1993;8:512–525. [PubMed] [Google Scholar]
- Lowenstam H. A., Margulis L. Evolutionary prerequisites for early Phanerozoic calcareous skeletons. Biosystems. 1980;12(1-2):27–41. doi: 10.1016/0303-2647(80)90036-2. [DOI] [PubMed] [Google Scholar]
- Towe K. M. Oxygen-collagen priority and the early metazoan fossil record. Proc Natl Acad Sci U S A. 1970 Apr;65(4):781–788. doi: 10.1073/pnas.65.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]