Abstract
The role of type IIA receptor protein tyrosine phosphatases (RPTPs), which includes LAR, RPTPσ and RPTPδ, in the nervous system is becoming increasingly recognized. Evidence supports a significant role for these RPTPs during the development of the nervous system as well as after injury, and mutations in RPTPs are associated with human disease. However, a major open question is the nature of the ligands that interact with type IIA RPTPs in the adult brain. Candidates include several different proteins as well as the glycosaminoglycan chains of proteoglycans. In order to investigate this problem, we used a receptor affinity probe assay with RPTPσ-AP fusion proteins on sections of adult mouse brain and to cultured neurons. Our results demonstrate that the major binding sites for RPTPσ in adult mouse brain are on neurons and are not proteoglycan GAG chains, as RPTPσ binding overlaps with the neuronal marker NeuN and was not significantly altered by treatments which eliminate chondroitin sulfate, heparan sulfate, or both. We also demonstrate no overlap of binding of RPTPσ with perineuronal nets, and a unique modulation of RPTPσ binding to brain by divalent cations. Our data therefore point to neuronal proteins, rather than CSPGs, as being the ligands for RPTPσ in the adult, uninjured brain.
Keywords: Protein Tyrosine Phosphatase, Receptor Affinity Probe Assay Chondroitin Sulfate, Heparan Sulfate Proteoglycan
Introduction
There is significant interest in the putative roles of the class II A (R2A) Receptor Protein Tyrosine Phosphatases (RPTPs), which includes the leukocyte common antigen receptor (LAR), as well as RPTPσ and RPTPδ, in brain function. Evidence has accumulated supporting a role for these RPTPs in the control of neuronal migration, axonal targeting, synaptic formation and neuronal plasticity (Chagnon et al., 2004; Dunah et al., 2005; Stoker and Dutta, 1998; Takahashi and Craig, 2013). Most recently, they have been identified as playing a role in restricting regeneration after neuronal injury (Fry et al., 2010; Shen et al., 2009). Mutations in RPTPs are increasingly recognized as causes of human disease (Nikolaienko et al., 2012; Stewart et al., 2013; Xu and Fisher, 2012). Less understood is the role that they play in the normal adult nervous system.
Members of the R2A class of phosphatases share features of their extracellular domain (ECD) common to many cell adhesion molecules, i.e., immunoglobulin and fibronectin type III repeats, as well as a membrane-spanning domain and two phosphatase domains, one of which (D1) is active, while the other (D2) is thought to control interactions with other intracellular proteins (Chagnon et al., 2004). The ECD binds to several different neuronal transmembrane proteins, including members of the Trk family of kinases, and molecules involved in synaptogenesis, including slitrak family members, neuroligin-3, and interleukin 1 receptor related protein (Kwon et al., 2010; Takahashi et al., 2011; Woo et al., 2009; Yoshida et al., 2011). However, the most studied interaction has been with proteoglycan glycosaminoglycan (GAG) chains: all members of the R2A class bind both heparan and chondroitin sulfate proteoglycans (Aricescu et al., 2002; Fox and Zinn, 2005).
These studies on the interactions of R2A class members with proteoglycan GAG chains have concluded that LAR, RPTPσ, and RPTPδ are receptors mediating the actions of proteoglycan GAG chains in the response to neuronal injury (Shen et al., 2009). Because these results were obtained through the use of knockout animals, these results may not generalize to the function of these receptors in the normal, uninjured brain, where proteoglycan levels are relatively low. To address this issue, we have used a receptor affinity probe assay to evaluate the location and cells in the adult mouse brain which bind RPTPσ. Our results indicate that binding sites in the adult mouse brain for RPTPσ are neuronal and do not depend upon the presence of either chondroitin or heparan sulfate GAG chains.
Materials and methods
Animals
All animal studies were approved by the Uniformed Services University of the Health Science (USUHS) Institutional Animal Care and Use Committee and were conducted in accordance with the NRC guide to the Care and Use of Laboratory Animals. A total of 8 mice were used for this study. Male mice (C57BL/6) of age 8-10 weeks (NCI, MD, USA) were kept under 12:12 light and dark cycle with access to food and water ad libitum. Mice were deeply anesthetized using ketamine/xylazine (100 mg·kg-ketamine/20 mg·kg-xylazine) and transcardially perfused with 4 % buffered formalin solution. Brains were carefully removed and post fixed in 4 % buffered formalin solution overnight at 4°C, then immersed in 30 % sucrose solution until they sank. 30 μm coronal sections were cut using a Leica SM2000 R sliding microtome with an attached freezing stage. Sections were kept in a PBS-based antifreeze solution containing ethylene glycol at −20°C until used for receptor affinity probe (RAP) assays.
Production of RPTPσ-AP
The extracellular domain of mouse RPTPσ (accession number: NM_001252453) was expressed as an alkaline phosphatase fusion protein. cDNA corresponding to the RPTPσ ECD (amino acids #1-847) was subcloned into the NheI/HindIII sites in pAPTAG5 (GeneHunter) which contains a sequence coding for alkaline phosphatase followed by a Myc epitope and a (His)6 tag at its C-terminus. Putative GAG binding residues (Lys68, Lys69, Lys71, Lys72) were mutated to Ala with site-directed mutagenesis. Stable transfectants were established in BHK cells under zeocin selection. Secreted fusion proteins (RPTPσ-AP, RPTPσ-ΔLys-AP or AP only) were purified by immobilized metal affinity chromatography (Profinity IMAC Ni-Charged Resin, Bio-Rad) via their (His)6 tags and their purity was determined by gel electrophoresis. Purified recombinant proteins were separated on a 10% SDS-PAGE and immunoblot analysis was performed with an anti-(His)6 antibody (Wang et al., 2008) (Supplementary Fig. 1).
In vitro binding of PTPσ -AP
PTPσ-AP binding to immobilized GAGs was examined in an ELISA format (Dickendesher et al., 2012). GAG chains were biotinylated according to the method described by Briani et al. (1998). ELISA plates (Immulon4, NUNC) precoated with streptavidin (Invitrogen, 5 μg/ml) and blocked with 5% BSA were incubated with biotinylated GAGs for 15 min and washed with BS (10 mM epes, 150 mM NaCl, p .3). Three nM of PTPσ-AP was incubated at 4 □ C for 2 h. Bound AP activity was measured with BluePhos (KPL) at 37°C under kinetics mode. The data were expressed as ΔmOD/min.
Receptor affinity probe assay
Sections were incubated overnight at 4 C with RPTPσ-AP, RPTPσ-ΔLys-AP or AP alone. The next day, sections were washed and bound AP was visualized with HistoMark RED (KPL). To evaluate the dependency of binding on divalent cations, incubation of RPTPσ-AP was done in Tris-buffered saline (pH 7.4, TBS) with the addition of either 5 mM Ca2+, 5mM Mg2+, 5mM EDTA, or 5mM EGTA. To verify whether RPTPσ binds to brain GAGs, sections were digested with either chondroitinase ACII (Seikagaku) and/or heparatinase III (Sigma) for 3 h at 3 C prior to incubation with RPTPσ-AP (Mizumoto and Sugahara, 2012). Parallel sections were stained with mouse monoclonal antibodies to native chondroitin-4-sulfate (2H6, Seikagaku), native heparan sulfate (HepSS-1, Seikagaku), cleaved chondroitin-4-sulfate (MAB2030, Millipore) or cleaved heparan sulfate (3G10, Seikagaku), followed by HRP-conjugated secondary antibodies. Widefield images were obtained on a Nikon Eclipse microscope equipped with a Zeiss Axiocam. Low magnification images spanning entire sections were taken using a 4× objective, while higher magnification images were obtained using a 20× objective. Montages of entire sections were created using Photoshop.
Immunofluorescence and confocal microscopy
For fluorescence staining for RPTPσ, the C-terminal (His)6 tag was used to detect bound RPTPσ on the sections. Free-floating sections were first incubated with RPTPσ-AP. After washing in PBS, the sections were blocked for 1 hour with 10% goat serum (Invitrogen) in PBS containing 0.1% Triton X-100 (Sigma) followed by incubation with an anti-(His)6 antibody (Abcam) and different antibodies: rabbit anti-GFAP (DAKO), NeuN (Millipore) or biotinylated sheep anti-Neurocan (R&D Systems), CS-56 (Sigma), 2B6 (Seikagaku) or anti-HS (HepSS-1), or biotinylated Wisteria Floribunda Agglutinin (WFA, Sigma) in the same solution. Following washes, sections were incubated with appropriate secondary antibodies: Alexa Fluor 488 goat anti-rabbit IgG or Alexa Fluor 568 anti-rabbit IgG (Life Technologies); FITC-conjugated goat F(ab’)2 anti-mouse IgM chain (Abcam); Alexa Fluor 488-streptavidin (Jackson Labs); Alexa Fluor 568-streptavidin (Life Technologies). Sections were mounted on silanated slides (KD Medical) and coverslipped using Fluoromount™ (Sigma) with or without DAPI. Confocal images were taken using a Zeiss LSM 510 UV microscope. 3-D co-localization analysis of confocal z-stacks was performed using routines incorporated in Imaris (Bitplane).
Results
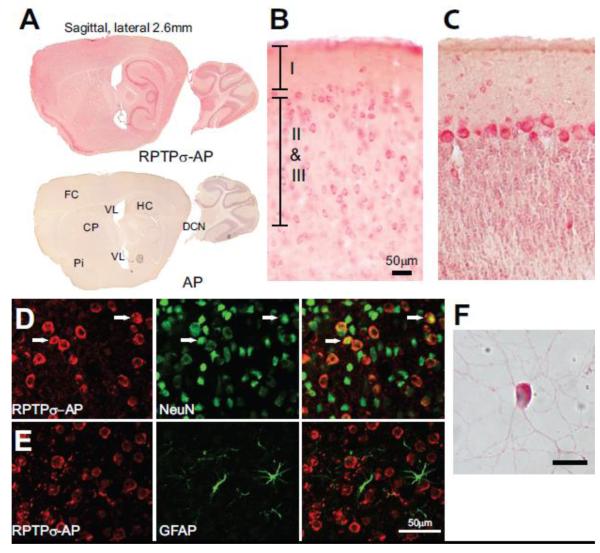
To determine the binding sites for RPTPσ within the adult mouse brain, a fusion protein was constructed consisting of the extracellular domain of RPTPσ attached to alkaline phosphatase (RPTPσ-AP). After incubation of this fusion protein with frozen sections of mouse brain, bound AP was visualized on the sections, indicating the sites of RPTPσ binding. There was significant AP activity above background to brain sections (Fig. 1A). The binding was not uniform across the brain, with the highest binding found in the piriform cortex, hippocampus, deep cerebellar nuceli and Purkinje cell layer of the cerebellum. However, in virtually all regions of the brain there were neurons that bound RPTPσ, albeit at lower levels. No reactivity was observed when brain sections were incubated with AP alone (Fig. 1A).
Fig. 1.
RPTPσ binds to neurons in adult mouse brain sections. (A) Montage of sagittal brain sections incubated with RPTPσ-AP or AP shows intense AP activity in the piriform cortex (Pi), hippocampus (HC), deep cerebellar nuclei (DCN), and frontal cortex (FC) of sections incubated with RPTPσ-AP as compared to AP alone. (B) Higher magnification view of the frontal cortex shows many neurons labeled with RPTPσ-AP. (C) Section through cerebellar cortex showing strong RPTPσ-AP labeling of Purkinje neurons and weaker labeling of basket and stellate cells in the molecular layer. (D) RPTPσ-AP binds to NeuN positive cells in the cerebral cortex (arrows). (E) RPTPσ-AP does not colocalize with GFAP in the cerebral cortex. (F) RPTPσ-AP binds to cerebellar granule neurons in culture. Scale Bars: B-E 50 μm, F 10 μm.
Higher magnification images of stained sections demonstrate that binding of RPTPσ-AP was confined to neuronal somata with a wide range of intensities. RPTPσ-AP bound to neurons within the superficial layers of the cerebral cortex (Fig. 1B) and more intensely to Purkinje neurons in the cerebellum (Fig. 1C) . Further confirmation of the neuronal binding of RPTPσ was obtained using co-localization of RPTPσ-AP with cell-type specific markers by immunofluorescence. RPTPσ-AP co-localized with the neuronal marker NeuN (Fig. 1D), but not with the astrocytic marker GFAP (Fig. 1E). RPTPσ-AP was reported to bind to dorsal root ganglion neurons in culture (Coles et al., 2011) and we found that RPTPσ-AP also bound to cultured cerebellar granule neurons (Fig. 1F). Thus, the major binding partners for RPTPσ are neuronal.
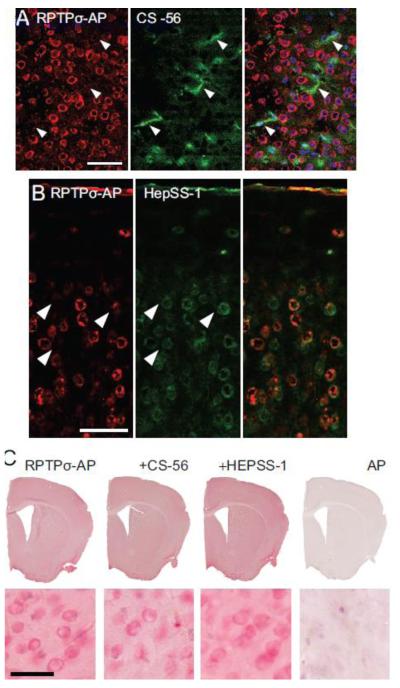
Considerable attention has been paid to the role of RPTPσ and other R2A subfamily members as putative receptors for proteoglycans during development and after injury to the mammalian CNS. We used double-labeling techniques to evaluate the co-localization of RPTPσ binding sites with both chondroitin and heparan sulfate proteoglycans. In the normal, uninjured, brain, the level of chondroitin sulfate proteoglycans in brain parenchyma is relatively low (Yi et al., 2012). When brain sections of cerebral cortex are stained with antibody CS-56, which recognizes CS GAG chains, the staining is very diffuse and patchy, and does not co-localize with cells (Fig. 2A). Moreover, there is no overlap with CS-56 staining and RPTPσ binding. While HSPGs are found to be associated with neuronal cell bodies as indicated by HepSS-1 antibody staining which recognizes HS GAG chains, many HepSS-1 positive neurons showed no PTPσ-AP bound (Fig. 2B). Moreover, in double-labeled neurons, the staining patterns for RPTPσ and HEPSS-1 were overall not the same. As a further test of binding, sections were incubated with RPTPσ-AP in the presence of CS-56 or HepSS-1 antibodies (Fig. 2C). Co-incubation of RPTPσ-AP with these anti-GAG chain antibodies (Fig. 2A, B) did not cause a reduction in binding of the recombinant protein. Thus, it would appear that the binding sites for RPTPσ in the normal mouse brain are not proteoglycan GAG chains.
Fig. 2.
RPTPσ does not bind to proteoglycans in the mouse brain. (A) RPTPσ-AP and anti-CS (CS-56) double labeling in the mouse cerebral cortex show no overlap. Arrowheads show areas of higher CSPG content in the cortex stained positively for anti-CS that are not binding RPTPσ-AP. (B) PTPσ-AP binds to mouse cerebral cortical neurons which also demonstrate immunoreactivity with anti-HS antibody HepSS-1, but no overlap in reactivity is observed. (C) RPTPσ-AP binding is not diminished by co-incubation with CS-56 or HepSS-1. Scale Bars: A-B 50 μm, C - E 25 μm.
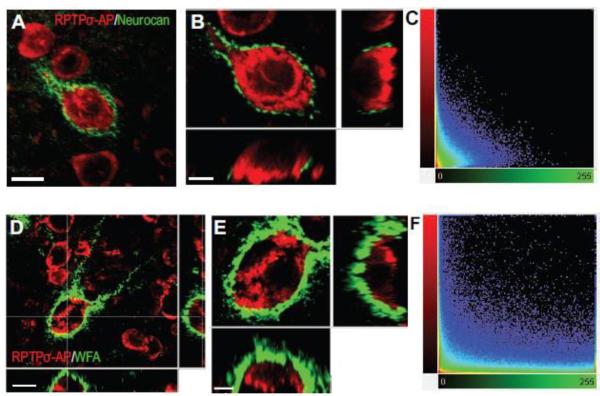
In the uninjured brain, the most intense CSPG staining is observed in perineuronal nets, which surround certain classes of neurons in the cerebral cortex (Celio et al., 1998). In naΔve mouse brain, antiserum raised against the CSPG neurocan intensely labels these perineuronal nets. Double-labeling of these brain sections with anti-neurocan antibody together with RPTPσ-AP shows no overlap of the RPTPσ binding sites with neurocan immunoreactivity, but instead RPTPσ-AP binds to the neurons surrounded by the perineuronal nets (Fig. 3A, B). Perineuronal nets can also be labeled with the wisteria floribunda lectin (WFA), which binds to the GAG chains of chondroitin sulfate proteoglycans (Hartig et al., 1992). Double-label experiments with WFA and RPTPσ again demonstrate that RPTPσ binds to neuronal somata, but not the perineuronal nets that surround them (Fig. 3D, E). The lack of overlap in each case was confirmed with 3D-colocalization analysis (Fig. 3C, F).
Fig. 3.
RPTPσ-AP does not bind to perineuronal nets in mouse brain. (A-B) Double labeling with RPTPσ-AP and neurocan. A) Labeling of neurons by RPTPσ-AP and perineuronal nets by anti-neurocan. B) Higher magnification Z-stack images of RPTPσ-AP and neurocan double labeling shows no overlap.C) 2D fluorogram showing no co-localization of RPTPσ-AP and neurocan. Correlation coefficient of -0.07. Perfect correlation =1, No correlation = 0, Inverse correlation = −1. D-E) Double labeling with RPTPσ-AP and WFA. D) Labeling of neurons by RPTPσ-AP and perineuronal nets by WFA. E) Higher magnification Z-stack images of RPTPσ-AP and WFA double labeling shows no overlap. F) 2D fluorogram showing no co-localization of RPTPσ-AP and WFA. Correlation coefficient of 0.00. Scale Bars: A, D 10 μm, B, E 5 μm.
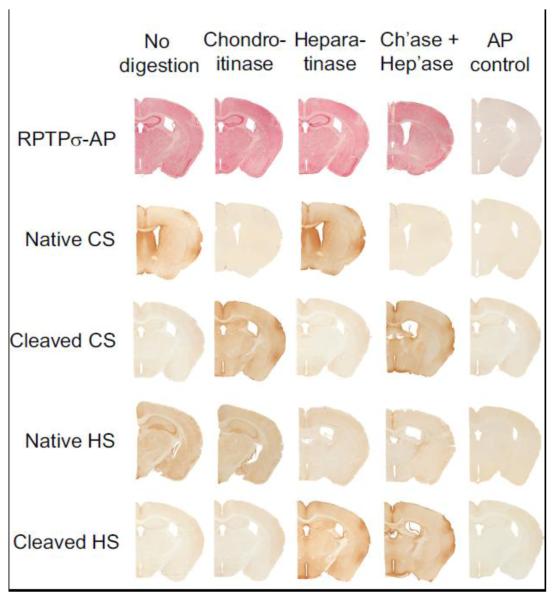
GAG chains can be removed from both heparan and chondroitin sulfate proteoglycans through the use of appropriate enzymes: chondroitinases remove GAG chains from CSPGs, while heparatinase removes GAG chains from HSPGs, with no reported cross-reactivity. To further examine whether RPTPσ was binding to any proteoglycan GAG chains in brain sections, we treated brain sections with either chondroitinase ACII, heparatinase, or a combination of both, and then evaluated the binding of RPTPσ-AP. Disappearance of staining with antibodies which react with native GAG chains and the concomitant appearance of immunoreactivity with antibodies to cleaved chains demonstrated that the enzymes were efficacious (Fig. 4). However, no change in binding was observed when these treated brain sections were incubated with RPTPσ-AP. Thus, RPTPσ binds to brain sections even after ablation of both HS and CS GAG chains.
Fig. 4.
Enzymatic removal of proteoglycan GAGs does not reduce RPTPσ binding. Predigestion of brain sections with chondroitinase or heparatinase or both removed native GAGs and created stub immunoreactivity, but did not affect the binding of RPTPσ-AP to the brain sections. Native chondroitin-4-sulfate (2H6); native heparan sulfate (HepSS1); cleaved chondroitin-4-sulfate (MAB2030); cleaved heparan sulfate (3G10).
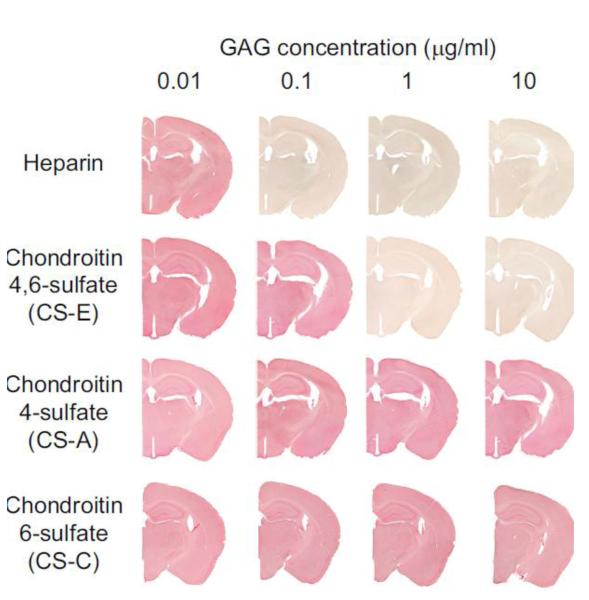
We have previously used ELISA assays to characterize the binding of RPTPσ to proteoglycan GAG chains in vitro (Dickendesher et al., 2012). The RPTPσ-AP fusion protein used here has the same binding specificity (Supplementary Fig. 2): RPTPσ-AP binds to highly sulfated GAGs, including heparin and heparan sulfate as well as the oversulfated chondroitin GAGs, CS-D and CS-E, but not the monosulfated GAGs, CS-A or CS-C. To determine if the sites on RPTPσ necessary for neuronal binding overlap with the binding sites for GAG chains, RPTPσ-AP was incubated with brain sections in the presence or absence of increasing concentrations of GAGs. Increasing concentrations of heparin or CS-E reduce the binding of RPTPσ to brain sections, suggesting that the binding sites in RPTPσ are capable of binding to both proteoglycan GAG chains and binding sites on brain sections (Fig. 5).
Fig. 5.
RPTPσ binding to brain sections is effectively blocked by highly sulfated GAG chains. RPTPσ-AP was incubated with brain sections with increasing concentrations of GAGs as indicated.
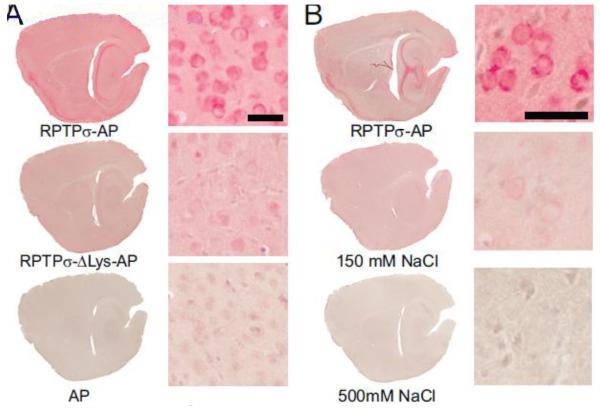
The binding of RPTPσ to GAG chains depends upon at least four lysine residues in the first immunoglobulin repeat of RPTPσ (Aricescu et al., 2002). To assay if this site was involved in binding to brain sections, we mutated all four lysines to alanine, and conducted RAP assays with the RPTPs-ΔLys-AP. Binding of RPTPσ-ΔLys-AP to mouse brain was significantly reduced as compared to binding of wild-type RPTPσ-AP (Fig. 6A), suggesting that a major portion of binding to mouse brain was dependent upon these charged amino acids.
Fig. 6.
RPTPσ binding to mouse brain is dependent on electrostatic interactions. (A) Mutation of lysines to alanines in the first immunoglobulin domain of RPTPσ reduces binding to mouse brain sections. (B) Binding of RPTPσ-AP to neurons in brain sections is reduced at 150 mM NaCl and eliminated at 500 mM NaCl. Scale bar = 20 μm.
The dependence of RPTPσ binding upon the positively charged amino acids in the first Ig domain suggests that the binding of RPTPσ to its ligand(s) is through an electrostatic interaction. To test this hypothesis, we incubated brain sections with RPTPσ-AP in the presence of increasing concentrations of NaCl. Indeed, RPTPσ binding to brain sections was displaced as ionic strength was increased from the normal 150 mM NaCl in TBS, with complete elimination of binding at 500 mM NaCl (Fig. 6B). Thus, the binding of RPTPσ to its ligand(s) is through electrostatic interactions.
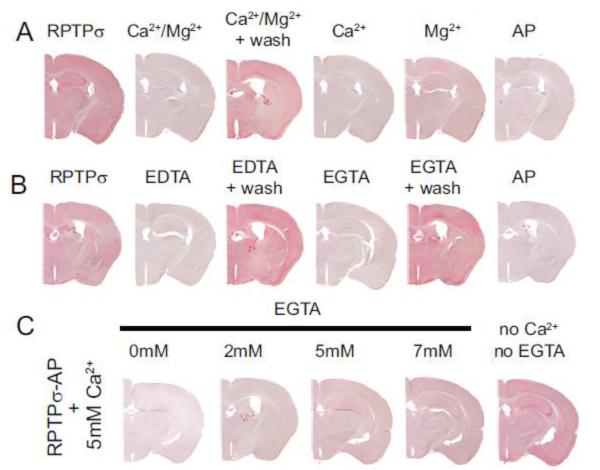
To characterize further the binding of RPTPσ-AP to mouse brain sections, we tested the effects of varying the concentration of divalent cations. Addition of 5 mM Ca2+ or 5 mM Mg2+, or a combination of both to TBS, reduced RPTPσ-AP binding to brain sections, suggesting that divalent cations can inhibit RPTPσ-ligand interaction (Fig. 7A). Surprisingly, addition of either 5 mM EDTA or 5 mM EGTA, chelating agents for divalent cations, also reduced binding. These changes in binding were only observed when RPTPσ-AP was incubated with brain sections in the presence of altered divalent cation concentration. When sections were pre-incubated with either divalent cations or chelating agents, but the RAP assay was conducted after a return to TBS, binding was restored to control levels (Fig. 7B). Further, decreasing free Ca2+ concentration by incrementally increasing the concentration of 5 mM EGTA in the presence of 5 mM Ca2+ progressively restores RPTPσ-AP binding to brain sections (Figure 7C), suggesting that an optimal concentration of free divalent cations is necessary for RPTPσ-AP binding. Thus, our results indicate a complex relationship between the concentration of divalent cations and RPTPσ binding.
Fig. 7.
Binding of RPTPσ-AP to mouse brain is altered by divalent cations. Brain sections were incubated with RPTPσ-AP in (A) TBS, TBS supplemented with 5mM Ca2+ or 5 mM Mg2+ or both, or TBS after an exposure to high Ca2+ and Mg2+ (Ca2+/Mg2+ + wash); (B) the presence of either EDTA or EGTA or TBS after exposure to EDTA or EGTA; (C) the presence of 5 mM Ca2+ without or with increasing concentrations of EGTA.
Discussion
R2A subfamily protein tyrosine phosphatases have been implicated in several different developmental processes related to axonal outgrowth and synaptic formation and stabilization as well as the response to central nervous system injury. Because much of the current data has been obtained using genetic tools to modify the levels of R2A subfamily members during development or after injury to the central nervous system, we sought to evaluate the binding of the extracellular domain of RPTPσ to normal adult brain using the receptor affinity probe assay. We found that RPTPσ bound exclusively to neurons in the brain, and that RPTPσ binding did not co-localize with proteoglycans, either in the brain parenchyma or in perineuronal nets. Enzymatic removal of CS/HS GAGs from brain sections did not impact RPTPσ binding, nor was binding to brain sections inhibited by addition of antibodies to CS-GAG or HS-GAG chains. Thus, these data indicate that the binding partners for RPTPσ in the normal adult mouse brain are produced by neurons and are not proteoglycan GAG chains. A similar result was obtained with LAR binding to syndecan-2 in the Drosophila nervous system: elimination of syndecan-2 abolished LAR binding to glia, with no change in neuronal binding (Fox and Zinn, 2005).
Solid phase assays using the extracellular domain of RPTPσ have demonstrated high-affinity binding to the chondroitin sulfate neurocan (Shen et al., 2009) as well as the heparan sulfate proteoglycans syndecan-2 (Coles et al., 2011), agrin and collagen XVIII (Aricescu et al., 2002). We also used solid phase assays to confirm that RPTPσ binds to S and CS GAG chains. In these earlier studies, binding in solid-phase assays was significantly reduced or eliminated by treatment with enzymes that remove GAG chains. In contrast, ECD binding to brain sections was not altered by enzymatic treatment. There are potential explanations for the difference between solid phase binding to GAG chains and binding to tissue. The first is that the level of GAG chains in normal brain is very low. A second possibility is that the affinity of RPTPσ-ECD constructs to GAG chains depends upon the sulfation composition of the GAG chains: RPTPσ binds with high affinity to HS and the highly sulfated CS-D and CS-E units, while there is low or no affinity for the singly-sulfated CS-A or CS-C (Dickendesher et al., 2012). CS-A and CS-C are the predominant species in the normal, uninjured mouse brain, while there is very little CS-D or CS-E (Maeda, 2010). Thus, RPTPσ would not have significant binding to CS GAG chains in the normal mouse brain. The third is that there exists some ligand(s) in normal brain that inhibit binding of the RPTPσ-ECD to GAG chains. These reasons may also account for the finding by Shen, et al. (2009) that RPTPσ-ECD-Fc did not bind to GAG chains in uninjured spinal cord.
The binding of RPTPσ and other R2A subfamily members to both heparin and chondroitin GAG chains has been localized to the first immunoglobulin domain of the protein (Lee et al., 2007). In solid phase assays, binding of receptor body constructs to GAGs is competed by either heparin or chondroitin sulfate GAG chains. In addition, mutation of four lysine residues in this domain causes a significant reduction in binding to GAGs (Aricescu et al., 2002). Our data indicate that this same region of RPTPσ is important for binding to neurons in mouse brain, as binding was reduced by addition of soluble heparin and chondroitin sulfate GAGs and the mutation of these lysines in the RPTPσ-ΔLys-AP fusion protein.
Because the elimination of the basic lysine residues drastically reduced RPTPσ binding to brain sections, we hypothesized that the interaction between RPTPσ and its binding partner(s) was electrostatic. Indeed, increasing the concentration of NaCl in the incubation medium reduced binding. A similar reduction in binding of RPTPσ to heparin has been reported (Aricescu et al., 2002). On the other hand, we found that binding to brain sections was critically dependent upon the concentration of free divalent cations, as binding was reduced with addition of Ca2+ or Mg2+ or EGTA or EDTA. This is unusual for cell adhesion molecules where the binding site resides in the Ig repeats, and the mechanisms of binding of RPTPσ to brain sections remain to be elucidated.
RPTPs have been suggested to mediate the inhibitory response to CSPGs after spinal cord injury, because recovery is enhanced in both RPTPσ and LAR knockout animals as compared to wild type animals (Fry et al., 2010; Shen et al., 2009). RPTPσ levels increase after nerve injury (Haworth et al., 1998). That the inhibitory response is actually mediated by CSPGs binding to RPTPσ has not been directly demonstrated. Peptides directed against the wedge domain of LAR alter intracellular signaling (Xie et al., 2006) and neurite outgrowth (Fisher et al., 2011), but the dependence of signaling upon receptor occupancy was not measured in these experiments. Additionally, deletion of LAR results in a change in EphA2 phosphorylation and cell migration (Lee and Bennett, 2013). However, the confirmation of the R2A subfamily members as signal transduction molecules depends upon directly showing a change in intracellular signaling following ligand binding, rather than by genetic disruption of RPTP levels. Moreover, the major increase in CSPG core proteins following injury is accompanied by a parallel increase in 4-sulfated GAG chains (Wang et al., 2008), which does not bind RPTPσ (Dickendesher et al., 2012).
Our data suggest that RPTPσ binding sites in the adult brain are not CSPGs; the question then arises as to what the binding partner(s) and functions of RPTPσ might be in the adult animal. Several different reports have supported a role for members of the R2A subfamily in synaptogenesis. Thus, RPTPσ has been implicated in modulation of synaptic plasticity by interactions with both TrkB and TrkC (Kurihara and Yamashita, 2012; Takahashi et al., 2011). Most recently, a specific role for RPTPσ in promoting excitatory and RPTPδ in promoting inhibitory synaptic development by interacting with specific slitraks has been reported (Takahashi et al., 2011; Yim et al., 2013). The interleukin-1-receptor accessory protein like 1 (IL1RAP1) has also been implicated in synapse formation by interacting with RPTPδ (Valnegri et al., 2011; Yoshida et al., 2011). Furthermore, the modulation of BDNF signal transduction by CSPGs was altered in RPTPσ-/- animals, supporting a role for CSPGs and RPTPσ in plasticity (Kurihara and Yamashita, 2012). Because each of the ligands for the R2A subfamily continues to be expressed at high levels in the adult CNS, their role in the adult may be in maintaining synaptic function and plasticity in addition to their developmental roles in establishing synaptic contacts. Because activation of RPTPσ is involved in several negative processes, such as inhibition of NGF signaling (Faux et al., 2007), it would make sense that the CNS would evolve a mechanism to avoid binding of RPTPσ to the CSPGs of the perineuronal nets to circumvent competition with these other protein ligands.
In summary, we have demonstrated that the binding sites for RPTPσ in adult brain are on neurons, and do not depend upon chondroitin or heparan sulfate proteoglycans. Because of the redundancies in binding and actions of the R2A family of protein tyrosine phosphatases, their binding partners and function in the adult brain is still a matter of active investigation.
Supplementary Material
Highlights.
A receptor affinity probe assay shows that RPTPσ binds to neurons in sections of adult mouse brain
RPTPσ binding does not overlap with CSPG staining in adult mouse brain cerebral cortex
Binding of RPTPσ remains when heparan sulfate or chondroitin sulfate are removed enzymatically
Binding of RPTPσ is displaced by incubation with CS-E and heparin but not CS-A or CS-C
Binding of RPTPσ is altered by changes in ionic strength
Acknowledgments
We also acknowledge the assistance of Radislav Junka, the NHLBI DIR light microscopy core and the members of CNRM mouse animal facility. This work has been supported by the CNRM grant to Aviva J. Symes and Herbert M. Geller (300604-11.0-60855) Je-Hyuk Yi was supported by a CNRM postdoctoral fellowship (G1709B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase σ. Mol Cell Biol. 2002;22:1881–1892. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briani C, Berger JS, Latov N. Antibodies to chondroitin sulfate C: a new detection assay and correlations with neurological diseases. J. Neuroimmunol. 1998;84:117–121. doi: 10.1016/s0165-5728(97)00209-9. [DOI] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De BS, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- Chagnon MJ, Uetani N, Tremblay ML. Functional significance of the LAR receptor protein tyrosine phosphatase family in development and diseases. Biochem Cell Biol. 2004;82:664–675. doi: 10.1139/o04-120. [DOI] [PubMed] [Google Scholar]
- Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension. Science. 2011;332:484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Hueske E, Wyszynski M, Hoogenraad CC, Jaworski J, Pak DT, Simonetta A, Liu G, Sheng M. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8:458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- Faux C, Hawadle M, Nixon J, Wallace A, Lee S, Murray S, Stoker A. PTPsigma binds and dephosphorylates neurotrophin receptors and can suppress NGF-dependent neurite outgrowth from sensory neurons. Biochim Biophys Acta. 2007;1773:1689–1700. doi: 10.1016/j.bbamcr.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol. 2005;15:1701–1711. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Fry EJ, Chagnon MJ, Lopez-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58:423–433. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- Hartig W, Brauer K, Bruckner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3:869–872. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Haworth K, Shu KK, Stokes A, Morris R, Stoker A. The expression of receptor tyrosine phosphatases is responsive to sciatic nerve crush. Molecular and cellular neurosciences. 1998;12:93–104. doi: 10.1006/mcne.1998.0707. [DOI] [PubMed] [Google Scholar]
- Kurihara D, Yamashita T. Chondroitin sulfate proteoglycans down-regulate spine formation in cortical neurons by targeting tropomyosin-related kinase B (TrkB) protein. J Biol Chem. 2012;287:13822–13828. doi: 10.1074/jbc.M111.314070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SK, Woo J, Kim SY, Kim H, Kim E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase δ (PTPδ), and PTPσ via specific domains regulate excitatory synapse formation. J Biol Chem. 2010;285:13966–13978. doi: 10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Bennett AM. Receptor protein tyrosine phosphatase-receptor tyrosine kinase substrate screen identifies EphA2 as a target for LAR in cell migration. Mol Cell Biol. 2013;33:1430–1441. doi: 10.1128/MCB.01708-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Faux C, Nixon J, Alete D, Chilton J, Hawadle M, Stoker AW. Dimerization of protein tyrosine phosphatase σ governs both ligand binding and isoform specificity. Mol Cell Biol. 2007;27:1795–1808. doi: 10.1128/MCB.00535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N. Structural variation of chondroitin sulfate and its roles in the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10:22–31. doi: 10.2174/187152410790780136. [DOI] [PubMed] [Google Scholar]
- Mizumoto S, Sugahara K. Glycosaminoglycan chain analysis and characterization (glycosylation/epimerization) Methods Mol. Biol. 2012;836:99–115. doi: 10.1007/978-1-61779-498-8_7. [DOI] [PubMed] [Google Scholar]
- Nikolaienko RM, Agyekum B, Bouyain S. Receptor protein tyrosine phosphatases and cancer: new insights from structural biology. Cell Adh Migr. 2012;6:356–364. doi: 10.4161/cam.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart K, Uetani N, Hendriks W, Tremblay ML, Bouchard M. Inactivation of LAR family phosphatase genes Ptprs and Ptprf causes craniofacial malformations resembling Pierre-Robin sequence. Development. 2013;140:3413–3422. doi: 10.1242/dev.094532. [DOI] [PubMed] [Google Scholar]
- Stoker A, Dutta R. Protein tyrosine phosphatases and neural development. BioEssays: news and reviews in molecular, cellular and developmental biology. 1998;20:463–472. doi: 10.1002/(SICI)1521-1878(199806)20:6<463::AID-BIES4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Arstikaitis P, Prasad T, Bartlett TE, Wang YT, Murphy TH, Craig AM. Postsynaptic TrkC and presynaptic PTPσ function as a bidirectional excitatory synaptic organizing complex. Neuron. 2011;69:287–303. doi: 10.1016/j.neuron.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Craig AM. Protein tyrosine phosphatases PTPdelta, PTPsigma, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 2013;36:522–534. doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valnegri P, Montrasio C, Brambilla D, Ko J, Passafaro M, Sala C. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPdelta and RhoGAP2. Hum Mol Genet. 2011;20:4797–4809. doi: 10.1093/hmg/ddr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. Journal of cell science. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, Sheng M, Kim E. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- Xie Y, Massa SM, Ensslen-Craig SE, Major DL, Yang T, Tisi MA, Derevyanny VD, Runge WO, Mehta BP, Moore LA, Brady-Kalnay SM, Longo FM. Protein-tyrosine phosphatase (PTP) wedge domain peptides: a novel approach for inhibition of PTP function and augmentation of protein-tyrosine kinase function. J Biol Chem. 2006;281:16482–16492. doi: 10.1074/jbc.M603131200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Fisher GJ. Receptor type protein tyrosine phosphatases (RPTPs) - roles in signal transduction and human disease. J Cell Commun Signal. 2012;6:125–138. doi: 10.1007/s12079-012-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Katagiri Y, Susarla B, Figge D, Symes AJ, Geller HM. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J Comp Neurol. 2012;520:3295–3313. doi: 10.1002/cne.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim YS, Kwon Y, Nam J, Yoon HI, Lee K, Kim DG, Kim E, Kim CH, Ko J. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2013;110:4057–4062. doi: 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Yasumura M, Uemura T, Lee SJ, Ra M, Taguchi R, Iwakura Y, Mishina M. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase δ. J Neurosci. 2011;31:13485–13499. doi: 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.