Abstract
Importin β1 is the nuclear-cytoplasmic transport receptor in eukaryotic cells. Its main function is to transport NLS (nuclear localization signal)-containing proteins from cytoplasm to nucleus. Our recent study found that AtKPNB1, a homolog of the human KPNB1, is an essential component of the classical nuclear import of the NLS-containing proteins in Arabidopsis and modulates plant development and ABA-mediated stress response. Human KPNB1 can also directly transport the nuclear proteins, such as ribosomal protein RPS7e, without the intervention of importin α proteins. However, we found that AtKPNB1 does not directly recognize and import the human RPS7e homologous proteins AtRPS7A, AtRPS7B and AtRPS7C into the nucleus like human KPNB1. These findings suggest that the importin β1 protein has the conserved function in translocating nuclear proteins to the nucleus, but their specific cargos may vary in different organisms.
Keywords: Arabidopsis thaliana, NLS-containing protein, importin α, importin β1, nuclear-cytoplasmic transport
Importin β1 (also known as Kayopherin β1) belongs to the karyopherin family and plays a key role in the protein nuclear import via association with adaptor protein importin α, which directly recognizes the NLS-containing cargoes.1 Importin β1 has a conserved function in nuclear localization signal (NLS)-mediated nuclear protein import pathway among different organisms, including animals,2-4 microbes5,6 and plants.7-10 In non-plant systems, interaction between importin α and NLS-containing nuclear proteins prompts complex formation with importin β1 in cytoplasm; then the importin β1:importin α:cargo complex is transported into the nucleus through importin β1 and nuclear pore proteins interaction; in nucleus, importin β1 binds to the Ran-GTP to release the substrates.1 In our recent study, we identified the importin β1 protein in Arabidopsis and named AtKPNB1. Like human KPNB1, AtKPNB1 interacts with several importin α proteins, such as AtIMPA1, AtIMPA2, AtIMPA4 and AtIMPA6. It also binds to nuclear pore protein AtNUP62 and Ran proteins RAN1, RAN2 and RAN3. Most importantly, AtKPNB1 was able to influence the nuclear localization of the NLS-GFP.9 These results suggest that there is also a conserved nuclear import pathway composed of formation of importin β1:importin α:cargo complex, translocation through nuclear pore and release of cargos in the nucleus in Arabidopsis. AtKPNB1 is an essential component in importin α-mediated translocation from cytoplasm to nucleus of some NLS-containing proteins.
The evidence has shown that importin β1:importin α-mediated classical nuclear transport pathway plays a critical role during development, disease and adaptation.11-13 Their cargos include various NLS-containing proteins, such as MRTF,14 mDia215 and so on, which nucleus localization is essential for their function in gene expression regulation of the downstream signaling pathways. It is apparent that AtKPNB1 modulates plant growth and response to ABA and stress.9 Identification of the direct cargos of AtKPNB1 and functional analysis of the proteins will help to understanding the roles of AtKPNB1 and the classical nuclear import of NLS-containing proteins.
Another important feature of human KPNB1 is that it can interact with the cargo proteins and directly transport them into nucleus, bypassing cargo adaptor importin α. For example, human KPNB1 is involved in cell cycle control via controlling CYCB1;1 nuclear translocation; it also mediates nuclear imports of H2A, H2B, H3 and H4 histones, as well as ribosomal proteins RPL5, RPS7e and RPL23A.16,17 To investigate whether AtKPNB1 is able to directly import proteins into nucleus like human KPNB1, we analyzed the homolog proteins of human ribosomal proteins RPS7 in Arabidopsis and three RPS7 proteins (AtRPS7A/AT1G48830, AtRPS7B/AT3G02560, AtRPS7C/AT5G16130) were found in Arabidopsis. Phylogenetic analysis (MEGA 5.2) shows that three proteins have close evolutionary relationship to human RPS7e (Fig. 1). To test whether AtKPNB1 directly transport these proteins from cytoplasm to nucleus in Arabidopsis, we first analyzed the interaction between AtKPNB1 and three ribosomal proteins AtRPS7A/AT1G48830, AtRPS7B/AT3G02560, AtRPS7C/AT5G16130 using the yeast two hybrid assay. To do this, we inserted AtKPNB1 to pGBKT7 and the ribosomal proteins to pGADT7 plasmids respectively. The constructs were cotransformed to the yeast strain Y2Hgold and the protein-protein interaction was analyzed by evaluating yeast growth on the selective medium lacking Leu/Trp/His/Ade (quadruple drop-out media). As shown in Figure 2A, no direct interactions between these proteins and AtKPNB1 were detected, suggesting that AtKPNB1 may not directly transport these ribosomal proteins into nucleus in Arabidopsis. To test the prediction, we then analyzed whether AtKPNB1 mutation affects the subcellular localization of three ribosomal proteins. We made constructs expressing the full length cDNA of the AtRPS7A, AtRPS7B and AtRPS7C fused to YFP in the plasmid P2GWY7 that were used to transform the protoplasts of wild type and atkpnb1 mutant. As shown in Figure 2B, the AtRPS7A, AtRPS7B and AtRPS7C proteins are all localized in the nucleus of the wild type protoplasts. When these proteins were expressed in the atkpnb1 protoplasts, no changes in their subcellular localization were detected. Taken together, the results confirmed that AtKPNB1 does not mediate nuclear import of the AtRPS7A, AtRPS7B and AtRPS7C proteins. Since there are 17 putative importin β proteins in Arabidopsis, it is possible that other importin β proteins function as specific receptors for recognizing and importing these ribosomal proteins to the nucleus. But we do not exclude the possibility that these proteins are translocated from cytoplasm to nucleus through the classical nuclear import pathway with involvement of importin α.
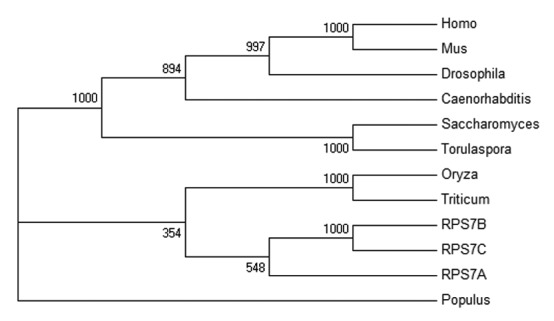
Figure 1. Phylogram of AtRPS7A, AtRPS7B, AtRPS7C and closely related proteins. An unrooted phylogenetic analysis was conducted using MEGA version 5.2. Bootstrap test of phylogeny was performed using the neighbor-joining method from 1,000 replications for each branch. The accession numbers are: AtRPS7A, AT1G48830; AtRPS7B, AT3G02560; AtRPS7C, AT5G16130; Homo sapiens, P62081; Caenorhabditis elegans, Q23312; Drosophila melanogaster, Q8IMI7; Oryza sativa, A2XFL8; Populus trichocarpa, A9PEW2; Saccharomyces cerevisiae, P26786; Triticum urartu, EMS56913; Torulaspora delbrueckii, XP_003682163; Mus musculus, AAB97861.1.
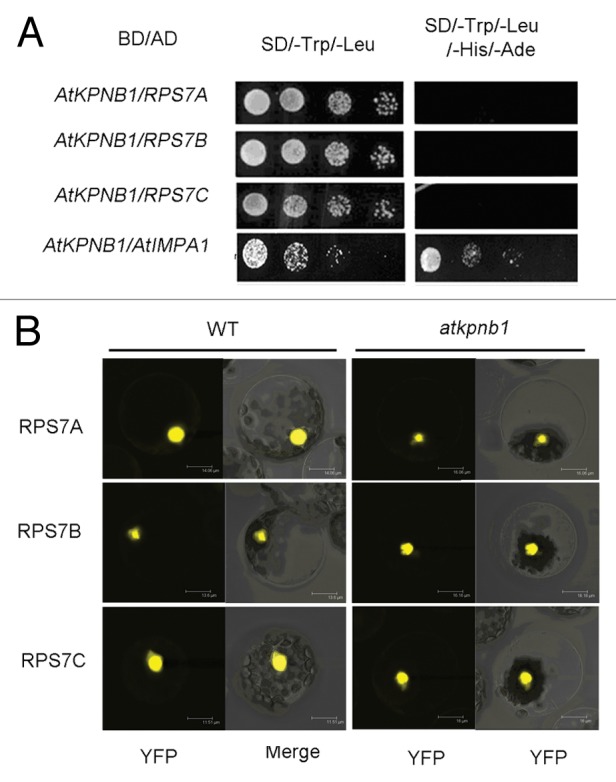
Figure 2. No interaction between the ribosomal proteins and AtKPNB1. (A) The interaction assay between AtKPNB1 and the ribosomal proteins using yeast two hybrid system. (B) The subcellular localizations of AtRPS7 in protoplasts of wild type (WT) and the mutant atkpnb1.
Conclusions
Our previous results confirmed that AtKPNB1 has conserved function in nuclear import of the NLS-containing proteins through the classical nuclear import pathway in Arabidopsis. AtKPNB1 may also import some nuclear proteins to nucleus without the intervention of importin α proteins, but it does not directly recognize and transport the ribosomal proteins AtRPS7A, AtRPS7B and AtRPS7C into the nucleus like human KPNB1. Thus, importin β1 may have conserved function in nuclear protein import in eukaryotes, but their substrates or cargos may vary in different organisms.
Acknowledgments
We would like to acknowledge support from the National Program on Key Basic Research Project (2012CB114300) and the National Transgenic Key Project of the Ministry of Agriculture of China (2011ZX08009-003-002).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25106
References
- 1.Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. Importin α: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–14. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Merkle T. Nucleo-cytoplasmic partitioning of proteins in plants: implications for the regulation of environmental and developmental signalling. Curr Genet. 2003;44:231–60. doi: 10.1007/s00294-003-0444-x. [DOI] [PubMed] [Google Scholar]
- 3.Conti E, Müller CW, Stewart M. Karyopherin flexibility in nucleocytoplasmic transport. Curr Opin Struct Biol. 2006;16:237–44. doi: 10.1016/j.sbi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Hu J, Wang F, Yuan Y, Zhu X, Wang Y, Zhang Y, et al. Novel importin-alpha family member Kpna7 is required for normal fertility and fecundity in the mouse. J Biol Chem. 2010;285:33113–22. doi: 10.1074/jbc.M110.117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koepp DM, Wong DH, Corbett AH, Silver PA. Dynamic localization of the nuclear import receptor and its interactions with transport factors. J Cell Biol. 1996;133:1163–76. doi: 10.1083/jcb.133.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peláez R, Fernández-García P, Herrero P, Moreno F. Nuclear import of the yeast hexokinase 2 protein requires α/β-importin-dependent pathway. J Biol Chem. 2012;287:3518–29. doi: 10.1074/jbc.M111.317230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang CJ, Imamoto N, Matsuki R, Yoneda Y, Yamamoto N. In vitro characterization of rice importin β1: molecular interaction with nuclear transport factors and mediation of nuclear protein import. FEBS Lett. 1998;437:127–30. doi: 10.1016/S0014-5793(98)01207-1. [DOI] [PubMed] [Google Scholar]
- 8.Matsuki R, Iwasaki T, Shoji K, Jiang CJ, Yamamoto N. Isolation and characterization of two importin-β genes from rice. Plant Cell Physiol. 1998;39:879–84. doi: 10.1093/oxfordjournals.pcp.a029448. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Wang Z, Ji H, Fang H, Wang S, Tian L, et al. An Arabidopsis homolog of importin β1 is required for ABA response and drought tolerance. Plant J. 2013 doi: 10.1111/tpj.12207. In press. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Cid A, Vega M, Herrero P, Moreno F. Yeast importin-β is required for nuclear import of the Mig2 repressor. BMC Cell Biol. 2012;13:31. doi: 10.1186/1471-2121-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur G, Delluc-Clavieres A, Poon IKH, Forwood JK, Glover DJ, Jans DA. Calmodulin-dependent nuclear import of HMG-box family nuclear factors: importance of the role of SRY in sex reversal. Biochem J. 2010;430:39–48. doi: 10.1042/BJ20091758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong Y, Wang Y, Yang H, Ballar P, Lee J-, Ye Y, et al. Importin beta interacts with the ER-associated degradation machinery and promotes ubiquitination and degradation of mutant alpha1-antitrypsin. J Biol Chem. 2011;286:33921–30. doi: 10.1074/jbc.M111.272906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–6. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajakyla EK, Vartiainen MK, Treisman R. An actin-regulated importin α/β-dependent extended bipartite NLS directs nuclear import of MRTF-A. EMBO J. 2004;29:3448–58. doi: 10.1038/emboj.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miki T, Okawa K, Sekimoto T, Yoneda Y, Watanabe S, Ishizaki T, et al. mDia2 shuttles between the nucleus and the cytoplasm through the importin-α/β- and CRM1-mediated nuclear transport mechanism. J Biol Chem. 2008;284:5753–62. doi: 10.1074/jbc.M806191200. [DOI] [PubMed] [Google Scholar]
- 16.Takizawa CG, Weis K, Morgan DO. Ran-independent nuclear import of cyclin B1-Cdc2 by importin β. Proc Natl Acad Sci USA. 1999;96:7938–43. doi: 10.1073/pnas.96.14.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jäkel S, Görlich D. Importin β, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 1998;17:4491–502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]


