Abstract
Background
Autism spectrum disorders (ASD) involve impairments in cognitive control. In typical development (TYP), neural systems underlying cognitive control undergo substantial maturation during adolescence. Development is delayed in adolescents with ASD. Little is known about the neural substrates of this delay.
Method
We used event-related functional magnetic resonance imaging (fMRI) and a cognitive control task involving overcoming a prepotent response tendency to examine the development of cognitive control in young (ages 12–15; n = 13 with ASD and n = 13 with TYP) and older (ages 16–18; n= 14 with ASD and n = 14 with TYP) adolescents with whole-brain voxel-wise univariate and task-related functional connectivity analyses.
Results
Older ASD and TYP showed reduced activation in sensory and premotor areas relative to younger ones. The older ASD group showed reduced left parietal activation relative to TYP. Functional connectivity analyses showed a significant age by group interaction with the older ASD group exhibiting increased functional connectivity strength between the ventrolateral prefrontal cortex (VLPFC) and the anterior cingulate cortex (ACC), bilaterally. This functional connectivity strength was related to task performance in ASD, whereas that between DLPFC and parietal cortex (BA 9 and BA 40) was related to task performance in TYP.
Conclusions
Adolescents with ASD rely more on “reactive” cognitive control, involving last minute conflict detection and control implementation by the ACC and VLPFC, versus “proactive” cognitive control requiring processing by DLPFC and parietal cortex. Findings await replication in larger longitudinal studies that examine their functional consequences and amenability to intervention.
Keywords: autism spectrum disorders, development, fMRI, cognitive control, adolescence, response inhibition
INTRODUCTION
Autism spectrum disorders (ASD) are life-long (1) neurodevelopmental disorders now diagnosed in 1 in 88 individuals (2). Alongside core social language and repetitive behavior symptoms, a growing body of literature suggests ASD involve impairments in cognitive control (3–13) --the class of mental operations that allow behavior to vary adaptively and flexibly depending on current goals (14). Cognitive control processes include goal or context representation and maintenance, and strategic processes such as attention allocation and stimulus-response mapping (15).
Adolescence (puberty to age 18 (16)) is a period of significant cognitive control development (15), thought to be shaped by the pruning of neural connections in cortical grey matter, and increases in white matter myelination (17). Both these changes result in wide scale reorganization of neural circuits and a shift from diffuse to progressively more specialized (18), or focalized (19) activation in brain networks implementing higher cognitive functions (20–26). Adopting the terminology of Belmonte et al. (27), who define short-range connectivity as that within a brain region, and long-range connectivity as that between brain regions, adolescent neurodevelopment produces alterations in network function leading to reduced short-range alongside increased long-range connectivity (28–32). Correlated activity between brain regions is referred to as functional connectivity (33). With maturation, neural activation and functional connectivity become more reliably related to performance on cognitive tasks (26, 34–36).
The implementation of cognitive control relies on a core network of brain regions in the prefrontal cortex (PFC) including those situated in dorsolateral PFC (DLPFC; BA 9, 46), ventrolateral PFC (VLPFC; BA 47), and dorsomedial PFC including the anterior cingulate cortex (BA 24); as well as regions of superior and inferior parietal cortex (BA 7 and BA 40) (37–38). Cognitive control also may involve recruitment of networks that include premotor regions (BA 6) of the PFC (for response execution), and cerebellar regions depending on task demands (39).
The functioning of networks involved in cognitive control is thought to reach adult levels by mid-adolescence (about age 15) (40–42), although the basic neural mechanisms required for simple tasks may mature by middle childhood (43–44), and the ability to complete tasks requiring the integration of multiple processes, may not consolidate until early adulthood (45–48). Cognitive control operates in at least two modes (49). “Proactive” control involves the early focus on goal-relevant information to optimally bias attention, perception, and action systems. It requires sustained PFC and parietal cortical activation. In “reactive” control, attention is recruited as a late correction, after the onset of conflict. It involves transient VLPFC activation, which signals the need for engagement of the ACC, and is optimal when behavior involves quick responding in novel conditions. Several recent studies suggest that reactive control is more characteristic of children, while proactive control develops through adolescence (50–51).
Behavioral studies suggest that cognitive control matures in children and adolescents with ASD but remains delayed (7, 52), but see (53). There are few functional neuroimaging (fMRI) studies examining cognitive control development throughout adolescence in those with ASD. Several studies of affected children and young adolescents have reported atypical patterns of neural activation in regions related to error monitoring and response conflict including the DLPFC and ACC (12–13). Our group found that adolescents aged 12–18 with ASD evidenced reduced fronto-parietal connectivity while engaged in a cognitive control task (9) – a pattern similar to that found in adults (4, 10, 54). These deficits in fronto-parietal connectivity are consistent with the under-connectivity theory of autism, which suggests that long range connectivity is impaired (27, 55–56). Interestingly, however, the only study of functional connectivity (between inferior PFC and pre-motor cortex) in 8–12 year olds with ASD did not find group differences (57).
The current study was undertaken to increase understanding of the neural correlates of cognitive control during early and late adolescence. Based on the extant literature (20–26), the first hypothesis was that there would be a main effect of diagnosis such that the ASD group would show overall reduced activation in the prefrontal and parietal regions versus TYP; and a main effect of age such that the older adolescents (ASD and TYP) would show reduced activation in brain regions not central to cognitive control (i.e. those used in sensorimotor processing), relative to the younger adolescents. Given the lack of prior studies, no hypothesis about the interaction of group and age was offered. Based on the under-connectivity theory of autism, the second hypothesis was that there would be reductions in shorter-range functional connectivity and increased long-range functional connectivity in the older versus the younger TYP group, but not in the ASD groups. Third, we hypothesized that, adolescents with ASD would exhibit a less mature more reactive style of cognitive control characterized by ACC involvement that was associated with task performance.
METHODS AND MATERIALS
Thirty-nine individuals with ASD and 37 with TYP development were enrolled in the study. Twenty two of those with ASD and 23 with TYP had been subjects in our previous study (9). Twelve subjects with ASD and 10 subjects with typical development were excluded due to poor task performance (2 with ASD; 1 with TYP), excess motion in the scanner (9 with ASD; 8 with TYP) or imaging values that represented outliers based on percent signal change (1 with TYP and 1 with ASD). This left a total sample of 27 subjects with ASD (Mean age=15.4 years) and 27 with TYP development (Mean age= 16.1 years) who are described in this manuscript. Subjects 12 to 15 years were considered young adolescents and subjects 16 to 18 years were considered older adolescents, based on reports in the literature that cognitive control generally reaches mature levels by about age 15 (40–42). Based on the male to female gender ratio of 4:1 in ASD (58), the study included 5 female subjects per group. Participants were recruited through psychiatrists, psychologists, neurologists, pediatricians, speech and language pathologists, and advocacy groups, and the M.I.N.D. Institute’s Subject Tracking System database. All subjects were right-handed and had a Full Scale IQ of at least 75 on the Wechsler Abbreviated Scales of Intelligence (WASI; (59)). Of the 27 participants with an ASD, 13 were diagnosed with Autistic Disorder (high functioning autism), and 14 were diagnosed with Asperger’s Disorder according to DSM-IV-TR (60), ADOS-G (61), and the Social Communication Questionnaire using an ASD cutoff score of 15 and above (SCQ; (62)). Individuals with both diagnoses are difficult to distinguish (e.g. (63–65)), and consequently are included in a single autism spectrum disorder diagnosis in DSM-5 (66). Exclusion criteria for ASD subjects included diagnoses of autism with known genetic etiologies (i.e. fragile X syndrome, tuberous sclerosis), and co-morbid psychopathology reported by parents. Despite this exclusion, 55% of participants with ASD, as is typical (67), and none of those with TYP met criteria for ADHD as assessed by the Conners’ Parent Rating Scale (68). Clinically significant anxiety was present on the Connors in 44% of participants with ASD, and 4% of TYP. Best efforts were used to recruit a sample not taking psychotropic medications. Three members of the ASD group were taking psychostimulants and were asked to discontinue them for 48 hours prior to scanning. Five participants with ASD were taking selective serotonin reuptake inhibitors (SSRIs). Individuals taking atypical antipsychotics were excluded due these medications’ potential impact on connectivity (69). TYP reported no neurodevelopmental, neuropsychiatric, or learning disorders and had a score of less than 11.0 on the Social Communication Questionnaire. See Table 1.
Table 1.
Participant Characteristics
| ASD | TYP | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 12–15 yrs. | 16–18 yrs. | Group | 12–15 yrs. | 16–18 yrs. | Group | |
| N | 13 | 14 | 27 | 13 | 14 | 27 |
|
| ||||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Age (Months) | 167.7 (13.4) | 201.4 (8.7) | 185.1 (20.4) | 174.5 (13.3) | 209.6 (10.5) | 192.7 (21.4) |
| VIQ | 108 (12.6) | 107.5 (14.9) | 107.7 (13.6) | 115.4 (15.9) | 112.6 (10.3) | 114 (13.1) |
| PIQ | 108.2 (14.8) | 107.4 (14) | 107.8 (14.1) | 109.5 (11.8) | 111.7 (12.7) | 110.6 (12.1) |
| FSIQ | 107.4 (12.3) | 108.1 (13.3) | 107.8 (12.6) | 113.1 (13.6) | 112.9 (10.2) | 113 (11.7) |
| SCQ | 21.3 (6.4) | 19.5 (6.4) | 20.4 (6.3) | 1.8 (1.9) | 3.1 (2.7) | 2.5 (2.4) |
| ADOS | 9.5 (3.1) | 10.9 (3) | 10.2 (3) | -- | -- | -- |
*2 subjects in the 12–15 yr old ASD group were taking SSRIs and 2 were taking psychostimulants.
*3 subjects in the 16–18 yr old ASD group were taking SSRIs and 1 was taking a psychostimulant.
All psychostimulants were washed out of the subject’s system 24–48 hours before participation.
Subjects gave written assent along with consent from their legal guardians to participate in this study, which was approved by the University of California, Davis’ Institutional Review Board.
Measures
Wechsler Abbreviated Scales of Intelligence (WASI; (59))
The WASI provides a short reliable estimate of intelligence in individuals aged 6 to 89 (70). It produces Verbal, Performance, and Full Scale IQ scores using four subtests: Vocabulary, Block Design, Similarities, and Matrix Reasoning.
Social Communication Questionnaire-Lifetime Form (SCQ: (71)
SCQ is a 40-item parent-report questionnaire for individuals aged 4 years and older containing parallel “yes/no” questions to those included on the ADI-R (72) -- the “gold standard” in the field. Berument and colleagues (62) reported that scores of 15 have high sensitivity and specificity for autism. TYPs with scores greater than 11 were excluded.
Autism Diagnostic Observation Schedule-Generic (ADOS-G; (61))
Participants with ASD were administered module 3 or 4 of the ADOS-G, a semi-structured interactive session and interview protocol that offers a standardized observation of current social-communication behavior. An algorithm score, that combines the communication and reciprocal social interaction domains, is the basis for diagnostic classification (61).
Preparing to Overcome Prepotency “POP” Task (8, 73–75)
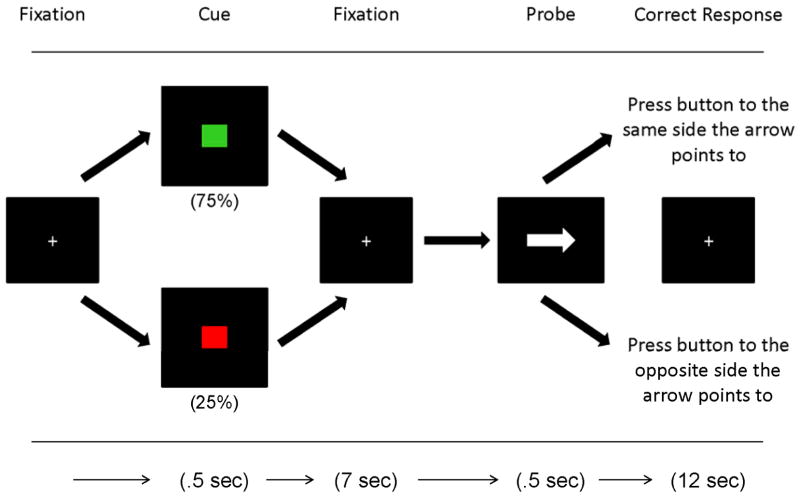
Subjects completed the POP task during fMRI. The POP assesses cognitive control necessary to maintain a cue over a delay and then to overcome a prepotent response tendency. As shown in Figure 1, subjects first are presented with a cue, followed by a delay, and then a probe (an arrow pointing either right or left). Easier “low control” green cues signal the participant to press the key on the same side as the probe arrow points to. “High control” red cues signal the participant to press the key on the opposite side as the probe arrow points to. Green trials occur more often (75% of the time), and are primed at the beginning of each block by three repeated presentations of the green stimulus. Red trials occur less often (25% of trials), and necessitate inhibition of a prepotent response tendency. Mean reaction times (trimmed for outliers more than two standard deviations from the mean), and error rates were calculated for each trial type. The contrast between red minus green trials during the cue phase of the task (considered the strongest measure of cognitive control) was used as the dependent variable.
Figure 1.
The POP task.
As shown in this schematic diagram of the preparing to overcome prepotency or POP task, participants view a fixation cross followed by either a low control green cue (75%) or a high control red cue (25%). In the probe phase of the task they either push the button on the same side as the arrow points to after a green cue or push to the opposite side as the arrow points to on a red trial. Our focus is on the cue phase of the task during the high control red minus low control green contrast.
The fMRI experiment was conducted using a slow event-related design. Each subject performed a brief practice block in the scanner followed by four runs of 24 trials (20 seconds/ trial), each lasting 480 seconds. There were a total of 24 red trials and 72 green trials. Subjects missing more than 60% of a trial type were excluded from the analysis.
Data Acquisition
Structural and functional images were acquired on a 3.0-T Siemens Trio magnetic resonance imaging scanner with an eight-channel phased array head coil. Cushions and pre-training in a mock scanner were used to minimize head motion. Earplugs and headphones were used to dampen scanner noise and to enable communication with the participants. Structural and functional images were acquired at each scan session. Thirty-six interleaved whole-brain axial slices (thickness = 4.0mm) were acquired in a plane parallel to the anterior commisure–posterior commisure (AC–PC) line. Each 20-s trial was sampled by ten 2.0 s functional volumes using a single-shot T2*-weighted echo-planar sequence (TR = 2000 ms, TE = 24 ms, flip angle = 90°,FOV=22cm, 64×64 voxels). A standard T1-weighted pulse sequence was used to obtain structural images in the same plane as the functional images. The task was presented on a desktop computer interfaced with a response box and color LCD projector using the E-prime software package. Stimuli were projected onto a screen viewed through a mirror attached to the head coil. Behavioral data were analyzed using error rates as dependent measures. The first three acquisitions of each run were discarded to allow for fMRI signal to reach steady-state.
Data Preprocessing and Analysis
Imaging data were preprocessed and then analyzed using SPM8 (76). Images underwent slice time acquisition correction; realignment to correct for motion; spatial normalization to the Montreal Neurological Institute (MNI) template; and then smoothing with a three-dimensional Gaussian kernel (8mm FWHM). They were then visually checked for quality. Data from participants exhibiting more than 3 mm of translational or 3 degrees of rotational movement across the session were excluded from the experiment. 23% of those with ASD and 21% of those with TYP were excluded due to excess motion defined this way. We also used the art_groupoutlier program from the ArtRepair toolbox of SPM8, which calculates each subject’s percent signal change for each voxel, to exclude outliers with respect to signal change. This resulted in the exclusion of one additional subject from each group. Comparisons of movement parameters across the age and diagnostic groups showed no differences in all rotations and translations in the remaining participants. Statistical analysis was performed using the general linear model (GLM) with multiple regression as implemented in SPM8. Estimations were made using the ordinary least squares (OLS) method, SPM’s canonical HRF, a high-pass filter of 100 s, and SPM’s AR(1) model. Temporal and dispersion derivatives, as well as motion estimates were included as covariates of non-interest in the model. The cue phase of the task was modeled separately from the probe phase. To correct for multiple comparisons in both univariate and functional connectivity analyses we thresholded for height at a T of 2.4 (p=.01) (77) and report results of between group analyses at the cluster level that are corrected at a FWE rate of p < .05.
Functional Connectivity Analysis
Functional connectivity analyses were conducted using the beta series correlation method (78) using custom-written Matlab (79) scripts. Every stage of every trial is modeled with a separate covariate to obtain trial-to-trial parameter estimates of stage-specific activity. The six head movement parameters were included as nuisance regressors. Betas from each trial were sorted according to task stage into sets of condition specific betas or “beta series.” These beta series were correlated across brain regions to obtain estimates of functional connectivity. All data was motion “scrubbed” using Power’s frame displacement derivative to prevent spurious connectivity findings caused by movement (80–81). After scrubbing, there were no significant group differences in root mean square motion displacement or the mean absolute value of displacement. The average trials removal rates were 9% in TYP and 13% in ASD. Seed regions were chosen from statistically significant clusters in brain regions shown in the literature to be involved in cognitive control (i.e. (82)). These included BA 10, BA 9, BA 46, BA 47, and BA 24. The Wake Forest University PickAtlas Software Toolbox (83) was used to define these regions, which were inflated by 1mm. Again, only correct trials were used. Stage-specific whole-brain correlation maps were obtained by calculating the correlations of the seed region beta series with that of all voxels in the brain using SPM8 and the MarsBaR Toolbox (84). To ensure that no artifactual anti-correlations were induced in seed regions due to global normalization (85), we examined all correlations between seed and other brain regions in the task-dependent functional connectivity analysis to verify that they were not negative. Fisher r-to-Z transformations were applied to the correlation coefficients of all brain voxels to normalize their distribution. Condition specific seed correlation maps were generated and Z-transformed maps were normalized into MNI space. These maps were then entered into group contrast analyses in SPM8.
RESULTS
Behavioral Performance
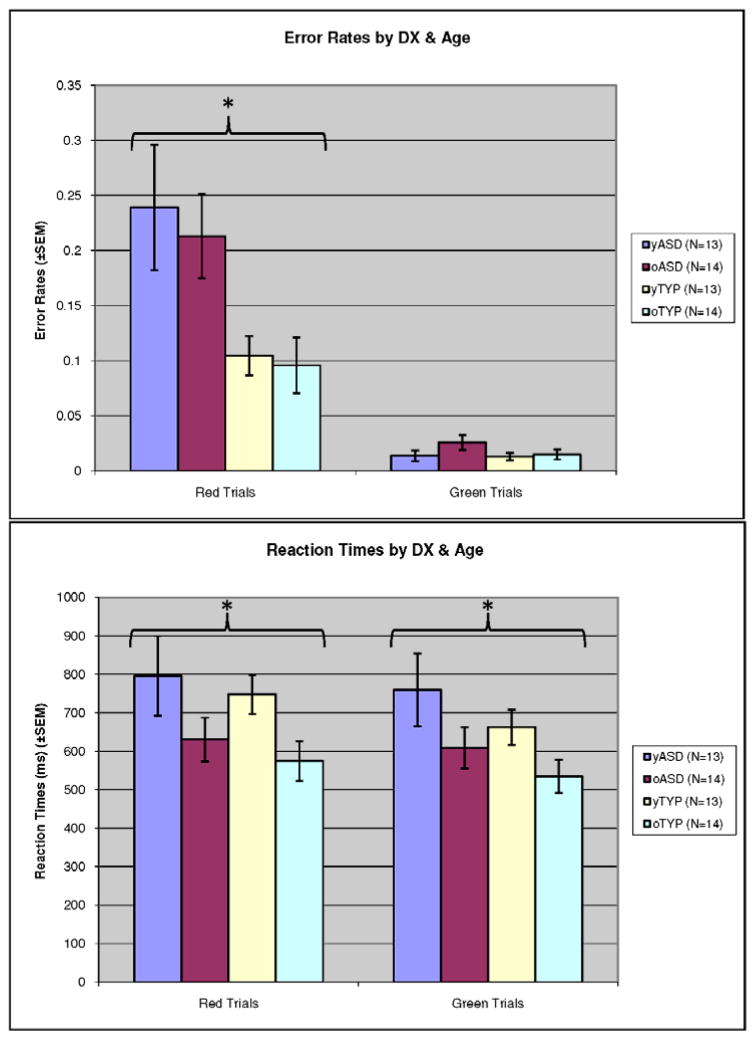
To examine differences in error rates for red minus green cues, a fixed-effects ANOVA was performed where age group and diagnosis were the fixed factors. See Figure 2. There was a main effect of diagnosis (F(1,50)=11.4, p=.001, ηp2 =.186), but a non-significant effect of age group and age by diagnostic group interaction. A similar fixed-effects ANOVA using response times (RTs) revealed a main effect of age group for both red trials (F(1,50)=6.15, p=.017, ηp2 =.110) and green trials (F(1,50)=5.05, p=.029, ηp2 =.092), such that RTs decreased for the older age group. The effect of diagnosis and interaction of age and diagnosis were not significant.
Figure 2.
Behavioral Findings: (A) Red and green trial error rates by age and diagnosis. (B) Red and green trial reaction times by age and diagnosis.
This figure shows behavioral findings for red and green trial error rates by age and diagnosis, and red and green trial reaction times by age and diagnosis. There is a significant main effect of diagnostic group on red trial error rates. (ASD perform worse than TYP), with both groups improving (non-significantly) over time. (B) There is a main effect of age such that reaction times on both red and green trials decrease with age.
Imaging results: Activation
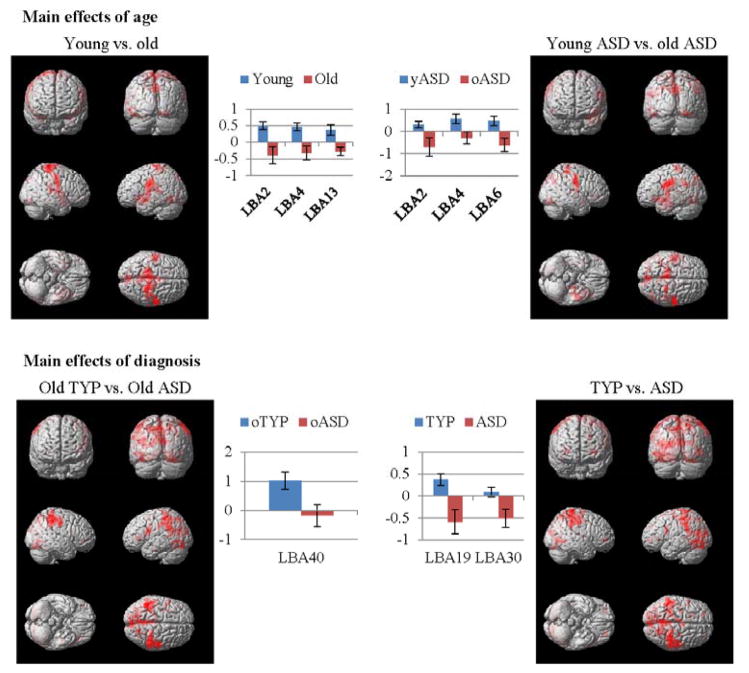
To examine our first hypotheses about the development of cognitive control in early and late adolescence, we used a multiple regression analysis and a full-factorial design with age and diagnostic groups as factors. Across the entire age range in this larger but overlapping sample, there were activation patterns and group differences for the entire adolescent age period in similar regions as found in Solomon et al., (2009) including L BA 19 ([−36, −68, 6]), and L BA 30 ([−29, −66, 10]) (t(52)=3.49, pFWE=.001). See Table 2, and also Supplemental Information Table S1. As shown in Table 2 and Figure 3, there were significant group differences in young versus old participants in sensorimotor brain regions including L BA 2 ([−42, −21, 30]) and L BA 4 ([−51, −16, 33]), and the insula ([−42 3 −5]) (t(52)=3.11, pFWE=.003). These were driven primarily by the ASD group who showed a significant difference in activation for the young minus old group (t(26)=2.70, pFWE=.012). There also were significant between group differences at the older age (ASD<TYP) in parietal cortex (L BA 40; [−44, −42, 34], t(26)=2.28, pFWE=.030).
Table 2.
| Between Group Comparisons | Marjorie Solomon | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Region | Cluster Size | Cluster p-value
|
Z-score | Talairach coordinatesb
|
|||
| FWE-correcteda | Uncorrected | x | y | z | |||
| Young - Old | |||||||
| LH Postcentral Gyrus (BA2) | 2183 | 0.003 | 0 | 3.90 | −42 | −21 | 30 |
| LH Postcentral Gyrus (BA4) | - | - | - | 3.81 | −51 | −16 | 33 |
| LH Insula (BA13) | - | - | - | 3.64 | −42 | 3 | −5 |
| Control - Autism | |||||||
| LH Posterior Cingulate (BA30) | 2814 | 0.001 | 0 | 4.00 | −29 | −66 | 10 |
| LH Lingual Gyrus | - | - | - | 3.53 | −31 | −73 | 6 |
| LH Middle Occipital Gyrus (BA19) | - | - | - | 3.58 | −36 | −68 | 6 |
| Young Autism - Old Autism | |||||||
| LH Postcentral Gyrus (BA2) | 1701 | 0.012 | 0.001 | 4.29 | −42 | −21 | 30 |
| LH Postcentral Gyrus (BA6) | - | - | - | 4.28 | −40 | 9 | 26 |
| LH Postcentral Gyrus (BA4) | - | - | - | 3.97 | −51 | −16 | 33 |
| Old Control - Old Autism | |||||||
| LH Supramarginal Gyrus (BA40) | 1395 | 0.031 | 0.001 | 3.48 | −44 | −42 | 34 |
Regions of activation for red trials minus green trials during the cue phase in controls (n = 27) and patients (n = 27) for T>2.4 (p=.01). There were no significant differences on this contrast between the young TYP and the old TYP or the young ASD and the young TYP groups.
Statistical values are cluster corrected at a FWE rate of p<.05. Regions were defined using Talairach Daemon and the “Nearest gray Matter” option (Lancaster et al., 1997; Lancaster et al., 2000; Maldjian et al., 2003).
Approximate Talairach coordinates were derived by using a MATLAB function written by Matthew Brett (http://www.imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/mni2tal.m) to convert from MNI space.
Figure 3.
Whole brain univariate analyses of younger versus older adolescents, and the ASD and TYP groups.
Beta plots and renderings for significantly different clusters in within and between group whole brain univariate analyses thresholded for height at a T of 2.4 (p=.01) and corrected at a FWE rate of p < .05. Renderings shown at p<.05 for easier visualization.
Imaging results: Functional connectivity analyses
To investigate our second hypothesis, we implemented functional connectivity analyses using cognitive control related seed regions and beta series correlations in a multiple regression analysis with a full-factorial design with age and diagnostic groups as factors. There was a main effect of age such that functional connectivity across both groups was greater between the bilateral BA 10 seed region and the left cerebellum [−24, −58, −38] in the young versus the older group (t(52)=2.93, pFWE=.003). There also were multiple significant within group findings of statistically significant co-activation between seed and other brain regions for young and older adolescent participants with TYP and ASD. See Supplemental Information Figure S1, which illustrates that only the younger TYP group exhibited significant within group functional connectivity between prefrontal, medial frontal, and parietal regions, while only the older ASD group showed significant co-activation between multiple regions of the prefrontal and medial frontal cortices in a pattern that resembled the one found in the young TYP group.
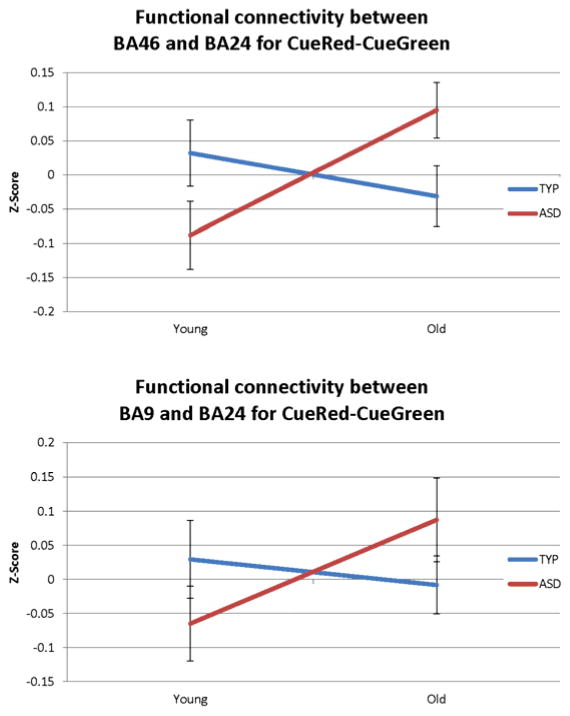
The third hypothesis about the TYP and ASD group’s use of proactive and reactive control, respectively, was tested by examining changes in the strength of functional connectivity (Fisher Z-scores of correlation coefficients) between prefrontal seed regions and the parietal cortex (for proactive control) and the ACC (for reactive control) using ANOVA where age group and diagnosis were the fixed factors. There were no main effects of diagnosis or group in either analysis, however, in the ASD group there were significant interactions in functional connectivity strength between the bilateral BA 24 seed and left and right BA 9 (F(1,50) = 10.67, p=.01) and BA46 (F(1,50) = 3.98, p=.05) such that connectivity between the ACC and the PFC regions declined for the TYP group and increased for the ASD group. See Figure 4.
Figure 4.
Significant interactions in connectivity strength between prefrontal seed regions and the ACC for the older versus the younger groups by diagnosis.
The development of functional connectivity strength between prefrontal seed regions and the ACC. Pictured are DLPFC brain regions showing a significant group by age interaction in connectivity strength with the ACC. Strength increases in the ASD group and declines in the TYP group.
Functional connectivity and behavior
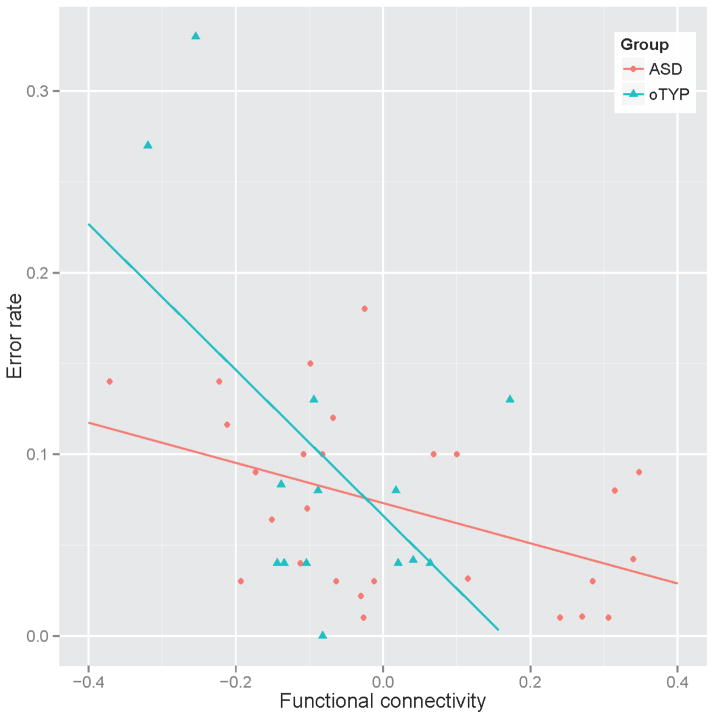
We examined relationships between error rates and functional connectivity on red-minus green trials between brain regions involved in proactive (DLPFC-Parietal or VLPFC-Parietal) and reactive (VLPFC-ACC) control in the ASD and TYP groups. Z-scored correlation coefficients between BA 24 and BA 47 bilaterally were negatively correlated with error rates in ASD for both the younger and older groups (r = −0.449, p=0.012), suggesting that stronger co-activation in these regions was associated with better task performance. The significant relationship was not present for the TYP group (r = −.081, p = .69). In old the TYP, but not the old ASD group, functional connectivity between DLPFC and parietal cortex (BA 9 and BA 40) was negatively related to task performance (r = −.549, p =.042), suggesting that better cognitive control in older TYPs was associated with lower error rates. Correlations for the ASD group were non-significant (r = .16, p=.58). See Figure 5.
Figure 5.
Functional connectivity between brain regions involved in reactive control in ASD and proactive control is TYP and task performance.
Synchronized activation in the ACC (BA 24) and VLPFC (BA 47) is related to task performance (error rates) in the autism group.
DISCUSSION
In support of our hypothesis, there was reduced activation in brain regions outside of core cognitive control areas (i.e. regions related to sensorimotor processing) in all old versus young adolescents. Effects were more pronounced for the ASD group, and activation in the parietal cortex also was reduced in older individuals with ASD. However, overall changes for both groups were subtle, perhaps because the task was relatively easy. Analyses of functional connectivity between cognitive control-related brain regions in the two groups did not show expected patterns of short and long-range connectivity development. Instead, both groups showed reduced connectivity between cognitive control related seed regions and the cerebellum at the older age, and within group analyses showed that TYP exhibited more functional connectivity between brain regions in the prefrontal, medial frontal, and parietal brain regions in early adolescence, with functional connectivity patterns in the older ASD group resembling these patterns. Supportive of our third hypothesis, there was a significant interaction in connectivity strength between cognitive control related prefrontal regions and the ACC bilaterally between early and late adolescence (increasing for ASD and decreasing for TYP). This signature of reactive control was positively associated with task performance in the ASD group, while fronto-parietal functional connectivity –the signature of proactive cognitive control—was associated with task performance in the old TYP group.
We found reductions between the younger and older ASD groups in the recruitment of sensory and motor regions, and reductions in fronto-cerebellar connectivity in older versus younger participants in both groups, suggesting maturation of the motor system. This supports the view that there is normative motor development in adolescence (86). Given that there are reports of permanent motor deficits are in ASD (87–89), and/or motor delays (90), findings of this study are hopeful and should be investigated further.
Both older adolescent groups exhibited increasingly specialized, patterns of task performance and brain activation relative to younger adolescents as shown by changes in error rates, activation patterns, functional connectivity, and the relationships between functional connectivity and performance. Older TYP engaged parietal cortex more robustly than those with ASD, while older individuals with ASD engaged a network including the PFC and the ACC more strongly. As suggested by Berl, Vaidya, & Gaillard (2006) (91), activation and functional connectivity in individuals with neurodevelopmental disorders reflect maturational and experiential factors altered by pathological processes. Networks can remain persistently immature, or permanently altered with respect to laterality, activated regions, or relative weighting of nodes. Although relatively little is known about developmental neuropathology in ASD, early brain overgrowth (92), and neurobiological insults (93–95) may set the stage for atypical patterns of adolescent maturation of neural circuits such that there are base rate reductions in fronto-parietal connectivity that produce sustained over-connectivity in nodes of the typical reactive control network. This would constitute a regionally-weighted compensatory strategy according to Berl and colleagues (56, 91). Indeed, there now are several studies suggesting atypical functioning of the ACC in adults with ASD. Furthermore, a recent human post-mortem study also found atypical development of axons and connectivity between the ACC and the PFC in ASD (96). This more nuanced view may help us more rigorously examine theories of under-connectivity. While receiving considerable support in TYP (97–98) and in ASD (33), such theories do not offer a complete explanation of ASD, perhaps due to methodological inconsistencies in the literature (99) or to the fact that compensatory over-connectivity also may develop (100–101).
Limitations of the study include that it was cross-sectional. Also, while close to sixty subjects participated, cells consisted of only 13–14, making it relatively small. Participants were high functioning, and it is not clear whether findings would generalize to an intellectually disabled group. Finally, a priori seed regions were defined using a brain atlas and the literature. While this is a principled approach, it is bound to an a priori regional hypothesis, and leaves open questions about the involvement of other brain regions, including those involved in visual processing, which may play a compensatory role in individuals with ASD (102).
It will be critical to initiate studies that examine development after age 18 in persons with ASD to clarify the degree to which cognitive and neural systems supporting cognitive control asymptote or continue to develop. Additionally, future studies are needed to identify under what conditions proactive control is possible in those with ASD, given the close relationship between cognitive ability level and adult functioning in TYP (103), and ASD (104–107). There now are multiple interventions to promote neuroplasticity (108). Refining our understanding of proactive and reactive cognitive control mechanisms holds the potential to advance our ability to intervene effectively.
Supplementary Material
Figure S1. Within group significant functional connectivity maps for the young and old ASD and TYP groups on red minus green trials.
Within group renderings showing functional connectivity maps of young and old ASD and TYP groups on red minus green trials using the beta series method are shown at a height threshold of T= 2.4 (p=01) and cluster corrected at FWE rate of p<.05.
Acknowledgments
The authors thank the adolescents and family members who participated in this study, and Jonathan Beck and John Matter for technical assistance with the manuscript. This work was supported by a K-08 Award from the National Institute of Mental Health (1-K-08 MH074967-01) and a Building Interdisciplinary Research Careers in Women’s Health Award (K12 HD051958) funded by the National Institute of Child Health and Human Development (NICHD), Office of Research on Women’s Health (ORWH), Office of Dietary Supplements (ODS), and the National Institute of Aging (NIA), and a Pilot Award from the National Alliance for Research on Schizophrenia and Depression (Atherton Foundation) to Marjorie Solomon. During the time of the study, Dr. Carter was supported by the National Institute of Mental Health (2R01 MH059883-05A1) and (1R24MH081807). Dr. Niendam was supported by the National Institutes of Mental Health (K23MH087708). Dr. Ragland was supported by the National Institutes of Mental Health (R01MH084895, Ragland, PI).
Footnotes
FINANCIAL DISCLOSURES
Dr. Carter reports having been a consultant for Pfizer, Merck, Lilly, and Servier. He also has received funding from Glaxo-Smith Kline. Drs. Solomon, Yoon, Ragland, Niendam, and Lesh and Ms. Fairbrother report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68:459–465. doi: 10.1001/archgenpsychiatry.2011.38. [DOI] [PubMed] [Google Scholar]
- 2.Baio J. Morbidity and Mortality Weekly Report. Surveillance Summaries: Centers for Disease Control; 2012. Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, United States, 2008. [PubMed] [Google Scholar]
- 3.Agam Y, Joseph RM, Barton JJ, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenet T, Orekhova EV, Bharadwaj H, Shetty NR, Israeli E, Lee AK, et al. Disconnectivity of the cortical ocular motor control network in autism spectrum disorders. Neuroimage. 2012;61:1226–1234. doi: 10.1016/j.neuroimage.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson MJ, South M, Clayson PE, Clawson A. Cognitive control and conflict adaptation in youth with high-functioning autism. J Child Psychol Psychiatry. 2012;53:440–448. doi: 10.1111/j.1469-7610.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 7.Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. International Journal of Developmental Neuroscience. 2008;26:239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in ASD. Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yerys BE, Wolff BC, Moody E, Pennington BF, Hepburn SL. Brief Report: Impaired Flexible Item Selection Task (FIST) in School-Age Children with Autism Spectrum Disorders. J Autism Dev Disord. 2012 doi: 10.1007/s10803-012-1443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg MC, Spinelli S, Joel S, Pekar JJ, Denckla MB, Mostofsky SH. Children with high functioning autism show increased prefrontal and temporal cortex activity during error monitoring. Dev Cogn Neurosci. 2011;1:47–56. doi: 10.1016/j.dcn.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaidya CJ, Foss-Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard WD. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and Autism Spectrum Disorders. Dev Sci. 2011;14:911–924. doi: 10.1111/j.1467-7687.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- 14.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 15.Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Current Opinion in Neurobiology. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Dulcan MK. Dulcan’s textbook of child and adolescent psychiatry. 1. Washington, DC: American Psychiatric Pub; 2010. [Google Scholar]
- 17.Giedd JN. The teen brain: insights from neuroimaging. Journal of Adolescent Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Lenneberg EH. Biological foundations of language. New York: Wiley; 1967. [Google Scholar]
- 19.Muller RA, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chugani HT. Developmental changes of cortical and cerebellar motor control: a clinical positron emission tomography study with children and adults. J Child Neurol. 1998;13:550–556. doi: 10.1177/088307389801301105. [DOI] [PubMed] [Google Scholar]
- 20.Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- 21.Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 23.Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Jolles DD, van Buchem MA, Crone EA, Rombouts SA. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb Cortex. 2011;21:385–391. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- 25.Jolles DD, Kleibeuker SW, Rombouts SA, Crone EA. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Dev Sci. 2011;14:713–724. doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- 26.Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 2012 doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. Public Library of Science: Computational Biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jolles DD, van Buchem MA, Crone EA, Rombouts SA. A Comprehensive Study of Whole-Brain Functional Connectivity in Children and Young Adults. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- 33.Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21:2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finn AS, Sheridan MA, Kam CL, Hinshaw S, D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J Neurosci. 2010;30:11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Supekar K, Menon V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Comput Biol. 2012;8:e1002374. doi: 10.1371/journal.pcbi.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braver TS, Cohen JD, Barch DM. The role of the prefrontal cortex in normal and disordered cognitive control: a cognitive neuroscience persp. Oxford: Oxford University Press; 2002. [Google Scholar]
- 38.D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- 42.Somsen RJ. The development of attention regulation in the Wisconsin Card Sorting Task. Developomental Science. 2007;10:664–680. doi: 10.1111/j.1467-7687.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 43.Crone EA, Ridderinkhof KR. The developing brain: from theory to neuroimaging and back. Dev Cogn Neurosci. 2011;1:101–109. doi: 10.1016/j.dcn.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll DC, Cohen JD. Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 46.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Ladouceur CD, Dahl RE, Carter CS. ERP correlates of action monitoring in adolescence. Annals of the New York Academy of Sciences. 2004;1134:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- 48.Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- 49.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6:e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Killikelly C, Szucs D. Delayed development of proactive response preparation in adolescents: ERP and EMG evidence. Dev Cogn Neurosci. 2013;3:33–43. doi: 10.1016/j.dcn.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozonoff S, McEvoy RE. A longitudinal study of executive function and theory of mind development in autism. Development and Psychopathology. 1994;6:415–431. [Google Scholar]
- 53.Happe F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn. 2006;61:25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Agam Y, Joseph R, Barton J, Manoach D. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Just M, Cherkassky V, Keller T, Minshew N. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 56.Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD, et al. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cereb Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nyden A, Hjelmquist E, Gillberg C. Autism spectrum and attention-deficit disorders in girls. Some neuropsychological aspects. Eur Child Adolesc Psychiatry. 2000;9:180–185. doi: 10.1007/s007870070041. [DOI] [PubMed] [Google Scholar]
- 59.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Harcourt Assessment; 1999. [Google Scholar]
- 60.APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 2000. Text Revised. [Google Scholar]
- 61.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- 62.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 63.Howlin P. Outcome in high-functioning adults with autism with and without early language delays: implications for the differentiation between autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2003;33:3–13. doi: 10.1023/a:1022270118899. [DOI] [PubMed] [Google Scholar]
- 64.Macintosh KE, Dissanayake C. Annotation: The similarities and differences between autistic disorder and Asperger’s disorder: a review of the empirical evidence. J Child Psychol Psychiatry. 2004;45:421–434. doi: 10.1111/j.1469-7610.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 65.Ozonoff S, Griffith EM. Neuropsychological function and the external validity of Asperger syndrome. In: Klin A, Volkmar FR, Sparrow SS, editors. Asperger Syndrome. New York: Guilford Press; 2000. pp. 72–96. [Google Scholar]
- 66.APA. DSM-5. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 67.Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome? J Atten Disord. 2009;13:117–126. doi: 10.1177/1087054708326261. [DOI] [PubMed] [Google Scholar]
- 68.Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 69.Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, et al. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stano JF. Test Review. Rehabilitation Counseling Bulletin. 2004;48:56–57. [Google Scholar]
- 71.Rutter M, Bailey A, Lord C. SCQ: Social communication questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 72.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 73.Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cerebral Cortex. 2005;15:899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- 74.Rosano C, Aizenstein H, Cochran J, Saxton J, De Kosky S, Newman AB, et al. Functional neuroimaging indicators of successful executive control in the oldest old. NeuroImage. 2005;28:881–889. doi: 10.1016/j.neuroimage.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 75.Snitz BE, MacDonald A, III, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode eschizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. American Journal of Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 76.Wellcome_Department_of_Cognitive_Neurology. SPM 8. London, UK: 2009. [Google Scholar]
- 77.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 78.Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 79.Natick M. The Math Works. MATLAB; 2000. [Google Scholar]
- 80.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 83.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- 85.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katic R, Bala G, Barovic Z. Gender differentiations of cognitive-motor functioning in prepubertal and pubertal children. Coll Antropol. 2012;36:563–572. [PubMed] [Google Scholar]
- 87.Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. J Autism Dev Disord. 2006;36:613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 88.Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- 89.Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nebel MB, Joel SE, Muschelli J, Barber AD, Caffo BS, Pekar JJ, et al. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berl MM, Vaidya CJ, Gaillard WD. Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage. 2006;30:679–691. doi: 10.1016/j.neuroimage.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 92.Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- 93.Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage. 2004;23:242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 94.Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55:1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- 95.Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154:1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- 96.Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finn AS, Sheridan MA, Kam CL, Hinshaw S, D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. Journal of Neuroscience. 2010;30:11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 99.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaldy Z, Kraper C, Carter AS, Blaser E. Toddlers with Autism Spectrum Disorder are more successful at visual search than typically developing toddlers. Dev Sci. 2011;14:980–988. doi: 10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. J Exp Psychol Hum Percept Perform. 2001;27:719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- 102.Dawson M, Soulieres I, Gernsbacher MA, Mottron L. The level and nature of autistic intelligence. Psychol Sci. 2007;18:657–662. doi: 10.1111/j.1467-9280.2007.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nat Rev Neurosci. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- 104.Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry. 2004;45:212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 105.Gillespie-Lynch K, Sepeta L, Wang Y, Marshall S, Gomez L, Sigman M, et al. Early childhood predictors of the social competence of adults with autism. J Autism Dev Disord. 2012;42:161–174. doi: 10.1007/s10803-011-1222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mawhood L, Howlin P, Rutter M. Autism and developmental receptive language disorder--a comparative follow-up in early adult life. I: Cognitive and language outcomes. Journal of Child Psychology and Psychiatry. 2000;41:547–559. doi: 10.1111/1469-7610.00642. [DOI] [PubMed] [Google Scholar]
- 107.Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: a retrospective study of high-IQ adolescents and adults. J Am Acad Child Adolesc Psychiatry. 1996;35:523–529. doi: 10.1097/00004583-199604000-00019. [DOI] [PubMed] [Google Scholar]
- 108.Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Within group significant functional connectivity maps for the young and old ASD and TYP groups on red minus green trials.
Within group renderings showing functional connectivity maps of young and old ASD and TYP groups on red minus green trials using the beta series method are shown at a height threshold of T= 2.4 (p=01) and cluster corrected at FWE rate of p<.05.