Abstract
The D-type cyclins (D1, D2 and D3) are components of the cell cycle machinery and govern progression through G1 phase in response to extracellular signals. Although these proteins are highly homologous and conserved in evolution, they contain distinct structural motifs and are differentially regulated in various cell types. Cyclin D1 appears to play a role in many different types of cancer, whereas cyclins D2 and D3 are less frequently associated with malignancy. In this study, we transiently expressed cyclin D1, D2 or D3 in hepatocytes and analyzed transcriptional networks regulated by each. All three D-type cyclins promoted robust hepatocyte proliferation and marked liver growth, although cyclin D3 stimulated less DNA synthesis than D1 or D2. Accordingly, the three D-type cyclins similarly activated genes associated with cell division. Cyclin D1 regulated transcriptional pathways involved in the metabolism of carbohydrates, lipids, amino acids, and other substrates, whereas cyclin D2 did not regulate these pathways despite having an equivalent effect on proliferation. Comparison of transcriptional profiles following 70% partial hepatectomy and cyclin D1 transduction revealed a highly significant overlap, suggesting that cyclin D1 may regulate diverse cellular processes in the regenerating liver. In summary, these studies provide the first comparative analysis of the transcriptional networks regulated by the D-type cyclins and provide insight into novel functions of these key cell cycle proteins. Further study of the unique targets of cyclin D1 should provide further insight into its prominent role in proliferation, growth and cancer.
Keywords: cyclin D1, cyclin D2, cyclin D3, liver regeneration
Introduction
The cell cycle is controlled by protein kinase complexes consisting of cyclins and cyclin-dependent kinases (cdks) which are sequentially activated at different stages of proliferation.1-4 A key component of cdk activity is binding to the appropriate cyclin partners, which are induced in different phases of the cell cycle. Extracellular mitogenic signals promote the expression of one or more D-type cyclins (D1, D2 or D3) that bind to cdk4 and cdk6 and control the transition through key checkpoints in G1 phase, after which the cell cycle can proceed autonomously. This is followed by the induction of cyclins E, A and B which regulate progression through late G1, S, G2 and M phases. Key targets of cyclin/cdk complexes during proliferation include the retinoblastoma (Rb) and related p107 and p130 proteins, which inhibit the cell cycle through their interaction with E2F transcription factors. Phosphorylation of Rb leads to activation of E2F target genes that drive proliferation.
The D-type cyclins, particularly cyclin D1, have been the focus of significant attention because of their pivotal role in normal and malignant cell proliferation.1-5 Cyclin D1 has been proposed to be a key link between mitogenic signaling and the cell cycle machinery during physiologic cell division. In addition, overexpression of cyclin D1 occurs in many cancers through constitutive activation of upstream signaling pathways, gene amplification, or gene rearrangement. This leads to a diminished requirement for mitogenic signals during proliferation, which is a key feature of malignant cells.6 Although cyclins D2 and D3 can similarly activate cdk4/6 and promote Rb phosphorylation, they are less frequently implicated in human cancers.1,7
The cause of the disproportionate role of cyclin D1 in cancer (relative to D2 and D3) has not been established, but possible explanations include distinct regulation by oncogenic signaling events, tissue-specific expression patterns, and the propensity for chromosomal abnormalities involving the cyclin D1 gene. In addition, the D-type cyclins may regulate normal and malignant cell physiology differently because they can affect unique downstream targets. For example, cyclin D1 regulates the activity of numerous transcription factors in a cdk-independent manner through motifs that are not shared by cyclins D2 and D3.7,8 However, relatively little is known about the distinct targets of the D-type cyclins.5
Significant insight into the role of cyclins D1, D2 and D3 has been provided by transgenic mouse models in which one or more of these genes have been deleted (reviewed in refs. 2–5). Knockout of individual D-cyclins leads to varied phenotypic effects including reduced body size and neurological abnormalities (cyclin D1), impaired pancreatic beta-cell and gonadal function (cyclin D2), and alterations in immune cell development (cyclin D3). Combined knockout of all 3 D-type cyclins (or both cdk4 and cdk6) leads to embryonic lethality, although development of most organs does occur and cell cycle progression is not strictly dependent on their expression.9,10 Interestingly, knock-in of either cyclin D2 or cyclin E into the cyclin D1 locus led to correction of most, but not all, of the abnormalities seen in cyclin D1–/– mice.11,12 In these knock-in mice, organ morphology was normal, suggesting that growth and proliferation proceeded normally. However, subtle defects in mammary, retinal and neurological function suggested that cyclin D1 has specific effects on cell physiology that cannot be replaced by other cyclins.11,12 Furthermore, the effect of gene ablation in knockout mice can be masked by compensatory expression of other genes during development,1-5 and thus it is possible that cyclin D1 normally regulates a broader range of cellular functions. Prior studies have indicated that cyclin D1 can affect diverse processes (including invasiveness, motility, angiogenesis, resistance to chemotherapeutic drugs, centrosome duplication and mitochondrial function),7,8,13,14 but the extent to which these functions are shared by cyclins D2 and D3 are unknown.
Unlike many other types of differentiated cells, quiescent hepatocytes retain a near-limitless capacity for replication, a property that underlies the remarkable ability of the liver to restore mass and function after injury.15,16 In addition to the induction of cell proliferation and growth, the liver undergoes a broad range of metabolic adaptations during this process. Although it is not expressed in quiescent liver, cyclin D1 is markedly induced in hepatocyte proliferation and liver regeneration and is thought to play a pivotal role in these processes.16-18 However, the role of cyclin D1 in regulating functions beyond cell cycle control in the liver has not been examined in detail.
In this study, we took an alternative approach to examine the cellular effects of the D-type cyclins. Using the well-established system of adenovirus transduction, we transiently expressed each D-type cyclin in hepatocytes in vivo and examined proliferation, growth and global gene expression using high density microarray analysis. As previously shown for cyclin D1,18 cyclins D2 and D3 promoted liver growth and hepatocyte proliferation, although with varying efficiency. As expected, these cyclins similarly induced genes associated with cell proliferation. Interestingly, a large number of genes regulated by the D-type cyclins were involved in functions distinct from the cell cycle, and there were marked differences in the transcriptional pathways modulated by each. Furthermore, the gene expression patterns seen after cyclin D1 transfection in vivo and 70% partial hepatectomy overlapped substantially, suggesting that this protein may regulate diverse physiologic processes in the regenerating liver. These results indicate that the D-type cyclins have broad effects on cell physiology when expressed in the adult animal, and provide further insight into the unique functions of these proteins.
Results
Induction of hepatocyte proliferation by the D-type cyclins
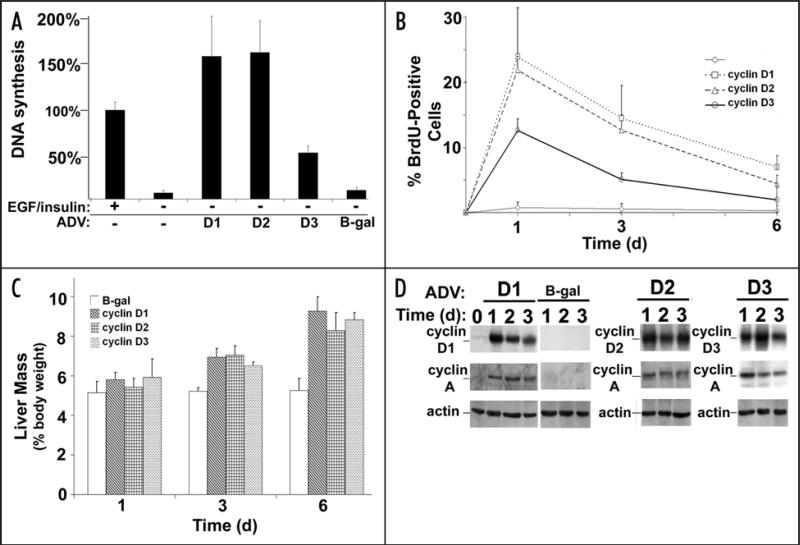
Overexpression of cyclin D1 is sufficient to trigger Cdk4 activation and hepatocyte proliferation in culture and in vivo.17,18 To determine whether each of the D-type cyclins had similar effects, hepatocytes were transduced with recombinant adenoviruses encoding each of these proteins (or a control adenovirus) (Fig. 1). Adenoviral vectors have been used extensively to study the effect of single-gene expression in the liver, and primarily target hepatocytes when injected intravenously.19 As previously shown for cyclin D1,17 cyclins D2 and D3 promoted cell cycle progression in cultured primary rat hepatocytes (as measured by DNA synthesis) in the absence of mitogens, although cyclin D3 was less than 50% as effective as cyclins D1 and D2. Expression of each of the D-type cyclins also induced substantial hepatocyte proliferation (Fig. 1B) and liver growth (Fig. 1C) in vivo. Similar to the effect seen in culture, cyclin D3 promoted less hepatocyte DNA synthesis in vivo than cyclins D1 and D2. Each of the transgenes was readily detectable in the liver after transduction, and induced expression of downstream cell cycle proteins including cyclin A (Fig. 1D), as previously shown for cyclin D1.18 These data indicate that transient expression of each of the D-type cyclins causes robust hepatocyte proliferation and marked liver growth in the absence of other stimuli. Cyclin D3, which is expressed at a low level in quiescent liver and other non-replicating tissues,20-22 was less mitogenic than cyclins D1 and D2 but had a similar effect on liver growth.
Figure 1.
Cyclins D1, D2 and D3 trigger hepatocyte proliferation and liver growth. (A) DNA synthesis in cultured rat hepatocytes. Cells were infected with recombinant adenoviruses (ADV) encoding cyclin D1, D2 or D3 (or a control vector) and 3H-thymidine uptake was determined at 72 hours. Cells treated with EGF and insulin were used as a positive control for proliferation. (B) Hepatocyte DNA synthesis in vivo. Mice were transduced with the indicated vectors and DNA synthesis was determined by BrdU immunohistochemistry at 1–6 days. (C) Liver mass as a percentage of body mass. (D) Western blot analysis of liver lysates.
Identification of cyclin D-regulated gene expression profiles in the liver
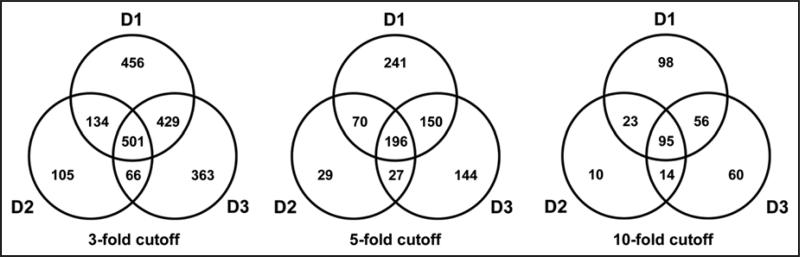
In addition to regulating the cell cycle, the D-type cyclins (especially cyclin D1) have been implicated in other cellular processes.3,7,8,13,14 To gain further insight into their role(s) in cellular physiology, we performed microarray analysis using RNA isolated from liver one day after transduction with cyclins D1, D2, D3, or the control vector. This analysis is unique because we studied the effect of acute transgene expression in differentiated cells in vivo that are highly responsive to the expression of these proteins (Fig. 1). For each condition, four different livers were separately evaluated to provide statistical power for the analysis. As is shown in Table 1, a large number of transcripts were up or downregulated at least 3-fold by each D-type cyclin. Cyclin D1 and cyclin D3 regulated the expression of a similar number of transcripts, in contrast to cyclin D2, which regulated half as many genes. The majority of affected genes showed increased expression, although a substantial number were downregulated.
Table 1.
Differentially-expressed genes
| Total | Upregulated | Downregulated | |
|---|---|---|---|
| Cyclin D1 | 1,520 | 1,132 | 388 |
| Cyclin D2 | 806 | 699 | 107 |
| Cyclin D3 | 1,359 | 1,069 | 290 |
The Venn diagrams in Figure 2 illustrate the overlap between the transcriptional profiles induced by the D-type cyclins at different expression levels (3-, 5- and 10-fold cutoffs). Notably, cyclins D1 and D3 altered the expression of substantially more unique genes (30%) than did cyclin D2 (13%). The majority of genes regulated by cyclin D2 were similarly controlled by all three D-type cyclins, suggesting that shared mechanisms (e.g., cdk4/6 activation and sequestration of cdk-inhibitor proteins) were responsible for many of the effects of cyclin D2. In contrast, the majority (58%) of transcripts regulated by cyclins D1 or D3 were not similarly affected by cyclin D2. These patterns were more pronounced when higher levels of gene expression were used for the analysis. These data suggest that cyclin D2 plays a more limited role in regulating gene expression in vivo than cyclins D1 and D3.
Figure 2.
Venn diagram showing the distribution of differentially expressed genes regulated by D-type cyclins. The number of genes with unique identifiers were counted and displayed as a Venn diagram to highlight relationships among differentially expressed genes. Venn diagrams depicting the distribution of three, five and ten-fold-regulated genes highlights differences in the proportions of these relationships.
The D-type cyclins bind and activate cdk4/6, which can phosphorylate Rb and trigger E2F-dependent transcription of cell cycle genes.1-5 Since their expression in hepatocytes triggered a robust proliferative response (Fig. 1), we expected that these proteins would activate downstream cell cycle genes. A list of representative cell cycle genes upregulated in the arrays, shown in Table 2, demonstrates marked induction by all three D-type cyclins. Interestingly, there was not a clear correlation between the induction of cell cycle genes and the degree of proliferation as measured by DNA synthesis, since cyclin D3 induced the highest expression of several genes despite having a lower proliferative effect. This may be due to differences in post-transcription regulation of the cell cycle proteins, which plays an important role in the control of cell division.1-5
Table 2.
Cell cycle genes
| Gene Symbol | Fold changes | ||
|---|---|---|---|
| Cyclin D1 | Cyclin D2 | Cyclin D3 | |
| Cyclins | |||
| Ccna2 | 10.24 | 20.96 | 28.93 |
| Ccnb1 | 6.55 | 19.42 | 54.99 |
| Ccnb2 | 20.26 | 62.13 | 92.99 |
| Ccne1 | 53.90 | 26.38 | 21.89 |
| Ccne2 | 59.51 | 36.89 | 23.11 |
| Ccnf | 18.64 | 12.72 | 25.34 |
| Ccng1 | 4.l0 | 3.97 | 3.76 |
| Cyclin-dependent kinase inhibitors | |||
| Cdkn1a | 94.95 | 39.87 | 87.98 |
| Cdkn2c | 8.00 | 15.52 | 11.58 |
| Cdkn3 | 3.25 | 6.61 | 12.01 |
| DNA replication | |||
| Cdc6 | 7.00 | 3.38 | 4.42 |
| Cdc20 | 70.82 | 131.24 | 174.25 |
| Cdc2a | 70.04 | 101.41 | 121.44 |
| Cdca5 | 23.30 | 31.61 | 22.93 |
| Cdca8 | 40.37 | 76.06 | 92.48 |
| Mcm2 | 49.91 | 23.30 | 34.78 |
| Mcm3 | 53.52 | 16.92 | 17.86 |
| Mcm4 | 53.71 | 27.86 | 25.94 |
| Mcm5 | 84.57 | 60.39 | 88.22 |
| Mcm6 | 138.82 | 77.23 | 64.14 |
| Mcm7 | 26.93 | 12.97 | 19.53 |
Unexpectedly, the genes showing the greatest degree of up or downregulation by each D-type cyclin were not classical cell cycle genes (Table 3). Markedly induced genes common to all three arrays included Lcn2 (lipocalin 2) and MT2 (metallothionein 2), which are multifunctional iron-binding proteins.23-25 Highly downregulated transcripts shared by each include Clec4f (C-type lectin domain family 4, member F) and Tm9sf2 (transmembrane 9 superfamily member 2), which are poorly characterized genes putatively involved in carbohydrate binding and endosome function, respectively.26,27 Notably, a number of highly regulated genes were differentially affected by the D-type cyclins. For example, Lgals3 (lectin, galactoside-binding, soluble, 3) was markedly induced by cyclin D3 but not cyclins D1 and D2. Similarly, Car3 (carbonic anhydrase 3) expression was substantially inhibited by cyclin D1 but not cyclin D2. A complete list of genes regulated by cyclins D1, D2 and D3 is presented in Tables 1–3 (Suppl. Material). The data suggest that in vivo expression of these cyclins distinctly regulate genes involved in a broad range of cellular processes.
Table 3.
Top 15 genes regulated by cyclin D1, cyclin D2 or cyclin D3
| Upregulated | Downregulated | ||
|---|---|---|---|
| Gene symbol | Fold change | Gene symbol | Fold change |
| Cyclin D1 | |||
| Lcn2 | 751.18 | D0H4S114 | −121.77 |
| Mt2 | 266.69 | Hsd3b5 | −71.91 |
| D17H6S56E-5 | 247.97 | Mup1 | −70.33 |
| Pap | 220.87 | Inmt | −55.84 |
| S100a8 | 190.16 | Clec4f | −39.18 |
| Mcm6 | 138.82 | AU018778 | −36.26 |
| Cdt1 | 138.34 | Cenpa | −29.70 |
| S100a9 | 130.42 | Cml4 | −28.73 |
| Tubb6 | 123.56 | Tm9sf2 | −26.38 |
| Rrm2 | 114.73 | Dct | −26.31 |
| MGC73635 | 104.84 | Agxt2l1 | −26.20 |
| Orm2 | 99.46 | Grem2 | −26.00 |
| LOC677168 | 96.61 | Car3 | −25.04 |
| Cdkn1a | 94.95 | 2810007J24Rik | −24.31 |
| Mcm5 | 84.57 | Slco1a1 | −23.63 |
| Cyclin D2 | |||
| Lcn2 | 466.27 | Clec4f | −36.51 |
| 7H6S56E-5 | 195.10 | Socs2 | −29.25 |
| Mt2 | 153.39 | Tm9sf2 | −25.78 |
| MGC73635 | 144.01 | Ugt2b38 | −23.37 |
| Cdc20 | 131.24 | D0H4S114 | −22.76 |
| Ube2c | 129.43 | Grem2 | −20.60 |
| Birc5 | 121.61 | Vsig4 | −20.15 |
| 2610305J24Rik | 111.20 | 5830411G16Rik | −18.68 |
| Mki67 /// LOC638774 | 106.45 | Cd5l | −13.23 |
| Orm2 | 101.83 | Onecut1 | −12.16 |
| Cdc2a | 101.41 | Csf1r | −11.40 |
| Rrm2 | 99.67 | Slc25a30 | −10.72 |
| Esco2 | 87.86 | Dct | −8.25 |
| Mcm6 | 77.23 | AV025504 | −7.93 |
| Cdca8 | 76.06 | D930010J01Rik | −7.62 |
| Cyclin D3 | |||
| Lcn2 | 562.62 | Ugt2b38 | −62.47 |
| Sprr1a | 367.86 | D0H4S114 | −49.22 |
| Afp | 277.63 | Clec4f | −38.70 |
| D17H6S56E-5 | 223.02 | Dct | −36.99 |
| Ube2c | 219.49 | Tm9sf2 | −36.08 |
| Mt2 | 184.96 | Mup1 | −29.01 |
| Cdc20 | 165.20 | Cenpa | −27.86 |
| Birc5 | 160.12 | Dio1 | −24.82 |
| Lgals3 | 147.75 | Grem2 | −24.58 |
| Tubb6 | 143.12 | Socs2 | −23.82 |
| Mki67 /// LOC638774 | 131.97 | Vsig4 | −21.44 |
| Cdc2a | 121.44 | Serpina4-ps1 | −20.80 |
| Egr1 | 120.77 | Cml4 | −20.51 |
| MGC73635 | 98.23 | Pdk1 | −19.74 |
| Anxa2 | 93.58 | 5830411G16Rik | −19.73 |
Identification of biological functions and pathways regulated by the D-type cyclins
To further explore the potential biological functions of each D-type cyclin, we performed pathway analysis. The most highly regulated molecular and cellular functional categories are listed in Table 4. As expected, cell cycle and DNA replication were among the most significantly affected functions for the three D-type cyclins. Each of these proteins also markedly regulated functions related to cell death and cellular assembly and organization. Surprisingly, cyclin D1 significantly regulated several different metabolic functions including carbohydrate, lipid and amino acid metabolism. In contrast, genes differentially regulated by cyclin D2 did not significantly associate with these metabolic functions.
Table 4.
Top molecular and cellular functions regulated by cyclins D1, D2 and D3
| Cyclin D1 Function | p-value | Cyclin D2 Function | p-value | Cyclin D3 Function | p-value | |
|---|---|---|---|---|---|---|
| 1 | Cell cycle | <0.001–0.049 | Cell cycle | <0.001–0.035 | Cell cycle | <0.001–0.035 |
| 2 | Cell death | 0.001–0.044 | DNA replication, recombination, repair | <0.001–0.035 | Cellular growth and proliferation | <0.001–0.035 |
| 3 | Cellular assembly and organization | 0.001–0.044 | Cellular assembly and organization | <0.001–0.035 | Cellular movement | <0.001–0.034 |
| 4 | Carbohydrate metabolism | 0.002–0.046 | Cellular movement | <0.001–0.042 | Cellular assembly and organization | <0.001–0.034 |
| 5 | Lipid metabolism | 0.002–0.048 | Cell death | <0.001–0.040 | DNA replication, recombination, repair | <0.001–0.034 |
| 6 | Molecular transport | 0.002–0.023 | Cell signaling | 0.002 –0.027 | Cell death | 0.001–0.035 |
| 7 | Small molecule biochemistry | 0.002–0.048 | Cellular growth and proliferation | 0.002–0.038 | Cell-to-cell signaling and interaction | 0.002–0.034 |
| 8 | Amino acid metabolism | 0.003–0.023 | Post-translational modification | 0.003–0.023 | Cellular development | 0.002–0.034 |
| 9 | DNA replication, recombination and repair | 0.003–0.048 | Cellular development | 0.037–0.042 | Lipid metabolism | 0.005–0.030 |
| 10 | Cell morphology | 0.007–0.026 | Small molecule biochemistry | 0.012–0.023 | Small molecule biochemistry | 0.005–0.030 |
The canonical pathways that were significantly regulated (p < 0.05) in the analysis are listed in Table 5. This again revealed that cyclin D1 modulated pathways involved in diverse metabolic processes involving energy utilization, amino acid metabolism and bile acid biosynthesis. The combined results of the analysis indicate that the D-type cyclins regulate genes associated with cell division in a similar manner, but have diverse and distinct effects on transcripts involved in other cellular processes. Cyclin D1 in particular appears to play a significant role in metabolism-related gene expression in hepatocytes in vivo. Further exploration of these novel functions may provide insight in the role of the D-type cyclins in normal physiology and cancer.
Table 5.
Top canonical pathways regulated by cyclins D1, D2 and D3
| Pathway | p-value |
|---|---|
| Cyclin D1 | |
| Butanoate metabolism | 0.002 |
| p53 signaling | 0.002 |
| Fatty acid metabolism | 0.003 |
| Valine,leucine, isoleucine degradation | 0.004 |
| Tryptophan metabolism | 0.005 |
| Arginine and proline metabolism | 0.013 |
| Bile acid biosynthesis | 0.018 |
| Interferon signaling | 0.019 |
| Ascorbate and alderate metabolism | 0.023 |
| Propanoate metabolism | 0.025 |
| Metabolism of xenobiotics by cytochrome P450 | 0.030 |
| Glycerolipid metabolism | 0.040 |
| Cyclin D2 | |
| Cell cycle: G2/M DNA damage checkpoint regulation | 0.0003 |
| p53 signaling | 0.0004 |
| Antigen presentation pathway | 0.012 |
| Pyrimidine metabolism | 0.016 |
| Nicotinate and nicotinamide metabolism | 0.019 |
| Sonic hedgehog signaling | 0.035 |
| cAMP-mediated signaling | 0.043 |
| Cyclin D3 | |
| Cell cycle: G2/M DNA damage checkpoint regulation | 0.014 |
Discrete functions regulated by the D-type cyclins
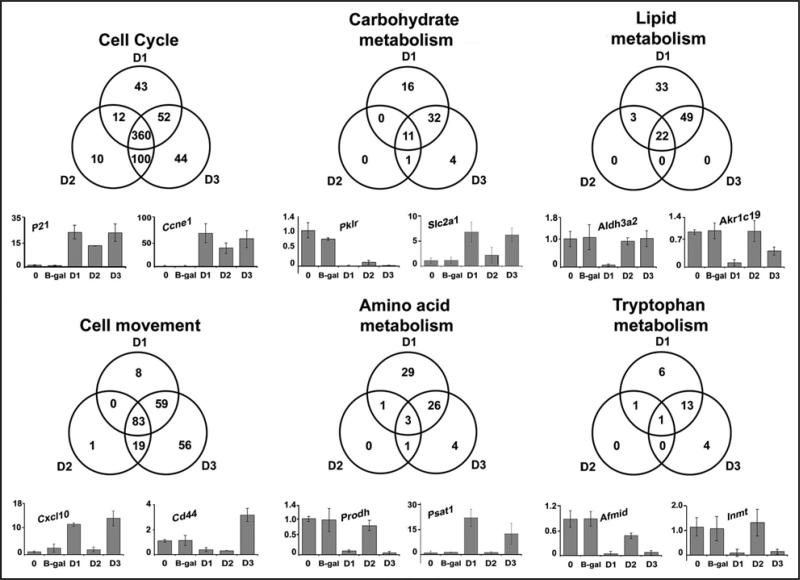
The large number of genes and pathways regulated by the D-type cyclins precludes a thorough analysis in this manuscript. In Figure 3, we focused on several discrete functions identified by the pathway analysis. Of the genes associated with cell cycle progression, most were commonly activated by all three D-type cyclins. RT-PCR of selected key genes, p21 and Ccne1 (cyclin E1) revealed similar induction in all three groups as predicted from the gene array analysis. In contrast, many of the genes involved in metabolic functions (carbohydrate, lipid, amino acid and tryptophan metabolism) were differentially expressed in the three groups. As shown in the Venn diagrams and RT-PCR figures, cyclin D1 substantially regulated a variety of metabolic genes, and these gene sets overlapped with cyclin D3 to a much greater degree than cyclin D2. Notably, cyclin D3 appeared to regulate genes associated with cell movement to a greater degree than cyclins D1 or D2. These data further suggest that the D-type cyclins have discrete effects on cell physiology in vivo.
Figure 3.
Cyclins D1, D2 and D3 regulate distinct molecular and cellular functions. Venn diagrams associated with highly regulated molecular and cellular functions were created from lists of genes generated by Ingenuity Pathways Analysis. mRNA was measured by real-time RT-PCR for select genes from untreated normal livers or livers overexpressing cyclins D1, D2, D3 or β-gal control. Data is expressed as relative change compared to untreated livers. P21, cyclin-dependent kinase inhibitor 1A (P21); Ccne1, cyclin E1; Pklr, pyruvate kinase liver and red blood cell; Slc2a1, solute carrier family 2 (facilitated glucose transporter), member 1; Aldh3a2, aldehyde dehydrogenase family 3, subfamily A2; Akr1c19, aldo-keto reductase family 1, member C19; Cxcl10, chemokine (C-X-C motif) ligand 10; Cd44, CD44 antigen; Prodh, proline dehydrogenase; Psat1, phosphoserine aminotransferase 1; Afmid, arylformamidase; Inmt, indolethylamine N-methyltransferase.
Comparison of transcriptional profiles induced by cyclin D1 and partial hepatectomy
Compensatory hepatocyte proliferation occurs after a wide variety of liver injuries and is an important adaptive response.15,16 In the best-studied model of liver regeneration, 70% partial hepatectomy (PH) in rodents, most of the remaining hepatocytes enter the cell cycle in a relatively synchronous manner, and liver mass is restored within 1–2 weeks. In addition to promoting cell cycle progression and tissue growth, PH also markedly regulates hepatic metabolism, presumably to maintain metabolic homeostasis in the setting of diminished functional liver mass.15,16 Previous studies have suggested that the induction of cyclin D1 plays an important role in liver regeneration by regulating cell cycle progression and growth.16-18,28 However, the influence of cyclin D1 on other functions in the regenerating liver has not been examined.
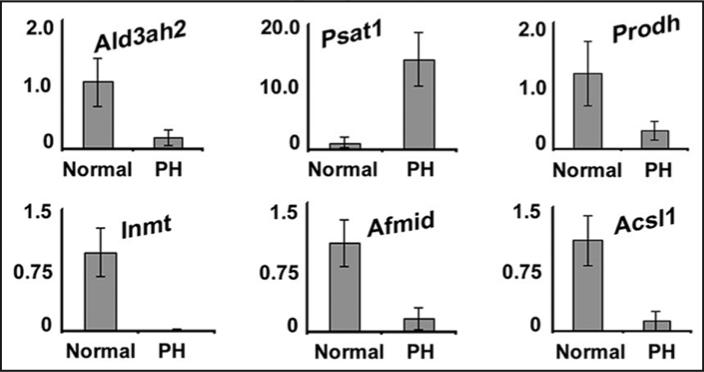
To explore this further, we compared our data to a prior study examining transcriptional networks regulated in regenerating mouse liver at 40 hours after PH, a time point at which cyclin D1 is abundantly expressed and cyclin D1/cdk4 activity is induced.20,29 The previous study in regenerating liver used a different gene chip,29 however 6637 discrete genes were common to both platforms. Strikingly, 32% of the genes that were upregulated and 25% of the genes that were downregulated after PH were similarly regulated by cyclin D1 (Table 6), and the association was highly significant (p < 0.000001). In Table 7, the top 15 up and downregulated genes at 40 hours after PH are listed. Notably, the most highly regulated transcripts after PH are not classical cell cycle genes.29 With few exceptions, these genes were similarly up or downregulated by cyclin D1 in the liver (Table 7). In Figure 4, we examined the expression of some of the metabolic genes shown in Figure 3, which had not been previously known to be regulated by cyclin D1. PH regulated transcripts associated with lipid and amino acid metabolism in a manner similar to cyclin D1. These data suggest that the induction of cyclin D1 in the regenerating liver not only promotes cell cycle progression and growth, but regulates genes involved in broad aspects of hepatic physiology.
Table 6.
Comparison of genes regulated in regenerating liver and cyclin D1
| Expression after PH | Total | Similarly regulated by cyclin D1 |
|---|---|---|
| Upregulated | 425 | 137 (32%) |
| Downregulated | 110 | 28 (25%) |
Table 7.
Top 15 genes regulated by PH and cyclin D1
| Upregulated | Downregulated | ||||
|---|---|---|---|---|---|
| Gene symbol | PH | Cyclin D1 | Gene symbol | PH | Cyclin D1 |
| Saa2 | 84.8 | 33.6 | Car3 | −15.7 | −25.0 |
| Lcn2 | 50.3 | 751.2 | Cyp2f2 | −6.9 | −3.8 |
| Fgl1 | 9.3 | 4.5 | 2810007J24Rik | −5.6 | −24.3 |
| Snrpb2 | 9.2 | 2.3 | Acsl1 | −3.6 | −12.7 |
| Ezh2 | 8.1 | 20.1 | Grb2 | −3.2 | 2.3 |
| Anxa1 | 6.8 | 3.4 | Ces6 | −2.5 | −2.5 |
| Wwp1 | 6.5 | −3.3 | Scd5 | −2.5 | −6.8 |
| Tuba2 | 5.5 | 4.7 | Serpina6 | −2.3 | −9.8 |
| Med28 | 5.4 | 3.6 | Gsta3 | −2.3 | −10.1 |
| Krt18 | 5.3 | 2.7 | Cyb5b | −2.3 | −2.6 |
| Wdr40a | 4.7 | 2.5 | Alas2 | −2.3 | −3.1 |
| Mt2 | 4.7 | 266.69 | Rbm39 | −2.1 | 2.6 |
| Cdc2a | 4.5 | 70.0 | Ces3 | −2.0 | −14.1 |
| Top2a | 4.4 | 47.9 | Tsc22d1 | −2.0 | 2.2 |
| Mt1 | 4.1 | 77.6 | Scp2 | −1.9 | −2.1 |
Figure 4.
Cyclin D1 and partial hepatectomy similarly regulate metabolic genes. RNA was prepared from livers harvested from normal mice and livers from mice 42 hours after partial hepatectomy. RT-PCR was performed for representative metabolic genes similar to Figure 3. The data are expressed as fold-change compared to normal mice.
Discussion
In this study, we showed that transient expression of cyclin D1, D2 or D3 was sufficient to trigger hepatocyte proliferation and liver growth in vivo, which supports the concept that these proteins regulate the Rb-E2F pathway in a similar manner.2-5 Analysis of global transcript expression in the transduced livers demonstrated a surprisingly large number of genes regulated by the D-type cyclins, suggesting that they may modulate a broader range of cellular processes than previously appreciated. Furthermore, each D-type cyclin induced unique transcriptional profiles that point to distinct biological activities in vivo. Cyclin D1 regulated genes involved in diverse metabolic pathways that may contribute to its prominent role in proliferation, growth and cancer.
Previous studies in cell culture systems have established a link between cyclin D1 and several different metabolic functions, in part through regulation of nuclear receptors such as PPARγ and the estrogen, androgen, and thyroid hormone receptors.7,8 Cyclin D promotes growth in a variety of organisms (including plants, fruit flies and mice), and this may be its primary function in some settings.3,28,30-32 Since growth requires significant metabolic adaptations, cyclin D1 must have the capacity to regulate metabolism.3 More direct evidence that cyclin D1 regulates metabolic function in vivo has recently been provided by Pestell and colleagues, who showed that expression of antisense cyclin D1 led to significant changes in genes associated with mitochondrial function and lipolysis in the mammary gland of transgenic mice with tissue-specific overexpression of oncogenic Erb2.33 In addition, cyclin D1–/– mice have hepatic steatosis at baseline,34 although it is not clear whether this is due to direct effects in hepatocytes or signaling from other tissues, since cyclin D1 is not significantly expressed in quiescent adult mouse liver.18,28 The current data suggest that transient induction of cyclin D1 in hepatocytes induces a pattern of gene expression consistent with decreased fatty acid production, and thus supports the hypothesis that this protein plays a role in lipid homeostasis.7,33,34 Conditions associated with fatty liver have a significant impact on liver regeneration, although the underlying mechanisms remain unclear.16,35,36 Further studies will be required to unravel the links between cyclin D1, lipid metabolism, proliferation and growth in the liver.
Our analysis found that cyclin D1 regulated a large number of transcripts involved in energy and substrate utilization (amino acid, carbohydrate and lipid metabolism), whereas cyclin D2 regulated substantially fewer of these genes. Cyclin D2 is not expressed in quiescent or regenerating adult liver.37,38 However, it caused robust proliferation, growth and upregulation of cell cycle genes, indicating that hepatocytes readily respond to its expression. The current data suggest that cyclin D2 may have fewer effects on metabolic functions than the other D-type cyclins. Cyclin D2–/– mice have pancreatic and gonadal dysfunction,39,40 but this appears to be the result of decreased beta and granulosa cell proliferation rather than direct metabolic regulation. Our data suggest that the regulation of metabolic pathways by cyclin D1 is not merely the result of activation of the Rb-E2F pathway, because cyclin D2 induced proliferation and growth had distinct effects on these other pathways.
Although cyclin D3 is induced in some types of replicating cells, it is distinct from the other D-type cyclins insofar as it is expressed in most adult tissues even in the absence of proliferation,21,22 suggesting a role in the physiology of quiescent cells. Indeed, induction of cyclin D3 has been associated with differentiation and cell cycle withdrawal in myocytes and adipocytes.41-43 Cyclin D3 is expressed in quiescent liver tissue and only modestly induced after PH.20 In the livers of aged mice, cyclin D3/cdk4 phosphorylates the transcription factor C/EBPα, enhancing its anti-proliferative effect.44 In our current studies, we find that overexpression of cyclin D3 in the livers of young adult mice leads to hepatocyte proliferation, although less effectively than cyclins D1 and D2. The cumulative data suggest that cyclin D3 plays a complex role in hepatocytes and other cells, and can either promote or inhibit proliferation depending on the cellular context. Of interest, transient cyclin D3 expression regulated genes associated with unexpected functions including cell movement and cell-to-cell interactions. Additional study of these findings may provide insight into the role(s) of cyclin D3 in non-replicating cells.
The current data suggest that induction of cyclin D1 may be responsible for some of the broad metabolic alterations seen during liver regeneration. Using data from a prior study by members of this group,29 we now show that a large percentage of genes up or downregulated in the regenerating liver are similarly regulated by activation of cyclin D1 alone. This was true not only for cell cycle genes, but also for transcripts involved in metabolic processes. The finding that cyclin D1 expression and PH produce overlapping effects on gene expression has several implications. First, this supports previous data suggesting that cyclin D1 plays a pivotal role in liver regeneration.16-18,28 Secondly, it suggests that some of the changes in hepatic metabolism seen after PH may not simply be a response to a deficit in functional liver mass, but may be the direct result of the induction of cyclin D1. Future studies to examine the effect of liver-specific ablation of cyclin D1 in the regenerating liver will be of significant interest.
The findings presented here are novel in several regards. This is the first study to examine the effects of the three D-type cyclins on global gene expression using a single model system, and demonstrated unique patterns of transcriptional regulation. The rapid induction of cyclin D following adenoviral transfection is comparable to that which occurs after mitogenic stimulation, and in fact led to hepatocyte proliferation similar to that seen after PH. This model was intended to mimic the induction of D-type cyclins in mitogen-stimulated cells under physiologic conditions, and thus provides a different perspective than transgenic knockout and overexpression models in which phenotypes can be muted by compensatory changes in gene expression during development. The current data demonstrate that cyclin D1 regulates a broader array of transcriptional pathways in differentiated hepatocytes than previously reported in a breast cancer cell line,45 possibly because the latter system cannot reproduce the tissue microenvironment and integrated physiology of the intact animal. We recognize that our study has several limitations, including: (i) The level of protein expression induced by adenoviral transduction is greater than that observed during physiologic proliferation; (ii) the effect of cyclin D expression on hepatocytes is likely to be distinct from other cell types, and thus some of these results may not apply to other systems; and (iii) transcript expression does not necessarily correlate with functional protein expression. Despite these limitations, the current study provides novel insight into the effects of the D-type cyclins in the intact animal.
In summary, our studies demonstrate that cyclins D1, D2 and D3 regulate genes involved in diverse functions when expressed in a single cell type in vivo, suggesting that these proteins play a more extensive role in cell physiology than previously recognized. As expected, genes associated with cell proliferation were regulated similarly, indicating that activation of the canonical Rb-E2F pathway occurs with each. However, the D-type cyclins differentially regulated transcriptional networks involved in metabolism and other cellular functions. These results provide further evidence that the effects of cyclins D1, D2 or D3 are determined not only by the tissue-specific expression patterns of these genes,3 but also by distinct biological activities of their cognate proteins. These data also indicate that cyclin D1 modulates key events in liver regeneration that are not limited to cell cycle progression. We anticipate that further study of the processes regulated by the D-type cyclins in vivo will provide insight into their role in development, organ function and cancer.
Materials and Methods
Animal procedures
All animal studies were completed following IACUC-approved techniques and National Institutes of Health guidelines. 8 week-old male BALB/c mice were injected with 5–6 × 109 plaque forming units via tail vein injection of recombinant adenoviruses encoding either cyclin D1, D2, D3 or β-galactosidase (control) as previously described,18 and livers were harvested at the indicated time points. 70% partial hepatectomy (PH) followed by liver harvest at 42 hours was performed as described.18,46 Primary rat hepatocytes were isolated and cultured in the absence or presence of EGF and insulin as previously described.17 Cells cultured in the absence of EGF/insulin were transduced with the indicated adenoviruses, and DNA synthesis was measured by 3H-thymidine uptake at 72 hours as previously described.17
Adenoviruses
Adenoviruses encoding cyclin D1 and β-galactosidase were prepared as previously described.17,18 An adenovirus encoding human cyclin D2 was constructed using the Bam H1-Xba1 fragment of the Rc-cyclin D2 plasmid, provided by Philip Hinds.47 The adenovirus encoding cyclin D3 was provided by Sanjoy K. Das.48 CsCl purification and viral titers were performed as previously described.17,18
Western blot and DNA synthesis studies
Protein isolated from liver tissue was used for western blot analysis as described previously.19 Antibodies used for western blot include cyclin D1 (UBI, Temecula CA), cyclins A and D2 (Santa Cruz Biotechnology, Santa Cruz CA), cyclin D3 (Lab Vision/Neomarkers, Fremont CA) and actin (Sigma, St. Louis, MO). DNA synthesis was determined by bromodeoxyuridine (BrdU) immunohistochemistry of hepatocyte nuclei following a 2-hour pulse of BrdU as described previously.19
RNA isolation, microarray expression profiling and data analysis
Livers from four mice transfected with D1, D2, D3 or three mice expressing β-gal for one day were harvested and snap frozen. Total liver RNA was extracted from frozen liver samples on the same day to minimize experimental error using the Qiagen RNeasy midi kit (Valencia, CA). Total RNA content was determined by measuring absorbance at 260 nm and purity assessed by an Agilent bioanalyzer (Agilent Biotechnologies, Inc., Palo Alto, CA). 5 ug of total liver RNA from mice expressing cyclin D1, D2, D3 or β-gal was used for cRNA target preparation. RNA from each mouse (15 total) was analyzed separately.
For microarray profiling cRNA was labeled with biotin and fragmented (Enzo Life Sciences, Inc., Farmingdale, NY). Labeled and fragmented cRNA was hybridized to the Affymetrix (Santa Clara, CA) mouse 430A gene chip by the Biomedical Genomics Center at the University of Minnesota (Minneapolis, MN). A total of 15 chips were used (one for each animal).
Affymetrix data were normalized using GCRMA in the R open source language environment for statistical computing (http://www.r-project.org/). The data were filtered to remove genes with expression levels flagged absent in both the control and the test condition for each comparison. Significant differential expression was determined using SAM (Significance Analysis of Microarrays, version 3.2, which determines false discovery rates (FDR) using the Q-value method.20 Genes were considered significantly differentially expressed with a FDR ≤10% and a fold change ≤-3 or ≥3 (Suppl. Tables 1–3).
The data used for differential gene expression following PH have been described previously.29 The data from this study were reanalyzed and the statistical analysis repeated to allow comparison with the Affymetrix data. In outline, median Cy5 (red) and Cy3 (green) intensities of each element on the array were normalized by the print tip Loess method using the BioConductor package “marray”.22 All statistical analysis of the array data was performed using the normalized M values for all non-control elements on the array. This M value represents the normalized ratio of the test sample over the reference sample. Direct comparisons were made between each time point (2 h, 16 h and 40 h) and the 0 h control samples, using SAM. Genes from the PH experiment were considered significantly differentially expressed with a FDR ≤20% and a fold change ≤-1.5 or ≥1.5, as had been experimentally validated previously.29 A comparison analysis of the overlap between genes differentially expressed following partial hepatectomy and cyclin D1 overexpression (fold change ≤-2 or ≥2) was performed using gene lists of probes common to the Mouse PancChip 5 (8,906 probes) and the Affymetrix MOE430_2 chip (22,005 probes). 6,637 genes were common to the two platforms. The gene list for the 182 overlapping genes is provided in Supplementary Table 4.
Pathway analysis
Biologically relevant molecular and cellular functions and canonical pathways affected by D-type cyclin over-expression were identified using Ingenuity Pathways Analysis (IPA; Ingenuity.com) associates biological functions and metabolic and signaling pathways based on input data. Genes with an expression value of ≤-3 or ≥3 above control were included in the D-type Cyclin input datasets and ≤-1.5 or ≥1.5 -fold for the partial hepatectomy dataset. Of the differentially expressed genes, 946 (cyclin D1), 538 (cyclin D2) and 1053 (cyclin D3) were associated with functional annotations in the IPA knowledge base and thus available for functions and pathways analysis. Top Molecular and Cellular Functions and Pathways are presented in order of p-value, by right-tailed Fisher's Exact Test, which represents the likelihood that an association between a set of Functional Analysis or Pathway molecules in the experiment and a given process or pathway is due to random chance.
Quantification of mRNA by Real Time RT-PCR
Total RNA from each liver was isolated and quantified as previously described.28 RNA (5 μg) was treated with DNase I (DNA-free™, Ambion) according to the manufacturers’ instructions. cDNA was synthesized from 5 μg of each RNA sample with a Taqman reverse transcriptase reagent kit (Applied Biosystems) primed with oligo-dT. Primers were purchased from Integrated DNA Technologies. Primer sequences are listed in Supplementary Table 5. Real-time PCR was performed using the Light Cycler DNA Master SYBR Green I kit (Roche Applied Sciences). Primers were used at a concentration of 0.5 μM and MgCl2 at 2.4 mM. Samples were denatured for 10 min at 95°C and then 40 cycles of 95°C for 20 s, 60°C for 20 s and 72°C for 20 s. Results were normalized to GAPDH. The RT-PCR data for each figure represents 3–4 samples, and a separate experiment using different specimens provided similar results (data not shown).
Supplementary Material
Acknowledgements
We thank Philip Hinds and Sanjoy Das for providing constructs used in these studies. This work was supported by NIH Grants DK54921 (J.H.A), F32DK074320 (L.K.M.), 2 R01 DK/CA56669 (L.E.G), DK-049210 (K.H.K).
Abbreviations
- BrdU
bromodeoxyuridine
- cdk
cyclin-dependent kinase
- PH
70% partial hepatectomy
- β-gal
β-galactosidase
Footnotes
Supplementary materials can be found at:
References
- 1.Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–95. [PubMed] [Google Scholar]
- 2.Santamaria D, Ortega S. Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci. 2006;11:1164–88. doi: 10.2741/1871. [DOI] [PubMed] [Google Scholar]
- 3.Pagano M, Jackson PK. Wagging the Dogma: Tissue-Specific Cell Cycle Control in the Mouse Embryo. Cell. 2004;118:535. doi: 10.1016/j.cell.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Berthet C, Kaldis P. Cell-specific responses to loss of cyclin-dependent kinases. Oncogene. 2007 doi: 10.1038/sj.onc.1210243. [DOI] [PubMed] [Google Scholar]
- 5.Kozar K, Sicinski P. Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle. 2005;4:388–91. doi: 10.4161/cc.4.3.1551. [DOI] [PubMed] [Google Scholar]
- 6.Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2:331–41. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- 7.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–47. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 8.Coqueret O. Linking cyclins to transcriptional control. Gene. 2002;299:35–55. doi: 10.1016/s0378-1119(02)01055-7. [DOI] [PubMed] [Google Scholar]
- 9.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, Akashi K, Sicinski P. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–91. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Carthon BC, Neumann CA, Das M, Pawlyk B, Li T, Geng Y, Sicinski P. Genetic Replacement of Cyclin D1 Function in Mouse Development by Cyclin D2. Mol Cell Biol. 2005;25:1081–8. doi: 10.1128/MCB.25.3.1081-1088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng Y, Whoriskey W, Park MY, Bronson RT, Medema RH, Li T, Weinberg RA, Sicinski P. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell. 1999;97:767–77. doi: 10.1016/s0092-8674(00)80788-6. [DOI] [PubMed] [Google Scholar]
- 13.Nelsen CJ, Kuriyama R, Hirsch B, Negron VC, Lingle WL, Goggin MM, Stanley MW, Albrecht JH. Short term cyclin D1 overexpression induces centrosome amplification, mitotic spindle abnormalities and aneuploidy. J Biol Chem. 2005;280:768–76. doi: 10.1074/jbc.M407105200. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Wang C, Prendergast GC, Pestell RG. Cyclin D1 functions in cell migration. Cell Cycle. 2006;5:2440–2. doi: 10.4161/cc.5.21.3428. [DOI] [PubMed] [Google Scholar]
- 15.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 16.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 17.Albrecht JH, Hansen LK. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ. 1999;10:397–404. [PubMed] [Google Scholar]
- 18.Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 2001;61:8564–8. [PubMed] [Google Scholar]
- 19.Becker TC, Noel RJ, Coats WS, Gomez-Foix AM, Alam T, Gerard RD, Newgard CB. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–89. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 20.Rickheim DG, Nelsen CJ, Fassett JT, Timchenko NA, Hansen LK, Albrecht JH. Differential regulation of cyclins D1 and D3 in hepatocyte proliferation. Hepatology. 2002;36:30–8. doi: 10.1053/jhep.2002.33996. [DOI] [PubMed] [Google Scholar]
- 21.Bartkova J, Lukas J, Strauss M, Bartek J. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene. 1998;17:1027–37. doi: 10.1038/sj.onc.1202016. [DOI] [PubMed] [Google Scholar]
- 22.Doglioni C, Chiarelli C, Macri E, Dei Tos AP, Meggiolaro E, Dalla Palma P, Barbareschi M. Cyclin D3 expression in normal, reactive and neoplastic tissues. J Pathol. 1998;185:159–66. doi: 10.1002/(SICI)1096-9896(199806)185:2<159::AID-PATH73>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Venkatesha S, Hanai J, Seth P, Karumanchi SA, Sukhatme VP. Lipocalin 2 antagonizes the proangiogenic action of ras in transformed cells. Mol Cancer Res. 2006;4:821–9. doi: 10.1158/1541-7786.MCR-06-0110. [DOI] [PubMed] [Google Scholar]
- 24.Cherian MG, Kang YJ. Metallothionein and Liver Cell Regeneration. Experimental Biology and Medicine. 2006;231:138–44. doi: 10.1177/153537020623100203. [DOI] [PubMed] [Google Scholar]
- 25.Lynes MA, Zaffuto K, Unfricht DW, Marusov G, Samson JS, Yin X. The Physiological Roles of Extracellular Metallothionein. Experimental Biology and Medicine. 2006;231:1548–54. doi: 10.1177/153537020623100915. [DOI] [PubMed] [Google Scholar]
- 26.Kaltner H, Gabius HJ. Animal lectins: from initial description to elaborated structural and functional classification. Adv Exp Med Biol. 2001;491:79–94. doi: 10.1007/978-1-4615-1267-7_6. [DOI] [PubMed] [Google Scholar]
- 27.Schimmoller F, Diaz E, Muhlbauer B, Pfeffer SR. Characterization of a 76 kDa endosomal, multispanning membrane protein that is highly conserved throughout evolution. Gene. 1998;216:311–8. doi: 10.1016/s0378-1119(98)00349-7. [DOI] [PubMed] [Google Scholar]
- 28.Nelsen CJ, Rickheim DG, Tucker MM, Hansen LK, Albrecht JH. Evidence that cyclin D1 mediates both growth and proliferation downstream of TOR in hepatocytes. J Biol Chem. 2003;278:3656–63. doi: 10.1074/jbc.M209374200. [DOI] [PubMed] [Google Scholar]
- 29.White P, Brestelli JE, Kaestner KH, Greenbaum LE. Identification of transcriptional networks during liver regeneration. J Biol Chem. 2005;280:3715–22. doi: 10.1074/jbc.M410844200. [DOI] [PubMed] [Google Scholar]
- 30.Edgar BA. Growth and Cell Cycle Control in Drosophila. In: Hall MN, Raff MC, Thomas G, editors. Cell Growth: Control of Cell Size. Cold Spring Harbor Laboratory Press; 2004. pp. 23–84. [Google Scholar]
- 31.Busk PK, Bartkova J, Strom CC, Wulf-Andersen L, Hinrichsen R, Christoffersen TE, Latella L, Bartek J, Haunso S, Sheikh SP. Involvement of cyclin D activity in left ventricle hypertrophy in vivo and in vitro. Cardiovasc Res. 2002;56:64–75. doi: 10.1016/s0008-6363(02)00510-2. [DOI] [PubMed] [Google Scholar]
- 32.Cockcroft CE, den Boer BG, Healy JM, Murray JA. Cyclin D control of growth rate in plants. Nature. 2000;405:575–9. doi: 10.1038/35014621. [DOI] [PubMed] [Google Scholar]
- 33.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, Jiao X, Li A, Zhang X, Lu Y, Wang C, Byers S, Nicholson R, Link T, Shemluck M, Yang J, Fricke ST, Novikoff PM, Papanikolaou A, Arnold A, Albanese C, Pestell R. Cyclin D1 determines mitochondrial function in vivo. Mol Cell Biol. 2006;26:5449–69. doi: 10.1128/MCB.02074-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Pattabiraman N, Zhou JN, Fu M, Sakamaki T, Albanese C, Li Z, Wu K, Hulit J, Neumeister P, Novikoff PM, Brownlee M, Scherer PE, Jones JG, Whitney KD, Donehower LA, Harris EL, Rohan T, Johns DC, Pestell RG. Cyclin D1 Repression of Peroxisome Proliferator-Activated Receptor {gamma} Expression and Transactivation. Mol Cell Biol. 2003;23:6159–73. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudnick DA. Trimming the fat from liver regeneration. Hepatology. 2005;42:1001–3. doi: 10.1002/hep.20931. [DOI] [PubMed] [Google Scholar]
- 36.Farrell GC. Probing Prometheus: fat fueling the fire? Hepatology. 2004;40:1252–5. doi: 10.1002/hep.20522. [DOI] [PubMed] [Google Scholar]
- 37.Albrecht JH, Hoffman JS, Kren BT, Steer CJ. Cyclin and cyclin-dependent kinase 1 mRNA expression in models of regenerating liver and human liver diseases. Am J Physiol. 1993;265:857–64. doi: 10.1152/ajpgi.1993.265.5.G857. [DOI] [PubMed] [Google Scholar]
- 38.Boylan JM, Gruppuso PA. D-type cyclins and G1 progression during liver development in the rat. Biochem Biophys Res Commun. 2005;330:722–30. doi: 10.1016/j.bbrc.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 39.Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–4. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- 40.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114:963–8. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cenciarelli C, De Santa F, Puri PL, Mattei E, Ricci L, Bucci F, Felsani A, Caruso M. Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblast differentiation. Mol Cell Biol. 1999;19:5203–17. doi: 10.1128/mcb.19.7.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarruf DA, Iankova I, Abella A, Assou S, Miard S, Fajas L. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2005;25:9985–95. doi: 10.1128/MCB.25.22.9985-9995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de La Serna IL, Roy K, Carlson KA, Imbalzano AN. MyoD Can Induce Cell Cycle Arrest but Not Muscle Differentiation in the Presence of Dominant Negative SWI/SNF Chromatin Remodeling Enzymes. J Biol Chem. 2001;276:41486–91. doi: 10.1074/jbc.M107281200. [DOI] [PubMed] [Google Scholar]
- 44.Wang GL, Shi X, Salisbury E, Sun Y, Albrecht JH, Smith RG, Timchenko NA. Cyclin D3 maintains growth-inhibitory activity of C/EBPalpha by stabilizing C/EBPalpha-cdk2 and C/EBPalpha-Brm complexes. Mol Cell Biol. 2006;26:2570–82. doi: 10.1128/MCB.26.7.2570-2582.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, Zahnow CA, Patterson N, Golub TR, Ewen ME. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell. 2003;114:323–34. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 46.Albrecht JH, Poon RY, Ahonen CL, Rieland BM, Deng C, Crary GS. Involvement of p21 and p27 in the regulation of CDK activity and cell cycle progression in the regenerating liver. Oncogene. 1998;16:2141–50. doi: 10.1038/sj.onc.1201728. [DOI] [PubMed] [Google Scholar]
- 47.Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 48.Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265:181–95. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.