Abstract
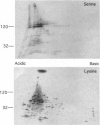
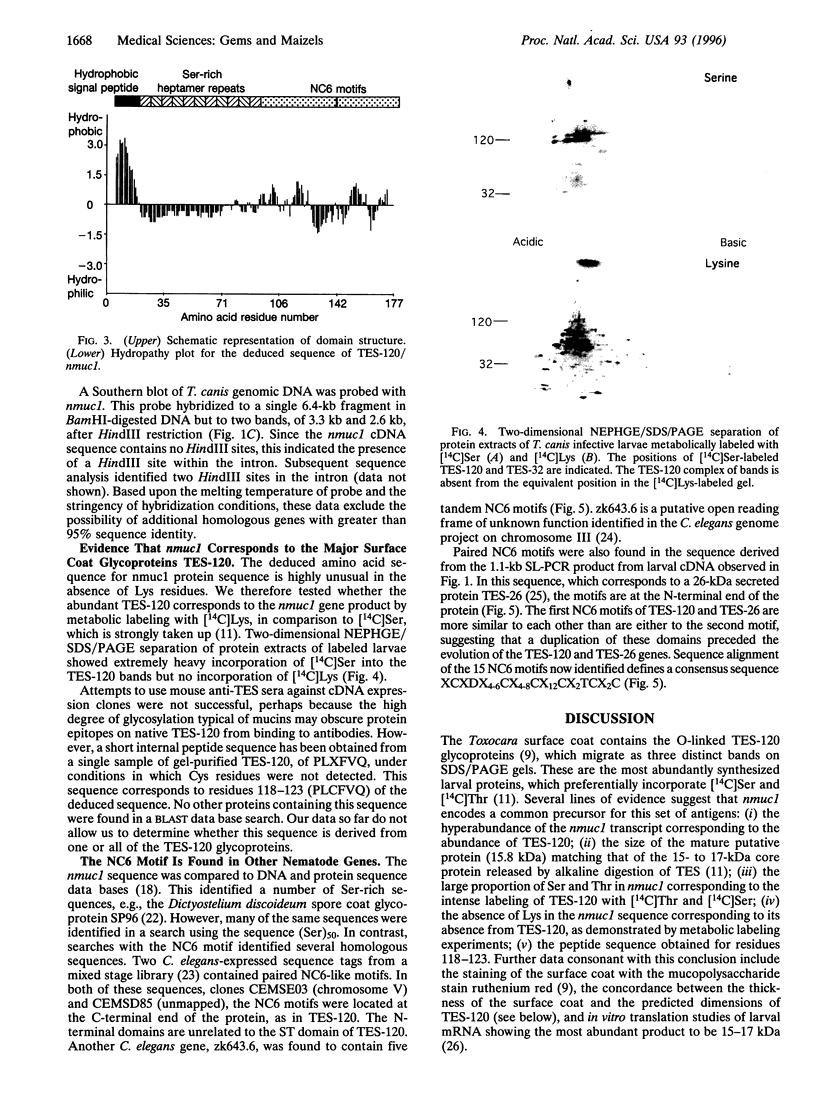
Evasion of host immunity by Toxocara canis infective larvae is mediated by the nematode surface coat, which is shed in response to binding by host antibody molecules or effector cells. The major constituent of the coat is the TES-120 glycoprotein series. We have isolated a 730-bp cDNA from the gene encoding the apoprotein precursor of TES-120. The mRNA is absent from T. canis adults but hyperabundant in larvae, making up approximately 10% of total mRNA, and is trans-spliced with the nematode 5' leader sequence SL1. It encodes a 15.8-kDa protein (after signal peptide removal) containing a typical mucin domain: 86 amino acid residues, 72.1% of which are Ser or Thr, organized into an array of heptameric repeats, interspersed with proline residues. At the C-terminal end of the putative protein are two 36-amino acid repeats containing six Cys residues, in a motif that can also be identified in several genes in Caenorhabditis elegans. Although TES-120 displays size and charge heterogeneity, there is a single copy gene and a homogeneous size of mRNA. The association of overexpression of some membrane-associated mucins with immunosuppression and tumor metastasis suggests a possible model for the role of the surface coat in immune evasion by parasitic nematodes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ardman B., Sikorski M. A., Staunton D. E. CD43 interferes with T-lymphocyte adhesion. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5001–5005. doi: 10.1073/pnas.89.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVER P. C. Toxocarosis (visceral larva migrans) in relation to tropical eosinophilia. Bull Soc Pathol Exot Filiales. 1962 Jul-Aug;55:555–576. [PubMed] [Google Scholar]
- Badley J. E., Grieve R. B., Rockey J. H., Glickman L. T. Immune-mediated adherence of eosinophils to Toxocara canis infective larvae: the role of excretory-secretory antigens. Parasite Immunol. 1987 Jan;9(1):133–143. doi: 10.1111/j.1365-3024.1987.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Behnke J. M., Barnard C. J., Wakelin D. Understanding chronic nematode infections: evolutionary considerations, current hypotheses and the way forward. Int J Parasitol. 1992 Nov;22(7):861–907. doi: 10.1016/0020-7519(92)90046-n. [DOI] [PubMed] [Google Scholar]
- Blaxter M. L., Page A. P., Rudin W., Maizels R. M. Nematode surface coats: actively evading immunity. Parasitol Today. 1992 Jul;8(7):243–247. doi: 10.1016/0169-4758(92)90126-m. [DOI] [PubMed] [Google Scholar]
- Bresalier R. S., Niv Y., Byrd J. C., Duh Q. Y., Toribara N. W., Rockwell R. W., Dahiya R., Kim Y. S. Mucin production by human colonic carcinoma cells correlates with their metastatic potential in animal models of colon cancer metastasis. J Clin Invest. 1991 Mar;87(3):1037–1045. doi: 10.1172/JCI115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Shotton D. M., Williams A. F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991 Apr;10(4):893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine P. L., McKenzie I. F. Mucins: structure, function, and associations with malignancy. Bioessays. 1992 Sep;14(9):619–625. doi: 10.1002/bies.950140909. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Zeng W. A comparison of trans-RNA splicing in trypanosomes and nematodes. Parasitol Today. 1990 Oct;6(10):327–334. doi: 10.1016/0169-4758(90)90177-6. [DOI] [PubMed] [Google Scholar]
- Gems D., Ferguson C. J., Robertson B. D., Nieves R., Page A. P., Blaxter M. L., Maizels R. M. An abundant, trans-spliced mRNA from Toxocara canis infective larvae encodes a 26-kDa protein with homology to phosphatidylethanolamine-binding proteins. J Biol Chem. 1995 Aug 4;270(31):18517–18522. doi: 10.1074/jbc.270.31.18517. [DOI] [PubMed] [Google Scholar]
- Ghosh I., Raghavan N., FitzGerald P. C., Scott A. L. Nucleoside diphosphate kinase from the parasitic nematode Brugia malayi. Gene. 1995 Oct 27;164(2):261–266. doi: 10.1016/0378-1119(95)00500-6. [DOI] [PubMed] [Google Scholar]
- Gillespie S. H. The epidemiology of Toxocara canis. Parasitol Today. 1988 Jun;4(6):180–182. doi: 10.1016/0169-4758(88)90156-1. [DOI] [PubMed] [Google Scholar]
- Glickman L. T., Schantz P. M. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981;3:230–250. doi: 10.1093/oxfordjournals.epirev.a036235. [DOI] [PubMed] [Google Scholar]
- Hayes D. F., Silberstein D. S., Rodrique S. W., Kufe D. W. DF3 antigen, a human epithelial cell mucin, inhibits adhesion of eosinophils to antibody-coated targets. J Immunol. 1990 Aug 1;145(3):962–970. [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Khoo K. H., Maizels R. M., Page A. P., Taylor G. W., Rendell N. B., Dell A. Characterization of nematode glycoproteins: the major O-glycans of Toxocara excretory-secretory antigens are O-methylated trisaccharides. Glycobiology. 1991 Mar;1(2):163–171. doi: 10.1093/glycob/1.2.163. [DOI] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Dowbenko D., Imai Y., Henzel W. J., Grimley C., Fennie C., Gillett N., Watson S. R., Rosen S. D. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992 Jun 12;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Buijs F., Vos H. L., Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992 Apr 15;52(8):2318–2324. [PubMed] [Google Scholar]
- Ligtenberg M. J., Kruijshaar L., Buijs F., van Meijer M., Litvinov S. V., Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem. 1992 Mar 25;267(9):6171–6177. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maizels R. M., Bundy D. A., Selkirk M. E., Smith D. F., Anderson R. M. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993 Oct 28;365(6449):797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- Maizels R. M., Kennedy M. W., Meghji M., Robertson B. D., Smith H. V. Shared carbohydrate epitopes on distinct surface and secreted antigens of the parasitic nematode Toxocara canis. J Immunol. 1987 Jul 1;139(1):207–214. [PubMed] [Google Scholar]
- Maizels R. M., de Savigny D., Ogilvie B. M. Characterization of surface and excretory-secretory antigens of Toxocara canis infective larvae. Parasite Immunol. 1984 Jan;6(1):23–37. doi: 10.1111/j.1365-3024.1984.tb00779.x. [DOI] [PubMed] [Google Scholar]
- McCombie W. R., Adams M. D., Kelley J. M., FitzGerald M. G., Utterback T. R., Khan M., Dubnick M., Kerlavage A. R., Venter J. C., Fields C. Caenorhabditis elegans expressed sequence tags identify gene families and potential disease gene homologues. Nat Genet. 1992 May;1(2):124–131. doi: 10.1038/ng0592-124. [DOI] [PubMed] [Google Scholar]
- Meghji M., Maizels R. M. Biochemical properties of larval excretory-secretory glycoproteins of the parasitic nematode Toxocara canis. Mol Biochem Parasitol. 1986 Feb;18(2):155–170. doi: 10.1016/0166-6851(86)90035-6. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W. Trans-splicing of nematode premessenger RNA. Annu Rev Microbiol. 1993;47:413–440. doi: 10.1146/annurev.mi.47.100193.002213. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Page A. P., Hamilton A. J., Maizels R. M. Toxocara canis: monoclonal antibodies to carbohydrate epitopes of secreted (TES) antigens localize to different secretion-related structures in infective larvae. Exp Parasitol. 1992 Aug;75(1):56–71. doi: 10.1016/0014-4894(92)90122-q. [DOI] [PubMed] [Google Scholar]
- Page A. P., Maizels R. M. Biosynthesis and glycosylation of serine/threonine-rich secreted proteins from Toxocara canis larvae. Parasitology. 1992 Oct;105(Pt 2):297–308. doi: 10.1017/s0031182000074229. [DOI] [PubMed] [Google Scholar]
- Page A. P., Rudin W., Fluri E., Blaxter M. L., Maizels R. M. Toxocara canis: a labile antigenic surface coat overlying the epicuticle of infective larvae. Exp Parasitol. 1992 Aug;75(1):72–86. doi: 10.1016/0014-4894(92)90123-r. [DOI] [PubMed] [Google Scholar]
- Savigny D. H. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J Parasitol. 1975 Aug;61(4):781–782. [PubMed] [Google Scholar]
- Smith H. V., Quinn R., Kusel J. R., Girdwood R. W. The effect of temperature and antimetabolites on antibody binding to the outer surface of second stage Toxocara canis larvae. Mol Biochem Parasitol. 1981 Dec;4(3-4):183–193. doi: 10.1016/0166-6851(81)90017-7. [DOI] [PubMed] [Google Scholar]
- Sugane K., Irving D. O., Howell M. J., Nicholas W. L. In vitro translation of mRNA from Toxocara canis larvae. Mol Biochem Parasitol. 1985 Mar;14(3):275–281. doi: 10.1016/0166-6851(85)90055-6. [DOI] [PubMed] [Google Scholar]
- Sulston J., Du Z., Thomas K., Wilson R., Hillier L., Staden R., Halloran N., Green P., Thierry-Mieg J., Qiu L. The C. elegans genome sequencing project: a beginning. Nature. 1992 Mar 5;356(6364):37–41. doi: 10.1038/356037a0. [DOI] [PubMed] [Google Scholar]
- Taylor M. R., Keane C. T., O'Connor P., Mulvihill E., Holland C. The expanded spectrum of toxocaral disease. Lancet. 1988 Mar 26;1(8587):692–695. doi: 10.1016/s0140-6736(88)91486-9. [DOI] [PubMed] [Google Scholar]
- Zorio D. A., Cheng N. N., Blumenthal T., Spieth J. Operons as a common form of chromosomal organization in C. elegans. Nature. 1994 Nov 17;372(6503):270–272. doi: 10.1038/372270a0. [DOI] [PubMed] [Google Scholar]
- van de Wiel-van Kemenade E., Ligtenberg M. J., de Boer A. J., Buijs F., Vos H. L., Melief C. J., Hilkens J., Figdor C. G. Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction. J Immunol. 1993 Jul 15;151(2):767–776. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]