Abstract
Background
Aseptic trauma engages the innate immune response to trigger a neuroinflammatory reaction that results in postoperative cognitive decline. We sought to determine whether high-mobility group box 1 protein (HMGB1), an ubiquitous nucleosomal protein, initiates this process through activation and trafficking of circulating bone marrow-derived macrophages to the brain.
Methods
The effects of HMGB1 on memory (using trace fear conditioning) were tested in adult C57BL/6J male mice; separate cohorts were tested after bone marrow-derived macrophages were depleted by clodrolip. The effect of anti-HMGB1 neutralizing antibody on the inflammatory and behavioral responses to tibial surgery were investigated.
Results
A single injection of HMGB1 caused memory decline, as evidenced by a decrease in freezing time (52 ± 11% vs. 39 ± 5%; n = 16-17); memory decline was prevented when bone marrow-derived macrophages were depleted (39 ± 5% vs. 50 ± 9%; n = 17). Disabling HMGB1 with a blocking monoclonal antibody, before surgery, reduced postoperative memory decline (52 ± 11% vs. 29 ± 5%, n = 15-16); also, hippocampal expression of monocyte chemotactic protein-1 (MCP-1) was prevented by the neutralizing antibody (n = 6). Neither the systemic nor the hippocampal inflammatory responses to surgery occurred in mice pre-treated with anti-HMGB1 neutralizing antibody (n = 6).
Discussion
Postoperative neuroinflammation and cognitive decline can be prevented by abrogating the effects of HMGB1. Following the earlier characterization of the resolution of surgery-induced memory decline, the mechanisms of its initiation are now described. Together, these data may be used to preoperatively test the risk to surgical patients for the development of exaggerated and prolonged postoperative memory decline that is reflected in delirium and postoperative cognitive dysfunction, respectively.
INTRODUCTION
Aseptic surgical trauma provokes a neuroinflammatory response, presumably, to defend the organism from further injury.1,2 When this homeostatic response is dysregulated, detrimental consequences can follow, including postoperative cognitive decline that can persist in up to 10% of surgical patients over the age of 65 yr.3,4 While it is possible that the cognitive response to surgery may also include enhancement (if the surgery “cures” a process that interferes with cognition) or no change (short-lived initiation and resolution of aseptic trauma-induced inflammation), we have explored, in rodent models, the process that mediates persistent postoperative cognitive decline.1,2,5 Following tissue injury the innate immune response is engaged resulting in penetration of bone marrow-derived macrophages (BM-DM) into the brain through a disrupted blood brain barrier.2 Within the hippocampus these activated macrophages release proinflammatory cytokines that are capable of attenuating long-term potentiation that is the neurobiologic correlate of learning and memory.6,7 These processes are reversed within days through inflammation-resolving mechanisms involving both neural and humoral pathways.2 Failure to resolve the neuroinflammatory response results in exaggerated and persistent postoperative cognitive decline.1,8,9 In an attempt to devise strategies that can detect and mitigate this risk, the most vulnerable patients need to be identified; in pursuit of this goal we sought to precisely define the initiating processes in order to devise a preoperative functional assay that is predictive of the patient's likely immune response to aseptic trauma.
Alarmins, a family of damaged-associated molecular patterns, are capable of activating the innate immune response through its interaction with pattern recognition receptors on circulating monocytes.10 In particular, high-mobility group box 1 protein (HMGB1) is an alarmin that is passively released into the circulation from traumatized necrotic cells; also, HMGB1 can be rapidly secreted by stimulated leukocytes and epithelial cells.10,11 We previously demonstrated that circulating HMGB1 increases after surgery in humans and also in a murine aseptic trauma model12,13; furthermore, we reported that this species of alarmin is required for trauma-induced exacerbation of the morphological and functional consequences of stroke.12
Now we describe data from experiments designed to test the hypothesis that the early release of HMGB1 triggers the neuroinflammatory and behavioral responses to trauma. These data set the stage for the development of a functional assay that assesses the initiation and resolution of inflammatory processes that are pivotal in postoperative cognitive decline.
MATERIALS AND METHODS
Animals
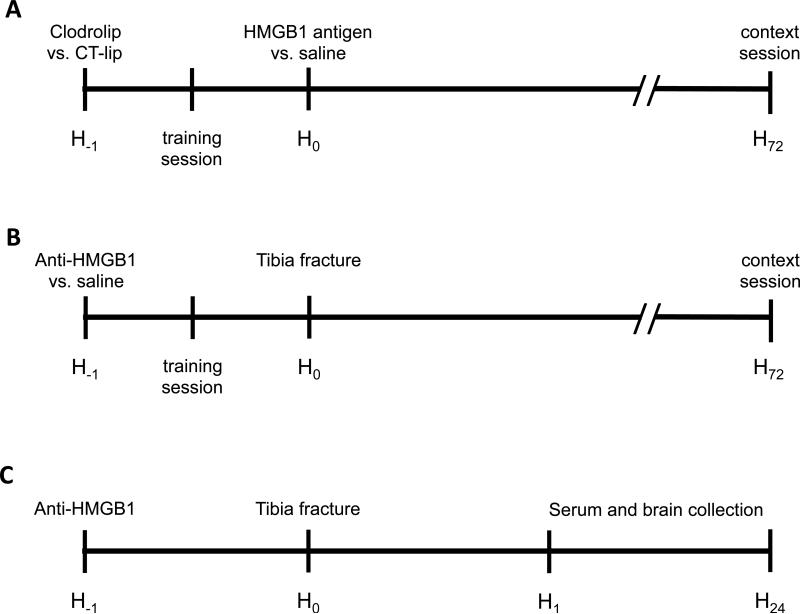
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco, and conformed to the National Institutes of Health Guidelines. All animals were fed standard rodent food and water ad libitum, and were housed (5 mice per cage) in sawdust-lined cages in an air-conditioned environment with 12-h light/dark cycles. Wild-type male mice (C57BL/6J, 12-14 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME) for the behavior tests (fig. 1A and B) and for the cytokine expression (fig. 1C).
Figure 1.
Study design. (A) First experiment; mice were divided in 5 groups treated with IP injection of control liposome versus clodrolip 1h before HMGB1 Ag versus saline injection. Control animals received saline injections. The training session of the memory test was performed 30 min after the clodrolip/control liposome injection and 30 min before HMGB1 Ag/saline injection; and the context session was performed 72 h later. (B) Second experiment; mice were divided in 4 groups treated with anti-HMGB1 versus saline 1 h before tibia fracture. The training session of the memory test was performed 30 min after the IP injection and 30 min before tibia fracture. (C) Third experiment; Mice were divided in 4 groups treated with anti-HMGB1 versus saline 1h before tibia fracture and sacrificed 1 h and 24 h after the tibia fracture. Anti-HMGB1 = neutralizing HMGB1 monoclonal antibody; CT-lip = control liposome; HMGB1 = high-mobility group box 1 protein; HMGB1 Ag = HMGB1 antigen; IP = intraperitoneal.
Animals were tagged and randomly allocated to each group before any treatment or procedure. Researchers were blinded to the group assignment that was revealed only after the analysis phase.
Body weight was measured before any procedure or treatment and 3 days later, following assessment of freezing behavior.
Surgical Trauma
Under aseptic conditions, groups of mice were subjected to an open tibia fracture of the left hind paw with an intramedullary fixation as previously described.2,12,14,15 Briefly, mice received general anesthesia with 2% isoflurane and analgesia was achieved with buprenorphine 0.1 mg/kg subcutaneously, immediately after anesthetic induction and before surgical insult. Warming pads and temperature-controlled lights were used to maintain body temperature at 37°C ± 0.5°C. The entire procedure from induction of anesthesia to end of surgery lasted 12 ± 5 min.
Trace-Fear Conditioning
Fear conditioning is used to assess learning and memory in rodents, which are trained to associate a conditional stimulus, such as a tone, with an aversive, unconditional stimulus, such as a foot-shock.16 Freezing behavior is an indicator of aversive memory that is measured when subjects are re-exposed to the conditional stimulus.
For this study we used a previously published paradigm.1,5,12,15,17 Briefly, the behavioral study was conducted using a conditioning chamber (Med. Associates Inc., St. Albans, VT) and an unconditional stimulus (two periods of 2-s foot-shock of 0.75 mAmp). Behavior was captured with an infrared video camera (Video Freeze, Med. Associates Inc.). Thirty minutes after a particular intervention animals underwent the training session after which they were returned to their housing cage. Three days after training, mice underwent a context test, during which no tones or foot-shocks were delivered. Freezing behavior, recognized as lack of movement, was recorded by video and analyzed by software. A decrease in the percentage of time spent freezing indicated impairment of memory.
Systemic Inflammatory response to surgery
One and 24 h after aseptic surgical trauma, blood was collected transcardially after thoracotomy under terminal isoflurane anesthesia and placed into heparin-coated syringes, Samples were centrifuged at 3,400 rotations/min for 10 min and plasma was collected and stored at −80°C until these were assayed. Blood samples taken from animals without intervention served as controls. Plasma interleukin (IL)-6 and HMGB1 were measured using commercially available enzyme-linked immunosorbent kits, according to the manufacturer's instructions (Invitrogen, Grand Island, NY and IBL international, Toronto, Ontario, Canada, respectively).
Neuroinflammatory response to surgery
Twenty four hours after surgery, mice were perfused with saline and the hippocampus was then rapidly extracted, placed in RNAlater™ solution (Qiagen, Valencia, CA) and stored at 4°C overnight. Total RNA was extracted using RNeasy Lipid tissue Kit (Qiagen). Extracted RNA was treated with recombinant DNase I using a RNase-Free Dnase set™ (Qiagen). Messenger RNA (mRNA) concentrations were determined with a ND-1000 spectrophotometer (NanoDrop® Thermo Fisher Scientific, Wilmington, DE) and mRNA was reverse transcribed to complementary DNA with a High Capacity RNA to-cDNA Kit (Applied Biosystems, Carlsbad, CA).
TaqMan Fast Advanced Master Mix (Applied Biosystems) and specific gene expression assays were use for qPCR as follows: actin beta (NM_007393.1), IL-6 (Mm00446190_m1), tumor necrosis factor (TNF)-α (Mm00443258_m1), IL-1β (Mm01336189_m1) and monocyte chemotactic protein-1 (MCP-1) (Mm00441242_m1). qPCR was performed using StepOnePlus™ (Applied Biosystems). Each sample was run in triplicate, and relative gene expression was calculated using the comparative threshold cycle ΔΔCt and normalized to beta-actin. Results are expressed as fold-increases relative to controls.
Interventions
(a) Depletion of bone-marrow derived macrophages
Clodrolip is a liposomal formulation of clodronate (dichloromethylene bisphosphonic acid), a nontoxic bisphosphonate. Liposomes (lipid vesicles consisting of concentric phospholipid bilayers surrounding aqueous compartments) encapsulate clodronate, which are then ingested and digested by phagocytes, followed by an intracellular release and accumulation of clodronate. At a certain intracellular concentration, clodronate induces apoptosis of the phagocytes. Clodrolip was obtained from clodronateliposomes.org (Vrije Universiteit, Amsterdam, Netherlands) at 7 mg/ml concentration and prepared as previously described.18,19 Clodrolip (200 μl, about 100 mg/kg) was injected intraperitoneally 60 min before aseptic surgical trauma. Control animals received 200 μl of control liposomal solution.
b. Administration of reagents to simulate or block the HMGB1 response to trauma
Fifty μg/kg (100 μl) of recombinant HMGB1 (R&D System, Minneapolis, MN) was administered intraperitoneally. To neutralize trauma-released HMGB1, 50 μg of anti-HMGB1 neutralizing monoclonal antibody (2G7, mouse IgG2b supplied by Dr. Tracey's Laboratory, Manhasset, NY) in 100 μl of saline was administered intraperitoneally, 60 min before bone fracture. Control animals received the same volume (100 μl) of the vehicle (saline).
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Normality was tested with the Kolmogorov-Smirnov normality test. Equality of variances was tested with the F-test. We applied a log transformation (ln(X)) to the response of HMGB1 and IL-6 blood concentrations and mRNA expression before performing analyses to better adhere to analysis of variance (ANOVA) model's assumptions of normally distributed residuals and homogeneity of variance.
For comparisons of more than two groups, means were compared using one-way ANOVA followed by t-tests with a Bonferroni-corrected alpha level. We used the two-way ANOVA procedure to determine whether or not time and antibody treatment were significant factors in predicting HMGB1 and IL-6 concentrations in the serum followed by Bonferroni post hoc analyses.
For our study, the primary outcome was the percentage of freezing time during the context session observed in anti-HMGB1 neutralizing monoclonal antibody and control groups. Based on previous freezing time data15, we estimated that a sample of 13 C57BL/6J surgical mice per group was necessary to demonstrate a 20% increase in percentage freezing time, with 80% power at the 0.0125 alpha level (after adjusting for four comparisons) to reach a significant difference.
A two-tailed p value < 0.05 was considered statistically significant for 2-group comparisons and the significance threshold was adjusted for multiple comparisons with a Bonferroni correction. Prism 6 (GraphPad Software Inc, La Jolla, CA) was used to conduct the statistical analyses.
RESULTS
HMGB1 antigen is sufficient to cause Cognitive Decline through the participation of Bone Marrow-Derived Macrophages
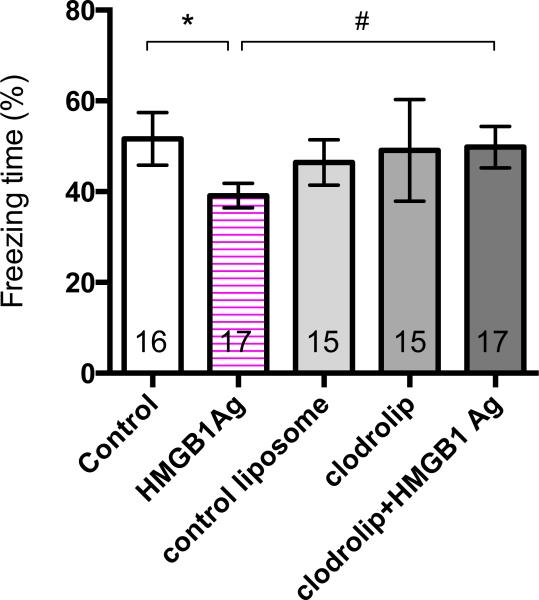
A single administration of HMGB1 produced cognitive decline as evidenced by a significant reduction in freezing time (52 ± 11% vs. 39 ± 5%, n = 16 in control group, n = 17 in HMGB1 antigen group, p = 0.012) (fig. 2).
Figure 2.
Effects of HMGB1 on Cognitive Decline. Contextual fear response reveals hippocampal-dependent memory impairment at postoperative day 3 (Arm A). Quantification of the freezing time percentage according to the five groups (n = 15-17; * p = 0.012 control versus HMGB1 Ag, and # p = 0.039 HMGB1 Ag versus clodrolip+HMGB1 Ag, with one-way ANOVA and Bonferroni post hoc analysis). HMGB1 = high-mobility group box 1 protein; HMGB1 Ag = HMGB1 antigen.
Recently, we showed that depletion of BM-DM by clodrolip exposure blocks surgery-induced neuroinflammation and cognitive decline.15 To determine whether HMGB1-induced cognitive decline requires the participation of BM-DM, mice were exposed to clodrolip or its vehicle prior to administration of HMGB1. Training was unaffected by the clodrolip exposure (data not shown). We lost one animal in the clodrolip group and another in the control liposome group on day 2. According to the manufacturer this may happen if there is transference of microorganisms from the skin or by injection of a nonhomogeneous suspension of liposome. We did not experience any death in the surgical groups. The HMGB1-induced decline in contextual freezing time was prevented by prior clodrolip exposure (39 ± 5% vs. 50 ± 9%, n = 17, p = 0.039) (fig. 2).
HMGB1 antibody prevented postsurgical cognitive decline
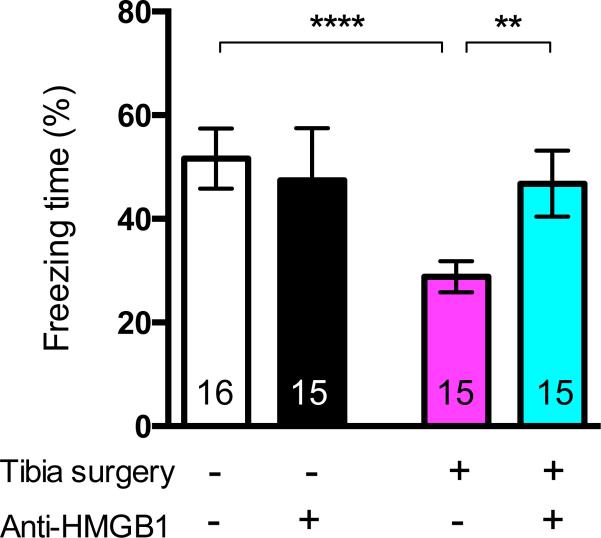
During the preoperative training period, learning was similar in the anti-HMGB1-exposed group and the control (nonexposed) groups (data not shown). Surgery significantly decreased the percentage of freezing time when compared to the control group (52 ± 11% vs. 29 ± 5%, n = 16 in control group, n = 15 in surgical group, p < 0.001); preoperative exposure to anti-HMGB1 attenuated the surgery-induced freezing behavior rendering the response to be no different from the nonsurgical control group (52 ± 11% vs. 47 ± 11%, n = 16 in control group, n = 15 in anti-HMGB1+surgery group, ns) (fig. 3).
Figure 3.
Effects of preoperative administration of HMGB1 neutralizing monoclonal antibody on postsurgical memory impairment. Quantification of the freezing time percentage on contextual testing according to the four groups at postoperative day 3 (Arm B). (n = 15-16; **** p < 0.001 control versus surgery, and ** p = 0.001 surgery versus anti-HMGB1+surgery and with one-way ANOVA and Bonferroni post hoc analysis). Anti-HMGB1 = neutralizing HMGB1 monoclonal antibody; HMGB1 = high-mobility group box 1 protein.
HMGB1 antibody reduces systemic and neuroinflammatory response to surgery
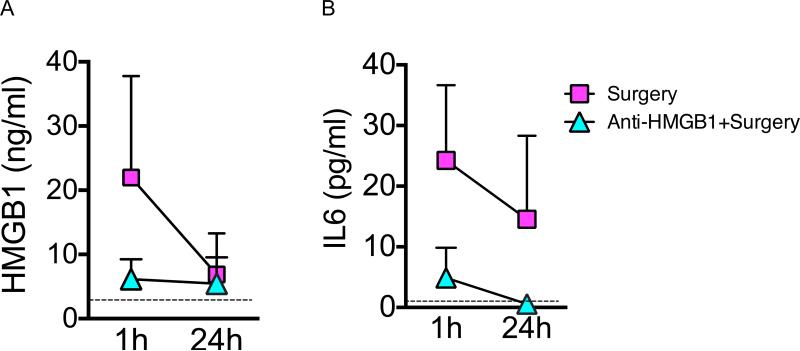
As expected12, we observed a significant decrease of the early increase of HMGB1 blood level between the first hour and 24 h after surgery (two way ANOVA, p = 0.002 for the time effect) (fig. 4). However, the neutralizing anti-HMGB1 reduced the systemic levels of HMGB1 (two way ANOVA, p = 0.005 for the treatment effect and p = 0.010 for interaction between time and treatment) and this reduction was significant one hour after the trauma (p = 0.04 with Bonferroni's post-hoc analysis) (fig. 4A). Concerning IL-6 increase following surgery, we observed a significant decreased within the first day (two way ANOVA, p = 0.015 for the time effect) HMGB1 neutralizing antibody was also a significant predicting factor for IL-6 concentration (two way ANOVA, p < 0.001 for treatment effect). Using HMGB1 neutralizing antibody, we observed a reduction in IL-6 concentration one hour after trauma (p = 0.006 with Bonferroni's post-hoc analysis) (fig. 4B).
Figure 4.
Effects of preoperative administration of HMGB1 neutralizing monoclonal antibody on the HMGB1 and IL-6 serum concentration at 1 and 24 h after tibia fracture (Arm C). The dotted lines represent the average values for respectively for HMGB1 and IL-6 concentration of 6 control mice without any treatment or surgery (n = 6). (A) Levels of HMGB1 serum concentration 1 h and 24 h after surgery. After log transformation of the raw data, we observed with two way ANOVA significant time and treatment effects (p = 0.002 for the time effect; p = 0.005 for the treatment effect and p = 0.010 for interaction between time and treatment) and a significant difference between the 2 groups at 1 h (22.00 ± 16.54 vs. 5.29 ± 4.13 surgical control vs. anti-HMGB1+surgery p = 0.040 with two way ANOVA with Bonferroni's post-hoc analysis). (B) Levels of IL-6 serum concentration 1 h and 24 h after surgery. After log transformation of the raw data, we observed with two way ANOVA significant time and treatment effects (p = 0.015 for the time effect; p < 0.001 for the treatment effect and p = 0.015 for interaction between time and treatment) and a significant difference between the 2 groups at 1 h (24.24 ± 12.87 vs. 4.90± 4.47 surgical control vs. anti-HMGB1+surgery p = 0.006 with two way ANOVA with Bonferroni's post-hoc analysis). Anti-HMGB1 = neutralizing HMGB1 monoclonal antibody; HMGB1 = high-mobility group box 1 protein; IL-6 = interleukin 6.
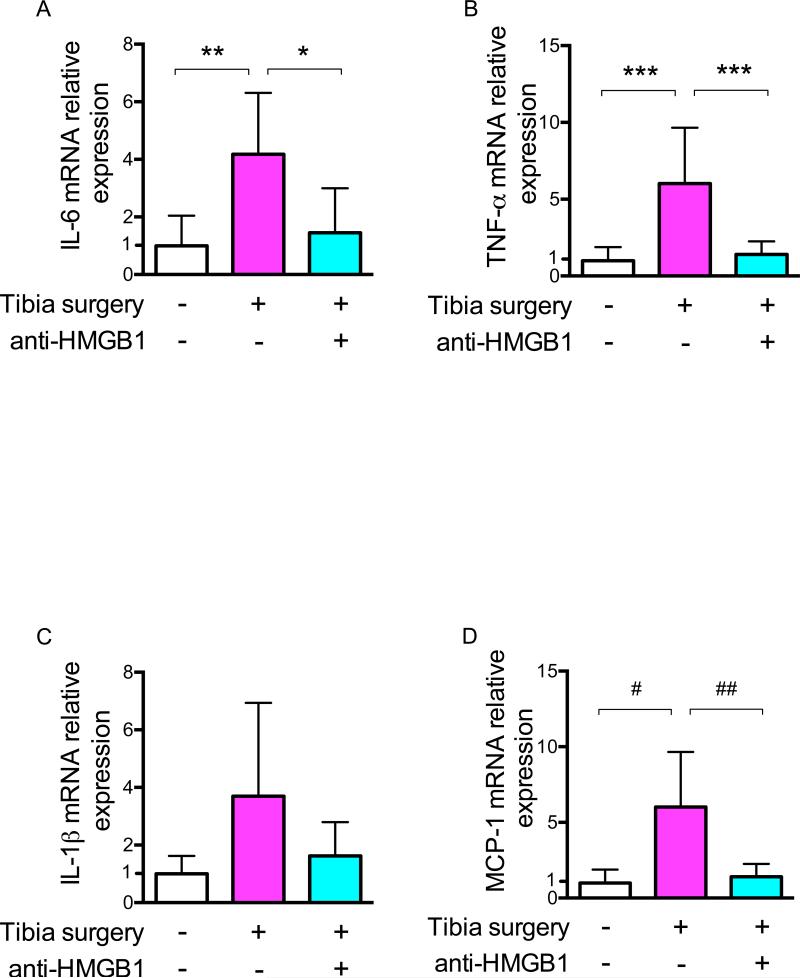
Twenty-four hours after surgery, the increase in hippocampal mRNA expression of IL-6 (n = 6; p = 0.009) and TNF-α (n = 6; p < 0.001) was blocked by exposure to the neutralizing anti-HMGB1 antibody. Treatment did not significantly change mRNA transcription of IL-1β (n = 6; p = 0.085) (fig. 5A–C).
Figure 5.
Effects of HMGB1 neutralizing monoclonal antibody on hippocampal transcription of IL-6, TNF-α, IL-1β and MCP-1 24 h after tibia surgery (Arm C). (A) IL-6 mRNA (B) TNF-α mRNA (C) IL-1β and (D) MCP-1 (n = 6; * p = 0.023; ** p = 0.009; *** p < 0.001, # p = 0.003, ## p = 0.005 with one-way ANOVA and Bonferroni post hoc analysis). Anti-HMGB1 = neutralizing HMGB1 monoclonal antibody; HMGB1 = high-mobility group box 1 protein; IL-1=interleukin 1; IL-6 = interleukin 6; TNF= tumor necrosis factor; MCP-1 = monocyte chemotactic protein-1.
Circulating CCR2-expressing BM-DM are recruited into the brain by the chemoattractant, MCP-1.12 After surgery there was an increase of hippocampal mRNA transcription of MCP-1 (n = 6; p = 0.003); treatment with the anti-HMGB1 neutralizing antibody prevented surgery-induced expression of MCP-1 (n = 6; p = 0.005) (fig. 5D).
DISCUSSION
This study posits that HMGB1, when released from a sterile traumatic injury, plays a pivotal role in postoperative memory dysfunction. Together with the detection of the cell type involved in the initiation of the surgery-induced inflammatory cascade these findings establish both the precise elements of the immune response that need to be interrogated for establishing risk of dysregulated trauma-induced inflammation as well as putative targets for interventions designed to limit or reverse persistent postoperative cognitive decline.
The independent role of the alarmin HMGB1 in disrupting cognitive processing was established by the fact that a single injection of HMGB1 was capable of reproducing a deficit in an hippocampal memory test similar to the surgical phenotype that we previously described.1 Furthermore, its dependence on bone marrow-derived monocytes was evidenced by the attenuation of HMGB1-induced cognitive decline if clodrolip is first administered to induce apoptosis of these circulating phagocytes (fig. 2); this is similar to that previously reported in the setting of surgery.1,5
After the aseptic trauma of elective surgery, either experimentally (fig. 4A) or clinically, HMGB1 is released into the circulation where it can interact with pattern recognition receptors (toll-like receptors 2 and 4 as well as receptor for advanced glycation end-products) on immunocytes.15 By neutralizing the early release of alarmins, HMGB1 antibody both decreased surgery-induced inflammation (fig. 4 and 5) as well as cognitive decline (fig. 3) further establishing the importance of hippocampal inflammation for the development of postoperative memory dysfunction.1,5
After peripheral surgery, CCR2+ expressing cells migrate to the brain, attracted by signaling from hippocampal MCP-1, a chemokine that regulates migration and infiltration of monocytes/macrophages15,20. Interestingly, by depleting BM-DM, the expression of MCP-1 in the surgical model remained unaffected, indicating that BM-DM are not the self-perpetuating source of this chemoattractant for its own recruitment.15 We now show that treatment with HMGB1 antibody was able to prevent the synthesis of MCP-1 in the hippocampus establishing the dependence of its increased expression on the release of HMGB1. Taking our previous report15 and our present findings together, it follows that HMGB1 is signaling to the hippocampus to produce the chemoattractant, MCP-1, through a mechanism that is independent of BM-DM; this HMGB1-dependent hippocampal expression of MCP-1 could involve either a neural or humoral pathway.
Accumulating evidence indicates that HMGB1 can stimulate migration of not only monocytes, but also various types of cells including neurite21, smooth muscle cells22, tumor cells23, mesoangioblast stem cells24, dendritic cells25,26, and neutrophils27,28. This could explain how blocking the effect of the release of HMGB1 may have blocked signaling of other HMGB1-derived factors. While BM-DM play a necessary role, they may not be sufficient to completely explain the cognitive decline seen after surgery; there may be other cells and factors involved in the genesis of postoperative cognitive dysfunction.
The following caveats apply when interpreting our findings. Our surgery model involves disruption of the bone marrow with an intramedullary pin for internal fixation of the broken bone. The bone marrow itself produces soluble factors that affect immune cells, such as a proliferation-inducing ligand A (APRIL), B-cell activating factor (BAFF), both belonging to the TNF family, CXCL12, IL-6, IL-7 and macrophage inhibitory factor.29 The possibility exists that surgical trauma that does not involve damage to the bone marrow may not utilize the same panoply of alarmins. In addition to its role as a primary lymphoid organ, the bone marrow can act as a host for various mature lymphoid cell types. Several subsets of bone marrow cells have been shown to support immune cell function.29
Given that HMGB1 resides in the nucleus and functions as an essential non-histone chromatin-binding protein, there are no HMGB1 knockout animals. For this study we then decided to use a pharmacologic strategy to quickly deplete the pool of systemic macrophages. However, because clodrolip is highly toxic to all phagocytes, it can increase the risk of postsurgical infections, generating a phenotype of its own.30 With a single dose, we did not observe loss of weight or other signs of sickness within three days. As this was a short-term study, focusing on the acute exaggeration phase of neuroinflammation, we are unable to extrapolate from these data the long-term effects and did not perform any long-term study with clodrolip. Clodrolip should be considered a tool for mechanistic studies.
In previous reports using this model of surgical trauma, we documented the effectiveness of preoperative administration of interventions such as IL-1 receptor antagonist, anti-TNF monoclonal antibody and activation of the α7 subtype of nicotinic acetylcholine receptor in preventing postoperative memory dysfunction.1,2,5 Each of these affect important host defense mediators and risk of infection needs to be evaluated before considering these agents.
These studies on the initiation of trauma-induced cognitive decline, coupled with our previous reports on the resolution of postoperative cognitive decline1,2,5 sets the stage for the development of an ex-vivo bioassay that can test the function of the innate immune response to trauma. Such an assay may be capable of prospectively identifying surgical patients at increased risk for the development of exaggerated and persistent cognitive decline; stratification of a surgical cohort, enriched for the development of cognitive decline, can result in a randomized trial to test with efficacy of interventions using fewer surgical patients.
MS #201307024 – Final Boxed Summary Statement.
What we already know about this topic:
Surgical trauma induces a neuroinflammatory response that contributes to cognitive dysfunction in rodent models
Penetration of bone marrow-derived macrophages into the brain with release of proinflammatory cytokines plays a major role in this neuroinflammatory response to injury
What this article tells us that is new:
Administration of the alarmin high-mobility group box 1 protein (HMGB1) produced memory dysfunction in mice
A neutralizing antibody to HMGB1 reduced memory dysfunction and prevented the inflammatory response following tibial surgery, indicating a major initiating role for this mediator in postoperative memory dysfunction
Acknowledgments
Supported by National Institutes of Health of Health R01GM104194, Bethesda, Maryland.
The authors thank members of the Hellman laboratory, University of California, San Francisco, San Francisco, California, and to Pedro Gambus, M.D., Staff Anesthesiologist, Department of Anesthesia, Hospital Clinic de Barcelona, Spain for their support and to Mitchell Marubayashi, M.Sc. and Stuart Dilg, B.A., volunteers in the Maze Laboratory, University of California, San Francisco, San Francisco, California, for their assistance in the behavior tests.
Footnotes
This work is presented by the Department of Anesthesia and Perioperative Care of the University of California, San Francisco, California.
The authors declare no competing interests.
References
- 1.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1β in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–8. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–95. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, Moller JT. Cognitive dysfunction 1-2 years after non-cardiac surgery in the elderly. ISPOCD group. International Study of Post-Operative Cognitive Dysfunction. Acta Anaesthesiol Scand. 2000;44:1246–51. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- 4.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 5.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-α triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–22. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011;36:979–92. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickering M, O'Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–54. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- 8.Feng X, Degos V, Koch LG, Britton SL, Zhu Y, Vacas S, Terrando N, Nelson J, Su X, Maze M. Surgery results in exaggerated and persistent cognitive decline in a rat model of the metabolic syndrome. Anesthesiology. 2013;118:1098–105. doi: 10.1097/ALN.0b013e318286d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su X, Feng X, Terrando N, Yan Y, Chawla A, Koch LG, Britton SL, Matthay MA, Maze M. Dysfunction of inflammation-resolving pathways is associated with exaggerated postoperative cognitive decline in a rat model of metabolic syndrome. Mol Med. 2013;18:1481–90. doi: 10.2119/molmed.2012.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. Alarmins: Awaiting a clinical response. J Clin Invest. 2012;122:2711–9. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MJ, Brohi K, Calfee CS, Rahn P, Chesebro BB, Christiaans SC, Carles M, Howard M, Pittet JF. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: Role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degos V, Maze M, Vacas S, Hirsch J, Guo Y, Shen F, Jun K, van Rooijen N, Gressens P, Young WL, Su H. Bone fracture exacerbates murine ischemic cerebral injury. Anesthesiology. 2013;118:1362–72. doi: 10.1097/ALN.0b013e31828c23f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terrando N, Brzezinski M, Degos V, Eriksson LI, Kramer JH, Leung JM, Miller BL, Seeley WW, Vacas S, Weiner MW, Yaffe K, Young WL, Xie ZC, Maze M. Perioperative cognitive decline in the aging population. Mayo Clin Proc. 2011;86:885–93. doi: 10.4065/mcp.2011.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harry LE, Sandison A, Paleolog EM, Hansen U, Pearse MF, Nanchahal J. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26:1238–44. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 15.Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, Young WL, Maze M. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–36. doi: 10.1097/ALN.0b013e3182834d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 17.Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, Feldmann M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15:178–85. doi: 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- 19.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 20.Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fages C, Nolo R, Huttunen HJ, Eskelinen EL, Rauvala H. Regulation of cell migration by amphoterin. J Cell Sci. 2000;113:611–20. doi: 10.1242/jcs.113.4.611. [DOI] [PubMed] [Google Scholar]
- 22.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–11. [PubMed] [Google Scholar]
- 24.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–9. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 26.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 27.van Zoelen MAD, Yang H, Florquin S, Meijers JCM, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of toll-Like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group Box-1 induced inflammation in vivo. Shock. 2009;31:280–4. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlova VV, Choi EY, Xie CP, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–39. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]