Abstract
The role of calcium (Ca2+) and its dependent protease calpain in Aeromonas hydrophila-induced head kidney macrophage (HKM) apoptosis has been reported. Here, we report the pro-apoptotic involvement of calmodulin (CaM) and calmodulin kinase II gamma (CaMKIIg) in the process. We observed significant increase in CaM levels in A. hydrophila-infected HKM and the inhibitory role of BAPTA/AM, EGTA, nifedipine and verapamil suggested CaM elevation to be Ca2+-dependent. Our studies with CaM-specific siRNA and the CaM inhibitor calmidazolium chloride demonstrated CaM to be pro-apoptotic that initiated the downstream expression of CaMKIIg. Using the CaMKIIg-targeted siRNA, specific inhibitor KN-93 and its inactive structural analogue KN-92 we report CaM-CaMKIIg signalling to be critical for apoptosis of A. hydrophila-infected HKM. Inhibitor studies further suggested the role of calpain-2 in CaMKIIg expression. CaMK Kinase (CaMKK), the other CaM dependent kinase exhibited no role in A. hydrophila-induced HKM apoptosis. We report increased production of intracellular cAMP in infected HKM and our results with KN-93 or KN-92 implicate the role of CaMKIIg in cAMP production. Using siRNA to PKACA, the catalytic subunit of PKA, anti-PKACA antibody and H-89, the specific inhibitor for PKA we prove the pro-apoptotic involvement of cAMP/PKA pathway in the pathogenicity of A. hydrophila. Our inhibitor studies coupled with siRNA approach further implicated the role of cAMP/PKA in activation of extracellular signal-regulated kinase 1 and 2 (ERK 1/2). We conclude that the alteration in intracellular Ca2+ levels initiated by A. hydrophila activates CaM and calpain-2; both pathways converge on CaMKIIg which in turn induces cAMP/PKA mediated ERK 1/2 phosphorylation leading to caspase-3 mediated apoptosis of infected HKM.
Author Summary
Aeromonas hydrophila is a natural fish pathogen and is known to induce apoptosis of HKM. Head kidney is an important immune-organ in fish and HKM are critical for immunity against the invading pathogen. The mechanisms of cell death induced by A. hydrophila are incompletely characterized. We have studied the role of Ca2+-dependent signalling pathways in the induction of A. hydrophila-induced HKM apoptosis. We observed that A. hydrophila infection led to increased CaM expression in infected HKM which was Ca2+-dependent. The inhibitor and siRNA studies suggested CaM to be pro-apoptotic and triggered CaMKIIg expression in the infected HKM. Calpain-2 appeared to influence CaMKIIg expression. However, further studies are needed to understand the process. We report that the CaM-CaMKIIg pathway is important for initiating cAMP production within the infected HKM. The pro-apoptotic activation of cAMP dependent PKA was quite evident. The activation of ERK 1/2 was observed in the HKM and results clearly suggested the pro-active role of cAMP/PKA in the process. Thus we conclude that CaM-CaMKIIg initiates the cAMP/PKA pathway that induces ERK 1/2 phosphorylation to promote caspase-3 mediated apoptosis of the A. hydrophila-infected HKM.
Introduction
Aeromonas hydrophila, a Gram-negative, rod-shaped, facultatively intracellular bacterium is commonly found as part of the normal microbial flora in the aquatic environment [1]. The pathogenicity of A. hydrophila is complex and multi-factorial. It induces a plethora of symptoms in fish characterized by severe open dermal ulcers, anaemia, visceral granulomata, septicaemia, failure of osmoregulatory balance and death, which together comprise the ulcerative disease syndrome or UDS [2]. A. hydrophila is also known for its wide range of host tropism that includes amphibians, reptiles as well as mammals [2]. In humans, this bacterium is frequently associated with individuals suffering from gastroenteritis, wound infections, septicemia and immunodeficiency disorders [1].
Pathogen-induced alterations in intracellular Ca2+ with varied effects have been well documented. In several instances an alteration in the intracellular Ca2+-levels was found to be a pre-requisite for pathogen-induced apoptosis of different cell types including macrophages [3]. Studies have also documented that initiation of Ca2+-influx delays the onset of apoptosis facilitating pathogen survival and growth inside the host macrophages [4]. In this context, it is worth mentioning that all such observations were based on studies in the mammalian systems and that there is scarcely any information on the role of Ca2+ or its dependent kinases in host-pathogen interactions in fish.
Calmodulin is one of the most abundant and well characterised Ca2+ sensor proteins [5]. It regulates numerous Ca2+-mediated cellular functions such as cell growth, differentiation, proliferation and apoptosis in divergent models including macrophages [6]. The increased cytosolic Ca2+ binds to CaM and the resulting Ca2+-CaM interaction leads to activation of several protein kinases including CaM-dependent kinases (CaMKs) [7]. The CaMK cascade includes CaMKII and CaMKK. CaMKII is one of the best characterised of CaMK family of kinases. It has been suggested that CaMKII, a serine/threonine kinase, is activated by Ca2+-CaM binding, followed by rapid autophosphorylation on Thr286, which abolishes its auto-inhibition [8]. As a consequence, a transient elevation in Ca2+ leads to a prolonged activation of CaMKII. Among the CaMKII isoforms, the gamma-isoform of CaMKII (CaMKIIg) has been reported in macrophage functioning [6], [9] and implicated both as pro- [6] and anti-apoptotic [10] in different studies.
The cyclic nucleotide, cAMP, is generated from ATP by adenylyl cyclases (ACs), whereas the phosphodiesterases (PDEs) catalyse its hydrolytic degradation. In eukaryotic cells cAMP requires the protein kinase A complex (PKA) as an intermediate to carry out its effects. Studies indicate that cAMP/PKA-pathway regulates a broad range of cellular responses that includes its central role as both a pro- and anti-apoptotic regulator [11]. There are varied pathways by which cAMP/PKA mediates its action and activation of MAPKs is one of them [12]. The MAPK family is composed of the extracellular signal-regulated kinase 1 and 2 (ERK 1/2), p38 and stress activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) pathways [13]. This family of kinases is important in a wide spectrum of cellular functions like proliferation, cytokine biosynthesis, cytoskeletal organization [14]. Amongst the MAPK family, activation of ERK 1/2 in pathogen-induced macrophage apoptosis is well characterized [15].
Mononuclear phagocytes such as macrophages are essential to the innate immune response against invading microorganisms. It has been suggested that one mechanism by which A. hydrophila induces pathogenicity in fish is through initiation of host macrophage apoptosis. In fish, especially teleosts, head kidney (HK) represents the anterior part of kidney. It is analogous to the mammalian bone marrow and the primary site of definitive hematopoiesis. The HK appears in the pre-hatching embryos on either side of the pharynx as renal units, each with a single glomerulus and short renal tubule. However, during the course of development the renal elements get degraded and it becomes primarily involved in hematopoiesis, antibody production and regulating immune-endocrine axis [16], [17]. The HKM serves as the first line of defence against invading pathogens. We have shown that HKM are crucial in the pathogenesis of A. hydrophila at the cellular level [18]. Recently, we demonstrated that live A. hydrophila infection leads to apoptotic death of HKM, involving calpain activation [19]. Here, we have identified and characterized other signalling effectors and executors that are involved in A. hydrophila-HKM interactions. Clarias gariepinus was selected as our model because of its availability round the year, ability to adapt to laboratory conditions and for having easily identifiable immune organs.
Results
CaM Expression Is Primal for A. hydrophila-Induced HKM Apoptosis
We earlier reported A. hydrophila-induced intracellular Ca2+ influx and subsequent calpain-2 activation in the infected HKM [19]. This study was designed to identify the role of other key molecules of Ca2+-pathway on A. hydrophila-induced HKM apoptosis and CaM was a rational candidate. At the outset, we checked for CaM expression at mRNA level following infection with A. hydrophila. In the absence of genome sequence of C. gariepinus degenerate primers for CaM were designed using the homologous stretch across vertebrates as the template. The PCR product was cloned and sequenced and the sequence showed 100% identity with CaM-mRNA sequence of channel catfish, Ictalurus sp. Primers for real time analysis were designed from this sequence and the real-time data revealed maximum induction of CaM at 2 h post-infection (p.i.) at the mRNA level (P<0.05) (Figure S1A). The next step was to check A. hydrophila-induced CaM protein expression using CaM assay kit. We observed maximum CaM protein expression at 2 h p.i. (P<0.05) (Figure S1B) and hence selected this time point for subsequent studies on CaM. At further time points CaM mRNA and protein levels decreased significantly reaching basal levels at 24 h p.i. (data not shown).
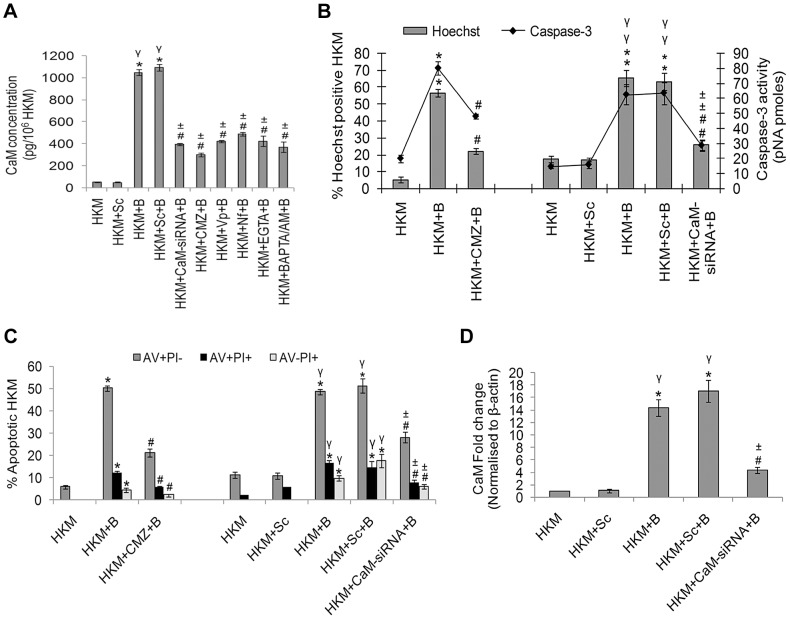
CaM activity was assayed in presence of the CaM antagonist-CMZ. This CaM antagonist binds reversibly to CaM thus inhibiting CaM-mediated enzyme activation [20]. Pre-treatment with CMZ inhibited CaM activation (P<0.05) in infected HKM (Figure 1A) which clearly suggested the role of CaM on A. hydrophila-induced HKM-pathology. Further, pre-treatment with Ca2+-chelators BAPTA/AM, EGTA and the Ca2+-channel blockers Nf and Vp led to significant reduction in CaM activity at the protein levels indicating CaM expression to be Ca2+-dependent in the infected HKM (Figure 1A).
Figure 1. A. hydrophila infection leads to increased CaM expression in HKM.
(A) HKM transfected separately with CaM-siRNA, scrambled siRNA or pre-treated with CMZ, Vp, Nf, EGTA, BAPTA/AM for different time periods were infected with A. hydrophila and CaM protein content measured in the lysates 2 h p.i. using EIA kit. HKM pre-treated with CMZ or transfected with CaM-siRNA or scrambled siRNA were infected with A. hydrophila and checked for (B) Hoechst 33342 positive cells and caspase-3 activity and (C) AV-PI staining 24 h p.i. (D) HKM transfected with CaM-siRNA or scrambled siRNA were infected with A. hydrophila and CaM mRNA expression detected by real time PCR 2 h p.i. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM; γP<0.05, compared to HKM+Sc; #P<0.05, compared to HKM+B; ±P<0.05, compared to HKM+Sc+B. HKM, control head kidney macrophage; HKM+Sc, HKM transfected with scrambled siRNA; HKM+B, HKM infected with A. hydrophila; HKM+Sc+B, HKM transfected with scrambled siRNA infected with A. hydrophila; HKM+CaM-siRNA+B, HKM transfected with CaM-siRNA infected with A. hydrophila; HKM+BAPTA/AM+B, HKM pre-treated with BAPTA/AM infected with A. hydrophila; HKM+EGTA+B, HKM pre-treated with EGTA infected with A. hydrophila; HKM+Nf+B, HKM pre-treated with Nf infected with A. hydrophila; HKM+Vp+B, HKM pre-treated with Vp infected with A. hydrophila; HKM+CMZ+B, HKM pre-treated with CMZ infected with A. hydrophila.
Our next step was to correlate CaM with HKM apoptosis. The HKMs were pre-treated with CMZ and apoptosis assessed 24 h p.i. by Hoechst, AV-PI staining and caspase-3 activation using specific assay kit. Pre-treatment with CMZ inhibited (P<0.05) apoptotic death and attenuated caspase-3 activity (P<0.05) (Figure 1B and 1C) in infected HKM implicating the role of CaM on initiating A. hydrophila-induced HKM apoptosis. These results were also confirmed using CaM-siRNA. Transfection with CaM-siRNA down-regulated CaM expression at mRNA level (Figure 1D), protein level (Figure 1A) at 2 h p.i. and also attenuated A. hydrophila-induced HKM apoptosis at 24 h p.i. (Figure 1B and 1C). Our results, for the first time, indicated the pro-apoptotic role of CaM in A. hydrophila-induced HKM apoptosis.
CaMKIIg Is the Likely CaM-Dependent Kinase Inducing HKM Apoptosis
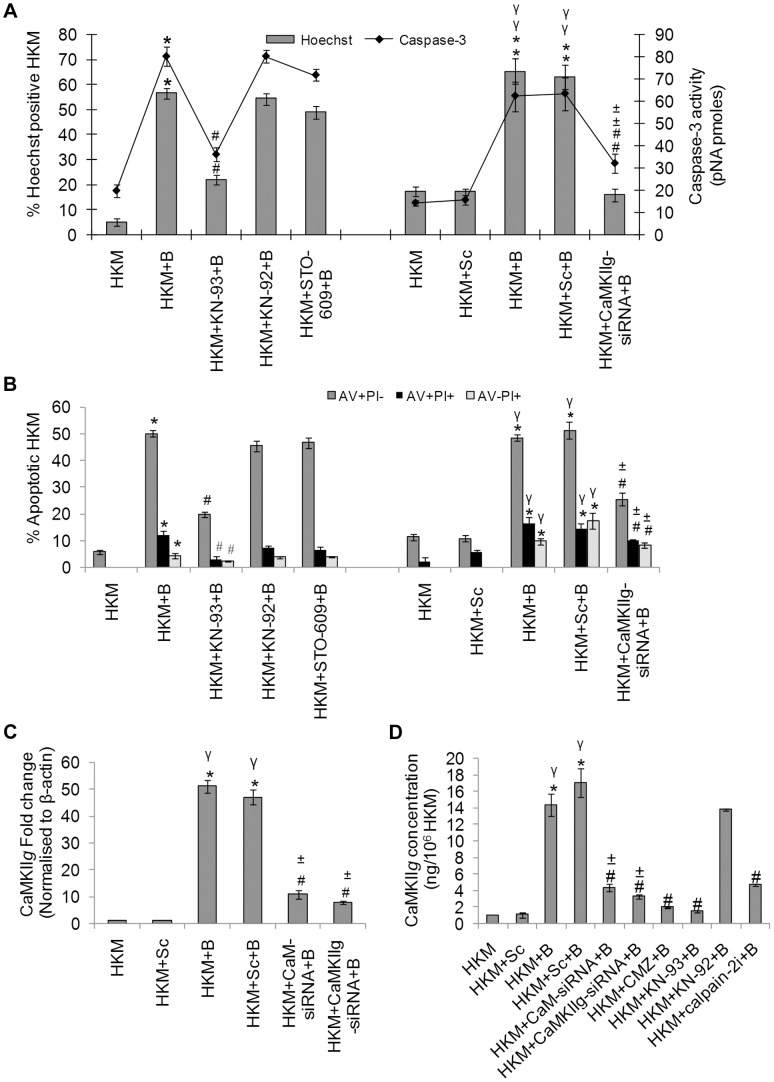
Among several downstream events that are initiated due to altered CaM activity the activation of CaMKs are believed to be important. We studied the role of CaMKK and CaMKII, two important CaMKs in our model. The HKM were pre-treated with specific pharmacological inhibitors of CaMKK and CaMKII and apoptosis was evaluated 24 h p.i. by Hoechst and AV-PI staining. First, we used the CaMKK inhibitor STO-609 which specifically inhibits the CaMKK activity by suppressing Ca2+-dependent signalling [21]. STO-609 had no preventive effect on A. hydrophila-induced HKM apoptosis (Figure 2A and 2B). Subsequently, we used the CaMKII specific inhibitor KN-93 and its structural analogue KN-92 to investigate the role of CaMKII on the process. KN-93 competitively blocks CaM binding to the kinase whereas KN-92 is a congener of KN-93 without CaM kinase inhibitory activity and is used as an experimental control [22]. We observed that pre-treatment with KN-93 attenuated caspase-3 activity and conferred significant protection to the infected HKM from A. hydrophila-induced apoptosis (Figure 2A and 2B). The inactive analogue KN-92 failed to inhibit HKM apoptosis suggesting a mediatory role of CaMKII on initiating apoptosis of infected HKM.
Figure 2. CaMKIIg is CaM-dependent and pro-apoptotic.
HKM were pre-treated separately with KN-93, KN-92, STO-609 or transfected with CaMKIIg-siRNA or scrambled siRNA then infected with A. hydrophila and checked for (A) Hoechst 33342 positive cells and caspase-3 activity and (B) AV-PI staining 24 h p.i. (C) HKM were transfected separately with CaM-siRNA, CaMKIIg-siRNA or scrambled siRNA then infected with A. hydrophila and CaMKIIg mRNA expression detected 2 h p.i. by real time PCR. (D) HKM were transfected with CaM-siRNA, CaMKIIg-siRNA, scrambled siRNA or pre-treated with CMZ, KN-93, KN-92, calpain-2i for different time periods then infected with A. hydrophila and CaMKIIg protein content detected 24 h p.i. using EIA kit. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM; γP<0.05, compared to HKM+Sc; #P<0.05, compared to HKM+B; ±P<0.05, compared to HKM+Sc+B. HKM, control head kidney macrophage; HKM+Sc, HKM transfected with scrambled siRNA; HKM+B, HKM infected with A. hydrophila; HKM+Sc+B, HKM transfected with scrambled siRNA infected with A. hydrophila; HKM+CaM-siRNA+B, HKM transfected with CaM-siRNA infected with A. hydrophila; HKM+CaMKIIg-siRNA+B, HKM transfected with CaMKIIg-siRNA infected with A. hydrophila; HKM+CMZ+B, HKM pre-treated with CMZ infected with A. hydrophila; HKM+KN-93+B, HKM pre-treated with KN-93 infected with A. hydrophila; HKM+KN-92+B, HKM pre-treated with KN-92 infected with A. hydrophila; HKM+STO-609+B, HKM pre-treated with STO-609 infected with A. hydrophila; HKM+calpain-2i+B, HKM pre-treated with calpain-2i infected with A. hydrophila.
To validate these observations qRT-PCR and EIA were done to evaluate the status of the specific transcript and protein. Degenerate primers for CaMKIIg were designed using the homologous stretch across vertebrates as the template. The PCR product was cloned, sequenced and the sequence showed 100% identity with CaMKIIg of zebrafish, Danio rerio. Primers for real time analysis were designed from this sequence for quantifying CaMKIIg levels. We observed that A. hydrophila infection led to significant increase in CaMKIIg mRNA expression with maximum levels being at 2 h p.i. (Figure S2A), which decreased thereafter reaching basal levels at 24 h p.i. (data not shown). We checked CaMKIIg concentrations using specific EIA kit 24 h p.i. as CaMKII is detected for prolonged period even after the Ca2+ signal has decayed [8]. A significant increase in CaMKIIg levels (P<0.05) was noted in the infected HKM which was inhibited (P<0.05) in presence of CMZ and KN-93, antagonists to CaM and CaMKIIg respectively (Figure 2D) but not by KN-92 in A. hydrophila-infected HKM. Using siRNAs specific to CaM and CaMKIIg that significantly down-regulated CaMKIIg at mRNA and protein levels (Figure 2C and 2D), significant attenuation of HKM apoptosis was also observed (Figure 2A and 2B). Taken together our data implicates Ca2+-induced CaM-CaMKIIg expression to be a critical event in A. hydrophila-induced HKM apoptosis.
CaM-CaMKII-Induced Activation of cAMP/PKA Pathway Is a Prime Event in A. hydrophila-Induced HKM Apoptosis
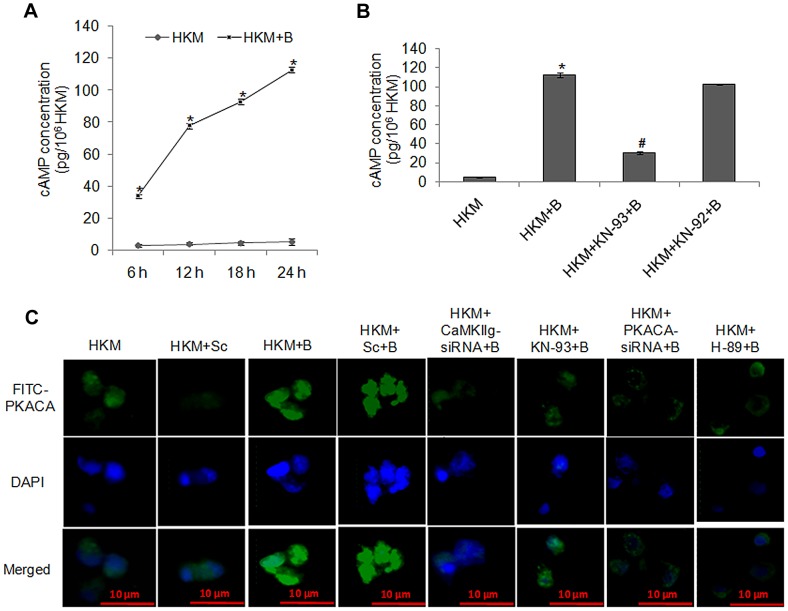
We studied cAMP/PKA signalling in A. hydrophila-induced HKM apoptosis as several macrophage activities appear to be controlled by this pathway [23]. Lysates from infected HKM were collected at different time intervals and assayed for intracellular cAMP production. It was observed that A. hydrophila-infection led to significant increase in intracellular cAMP levels at all time points studied with maximum production recorded at 24 h p.i. (Figure 3A). The cell-permeable cAMP analogue (8-Br-cAMP) when used as positive control elevated intracellular cAMP level and induced HKM apoptosis (data not shown). Our results thus implicate the role of cAMP in the pathophysiology associated with A. hydrophila infection.
Figure 3. A. hydrophila-infection triggers cAMP release, induces activation and nuclear translocation of PKACA.
(A) Cell lysates of HKM infected with A. hydrophila were checked for intracellular cAMP release at indicated time point p.i. using EIA kit. (B) HKM pre-treated with KN-93 or KN-92 were infected with A. hydrophila and cAMP release was measured in the cell lysates at 24 h p.i. (C) HKM were transfected with CaMKIIg-siRNA, PKACA-siRNA or scrambled siRNA or pre-treated with KN-93 or H-89 then infected with A. hydrophila and PKACA activation and nuclear translocation checked by immunofluorescence. The images are representative of three independent experiments. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM; #P<0.05, compared to HKM+B. HKM, control head kidney macrophage; HKM+Sc, HKM transfected with scrambled siRNA; HKM+B, HKM infected with A. hydrophila; HKM+Sc+B, HKM transfected with scrambled siRNA infected with A. hydrophila; HKM+CaMKIIg-siRNA+B, HKM transfected with CaMKIIg-siRNA infected with A. hydrophila; HKM+PKACA-siRNA+B, HKM transfected with PKACA-siRNA infected with A. hydrophila; HKM+KN-93+B, HKM pre-treated with KN-93 infected with A. hydrophila; HKM+KN-92+B, HKM pre-treated with KN-92 infected with A. hydrophila; HKM+H-89+B, HKM pre-treated with H-89 infected with A. hydrophila.
We next studied the role of CaMKIIg on initiating the production of cAMP in infected HKM since Ca2+-dependent signalling molecules often converge on cAMP [24]. As the cAMP levels were maximally induced at 24 h p.i., this time point was chosen for subsequent studies. It is evident from Figure 3B that A. hydrophila-induced cAMP levels were significantly reduced following pre-treatment with CaMKII specific inhibitor-KN-93. This suggests CaM-activated CaMKIIg plays an important role in cAMP generation in the infected HKM.
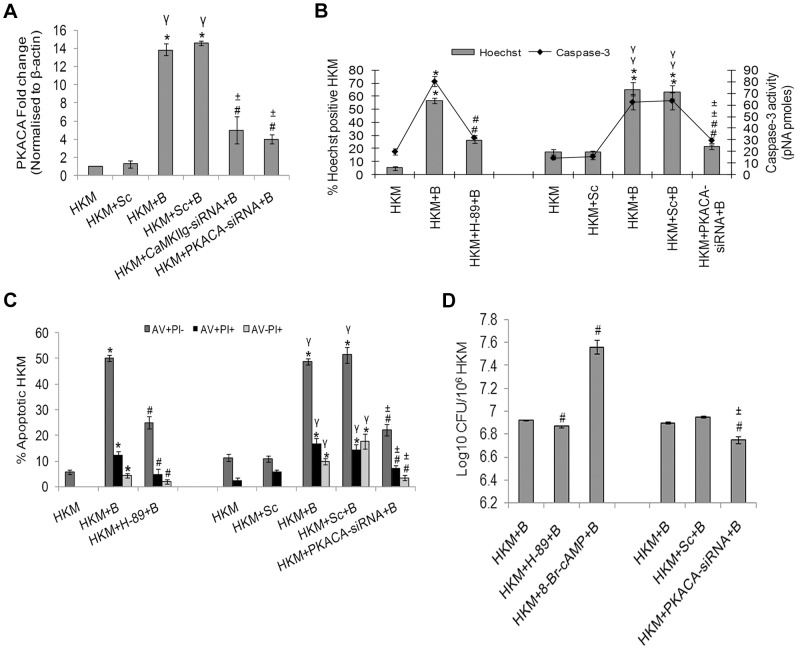
Protein kinase A is an important mediator of the cAMP dependent signalling pathway. The cyclic nucleotide cAMP causes PKA activation by binding to the regulatory subunit, triggering release, activation and nuclear translocation of PKACA [25], [26]. Therefore, our next step was establishing the involvement of PKACA in the pathogenicity of A. hydrophila. Degenerate primers specific for PKACA were designed the PCR product cloned, sequenced and nBLAST analysis suggested 100% identity with PKACA of Ictalurus sp. We used real time primers based on the sequence obtained and observed that A. hydrophila-infection induced a several fold increase in PKACA transcripts with maximum expression recorded by 2–6 h p.i. (P<0.05) (Figure S2B) and thereafter it started declining (data not shown) though significant level of PKACA mRNA expression was noted till 24 h p.i. This decline in PKACA transcripts at later time points probably serves as controlling mechanism to prevent the overshoot of cAMP dependent downstream events in the infected cells.
CaMKIIg and PKACA targeted siRNAs also led to significant inhibition of PKACA at the transcript level (Figure 4A). We then studied PKACA expression and nuclear translocation by immunofluorescence 24 h p.i. in infected HKM. The results with anti-PKACA antibody clearly suggested increased PKACA expression in nuclei of A. hydrophila-infected HKM (Figure 3C).
Figure 4. PKA is pro-apoptotic in A. hydrophila-infected HKM.
(A) HKM were transfected with CaMKIIg-siRNA, PKACA-siRNA or scrambled siRNA then infected with A. hydrophila and PKACA mRNA expression detected by real time PCR 2 h p.i. HKM were pre-treated with H-89 or transfected with PKACA-siRNA or scrambled siRNA then infected with A. hydrophila and checked for (B) Hoechst 33342 positive cells and caspase-3 activity and (C) AV-PI staining 24 h p.i. (D) A. hydrophila viability was checked following either pre-incubation of HKM with H-89, co-incubation with 8-Br-cAMP or transfection with PKACA-siRNA. The intracellular bacterial number was determined by dilution plating on nutrient agar plate. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM; γP<0.05, compared to HKM+Sc; #P<0.05, compared to HKM+B; ±P<0.05, compared to HKM+Sc+B. HKM, control head kidney macrophage; HKM+Sc, HKM transfected with scrambled siRNA; HKM+B, HKM infected with A. hydrophila; HKM+Sc+B, HKM transfected with scrambled siRNA infected with A. hydrophila; HKM+CaMKIIg-siRNA+B, HKM transfected with CaMKIIg-siRNA infected with A. hydrophila; HKM+PKACA-siRNA+B, HKM transfected with PKACA-siRNA infected with A. hydrophila; HKM+H-89+B, HKM pre-treated with H-89 infected with A. hydrophila; HKM+8-Br-cAMP+B, HKM exposed to 8-Br-cAMP infected with A. hydrophila.
We extended the study by including PKA specific inhibitor H-89, CaMKII specific inhibitor-KN-93 or transfected the HKM with CaMKIIg-siRNA and PKACA-siRNA. The inhibitor H-89 blocks PKA actions through competitive inhibition of the adenosine triphosphate (ATP) site on the PKA catalytic subunit [27]. We observed decreased expression and restricted nuclear movement of PKACA in the infected HKM at 24 h p.i. in presence of H-89, KN-93, CaMKIIg-siRNA and PKACA-siRNA (Figure 3C). Our results for the first time established the role of CaMKIIg on PKACA activation in A. hydrophila-infected HKM. The presence of H-89 and PKACA-siRNA also led to significant alleviation (P<0.05) of apoptotic death and diminished caspase-3 activity in the infected HKM (Figure 4B and 4C) suggesting the critical role of PKA pathway on the pathogenicity induced by the bacteria.
We next studied the link between the activation of PKA and intra-cellular survival of A. hydrophila. Pre-incubation of HKM with H-89 or PKACA-siRNA reduced (P<0.05) recovery of intracellular bacteria (Figure 4D). Besides, we found that H-89 addition till 60 mins p.i. reduced the bacterial load and beyond that exhibited little inhibitory effect (Figure S3). No direct effect of H-89 on A. hydrophila was observed as adding the inhibitor at the particular concentration did not affect the viability, growth and initial uptake by HKM (data not shown).
ERK 1/2 Activation Is Pro-Apoptotic in A. hydrophila Infection and Depends on cAMP/PKA Pathway
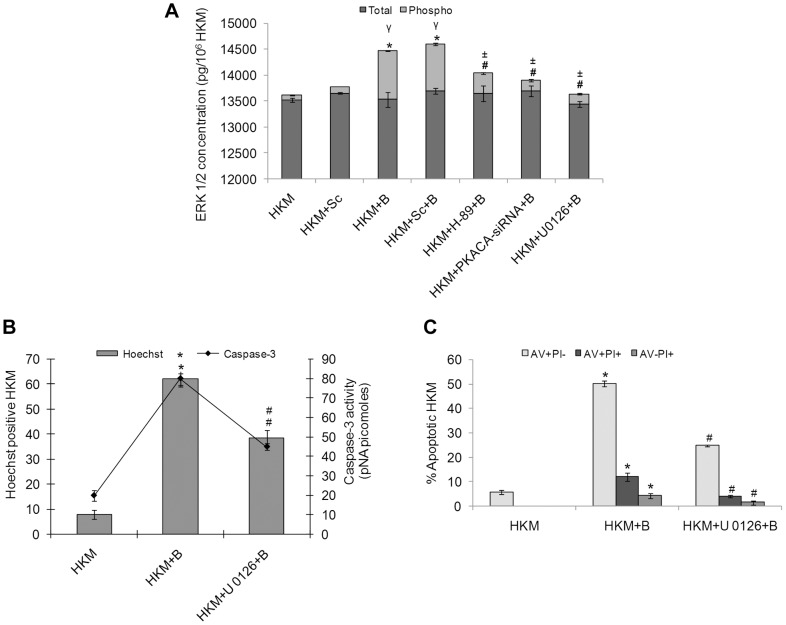
The ERK 1/2 pathway has been implicated both as pro-and anti-apoptotic in host macrophage responses. We aimed to investigate the involvement of ERK 1/2 activation in A. hydrophila-induced HKM apoptosis. The HKM were infected with A. hydrophila in presence or absence of specific inhibitors, lysed at indicated time intervals and the changes in the levels of total and phosphorylated ERK 1/2 measured using specific EIA kits. It is evident from Figure 5A that there was significant increase in pERK 1/2 levels in the A. hydrophila-infected HKM though no noticeable change was observed in total-ERK 1/2 levels. The role of cAMP/PKA on ERK 1/2 activation has been reported [28]. To investigate this possibility the HKM were pre-treated with PKA specific inhibitor H-89 and changes in total and pERK 1/2 levels studied. We observed that H-89 significantly inhibited pERK 1/2 levels in the infected HKM implicating the role of cAMP/PKA pathway in initiating the kinase activity. U0126 is a non-ATP competitive inhibitor of mitogen-activated protein kinase kinase (MEK) which exerts its effect by inhibiting the ability of MEK to phosphorylate downstream ERK 1/2 [29]. Hence it served as an effective negative control in the study. To further substantiate the role of cAMP/PKA pathway on ERK 1/2 activation we used PKACA-siRNA and our results (Figure 5A) confirmed ERK 1/2 activation to be downstream to cAMP/PKA in A. hydrophila-infected HKM.
Figure 5. A. hydrophila-induced ERK 1/2 phosphorylation is PKA mediated and is pro-apoptotic in HKM.
(A) HKM were transfected with PKACA-siRNA, scrambled siRNA or pre-treated with H-89, U 0126 then infected with A. hydrophila and checked for total ERK and pERK activity 24 h p.i. using EIA based kits. HKM were pre-treated with or without U 0126 then infected with A. hydrophila and checked for (B) Hoechst 33342 positive cells and caspase-3 activity and (C) AV-PI staining 24 h p.i. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM; γP<0.05, compared to HKM+Sc; #P<0.05, compared to HKM+B; ±P<0.05, compared to HKM+Sc+B. HKM, control head kidney macrophage; HKM+Sc, HKM transfected with scrambled siRNA; HKM+B, HKM infected with A. hydrophila; HKM+Sc+B, HKM transfected with scrambled siRNA infected with A. hydrophila; HKM+PKACA-siRNA+B, HKM transfected with PKACA-siRNA infected with A. hydrophila; HKM+H-89+B, HKM pre-treated with H-89 infected with A. hydrophila; HKM+U 0126+B, HKM pre-treated with U 0126 infected with A. hydrophila.
We next investigated the contribution of ERK 1/2 activation on HKM apoptosis. For that HKM were pre-treated with or without U 0126 prior to infection with A. hydrophila and checked for apoptotic death using Hoechst 33342, AV-PI staining and caspase-3 activity 24 h p.i. It is clear from Figure 5B and 5C that inhibition of ERK 1/2 significantly inhibited (P<0.05) A. hydrophila-induced HKM apoptosis and lowered caspase-3 activity. Taken together our observations suggest ERK 1/2 activation is downstream event of cAMP/PKA pathway and is pro-apoptotic in A. hydrophila-infected HKM.
Discussion
Calmodulin plays a significant role in microbial pathogenicity. Its role has been implicated in the pathogenicity of Mycobacterium sp. [28], Clostridium perfingens [30] and for survival of Pneumocystis-infected alveolar macrophages [31]. The presence of well conserved CaM is well documented in fish [32], [33]. As we observed increased intracellular Ca2+-levels in infected HKM [19] and CaM being a well-known Ca2+-sensor, we hypothesised a role of CaM on the pathogenicity of A. hydrophila. Indeed, results obtained from qRT-PCR, specific EIA assays, siRNA and pharmacological inhibitors conclusively demonstrated the importance of Ca2+-induced CaM expression in the initiation of A. hydrophila-induced HKM apoptosis. The kinetics of CaM expression, both at transcript and protein level demonstrated maximum expression at 2 h p.i. This suggested CaM expression at protein level closely follows pattern at transcript level, a phenomenon observed for many signalling molecules [34]. To our knowledge, this is the first report that clearly documents the pro-apoptotic involvement of CaM in A. hydrophila infections. We had earlier reported the role of calpain-2 in A. hydrophila-induced HKM apoptosis. We questioned whether the two Ca2+-dependent molecules crosstalk to initiate HKM apoptosis. Contrary to our expectations we did not observe any crosstalk between calpain-2 and CaM activity (data not shown), which suggests two possibilities: (a) CaM is resistant to the lytic action of calpains [35] and (b) CaM and calpain activity are independent to each other in our model.
Although there is little information regarding the mechanisms of CaM involvement in apoptosis, there are reports suggesting pro-apoptotic roles of CaM-dependent enzymes including CaMKs [8]. We reasoned that downstream activation of CaMKs is important for the initiation of apoptosis and inhibiting the kinase activities would confer protection to the infected HKM. To investigate this, we investigated the roles of CaMKK and CaMKII in initiating A. hydrophila-induced HKM apoptosis. We selected these two kinases as they are conserved, well characterised, have wide tissue distribution including macrophages and work through distinct pathways regulating diverse biological functions [8]. Our inhibitor studies indicated CaMKII involvement and clearly ruled out the involvement of CaMKK in the process. This observation also ruled out the involvement of CaMKI and CaMKIV in the process as both kinases require upstream phosphorylation of CaMKK for getting activated [7]. CaMKII has been reported to be both pro-apoptotic [6] and anti-apoptotic [10]. To investigate the role of CaMKII in A. hydrophila-induced HKM apoptosis we selected the gamma-isoform, CaMKIIg as it is largely reported in macrophage functioning [6], [9]. Use of siRNAs confirmed the involvement of CaMKIIg and its modulation by CaM in HKM due to A. hydrophila infection. It is worth mentioning that there is little information on the involvement of CaMKIIg in pathogen-induced apoptosis except for its role in the pathogenicity induced by Mycobacterium sp. [28]. These results clearly indicated CaMKIIg to be important in Ca2+-mediated apoptotic signalling downstream of CaM in A. hydrophila-infected HKM.
CaMKs are considered to be the likely substrates for calpain proteolysis [35]. Calpain-mediated CaMKIV activation is well documented in the apoptosis of SH-SY5Y cells [36] and cerebellar granule cells [37]. In contrast, there is no report on the role of calpains on CaMKIIg expression especially in fish macrophages. In this connection, it is worth mentioning that pre-treatment with calpain-2 inhibitor led to significant decline in CaMKIIg expression in infected HKM. Our finding for the first time suggested the existence of an alternate or calpain-mediated pathway for CaMKIIg expression in fish macrophages following A. hydrophila-infection. Further studies are needed to understand the crosstalk between CaMKIIg and activated calpains.
The role of cAMP varies between the prokaryotic and eukaryotic system. In eukaryotes cAMP signalling is important in diverse biological processes such as metabolism, memory formation and apoptosis [38]. To begin with, we initially looked for the changes of cAMP and observed significant increase in cAMP levels following A. hydrophila infection. Earlier studies with Brucella suis [39], Bordetella pertussis [40], Bacillus anthracis [41], Mycobacterium sp [28], Pseudomonas aeruginosa [42] and Porphyromonas gingivalis [43] also reported increased cAMP production via different mechanisms implicating cAMP in the pathogenicity of these microbes. It is interesting to note that the levels of A. hydrophila-induced cAMP observed by us is comparable to those recorded in many of these microbes which make us believe that such an A. hydrophila-induced effect could also be important for the pathogenicity of this bacteria. Besides, we also observed a time-dependent increase in cAMP production following A. hydrophila infection which corroborates with the earlier reports of Chopra et al., (2000) [44] in which a gradual increase in cAMP production was seen in the murine macrophage cell line RAW 264.7, treated with the A. hydrophila-toxin Act. There are also reports suggesting cAMP to be both pro- and anti-apoptotic [11]. A time-dependent increase of cAMP levels in infected HKM suggested that downstream cAMP-dependent pathways ultimately determine the outcome of infection in infected cell. This also led us to the identification that elevated cAMP levels are indeed due to CaM-dependent CaMKIIg activity in the A. hydrophila-infected HKM. Determining how the CaM-CaMKIIg axis is connected with cAMP production would be an interesting direction of the future. Previous studies have suggested that some isoforms of AC can be activated by Ca2+-CaM-CaMK by phosphorylation of the serine residues on AC [45]. It remains to be seen if this is also true in our system.
On establishing the role of cAMP we looked for its downstream targets in the infected HKM. Protein kinase A being an important intermediary in cAMP-dependent signalling, was an attractive candidate. Our observations using qRT-PCR, siRNA, cAMP/PKA-specific inhibitor, H-89 and PKACA antibody suggested that activation of cAMP/PKA is a downstream consequence of CaMKIIg activation. Pre-treatment with H-89 did not influence CaMKIIg activation in the infected HKM (data not shown). The cAMP/PKA pathway attributed to the virulence of A. hydrophila as inhibition of PKA activity led to increased HKM viability with concomitant decline in the number of intracellular bacteria. If PKA activation is essential for survival of A. hydrophila inside the HKM at what stage of infection is it important? We suggest PKA activation is critical at initial stages of infection as inhibiting PKA activity at late stages of infection had little effect on the replication of intra-cellular A. hydrophila. Our observations are similar to those reported by Gross et al., (2003) [39] for Brucella suis and we believe that A. hydrophila alike B. suis initiates an early cAMP/PKA-mediated virulence trait that helps in eluding macrophage-microbicidal responses critical at the onset of infection. Although, it is not possible from this study to conclude on how A. hydrophila affects cAMP/PKA pathway; we speculate that the bacteria could be releasing toxins which directly phosphorylate ACs or inhibit the activation of PDEs leading to increased accumulation of the cyclic nucleotide inside the HKM.
The participation of MAPKs has been widely observed during pathogenic invasion [46]. The genes encoding the MAPK family proteins are well conserved and have been reported in fish to be regulating various downstream targets such as transcription factors and heat shock proteins [47]. The involvement of MAPK cascade is beginning to be understood in the pathogenicity of A. hydrophila in fish [48]. Amongst the MAPK family members, ERK 1/2 has been reported to act both as pro- and anti-apoptotic factor under different conditions of pathogenic-stress [46]. We sought to understand its role in A. hydrophila-infection and our results conclusively demonstrate pro-apoptotic role of ERK 1/2 in A. hydrophila-infected HKM. ERK 1/2 activation is also reported in A. hydrophila pathogenicity though in mammalian system [1]. Interestingly, we also noted pro-apoptotic role of JNK and p38, the other members of the MAPK family in initiating A. hydrophila-induced HKM apoptosis (unpublished observations). This suggests that A. hydrophila uses a common signalling mechanism to activate the MAPK cascade to induce apoptosis in different hosts. Thus, our observations contradict those reported in Mycobacterium [27], Pseudomonas [49] or Yersinia [50] where the differential activation of MAPK family was reported to be important for pathogenicity.
Caspases are members of a family of cysteine proteases that are involved in apoptosis induced by various stimuli in different cell types, including macrophages. We recently reported A. hydrophila-induced HKM apoptosis to be caspase-3 mediated [19]. Presently, we aimed to determine what other signals initiated out of Ca2+ wave have a role in the activation of caspase-3 following A. hydrophila infections. From the available literature it is evident that a relationship between MAPKs and caspase-3 exist with reports suggesting MAPK activating caspase-3 [51] and vice versa [52]. Interestingly, our results clearly showed caspase-3 to be downstream of MAPK (ERK 1/2) in A. hydrophila-infected HKM. The means by which MAPKs regulate caspase-3 activity remain unclear and further investigation in this direction will aid in understanding the pathogenicity of this bacteria.
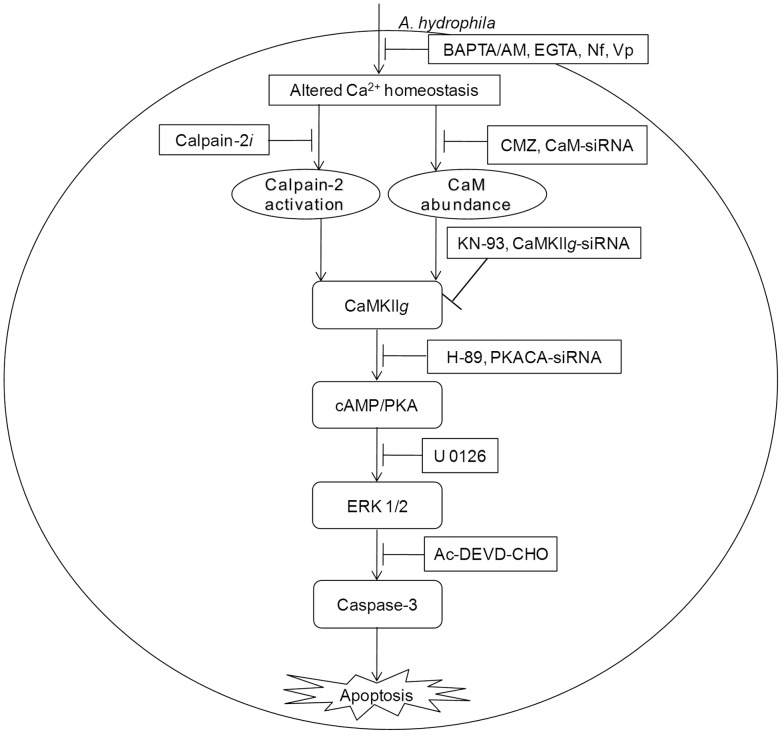
To conclude, our findings emphasise the unrecognized role of CaM in initiating A. hydrophila-induced HKM apoptosis. We propose that the Ca2+-influx initiated by A. hydrophila activates pro-apoptotic CaM and downstream CaMKIIg in infected HKM. Calpains also appear to have some role on CaMKIIg expression. CaMKIIg in turn initiates a network of signals resulting in the downstream activation of cAMP/PKA and MAPK pathways respectively and the cascade of events culminate in caspase-3 activation and apoptosis of infected HKM (Figure 6).
Figure 6. CaM-CaMKII-cAMP/PKA-ERK 1/2 axis leads to caspase-3 mediated apoptosis of A. hydrophila-infected HKM.
A. hydrophila-elevated Ca2+ leads to downstream calpain-2 activation and CaM expression. The pathways converge at CaMKIIg to induce cAMP/PKA mediated activation of ERK 1/2 leading to caspase-3 mediated apoptosis of A. hydrophila-infected HKM.
Materials and Methods
All the chemicals used in this study were purchased from Sigma unless otherwise stated.
Ethics Statement
All animal experiments described in the present study were approved by the Animal Ethics Committee of University of Delhi (DU/ZOOL/IAEC-R/2013/33) and carried out in accordance with animal experimentation protocols approved by The Prevention of Cruelty to Animals Act, Govt. of India.
Animal Care and Maintenance
Catfish (Clarias gariepinus, Siluriformes, 100±20 g) were maintained in 50-L glass tanks (2–3 fish per tank) under natural conditions. The water quality, dissolved oxygen content and pH were monitored regularly in each tank. The fish were fed boiled chicken liver ad libitum and acclimatized to laboratory conditions for 15 days before use for experimental purpose. During this period and when the experiments were conducted, fish health was routinely monitored by appearance and pathological examinations as mentioned earlier [53].
Bacterial Preparations
The A. hydrophila (Strain 500297) was a gift from Dr. T. Ramamurthy, National Institute of Cholera and Enteric Diseases (NICED), India. The bacteria were grown to late log phase (12 h) in brain heart infusion broth (BHI, HiMedia) containing 100 μg mL-1 ampicillin at pH 7.4 overnight at 30°C with aeration and maintained in nutrient agar slants at 4°C. The pathogenicity of the strain was confirmed in vitro by hemolysin and cytotoxicity assays and by the ability to induce characteristic UDS lesions when injected into the fish [18].
Isolation of HKM
Head kidneys were aseptically removed and placed in RPMI-1640 (Gibco-Invitrogen) with phenol-red indicator and supplemented with 25 mM HEPES (Gibco-Invitrogen) containing 1% penicillin-streptomycin (complete-RPMI). Single cell suspensions of each pair of head kidney were prepared using 100 μm wire mesh, diluted and the total cell population pelleted by centrifugation at 400×g for 10 mins at 4°C. The supernatant was discarded and the re-suspended pellet layered on discontinuous percoll density gradient (34/51) and centrifuged at 400×g for 20 mins at 4°C. The phagocyte rich fraction appearing above the 34/51 interface was collected, washed and enriched for macrophages as described earlier [19]. The purity of the HKM was checked by staining with Wright Giemsa Stain and viability determined using 0.4% trypan blue dye exclusion method.
Infection Procedure
The HKM were seeded in 24 well plates. The A. hydrophila were grown to mid log phase, harvested and infected into HKM at a multiplicity of infection (MOI) of 50 for 60 mins. To kill extracellular bacteria, the infected HKM cultures were incubated for a further 1 h in RPMI containing 30 μg mL−1 chloramphenicol. Exposure to 30 μg mL−1 chloramphenicol for 1 h was sufficient to kill 100% of microorganisms but had no effect on macrophage viability. Uninfected HKM served as control and maintained in complete-RPMI for the same time.
The siRNA transfection was done using HiPerFect Transfection Reagent (Qiagen) as per manufacturer's instructions. The siRNA-Hiperfect complex (5 μl each) was mixed gently in Opti-MEM (Invitrogen) and incubated for 20 mins to allow complex formation. This complex was added to the isolated HKM maintained in Opti-MEM, mixed properly and incubated at 30°C and 5% CO2. Cell viability was continuously monitored and after 5 h of incubation, media was changed to complete-RPMI. At 24 h post-transfection, HKM were washed, placed in fresh complete-RPMI and infected with A. hydrophila as mentioned earlier and proceeded for apoptotic studies, qRT-PCR and protein assays to confirm knockdown. Targeted [(CaM, SENSE-5′-CCAUUACGACCAAAGAGUU-3′ and ANTISENSE-5′-AACUCUUUGGUCGUAAUGG-3′); (CaMKIIg, SENSE-5′-GGACAUUUGGGCUUGUGGA-3′ and ANTISENSE-5′-UCCACAAGCCCAAAUGUCC-3′); (PKACA, SENSE-5′-CAGUAAAGGCUACAAUAAA-3′ and ANTISENSE-5′-UUUAUUGUAGCCUUUACUG-3′)] plus siCONTROL non-targeting siRNA pool (HS Number 29349990700) 5 nM each were used.
For inhibitor studies, HKM were pre-treated with or without 10 μM caspase-3 inhibitor (Acetyl-Asp-Glu-Val-Asp-aldehyde, Ac-DEVD-CHO, Promega), 50 μM calpain-2 inhibitor (N-acetyl-leucyl-leucyl-methioninal, calpain-2i), 4 nM CaM inhibitor (calmidazolium chloride, CMZ), 20 μM CaMKII inhibitor (KN-93, Calbiochem), 20 μM inactive analogue of KN-93 (KN-92), 20 μM cAMP dependent PKA inhibitor (H-89) for 1 h or 5 mM intracellular calcium chelator {[1,2-bis-(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetra (acetoxymethyl) ester], BAPTA/AM)}, 10 mM extracellular calcium chelator [Ethyleneglycol-bis (β-aminoethyl-N,N,N′,N′-tetraacetic acid), EGTA], 10 μM calcium channel blocker nifedipine (Nf), 5 μM calcium channel blocker verapamil (Vp), 100 μM CaMKK inhibitor (STO-609), 20 μM ERK 1/2 inhibitor (U 0126, Calbiochem) for 2 h followed by A. hydrophila infection as mentioned above and checked for apoptotic studies and protein assays. The doses of different inhibitors were selected on the basis of inhibitor specificity and cytotoxicity. The HKM treated with the indicated concentrations of the inhibitors remained as viable as control HKM at all time points as determined by the trypan blue (0.4%) dye exclusion method and were maintained during the entire course of the experiment.
Apoptosis Study
For apoptosis studies the HKM (1×106) were pre-treated separately with or without indicated concentrations of targeted or scrambled siRNAs, indicated concentrations of inhibitors for different time periods then infected with A. hydrophila at an MOI of 50 as described above. The HKM were subjected to Hoechst 33342 and Annexin-V-fluorescein isothiocyanate-Propidium Iodide (AV-FITC-PI, BD Pharmingen) staining 24 h p.i. as mentioned earlier [19]. For the Hoechst 33342 study, HKM were collected, washed and fixed with 3.7% paraformaldehyde solution at room temperature. After fixation, the HKM were stained with Hoechst 33342 (2 μg mL−1 in 1×PBS) and visualized under fluorescence microscope (×40, Nikon Eclipse 400) within 30 mins of adding the stain. A total of 100 cells were studied in each field and three such fields were included to determine the percentage of apoptotic HKM. For the AV-FITC-PI study the HKM were collected, washed, fixed with 3.7% paraformaldehyde solution and stained with AV-FITC-PI following manufacturer's instructions. The HKM were observed under the fluorescence microscope (×40, Nikon Eclipse 400) within 30 mins of adding the dye. A total of 100 cells were studied in each field and three such fields were included to determine the percentage of apoptotic HKM.
RNA Isolation, Reverse Transcriptase (RT)-PCR, Cloning, Amplification, Sequencing and Quantitative Real-Time PCR
The HKM (2×107) transfected separately with or without indicated concentrations of targeted or scrambled siRNA were infected with A. hydrophila and at indicated time point p.i., culture was terminated, total RNA was isolated using TRIZOL according to manufacturer's instructions. Total RNA dissolved in diethylpyrocarbonate (DEPC) water was treated with deoxyribonuclease I (RNase-free, MBI Fermentas) at 37°C for 30 mins and the DNase was inactivated by incubation with 50 mM EDTA at 65°C for 10 mins. One microgram of total RNA from each sample was reverse transcribed using first strand cDNA synthesis kit as per manufacturer's instructions (MBI Fermentas). The resulting cDNA was then subjected to PCR amplifications using degenerate primers for each gene (Table 1). The amplified product was then gel extracted using QIA quick Gel Extraction Kit (Qiagen) and cloned into pGEM-T EASY vector (Promega) and sequenced (Macrogen). The sequences obtained (Table 2) were aligned to nBLAST and have been submitted to NCBI database.
Table 1. List of degenerate primers.
| CaM | |
| Forward Primers | Reverse Primers |
| FP1 5'- GAR GAR CAR ATY GCW GAR TTC -3' | RP1 5'- YTT CAT YTT YCT DGC CAT CAT -3' |
| FP2 5'- CAG GAY ATG ATY AAY GAR GT -3' | RP2 5'- CAT CAT YTG KAC RAA YTC TTC -3' |
Table 2. Gene sequences.
| >CaM (Accession No. KF892532) |
| GAGGAGCAAATCGCAGAGTTCAAAGAGGCATTCTCACTCTTTGACAAAGATGGAGATGGCACCATTACGACCAAAGAGTTGGGGACTGTCATGCGCTCTCTTGGCCAGAACCCAACAGAGGCTGAGCTGCAGGACATGATCAATGAAGTGGATGCTGATGGGAATGGGACGATAGACTTCCCAGAGTTCCTGACCATGATGGCAAGGAAGATGAAG |
| >CaMKII g (Accession No. KF892533) |
| CATATTCACCAGCATGACATCGTGCACAGAGACCTCAAGCCTGAGAACCTGCTGTTGGCCAGTAAGATGAAAGGAGCAGCAGTTAAGCTGGCTGACTTTGGACTGGCGATTGAGGTACAGGGAGACCAGCAGGCATGGTTTGGCTTTGCAGGCACACCTGGTTACTTATCTCCAGAAGTCCTGAGAAAGGACCCTTATGGCAAACCTGTGGACATTTGGGCTTGTGGAGTTATCCTCTATATTCTTCTTGTGGGATATCCTCCATTCTGGGATGAAGATCAGCATAGGCTCTACCAGCAAATTAAGGCCGGAGCTTATGATTTTCCTTCTCCTGAGTGGGACACCGTTACCCCCGAGGCCAAGAACCTCATCAACCAGATGCTGACCATCAACCCAGCCAAGAGGATCACCGCTGAGCAGGCCCTCAAGCACCCGTGGGTCTG |
| >PKACA (Accession No. KF892534) |
| CAGCAGGGCTACATACAGGTTACGGATTTCGGCTTTGCCAAGCGTGTAAAGGGCCGGACCTGGACGTTGTGCGGCACTCCTGAGTACCTGGCTCCAGAGATTATCCTCAGTAAAGGCTACAATAAAGCAGTAGATTGGTGGGCTCTGGGTGTGCTGATTTATGAAATGGCTGCAGGATATCCCCCGTTCTTTGCAGACCAGCCCATTCAGATCTACGAGAAGATCGTCTCGGGCAAGGTGCGCTTCCCTTCTCACTTCAGCTCAGACCTGAAGGACCTGCTAAGGAACCTGCTACAGGTAGACCTGACAAAGCGCTTTGGCAACCTCAAGAATGGTGTCAACGACATCAAAGGCCACAAGTGGTTCGCCACCACTGACTGGATTGCCATCTACCAGA |
Real-Time PCR for quantitation was done for CaM, CaMKIIg and PKACA using SYBR green PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions (ABI ViiA, Applied Biosystems) using gene specific primers (CaM forward: 5′-AAGATGGAGATGGCACCATTA-3′ and reverse: 5′-TGGTCAGGAACTCTGGGAAG-3′; CaMKIIg forward: 5′- TTGTTGACATCTGGGCATGT-3′ and reverse: 5′- CATAAGCTCCGCTTTGATCT-3′; PKACA forward: 5′-AGGTTACGGATTTCGGCTTT-3′ and reverse: 5′-GATCTGAATGGGCTGGTCTG-3′; β-actin forward: 5′- CGAGCAGGAGATGGGAACC-3′ and reverse: 5′-CAACGGAAACGCTCATTGC-3′). The PCR mixture (total volume 10 μl) contained 5 μl of Power SYBR Green, 1 μl of the first-strand cDNA (diluted to 1/100 of the original concentration) and 0.20 μM of forward and reverse primers. Amplification and detection of all genes was performed with ABI ViiA using the following thermal cycling conditions: one cycle of 95°C for 10 mins, 40 cycles of 95°C for 15 s, 60°C for 1 mins. Reactions were performed with cDNAs from six independent experiments and the expression of each transcript was quantified by the comparative ΔΔCT method and normalized to those of β-actin chosen as endogenous control.
CaM Assay
The cell lysates of HKM (1×106) were pre-treated separately with or without indicated concentrations of CaM-siRNA, scrambled siRNA and inhibitors including BAPTA/AM, EGTA, Vp, Nf for different time periods then infected with A. hydrophila at an MOI of 50 as described earlier. The HKM were collected at indicated time point p.i. and checked for CaM concentration by Enzyme Immunoassay Kit (EIA, USCN Life Sciences) as per manufacturer's instruction with brief modifications. Briefly, the lysates along with the standards provided were diluted with diluents, placed at the bottom of each well in triplicate and incubated overnight at 4°C. The liquid of each well was removed carefully and 100 μL of Detection Reagent A was added to each well and incubated for 1 h at 30°C. The solutions were aspirated carefully and each well washed with 200 μL of wash buffer. The Detection Reagent B (100 μL) was added to the wells for 30 mins at 30°C and washed with 200 μL of wash buffer. Ninety microliters of substrate solution was added to each well and the reaction terminated with the addition of 50 μL of stop solution after 25 mins of incubation and the readings taken at 450 nm. The amount of CaM was interpolated from the standard curves obtained by plotting the O.D. of the standards. All chemicals used in the assay were provided with the kit.
CaMKIIg Assay
The HKM (1×106) were pre-treated separately with or without indicated concentrations of CaM-siRNA, CaMKIIg-siRNA, scrambled siRNA and inhibitors including CMZ, KN-93 and KN-92 for different time periods then infected with A. hydrophila at an MOI of 50 as described earlier. The HKM were collected at 24 h p.i. and quantitative estimation of CaMKIIg was done in cell lysates using EIA Kit for CaMKIIg (USCN) as per manufacturer's instruction. Briefly, HKM were collected by centrifugation, re-suspended in chilled lysis buffer provided with the kit and incubated on ice for 30 mins. Following incubation the cell lysates were collected by centrifugation at 16,000×g for 20 mins at 4°C. Hundred microlitres each of standards and cell lysates were added into the wells and incubated for 5 h at 30°C. Following incubation the liquid from each well was removed and 100 μl of Detection Reagent A added to each well and further incubated for 1 h at 30°C. The Detection Reagent A was removed and the wells washed several times with 200 μl of 1× wash solution followed by addition of 100 μl of Detection Reagent B to each well and incubated for 30 mins at 30°C. The wells were washed, 90 μl of substrate added to each well and incubated for 30 mins at room temperature. The reaction was stopped by adding 50 μl stop solution, the readings taken at 450 nm in ELISA plate reader (BMG labtech) and the amount of CaMKIIg interpolated from the standard curves obtained by plotting the O.D. of the standards. All chemicals used in the assay were provided with the kit.
cAMP Assay
The cell lysates of HKM (1×106) were pre-treated separately with or without indicated concentrations of inhibitors including KN-93 and KN-92 for different time periods then infected with A. hydrophila at an MOI of 50 as described and checked for intracellular cAMP level by ELISA kit (Enzo Life Sciences) as per manufacturer's instruction. Briefly, HKM were collected by centrifugation and resuspended in 100 μl of 0.1 N HCl for 10 mins at room temperature followed by centrifugation at 800×g to pellet cellular debris. Hundred microlitres each of standards and supernatant was added to the bottom of appropriate wells, 50 μL each of conjugate and antibody then added and incubated on a plate shaker at room temperature for 4 h. The plate was washed, 200 μL of substrate added to each well and incubated for 1 h at room temperature. The reaction was finally stopped by adding 50 μl of stop solution and reading taken at 405 nm (BMG Labtech). All chemicals used in the assay were provided with the kit.
Immunofluorescence for PKACA
The HKM (4×106) were pre-treated separately with or without indicated concentrations of CaMKIIg-siRNA, PKACA-siRNA, scrambled siRNA and inhibitors including KN-93 and H-89 for different time periods then infected with A. hydrophila at an MOI of 50 as described earlier. The HKM were collected at 24 h p.i., fixed in methanol/acetic acid (1∶1, v/v) for 30 mins on ice and subsequently incubated with blocking and permeabilizing solution (PBS, 2 mg/ml BSA, 0.2 mg/ml saponin). The cells were washed and incubated with primary antibody for PKACA (1: 200, Sc-903, anti-PKACA raised in rabbit, Santacruz) overnight at 4°C. The HKM were washed, incubated with secondary antibody (1: 250, FITC-conjugated, anti-rabbit, Cell Signalling Technology) for 1 h at room temperature and mounted on microslide with cover slips using fluoroshield. Nuclear staining was done with DAPI (1 μg/ml). The expression and nuclear translocation of PKACA was studied under fluorescence microscope (×100 oil immersion, Zeiss).
ERK-1/2 Assay
Total ERK was measured using ERK 1/2 EIA (Enzo Life Sciences) and pERK was measured with pThr202/Tyr204 ERK 1/2 EIA kit (Enzo Life Sciences) [54] using chemicals supplied with the kit. Briefly, HKM (1×106) were pre-treated separately with or without indicated concentrations of PKACA-siRNA, scrambled siRNA and inhibitors including H-89 and U 0126 for different time periods then infected with A. hydrophila at an MOI of 50 as described earlier. Total ERK 1/2 and pERK 1/2 activities were measured 24 h p.i. The HKM were collected by centrifugation and re-suspended in chilled lysis buffer and incubated on ice for 30 mins. The cell lysate was collected by centrifugation at 16,000×g for 20 mins at 4°C. The assay was performed in a total volume of 100 μl in microtiter plate coated with mouse monoclonal antibody specific to ERK 1/2 provided with the kit. Hundred microliters of the samples were pipetted to the wells and incubated for 2 h at room temperature with shaking. The contents of the wells were washed and 100 μl of polyclonal ERK 1/2 and pERK 1/2 antibody were added separately into respective well and incubated overnight at 4°C. The plates were washed, incubated for 45 mins with HRP-conjugated secondary antibody at room temperature following which 100 μl of TMB substrate containing hydrogen peroxide added to each well and incubated for 30 mins. Finally, 100 μl of stop solution was added and colour development studied at 450 nm. Triplicate sets were prepared containing serially diluted standards, blank (no cell extract), negative control (extract from untreated cells) and A. hydrophila-infected HKM. The amounts of ERK 1/2 and pERK 1/2 in the cell lysates were interpolated from the standard curves obtained by plotting the O.D. of the standards. All chemicals used in the assay were provided with the kit.
Caspase-3 Assay
The caspase-3 (DEVDase) assay was performed using caspase-3 assay kit (Promega) [19]. Briefly, HKM (1×106) were pre-treated separately with or without indicated concentrations of CaM-siRNA, CaMKIIg-siRNA, PKACA-siRNA, scrambled siRNA and inhibitors including CMZ, KN-93, KN-92, STO-609, H-89 and U 0126 for different time periods then infected with A. hydrophila at an MOI of 50 as described earlier. The HKM were collected 24 h p.i. and re-suspended in 50 μL of chilled cell-lysis buffer followed by incubation on ice for 10 mins. The cell lysate were then collected by centrifugation at 10, 000×g for 5 mins at 4°C. The caspase-3 (DEVDase) assay was performed in a total volume of 100 μL in 96 well plates. Triplicate wells were prepared containing blank (no cell extract), negative control (extract from untreated cells) and A. hydrophila-infected cells. In 10 μL cell extract 32 μL caspase buffer, 2 μL DMSO, 10 μL DTT (100 mM) and 2 μL of the DEVD-pNA substrate were added. The plates were incubated at 37°C for 5 h and absorbance read at 405 nm (BMG Labtech). All chemicals used in the assay were provided with the kit.
Assay to Detect Intracellular Load of A. hydrophila
The PKA specific inhibitor, H-89 was added to HKM (1×106) at different time point viz., pre-infection, simultaneous as well as post-infection to A. hydrophila infection. In another set of experiment, HKM were transfected with PKACA-siRNA or scrambled siRNA then infected with A. hydrophila as described earlier. To quantify the number of intracellular A. hydrophila, the HKM were lysed 24 h p.i. with Triton X-100 at a final concentration of 0.1% (v/v) in sterile distilled water. Serial dilutions of lysate from each well were prepared and 0.1 mL of each dilution was plated on nutrient agar and CFU determined after 24 h of incubation at 30°C.
Statistical Analysis
Data are presented as mean ± SE of number of experiments performed, as indicated in the corresponding figure. Pair-wise comparison was done between group employing paired t-test with P<0.05 as the minimum significant level.
Supporting Information
A. hydrophila increases CaM expression in HKM. HKM were infected with A. hydrophila and at indicated time point p.i. CaM expression detected by (A) real time PCR and (B) EIA. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM. HKM, control head kidney macrophage; HKM+B, HKM infected with A. hydrophila.
(TIF)
Expression of CaMKII g and PKACA at transcript level in A. hydrophila -infected HKM. HKM were infected with A. hydrophila and at indicated time point p.i. (A) CaMKIIg and (B) PKACA expression detected by real time PCR. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM. HKM, control head kidney macrophage; HKM+B, HKM infected with A. hydrophila.
(TIF)
Effect of PKA inhibition on intracellular A. hydrophila multiplication. Intracellular load of A. hydrophila was checked in HKM following H-89 addition simultaneous with chloramphenicol and other sets where H-89 was added 15 mins, 30 mins, 60 mins and 120 mins p.i. A. hydrophila number was determined by dilution plating on nutrient agar plate. Vertical bars represent mean ± SE (n = 6). #P<0.05, compared to HKM+B. HKM+B, HKM infected with A. hydrophila; HKM+B+H-89 (Simultaneous with Chl), H-89 was added at the time of addition of chloramphenicol; HKM+B+H-89 (15 mins p.i.), H-89 was added 15 mins after the addition of chloramphenicol; HKM+B+H-89 (30 mins p.i.), H-89 was added 30 mins after the addition of chloramphenicol; HKM+B+H-89 (60 mins p.i.), H-89 was added 60 mins after the addition of chloramphenicol; HKM+B+H-89 (120 mins p.i.), H-89 was added 120 mins after the addition of chloramphenicol.
(TIF)
Acknowledgments
The authors are grateful to S. Das, North Shore University Health System and S. Barik, Cleveland State University for helpful discussions and critically analyzing the manuscript. We thank U. Rai, University of Delhi for the help in fluorescence microscopy and A. K. Pal for help in maintaining fish.
Funding Statement
The research work was supported by Department of Biotechnology (DBT), Govt of India Grant (BT/PR13883/AAQ/03/516/2010), Delhi University R&D grant and NAIP, Indian Council of Agricultural Research, Govt of India. CB was supported by DST-INSPIRE Fellowship (Govt of India) and HB was supported by CSIR Fellowship (Govt of India). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Galindo CL, Fadl AA, Sha J, Gutierrez Jr C, Popov VL, et al. (2004) Aeromonas hydrophila cytotoxic enterotoxin activates mitogen-activated protein kinases and induces apoptosis in murine macrophages and human intestinal epithelial cells. J Biol Chem 279: 37597–37612. [DOI] [PubMed] [Google Scholar]
- 2.Gosling PJ (1996) Aeromonas species in diseases of animals. In: Austin B, Altwegg M, Gosling PJ, Joseph SW, editors. The Genus: Aeromonas. First edition. Chicester: John Wiley & Sons, Ltd. p.175.
- 3. Nhieu GTV, Clair C, Grompone G, Sansonetti P (2004) Calcium signalling during cell interactions with bacterial pathogens. Biol Cell 96: 93–101. [DOI] [PubMed] [Google Scholar]
- 4. Fettucciari K, Fetriconi I, Mannucci R, Nicoletti I, Bartoli IA, et al. (2006) Group B Streptococcus induces macrophage apoptosis by calpain activation. J Immunol 176: 7542–7556. [DOI] [PubMed] [Google Scholar]
- 5. Ikura AM, Ames JB (2006) Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: Two ways to promote multifunctionality. Proc Nat Aca Sci USA 103: 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Timmins JM, Oczan L, Seimon TA, Li G, Malagelada C, et al. (2009) Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Investig 119: 2925–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmitt JM, Guire ES, Saneyoshi T, Soderling TR (2005) Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J Neurosci 25: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodge JJL, Mullasseril P, Griffith LC (2006) Activity-dependent gating of CaMKII autonomous activity by Drosophila CASK. Neuron 51: 327–337. [DOI] [PubMed] [Google Scholar]
- 9. Hojabrpour P, Waissbluth I, Ghaffari M, Cox ME, Duronio V (2012) CaMKII-γ mediates phosphorylation of BAD at Ser170 to regulate cytokine-dependent survival and proliferation. Biochem J 442: 139–149. [DOI] [PubMed] [Google Scholar]
- 10. Oloffsson MH, Havelka AM, Brnjic S, Shoshan MC, Linder S (2008) Charting calcium-regulated apoptosis pathways using chemical biology: role of calmodulin kinase II. BMC Chem Biol 8: 2 doi: 10.1186/1472-6769-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC (2011) Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol 204: 277–287 doi: 10.1111/j.1748-1716.2011.02273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turk BE (2007) Manipulation of host signalling pathways by anthrax toxins. Biochem J 402: 405–417. [DOI] [PubMed] [Google Scholar]
- 13. Seger R, Krebs EG (1995) The MAPK signaling cascade. FASEB J 9: 726–735. [PubMed] [Google Scholar]
- 14. Wada T, Penninger JM (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23: 2838–2849. [DOI] [PubMed] [Google Scholar]
- 15. Ashida H, Mimuro H, Ogawa M, Kobayashi T, Sanada T, et al. (2011) Cell death and infection: A double-edged sword for host and pathogen survival. J Cell Biol 195: 931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Becker BK, Fishelson L, Amselgruber WM (2001) Cytological ontogenesis and involution of the thymus and head-kidney in juvenile and old domestic carp: Is ageing in fish a chronological or growth-related phenomenon? J Appl Ichthyol 17: 1–13. [Google Scholar]
- 17. Verburg-van Kemenade BML, Ribeiro CMS, Chadzinska M (2011) Neuroendocrine-immune interaction in fish: Differential regulation of phagocyte activity by neuroendocrine factors. Gen Comp Immunol 172: 31–38. [DOI] [PubMed] [Google Scholar]
- 18. Majumdar T, Ghosh D, Datta S, Sahoo C, Pal J, et al. (2007) An attenuated plasmid-cured strain of Aeromonas hydrophila elicits protective immunity in Clarias batrachus L. Fish Shellfish Immunol. 23: 223–230. [DOI] [PubMed] [Google Scholar]
- 19. Banerjee C, Goswami R, Verma G, Datta M, Mazumder S (2012) Aeromonas hydrophila induced head kidney macrophage apoptosis in Clarias batrachus involves the activation of calpain and is caspase-3 mediated. Dev Comp Immunol 37: 323–333. [DOI] [PubMed] [Google Scholar]
- 20. Illiano S, Nagao T, Vanhoutte PM (1992) Calmidazolium, a calmodulin inhibitor, inhibits endothelium-dependent relaxations resistant to nitro-L-arginine in the canine coronary artery. Br J Pharmacol 107: 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, et al. (2002) STO-609, a specific inhibitor of the Ca2+/calmodulin-dependent protein kinase kinase. J Biol Chem 277: 15813–15818. [DOI] [PubMed] [Google Scholar]
- 22. Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, et al. (1998) KN-93, an inhibitor of multifunctional Ca2+/calmodulin-dependent protein kinase, decreases early after depolarizations in rabbit heart. J Pharmacol Exp Ther 287: 996–1006. [PubMed] [Google Scholar]
- 23. de Bagues Maria-Pilar J, Dudal S, Dornan J, Gross A (2005) Cellular bioterrorism: how Brucella corrupts macrophage physiology to promote invasion and proliferation. Clin Immunol 114: 227–238. [DOI] [PubMed] [Google Scholar]
- 24. MacDonald LJ, Kurten RC, Voth DE (2012) Coxiella burnetii alters cyclic AMP-dependent protein kinase signaling during growth in macrophages. Infect Immun 80: 1980–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hedrick ED, Agarwal E, Leiphrakpam PD, Haferbier KL, Brattain MG (2013) Differential PKA activation and AKAP association determines cell fate in cancer cells. J Mol Signalling 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tasken K, Aandahl EM (2004) Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 84: 137–167. [DOI] [PubMed] [Google Scholar]
- 27. Murray AJ (2008) Pharmacological PKA inhibition: All may not be what it seems. Science Signal 1 (22): re4 doi: 10.1126/scisignal.122re4 [DOI] [PubMed] [Google Scholar]
- 28. Yadav M, Roach SK, Schorey JS (2004) Increased mitogen-activated protein kinase activity and TNF-α production associated with Mycobacterium smegmatis-but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/Protein Kinase A pathways. J Immunol 172: 5588–5597. [DOI] [PubMed] [Google Scholar]
- 29. Quan H, Liu H, Li C, Lou L (2009) 1,4-diamino-2,3-dicyano-1,4-bis (methylthio) butadiene (U0126) enhances the cytotoxicity of combretastatin A4 independently of mitogen-activated protein kinase kinase. J Pharmacol Exp Ther 330: 326–333. [DOI] [PubMed] [Google Scholar]
- 30. Chakrabarti G, McClane MA (2005) The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol 7: 129–146. [DOI] [PubMed] [Google Scholar]
- 31. Lasbury ME, Durant PJ, Liao CP, Lee CH (2009) Effects of decreased calmodulin protein on the survival mechanisms of alveolar macrophages during Pneumocystis pneumonia . Infect Immun 79: 3344–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cox RL, Mariano T, Heck DE, Laskin JD, Stegeman JJ (2001) Nitric oxide synthase sequences in the marine fish Stenotomus chrysops and the sea urchin Arbacia punctulata, and phylogenetic analysis of nitric oxide synthase calmodulin-binding domains. Comp Biochem Physiol Part B 130: 479–491. [DOI] [PubMed] [Google Scholar]
- 33. Friedberg F, Taliaferro LT (2005) Calmodulin genes in zebrafish (revisited). Mol Biol Rep 32: 55–60. [DOI] [PubMed] [Google Scholar]
- 34. Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang KKW, Villalobo A, Roufogalis BD (1989) Calmodulin-binding proteins as calpain substrates. Biochem J 262: 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kruidering M, Schouten T, Evan GI, Vreugdenhil E (2001) Caspase-mediated cleavage of the Ca2+/calmodulin-dependent protein kinase-like kinase facilitates neuronal apoptosis. J Biol Chem 42: 38417–38425. [DOI] [PubMed] [Google Scholar]
- 37. Tremper-Wells B, Vallano ML (2005) Nuclear calpain regulates Ca2+-dependent signaling via proteolysis of nuclear Ca2+/calmodulin-dependent protein kinase type IV in cultured neurons. J Biol Chem 280: 2165–2175. [DOI] [PubMed] [Google Scholar]
- 38. Xiaodong C, Zhenyu J, Tamara T, Fang M (2008) Epac and PKA: a tale of two intracellular cAMP receptors. Acta Bioch Bioph Sin 40: 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gross A, Bouaboula M, Casellas P, Liautard JP, Dornand J (2003) Subversion and utilization of the host cell cyclic adenosine 5′ -monophosphate/protein kinase A pathway by Brucella during macrophage infection. J Immunol 170: 5607–5614. [DOI] [PubMed] [Google Scholar]
- 40. Khelef N, Zychlinsky A, Guiso N (1993) Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun 61: 4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crawford MA, Aylott CV, Bourdeau RW, Bokoch GM (2006) Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J Immunol 176: 7557–7565. [DOI] [PubMed] [Google Scholar]
- 42. Kanack KJ, Runyen-Janecky LJ, Ferrell EP, Suh SJ, West SHE (2006) Characterization of DNA-binding specificity and analysis of binding sites of the Pseudomonas aeruginosa global regulator, Vfr, a homologue of the Escherichia coli cAMP receptor protein. Microbiol 152: 3485–3496. [DOI] [PubMed] [Google Scholar]
- 43. Hanazawa S, Murakami Y, Takeshita A, Kitami H, Ohta KA, et al. (1992) Porphyromonas gingivalis fimbriae induce expression of the neutrophil chemotactic factor KC gene of mouse peritoneal macrophages: role of protein kinase C. Infect Immun. 60: 1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chopra AK, Xu XJ, Ribardo D, Gonzalez M, Kuhl K, et al. (2000) The cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infect Immun 68: 2808–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu X, McMurray CT (2001) Calmodulin Kinase II attenuation of gene transcription by preventing cAMP response element-binding protein (CREB) dimerization and binding of the CREB-binding protein. J Biol Chem 276: 1735–1741. [DOI] [PubMed] [Google Scholar]
- 46. Krachler AM, Woolery AR, Orth K (2011) Manipulation of kinase signaling by bacterial pathogens. J Cell Biol 195: 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leal RB, Ribeiro SJ, Posser T, Cordova FM, Rigon Filho EZ, et al. (2006) Modulation of ERK 1/2 and p38 MAPK by lead in the cerebellum of Brazilian catfish Rhamdia quelen . Aqua Toxicol 77: 98–104. [DOI] [PubMed] [Google Scholar]
- 48. Mu Y, Ding F, Cui P, Ao J, Hu S, Chen X (2010) Transcriptome and expression profiling analysis revealed changes of multiple signaling pathways involved in immunity in the large yellow croaker during Aeromonas hydrophila infection. BMC Genomics 11: 506 doi: 10.1186/1471-2164-11-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jia J, Alaoui-El-Azher M, Chow M, Chambers TC, Baker H, et al. (2003) c-Jun NH2-terminal kinase-mediated signaling is essential for Pseudomonas aeruginosa ExoS-induced apoptosis. Infect Immun 71: 3361–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grassl GA, Kracht M, Wiedemann A, Hoffmann E, Aepfelbacher M, et al. (2003) Activation of NF-κB and IL-8 by Yersinia enterocolitica invasin protein is conferred by engagement of Rac1 and MAP kinase cascades. Cell Microbiol 5: 957–971. [DOI] [PubMed] [Google Scholar]
- 51. Yang G, Sun X, Wang R (2004) Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen activated protein kinases and caspase-3. FASEB J 18: 1782–1784. [DOI] [PubMed] [Google Scholar]
- 52. Widmann C, Gerwins P, Johnson NL, Jarpe MB, Johnson GL (1998) MEK kinase 1, a substrate for DEVD directed caspases, is involved in genotoxin-induced apoptosis. Mol Cell Biol 18: 2416–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goswami R, Ghosh D, Saha DR, Padhy PK, Mazumder S (2011) Effect of acute and chronic arsenic exposure on growth, structure and virulence of Aeromonas hydrophila isolated from fish. Microb Pathog 50: 63–69. [DOI] [PubMed] [Google Scholar]
- 54. Banerjee C, Goswami R, Datta S, Rajagopal R, Mazumder S (2011) Arsenic-induced alteration in intracellular calcium homeostasis induces head kidney macrophage apoptosis involving the activation of calpain-2 and ERK in Clarias batrachus . Toxicol Appl Pharmacol 256: 44–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. hydrophila increases CaM expression in HKM. HKM were infected with A. hydrophila and at indicated time point p.i. CaM expression detected by (A) real time PCR and (B) EIA. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM. HKM, control head kidney macrophage; HKM+B, HKM infected with A. hydrophila.
(TIF)
Expression of CaMKII g and PKACA at transcript level in A. hydrophila -infected HKM. HKM were infected with A. hydrophila and at indicated time point p.i. (A) CaMKIIg and (B) PKACA expression detected by real time PCR. Vertical bars represent mean ± SE (n = 6). *P<0.05, compared to HKM. HKM, control head kidney macrophage; HKM+B, HKM infected with A. hydrophila.
(TIF)
Effect of PKA inhibition on intracellular A. hydrophila multiplication. Intracellular load of A. hydrophila was checked in HKM following H-89 addition simultaneous with chloramphenicol and other sets where H-89 was added 15 mins, 30 mins, 60 mins and 120 mins p.i. A. hydrophila number was determined by dilution plating on nutrient agar plate. Vertical bars represent mean ± SE (n = 6). #P<0.05, compared to HKM+B. HKM+B, HKM infected with A. hydrophila; HKM+B+H-89 (Simultaneous with Chl), H-89 was added at the time of addition of chloramphenicol; HKM+B+H-89 (15 mins p.i.), H-89 was added 15 mins after the addition of chloramphenicol; HKM+B+H-89 (30 mins p.i.), H-89 was added 30 mins after the addition of chloramphenicol; HKM+B+H-89 (60 mins p.i.), H-89 was added 60 mins after the addition of chloramphenicol; HKM+B+H-89 (120 mins p.i.), H-89 was added 120 mins after the addition of chloramphenicol.
(TIF)