Abstract
Wnt signaling affects both bone modeling, which occurs during development, and bone remodeling, which is a lifelong process involving tissue renewal. Wnt signals are especially known to affect the differentiation of osteoblasts. In this review, we summarize recent advances in understanding the mechanisms of Wnt signaling, which is divided into two major branches: the canonical pathway and the noncanonical pathway. The canonical pathway is also called the Wnt/β-catenin pathway. There are two major noncanonical pathways: the Wnt-planar cell polarity pathway (Wnt-PCP pathway) and the Wnt-calcium pathway (Wnt-Ca2+ pathway). This review also discusses how Wnt ligands, receptors, intracellular effectors, transcription factors, and antagonists affect both the bone modeling and bone remodeling processes. We also review the role of Wnt ligands, receptors, intracellular effectors, transcription factors, and antagonists in bone as demonstrated in mouse models. Disrupted Wnt signaling is linked to several bone diseases, including osteoporosis, van Buchem disease, and sclerosteosis. Studying the mechanism of Wnt signaling and its interactions with other signaling pathways in bone will provide potential therapeutic targets to treat these bone diseases.
Keywords: Beta-catenin, noncanonical Wnt signaling, osteoblast, osteoclast, osteoporosis, PTH/PTHrP, sclerosteosis, Wnt antagonists, Wnt ligands, Review
2. INTRODUCTION
Bone is the rigid tissue that functions to move, support, and protect various organs of the body. It is made mostly of collagen and calcium phosphate. Physiological bone turnover can be divided into two temporal phases: modeling, which occurs during development, and remodeling, a lifelong process involving tissue renewal (1). Bone development has two stages (2): intramembranous ossification and endochondral ossification. Intramembranous ossification occurs in the formation of flat bones. This begins with the condensation of mesenchymal stem cells, which then differentiate into osteoprogenitors and become mature osteoblasts. Later, these osteoblasts will either undergo apoptosis or change into osteocytes. Endochondral ossification, which takes place on the long bone, also begins with mesenchymal stem cell condensation. Unlike intramembranous ossification, cartilage is present during endochondral ossification. Osteoblasts and osteoclasts are two major bone cells that affect the remodeling process. There is a balance between osteoclastic bone resorption and osteoblastic bone formation. Most adult skeletal diseases are due to the disturbance of this balance, including osteoporosis, multiple myeloma, and cancer metastases. Therefore, study of the proliferation and differentiation of osteoblasts and osteoclasts can help us to deeply understand these diseases and develop better treatments.
The Wnt family consists of a number of highly conserved genes that regulate gene expression, cell behavior, cell adhesion, and cell polarity, including 19 genes in humans and mice, 7 in Drosophila, and 5 in C. elegans. The term “Wnt” is derived from the terms wingless and int. The Int oncogenes, including Int1, were first identified in the mouse mammary tumor. In 1987, investigators sequenced wingless in Drosophila and found it was the homolog of int-1(3). In mammals, the complexity and specificity in Wnt signaling are in part achieved through Wnt ligands, R-spondin proteins, and norrin. Receptors on the cell surface and a multi-step process within the cell trigger downstream gene expression.
The production and secretion of Wnt ligands requires lipid modification by the acyltransferase Porcupine (Porcn) followed by the binding of Wntless (Wls), which serves as a Wnt chaperone and facilitates the transport of lipid-modified Wnt to the plasma membrane (4–10). The Wnt pathway is divided into two major branches: the canonical pathway and the noncanonical pathway. The canonical pathway is also called the Wnt/β-catenin pathway (11). There are two major noncanonical pathways: the Wnt-planar cell polarity pathway (Wnt-PCP pathway)(12) and the Wnt-calcium pathway (Wnt-Ca2+ pathway)(13). The effect of Wnt ligands, receptors, intracellular effectors, transcription factors, and antagonists on both the bone modeling and remodeling processes have been studied in mouse models (Table 1).
Table 1.
Mouse Model of Wnt ligands and its signaling effectors regulate skeletogenesis
| Model | Phenotype | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Bone resorption | Bone formation | Chondrocyte differentiation | BMD change | Cortical thickness | Trabecular thickness | Others Features | ||
| Functional Group: Wnt ligands | ||||||||
| Wnt3a+/− | nd | ↓ | nd | ↓ | nd | Number (down) | Low bone mass | (32) |
| Wnt4 transgenic (Col2a1-cre) | nd | ↓ | Increased hypertrophic chondrocytes | — | nd | nd | Dwarfism | (29) |
| Wnt5a+/− | nd | ↓ | nd | ↓ | nd | Number (down) | Low bone mass | (32) |
| Wnt5a transgenic (Col2a1-cre) | nd | ↓ | ↓ | nd | nd | nd | Short long bones and reduce ossification | (30) |
| Wnt5b transgenic (Col2a1-cre) | nd | ↓ | ↓ | nd | nd | nd | Open skull, short long bones and reduce ossification | (30) |
| Wnt7 conditional knockout (dermo-cre) | nd | ↓ | ↓ | nd | nd | nd | Bone development defects | (44) |
| Wnt9a/Wnt14−/− | nd | nd | ↓ | ↓ | nd | nd | Reduced the length of long bone and lead to ectopic differentiation of cartilage | (28) |
| Wnt9a/Wnt14 transgenic | Nd | nd | ↓ | ↑ | nd | nd | Enhanced ossification and reduced joint formation | (26) |
| Wnt10b−/− | — | ↓ | nd | ↓ | nd | Number (down) | Low bone mass | (34) |
| Wnt10b+/− | — | ↓ | nd | ↓ | nd | ↓ | Osteopenia and has less osteoprogenitors | (36) |
| Wnt10b transgenic (osteocalcin) | nd | ↑ | nd | ↑ | nd | ↑ | Increase mandibular bone, trabecular bone and delayed incisor development | (35) |
| Wnt10b transgenic (FABP4 promoter) | nd | ↑ | nd | ↑ | — | ↑ | Increased bone mass | (34) |
| Wnt16−/− | nd | nd | nd | ↓ | ↓ | — | Low bone mass and increase risk of fracture | (45) |
| Functional Group: Wnt receptors | ||||||||
| Fzd8−/− | ↑ | — | nd | ↓ | nd | ↓ | Low trabecular bone volume | (59) |
| Fzd9−/− | nd | ↓ | nd | ↓ | nd | Number (down) | osteopenia | (60) |
| Lrp4−/− | nd | nd | Disrupted | nd | nd | nd | Polysyndactyly, fused digital bones, and tooth development abnormalities | (288) |
| Lrp5−/− | — | ↓ | nd | ↓ | nd | nd | Low bone mass | (79) |
| Lrp5+/− | nd | ↓ | nd | ↓ | nd | nd | Low bone mass | (79) |
| Lrp5 conditional knockout (Dmp1-cre) | nd | ↓ | nd | ↓ | nd | nd | Low bone mass | (289) |
| Lrp5 a point mutation (A214V) | — | ↑ | nd | ↑ | ↑ | ↑ | Increased bone mass, bone strength and bone formation rate | (289, 290) |
| Lrp5 a point mutation (G171V) | — | ↑ | nd | ↑ | ↑ | ↑ | Increased bone mass, bone strength and bone formation rate | (289, 290) |
| Lrp6 a point mutation (R886W) | nd | nd | nd | ↓ | ↓ | Number (down) | Delayed ossification at birth and osteoporosis in adult | (82) |
| Functional Group: Wnt antagonist | ||||||||
| Dkk1+/− | — | ↑ | nd | ↑ | nd | ↑ | High bone mass | (90) |
| Dkk1 transgenic (Col1A1) | — | ↓ | nd | ↓ | ↓ | ↓ | Systemic osteopenia | (89) |
| Dkk2−/− | ↑ | ↓ | nd | ↓ | ↓ | ↓ | osteopenia | (97) |
| Sfrp1−/− | — | ↑ | nd | — | — | ↑ | Increase trabecular bone formation | (63) |
| Sfrp2−/− | nd | ↓ | ↓ | nd | nd | nd | Brachydactyly, mild mesomelic shortening and posterior soft-tissue syndactyly | (68) |
| Sfrp4 transgenic (col1a1) | nd | ↓ | nd | ↓ | nd | Number (down) | Low bone mass | (70) |
| Sost transgenic (human SOST) | — | ↓ | ↓ | ↓ | ↓ | ↓ | Low bone mass | (108) |
| Functional Group: Effectors in cytoplasm | ||||||||
| GSK3β−/+ | ↑ | ↑ | nd | ↑ | ↑ | ↑ | High bone mass | (291) |
| GSK3α−/−; GSK3β+/− | nd | ↓ | ↓ | nd | nd | nd | Dwarfism with significantly shortened long bone and vertebra. | (139) |
| Axin2−/− | nd | ↑ | ↑ | ↑ | nd | nd | Craniosynostosis | (133, 134) |
| Apc−/− conditional knockout (osteocalcin) | ↓ | ↑ | nd | ↑ | nd | Nd | Increased bone deposition and a disappearance of osteoclasts | (128) |
| Apc conditional knockout (Col2a1) | nd | ↓ | ↓ | ↓ | nd | nd | Perinatally lethal; craniofacial abnormalities, short trunk, an incomplete closure of both thoracic and abdominal cavities | (127) |
| Functional Group: Transcription regulation | ||||||||
| β-catenin conditional knock out (Prx) | — | ↓ | ↓ | ↓ | nd | nd | Bone development defect. | (146) |
| β-catenin conditional knockout (Dermo1) | ↓ | nd | ↑ | nd | nd | nd | Long bone shortened, thickened, bowed, and ectopic cartilage formation | (148) |
| β-catenin conditional knockout (Col2a1) | nd | ↓ | ↓ | nd | nd | nd | Died shortly after birth. Limbs were shortened and head was dome shaped. Joints between the future tarsal bones were either missing or incompletely formed. | (26, 147) |
| β-catenin conditional knockout (Col1a1) | ↑ | — | nd | ↓ | nd | nd | Low bone mass | (153) |
| β-catenin conditional knockout (Osterix) | nd | ↓ | ↓ | nd | nd | nd | Lack the membranous bone of cranial ossification center and complete loss of bone deposition | (190) |
| β-catenin conditional knockout (osteocalcin) | ↑ | nd | nd | ↓ | ↓ | ↓ | Occasionally paralysis, consistent with osteoporotic-related fracture | (128) |
| β-catenin+/− conditional knockout (PPARγ) | ↑ | — | nd | ↓ | ↓ | ↓ | Osteoporosis | (154) |
| β-catenin conditional knockout (PPARγ) | ↓ | — | nd | ↑ | ↑ | ↑ | Osteopetrosis | (154) |
| β-catenin transgenic (Prx) | nd | ↓ | ↓ | nd | nd | nd | Limbs contain only tiny remnants of skeletal elements and skull bones are lost. | (146) |
| β-catenin transgenic (Col2 A1) | nd | nd | ↓ | nd | nd | nd | Perinatal lethal, ectopic joint formation and endochondral ossification | (26) |
| β-catenin transgenic (Col1A1) | ↓ | — | nd | ↑ | nd | nd | osteopetrosis | (153) |
| β-catenin+/− transgenic (Osterix) | nd | ↑ | — | nd | nd | nd | Died at birth, shorter limbs, intense and broader ossification center in long bone. | (190) |
| β-catenin+/− transgenic (PPARγ) | ↓ | — | nd | ↑ | ↑ | ↑ | Osteopetrosis | (154) |
| Tcf1−/− | ↑ | — | nd | ↓ | nd | nd | Low bone mass | (153) |
| Tcf1 Dominant negative (Col2a1) | nd | ↓ | ↓ | nd | nd | nd | Dwarfism, retarded mineralization in limbs, ribs and vertebrae | (160) |
| Lef1ΔN Transgenic (Col1a1) | nd | ↑ | nd | ↑ | nd | ↑ | High bone mass | (161) |
| β-catenin+/− conditional knockout (Col1A1); Tcf+/− | ↑ | — | nd | ↓ | nd | nd | Low bone mass | (153) |
BMD, bone mineral density; ↑ promotion; ↓ inhibition; — not changed; nd, not detected
Wnt signaling proteins participate in multiple developmental events during embryogenesis and adult tissue homeostasis. Wnt signals have multiple functions, including mitogenic stimulation, cell fate determination, and differentiation (1). Wnt signals also affect bone development, especially the differentiation of osteoblasts. It has always been a hot spot of research since the first Wnt family protein was identified. This review summarizes various Wnt signaling pathways and discusses how the Wnt signaling pathway influences bone development and bone diseases.
3. WNT CANONICAL SIGNALING PATHWAY IN SKELETOGENESIS
3.1. Wnt ligands and Wnt agonists in bone
Wnt ligands, which are cysteine-rich proteins of approximately 350–400 amino acids that contain an N-terminal signal peptide for secretion (14), have distinct effects on different phases of bone development, including chondrogenesis, osteoblastogenesis, and osteoclastogenesis. A recent study reported that Wnt1 mutations were found in four children who have osteogenesis imperfecta (15), a genetic disorder of increased bone fragility, low bone mass, and other connective-tissue manifestations (16). The Wnt1 knockout mouse model has severe mid- and hindbrain deficiencies (17, 18). Wnt1 and Wnt3a control expression of dorsal genes and suppression of the ventral programs though the Wnt canonical pathway and gliotactin (Gli) activity (19, 20). Wnt2b functions with T-box 5b (Tbx5b) to initiate forelimb outgrowth and identity through fibroblast growth factor 10 (fgf10) (21). Wnt3a regulates dorsal mesoderm fate (22) and is required at the earliest stages of limb formation (23, 24) and craniofacial development (25). Wnt4, Wnt6, Wnt9a, and Wnt16 are required for joint formation (26–28). Conditional expression of Wnt4 during chondrogenesis in R26floxneoWnt4; Col2a1-Cre mutant mice resulted in dwarfism and an increased number of hypertrophic chondrocytes (29). Yingzi Yang’s et al. revealed that although Wnt5a and Wnt5b are the closest Wnt relatives to each other, they exhibit distinct activities in coordinating chondrocyte proliferation and differentiation (30, 31). A recent study showed that Wnt3a+/− and Wnt5a+/− mice have a low bone mass phenotype (32). Wnt6, Wnt10a, and Wnt10b stimulate osteoblastogenesis and inhibit adipogenesis (33–36). Mutations in Wnt7a result in defects in limb development (37–41). Wnt7b and Wnt11 are identified as endogenous ligands regulating chondrocyte and osteoblast differentiation (42–44). Wnt16 deficiency decreases bone mineral density and increases fracture risk (45).
Norrin, a highly divergent member of the transforming growth factor-beta superfamily, is a kind of Wnt agonist. It exhibits highly-binding-affinity with Frizzled-4(46). Together with low-density lipoprotein receptor-related protein (LRP), Norrin and Frizzled-4 can activate the canonical Wnt signaling pathway (46). Thus, Norrin is related to several inherited disorders, including osteoporosis-pseudoglioma syndrome (47).
The other class of Wnt agonists, the R-spondin family, consists of 4 types of R-spondins (i.e. R-spondin1–4) which stimulate β-catenin-dependent signaling. R-spondins were discovered by Kazanskaya et al. and identified as a novel family of secreted Wnt agonists (48). All R-spondins contain an N-terminal signal peptide, two furin-like domains, one thrombospondin type 1 domain, and a C-terminal low complexity region enriched with positively charged amino acids (48). R-spondins promote osteoblast differentiation and are highly expressed in skeletal tissues during development and postnatally (49). R-spondin1 promotes osteoblast differentiation and bone formation while blocking osteoclastogenesis (50). R-spondin2 deficiency results in skeletal developmental defects (49). R-spondin3 is required for head cartilage morphogenesis through Wnt/PCP signaling pathway (51). R-spondin4 mutations result in anonychia, which is the absence or hypoplasia of nails on fingers and toes (52).
3.2. Wnt canonical signaling pathway
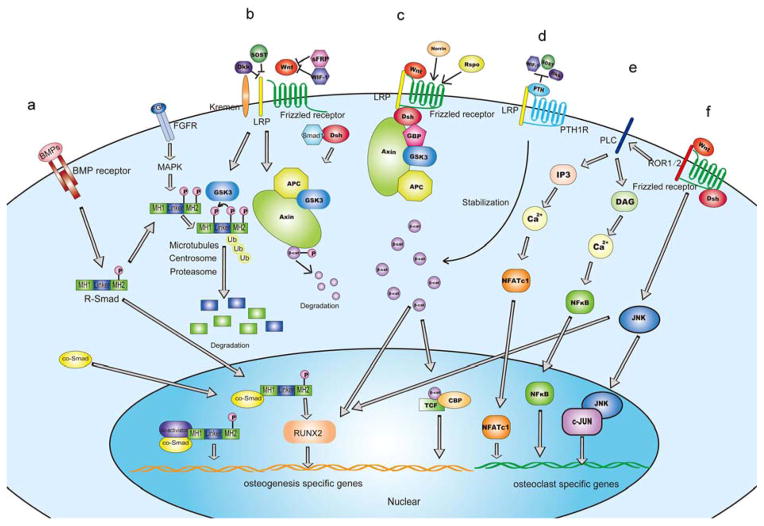
The Wnt/β-catenin signaling pathway is the best studied of the Wnt pathways. Although Wnt signals through several pathways to regulate cell growth, differentiation, function, and death; it is central to the bone development and homeostasis in adults (53, 54). WNT signaling has been studied primarily in developing embryos, in which cells respond to WNTs in a context-dependent manner, but WNTs also have important functions in adults (55). The current model of how Wnt signals are transduced in the Wnt canonical pathway is shown in Figure 1B. Wnt proteins, following their binding to a frizzled receptor and a Lrp co-receptor (most likely LRP6), activate the canonical Wnt signaling pathway. These receptors transduce a signal to several intracellular proteins that include Dishevelled (Dsh), glycogen synthase kinase-3β (GSK-3), Axin, Adenomatous Polyposis Coli (APC), and the transcriptional regulator, β-catenin. This results in the translocation to nucleus of β-catenin, the association of β-catenin with members of the Lef1/Tcf nuclear protein family, and the activation of a specific program of gene expression (See Figure 1B, C)
Figure 1.
The Wnt pathway and its interactions with other pathways in bone. (a)Wnt signaling and the TGF-β/BMP-Smad pathway influence each other during bone development. The Wnt pathway participates in R-Smad degradation and so does the Bmp pathway in β-catenin degradation. (b. c)The Wnt canonical pathway: Wnt proteins, following their binding to a frizzled receptor and a Lrp co-receptor (most likely LRP6), activate the canonical Wnt signaling pathway. These receptors transduce a signal to several intracellular proteins that include Dishevelled (Dsh), glycogen synthase kinase-3β(GSK-3), Axin, Adenomatous Polyposis Coli(APC), and the transcriptional regulator, β-catenin. This results in the translocation to nucleus of β-catenin, β-catenin’s association with members of the Lef1/Tcf family of nuclear proteins, and activation of a specific program of gene expression. (d) Wnt interacts with PTH1R, decreases wnt antagonists, Sost, WIF1 and Dkk expression, and sustains β-catenin stabilization. (e, f) The Wnt noncanonical pathway. The Ca2+ pathway and PCP pathway affect osteoblastogenesis and osteoclastogenesis.
3.3. Wnt receptors and their inhibitors in bone
3.3.1. Frizzled protein and its antagonists (sFRPs) regulate osteoblastogenesis and osteoclastogenesis
Genetic and biochemical data have demonstrated that the Frizzled (Fz) proteins are primary receptors for the Wnts (56). Frizzled proteins transmit signaling through both β-catenin-dependent and β-catenin independent pathways. All members of the Fz family are characterized by the following features: a putative signal sequence followed by a sequence of 120 amino acids (aa) containing 10 highly conserved cysteine-rich domains(CRD), a highly divergent region of 40–100 aa predicted to form a flexible linker, seven transmembrane segments separated by short extracellular and cytoplasmic loops, and a cytoplasmic tail (57, 58). The CRD appears to be the ligand-binding site of Frizzled proteins. Osteoclastogenesis is promoted independently of osteoblasts in Fzd8 deficient mice (59). Fzd9 is required for bone formation (60).
Secreted Frizzled-related proteins(sFRPs), the largest family of Wnt inhibitors, share sequence similarity with the cysteine-rich domain found in the extracellular region of Frizzled (61). sFRPs bind the Wnt ligands through their CRD, thereby preventing their binding to the Frizzled receptor (62). Bodine et al. demonstrated that sFRP-1 is an important regulator of osteoblast and osteocyte survival in vitro and in vivo. They developed a sFRP1−/− mouse line and show that deletion of sFRP-1 not only reduces osteoblast and osteocyte apoptosis, but also potentiates osteoblast proliferation and differentiation, and increases trabecular bone formation (63–65). sFRP1 transgenic mice exhibit blocked bone formation and decreased trabecular bone mass (66). According to Gillespie et al., sFRP-1 also plays a role in receptor activator of NF-kappaB ligand (RANKL)-dependent osteoclast formation (67). sFRP-2 and sFRP-4 are required for limb development (68) and bone formation (69, 70).
3.3.2. Low-density lipoprotein receptor-related proteins regulate osteoblast differentiation through Wnt signaling
The low-density lipoprotein receptor-related protein (LRP) family is a single-pass transmembrane molecule family involved in Wnt signaling. In addition to Wnt/Frizzled interactions, LRP5/6 is required for Wnt signalling in invertebrates (71, 72). The gene arrow in Drosophila encodes a transmembrane protein that is homologous to LRP5/6 (73). All members of the family contain highly conserved motifs. Most notably, all contain several complement-type and epidermal growth factor (EGF) precursor-like repeats, as well as single transmembrane and cytoplasmic domains (74). LRP5 and LRP6 are 71% homologous and they have overlapping roles during bone mass accrual (75). Investigators proposed that Wnt protein binds to Fz and LRP to form a complex, although it hasn’t been observed in Drosophlia (76). Extensive evidence indicates the importance of low-density lipoprotein receptor-related proteins in Wnt signaling and the regulation of bone formation. In situ hybridization on skeletal elements in developing mice to determine LRP5 is expressed in osteoblast and transducing Wnt signaling (77). The intracellular domain of LRP5 can interact with Axin, stabilize β-catenin, and induce LEF activation (78). Embryos homozygous for an insertion mutation in the LRP6 gene exhibit developmental defects that are a striking composite of those caused by mutations in individual Wnt genes. This indicates a broad role for LRP6 in the transduction of several Wnt signals in mammals (71). Moreover, mice with a targeted disruption of LRP5 develop a low bone mass phenotype. In vivo and in vitro analyses indicate that this phenotype becomes evident postnatally, and demonstrate that it is secondary to decreased osteoblast proliferation and function in a RUNX-2-independent manner (79). Humans and mice lacking LRP5 exhibit low bone mineral density (BMD). Compound mutants have dose-dependent deficits in BMD, suggesting functional redundancy between LRP5 and LRP6 in bone development (75). Conversely, a gain of function mutation in LRP5(Gly171Val) causes a hereditary high bone mass trait in humans, and transgenic mice expressing this mutation in osteoblasts display greater bone formation and density (80, 81). LRP6 showed similar function as the LRP5 in a spontaneous point mutation mouse model, the ringelschwanz(rs). This model, with a point mutation that leads to an amino acid substitution of tryptophan for the conserved residue arginine at codon 886(R886W) and cannot efficiently transduce signals through Wnt signaling, exhibited delayed ossification at birth and a low bone mass phenotype in adults (82). Disrupting LRP5/6 could affect osteoblastogenesis, then bone formation, and ultimately trigger bone diseases. Mutation in LRP5 causes the autosomal recessive disorder osteoporosis-pseudoglioma syndrome (OPPG), whose carriers have reduced bone mass when compared to age- and gender-matched controls (77). Recent studies show that missense mutations in LRP6 can lead to osteoporosis (82, 83). In a duodenal-specific Lrp5 activating mouse model, it was demonstrated that Lrp5 regulates bone mass by affecting serotonin synthesis (84). N-cadherin interacts with LRP5/6 and suppresses Wnt signaling and bone formation, which can be disrupted by a competitor peptide (85). This finding provides a new strategy to promote osteoblast function and bone formation through Wnt signaling. Another member of the LRP family, LRP4, shares structure elements within the extracellular ligand binding domain with LRP5 and LRP6. LRP4 is expressed in bone and is a novel osteoblast expressed Dkk1 and SOST receptor with a physiological role in the regulation of bone growth and turnover (86). LRP4 also can bind Wise, a secreted Wnt modulator and Bone morphogenetic protein (BMP) antagonist. Recently, a group in the Netherlands identified a new member of the low-density lipoprotein receptor-related protein family: Lrp8 (87). Knockdown of Lrp8 results in a decrease in the level of β-catenin (87). These results indicate that Lrp8 is a novel positive factor of Wnt signaling and may play a role in controlling osteoblast differentiation.
3.3.3. Dkk and Kremen regulate bone mass by modulating Lrp5/6
Among the several known modulators of Lrp5 activity, Dkk proteins are the best characterized secreted Wnt-signaling inhibitors. Dickkopf-1(DKK1), a member of the Dickkopf family, is indispensable for embryonic head induction and limb development in mice (88). Endogenous Dkk1 expression was detected primarily in osteoblasts and osteocytes (89). While Dkk1 null mice die at birth due to a lack of head structure, Dkk1 heterozygous mutants (Dkk1+/−) display increased bone formation and high bone mass phenotype (90). Conversely, the Dkk1 transgenic mouse [collagen, type I, alpha 1 (Col1A1)] showed systemic osteopenia, decreased osteoblastic bone formation, and unaffected osteoclastogenesis (89). Dkk1 is over-produced in human cancer cells while developing osteolytic lesions associated with metastatic bone disease (91–94). Knockdown of Dkk1 expression by end-capped phosphorothioate Dkk1 antisense oligonucleotide (Dkk1-AS) abrogated dexamethasone suppression of alkaline phosphatase activity and osteocalcin expression in MC3T3-E1 preosteoblasts. Exogenous Dkk1-AS treatment alleviated dexamethasone suppression of mineral density, trabecular bone volume, osteoblast surface, and the rate of bone formation in bone tissue and ex vivo osteogenesis of primary bone marrow mesenchymal cells (95). Notably, Dkk1 inhibits Wnt signaling by binding to the LRP6 component of the receptor complex, instead of exerting an inhibitory effect by molecular mimicry of Fz or Wnt sequestration like most other Wnt antagonist (96). LRP5 gain-of-function mutations which alter the first epidermal growth factor (EGF)–like domain (i.e. LRP5 -propeller 1 region) can prevent DKK1-LRP5 interaction and are the cause of high bone mass (HBM) and mandibular, buccal, and lingual exostoses (80, 91). The Dkk2−/− mice represent decreasing bone formation by affecting terminal osteoblast differentiation and mineralized matrix formation (97). This result suggests that Dkk2 plays an opposite role with Dkk1 in osteoblastogenesis. Dkk2 deficiency led to a substantial increase in the number of osteoclasts by delayed mineralization of osteoblasts (97, 98). Dkk1 antagonizes LRP5/6 by competitively binding to LRP with high affinity (99), and its antagonistic function is significantly enhanced by Kremens, which are another type of transmembrane molecule (100). Kremen1 (Krm1) and Kremen2 (Krm2) are high-affinity Dkk1 receptors that functionally cooperate with Dkk1 to block Wnt canonical signaling (101). Kremen2 forms a ternary complex with Dkk1 and LRP6, and induces rapid endocytosis and removal of the Wnt receptor LRP6 from the plasma membrane. As we described before, the R-spondin (RSpo) family of secreted proteins act as potent activators of the Wnt signaling pathway (102). Although RSpo1 does not directly activate LRP6, it interferes with DKK1/Kremen-mediated internalization of LRP6 through an interaction with Kremen, resulting in increased LRP6 levels on the cell surface (102). Krm2, unlike Krm1, is predominantly expressed in bone. Specific overexpression of Krm2 in osteoblasts in transgenic mice(Col1a1-Krm2) results in severe osteoporosis (103). Dexamethasone, an agent known to induce osteoporosis, upregulates Dkk1 expression in primary human osteoblasts and provides a molecular explanation for osteoporosis caused by long-term glucocorticoid use (104).
3.3.4. Other Wnt antagonists
Sclerostin (SOST), which is a member of the cysteine-knot superfamily, has been localized to the chromosome region 17q12-q21 (105). SOST is expressed exclusively in mouse and human bone by osteocytes embedded within the mineralized matrix (106, 107). At first, sclerostin was considered a BMP signaling antagonist because it competed with type I and type II bone morphogenetic protein receptors for binding to BMPs, decreased BMP signaling, and suppressed mineralization of osteoblastic cells (108). However, subsequent studies have shown that it is also a Wnt signaling antagonist by binding LRP5/6(109, 110). Sclerostin binding to the six-bladed β-propeller domain of LRP5/6 is mediated by the central core of sclerostin but not the amino- and carboxyl- terminal flexible arm region (111). Sclerostin also binds to Lrp4 and functions in bone formation and turnover (86, 112). Mouse genetics have demonstrated the link between SOST and bone formation. In vivo, SOST−/− mice showed a high bone mass state, and transgenic mice overexpressing SOST exhibited low bone mass and decreased bone strength (108, 113, 114).
Wise shares 38% amino acid identity with sclerostin and appears to be a context-dependent regulator of Wnt signaling; it may inhibit or stimulate Wnt signaling. Data from Itasaki et al. shows that Wise is an inhibitor of Wnt signaling by binding to the Wnt co-receptor, lipoprotein-related protein 6, LRP6 and thus competing with Wnt8 for binding to LRP6(115).
Wnt-inhibitory factor-1(WIF-1) is a secreted protein that binds to Wnt proteins and inhibits their activities (116). The deduced 379-amino acid WIF-1 secreted protein contains an N-terminal signal sequence, a 150-amino acid WIF domain, 5 epidermal growth factor (EGF; 131530)-like repeats that are similar to those of tenascin, and a C-terminal hydrophilic domain of approximately 45 amino acids (116). WIF-1 is present in fish, amphibians, and mammals, and is expressed during Xenopus and zebrafish development in a complex pattern that includes paraxial presomitic mesoderm, notochord, branchial arches and neural crest derivatives. In vitro, WIF-1 binds to Drosophila Wingless and Xenopus Wnt8 produced by Drosophila S2 cells.
3.4. Dishevelled and Axin proteins relay Wnt signals from receptors to downstream effectors
Dishevelled (Dvl-1, -2, and -3 in mammalian, Dsh in Drosophila) is composed of an amino-terminal DIX domain, a PDZ domain in the middle, and a carboxy-terminal DEP domain (117). Dsh can interact with Fz directly (118) through the conserved the motif (Lys-Thr-X-X-X-Trp) located two amino acids after the seventh transmembrane domain in Fz (119). Investigators have identified PAR-1 as a Dsh-associated kinase. PAR-1 potentiates Wnt activation of the β-catenin pathway. Suppressing endogenous PAR-1 function inhibits Wnt signaling through β-catenin in mammalian cells, and Xenopus and Drosophila embryos. PAR-1 seems to be a positive regulator of the β-catenin pathway.
Similar to Dsh, cytosolic protein Axin (a scaffolding protein controlling beta-catenin stability) interacts with LRP. Wnts stimulate phosphorylation of LRP on the Pro-Pro-Pro-(SerTrp)Pro[PPP(S/T)P] motif, which creates an inducible docking site for Axin (78, 120, 121). The Dvl and Axin proteins each contain a conserved DIX domain in their sequences (122). Though their DIX domain, Dvl-1 directly binds to Axin and Dvl-1 inhibits Axin-promoted GSK-3β-dependent phosphorylation of β-catenin and APC. Furthermore, deletion of the DIX domains of Dvl-1 and Axin destroys their abilities to accumulate and to degrade β-catenin (123). Possibly, Wnt binding of Fz and LRP promotes direct interactions between Axin and Dvl through their domains, reconfiguring the protein complex that regulates the level of β-catenin in the cell (11).
3.5. Wnt signaling in cytoplasm
In the absence of Wnt ligands, a master complex comprising APC, GSK-3β, Axin, and Casein kinase I (CKI) phosphorylates cytoplasmic β-catenin, marking it for ubiquitination and subsequent proteasomal degradation (124). Wnt ligands binding to the membrane co-receptors (LRP5/6 and Frizzled) inhibit this complex, allowing nuclear translocation of dephosphorylated β-catenin, where it activates a large number of context-dependent target genes (125).
The Apc (adenomatous polyposis coli) tumor suppressor gene is involved in the initiation and progression of colorectal cancer (126). Conditional homozygous Apc mutation mice died perinatally showing greatly impaired skeletogenesis. The majority of the precursor cells lacking APC-mediated control of β-catenin level failed to differentiate into chondrocytes or osteoblasts (127). Also, APC is suggested to regulate the function of chondrocytes, osteoblasts, and osteoclasts though catenin-cadherin interactions. Conditional knockout of Apc with the osteocalcin promoter disclosed dramatic defects in bone development, a significant accumulation of bone matrix, disturbance in bone architecture, rapidl rate of bone formation, and lack of osteoclasts (128). Conditional knockout of Apc with the Col2a1 promoter is embryonic lethal and it causes the majority of the precursor cells lacking Apc to fail to differentiate into chondrocytes or osteoblasts (127). Mice carrying osteoblast-specific deletion of both the Apc and β-catenin genes display growth and survival characteristics similar to those lacking only the β-catenin gene, suggesting that the severe phenotype induced by loss of Apc is due to dysregulation of β-catenin signaling (128).
Axin acts as a scaffold in the Axin-APC-GSK3β-CKI complex to assemble β-catenin substrate and kinases (GSK3β and CKI)(129). Axin has several domains. The RGS (Regulators of G protein signaling) domain interacts with APC(78). The DIX domain can interact with Dishevelled as discussed before. There are two vertebrate Axin genes, which act as negative regulators (130). Axin1 is constitutively expressed, but Axin2 (Axil) is a direct target of the Wnt pathway and mediated through Tcf/LEF factors. This suggests that Axin2 participates in a negative feedback loop, which could serve to limit the duration or intensity of a Wnt-initiated signal (131). Mice with deletion of Axin1 exhibit defects in axis determination and brain patterning during early embryonic development (132). Axin2−/− mice display enhanced expansion of osteoprogenitors, accelerated ossification, stimulated expression of osteogenic markers, and increased mineralization (133). Axin2-null mice exhibit a phenotypic defect resembling craniosynostosis in humans (133). Recently, another group revealed that disruption of Axin2−/− expression not only played a critical role in intramembranous bone formation, but also accelerated chondrocyte maturation and influenced the endochondral bone formation (134).
Glycogen synthase kinase 3(GSK3) has two highly conserved isoforms α and β originally identified in 1980(135). In the Wnt pathway, GSK3β is recruited to form a complex via interaction with Axin, where it phosphorylates three serine(S)/threonine(T)residues(S33, S37, T41) at the amino-terminal region of β-catenin (130, 136). These phosphorylated S/T residues are critical for its recognition by the F-box β-Trcp (130). Hyperphosphorylated β-catenin is subjected to ubiquitylation by the F-box β-Trcp E3 ligase complex followed by degradation via the 26S proteasome (137). Hoeflich et al. found that lithium treatment, which inhibits GSK-3, can inhibit transactivation of NF-κB (a key transcription factor of osteoclasts) without affecting degradation of I-κB and translocation of NF-κB to the nucleus (138). Thus, NF-κB is regulated by GSK-3 at the level of the transcriptional complex (138). GSK3α−/−;GSK3β+/− mice exhibit a dwarfism phenotype with significantly shortened long bones and vertebra, while GSK3α+/− and GSK3β+/− mice display normal skeleton development (139).
Casein kinase Ialpha (CKIα) is another Axin-associated kinase, whose phosphorylation of β-catenin is required for subsequent phosphorylation of β-catenin by GSK3(140). Wnt signaling inhibits GSK3β, but not CKIα phosphorylation of β-catenin (130, 141). Therefore, CKIα may represent a node at which other signaling pathways regulate β-catenin protein (130, 141).
Rac1, a Rho-family small GTPase, can accumulate β-catenin via Gαq/11βγ signaling involving phosphatidylinositol-3 kinase(PI-3K)(142). The role of Rac1 depends on the phosphorylation of β-catenin at Ser191 and Ser605, an event chiefly mediated by c-Jun NH2-terminal kinase 2(JNK2) in the stromal cell line ST2 (142). Mutations of these residues significantly affect β-catenin nuclear accumulation in response to Wnt (142).
SRY-box containing gene 9 (Sox9) is an intrinsic transcription factor that is inhibited by the Wnt canonical signaling pathway (143). It can antagonize Wnt/β-catenin signaling in chondrocyte differentiation in two distinct mechanisms: the Sox9 N-terminus is necessary and sufficient to promote β-catenin degradation, whereas the C terminus is required to inhibit β-catenin transcriptional activity without affecting its stability (143).
There are generally two pools of β-catenin: one is associated with cadherins while the other is “free” in the cytosol/nucleus. The latter pool is involved in gene transcription regulation (137). Phosphorylated β-catenin is specifically recognized by beta-transducin repeat containing protein (β-Trcp), an F-box/WD40-repeat protein that also associates with S-phase kinase-associated protein 1 (Skp1), which is an essential component of the ubiquitination apparatus (144). Mutations at the critical phosphoserine residues of β-catenin results in the loss of recognition by β-Trcp and in the accumulation of β-catenin (144). Inhibition of endogenous β-Trcp function by a dominant negative mutant stabilizes β-catenin and activates the Wnt canonical pathway (144). Activating mutations in the human β-catenin gene have been found in human colon cancer and melanomas (145).
β-catenin through Wnt signaling plays a very important role in skeletal development by regulating chondrogenesis, osteoblastogenesis, osteoclastogenesis, and limb patterning. First, β-catenin regulates chondrocyte differentiation (26, 146, 147). The transgenic mouse of β-catenin under Col2A1 promoter control reveals that cartilage formation and endochondral ossification were greatly reduced (26). In the β-cateninc/c, Dermo-1-Cre mice, the long bones were greatly shortened, thickened, and bowed, and cartilage formation was ectopic due to the ectopic chondrocyte differentiation at the expense osteoblasts (148). Detailed in vivo and in vitro loss- and gain-of-function analysis reveals that β-catenin activity is necessary and sufficient to repress the differentiation of mesenchymal cells into Runx2- and Sox9-positive skeletal precursors (146). These results suggest that β-catenin is required for the suppression of chondrocyte differentiation and the allowance of osteoblast formation during both intramembranous and endochondral ossification (148). Recent studies of inducible cartilage-derived β-catenin uncovered that β-catenin also affects chondrocyte maturation, primary and secondary ossification center development, and perichondral bone formation (149, 150). Furthermore, β-catenin could affect osteoblast differentiation (146, 147). Global inactivation of β-catenin results in early embryonic death. Conditionally inactivatable β-catenin mice expressing cre under the control of the osteocalcin promoter displayed striking reductions in both the trabecular and cortical bone compartments (128). Study of the calvarial osteoblasts of the conditional knockout mice in vitro revealed that β-catenin is not required for the initial commitment of cells to the osteoblast lineage, but that it appears to be essential for the performance of more mature osteoblast (128). Interestingly, Long et al. found that conditional knockout of β-catenin in osterix expressing osteoblasts promotes osteoblast formation and suppresses bone resorption (151). This finding indicates a complicated role for β-catenin in bone homeostasis. Recent studies demonstrate that BMP-2 acts synergistically with β-catenin to promote osteoblast differentiation. The Wnt autocrine loop mediates the induction of alkaline phosphatase and mineralization by BMP-2 in pre-osteoblastic cells (152). Additionally, alterations in β-catenin signaling in osteoblasts brought about by each mutation leads to marked disturbances in osteoclast differentiation (128, 148), as evidenced by the dramatic increase in osteoclast numbers and severe osteopenia in β-catenin conditional knockout mice (128). Stabilizing expressed β-catenin in mice could cause osteopetrosis through osteoclast defects (153, 154). Constitutive activation of β-catenin in osteoclast cells causes severe osteopetrosis (154). Dosage-dependent inhibition of β-catenin expression shows an opposite phenotype of mice. β-catenin heterozygosity in osteoclast lineage causes osteoporosis while β-catenin deletion in osteoclasts causes osteopetrosis (154). Other studies have shown that the osteoprotegerin (OPG) gene, a major inhibitor of osteoclast differentiation, may be a direct transcriptional target for complexes containing the β-catenin protein (153, 155). Mesenchymal β-catenin has multiple roles during limb patterning (156). Abnormal expression of mesenchymal β-catenin causes limb truncation and apical ectodermal ridge (AER) defects (156). In vitro, osteoblasts lacking the β-catenin gene exhibited impaired maturation and mineralization with elevated expression of the osteoclast differentiation factor, RANKL, and diminished expression of the RANKL decoy receptor, osteoprotegerin (128). According to these findings, we know that β-catenin regulates bone development during different phases and that abnormal β-catenin may cause bone diseases (e.g. osteoporosis and osteopetrosis).
3.6. Wnt signaling in nucleus
In vertebrates, β-catenin acts astranscriptional activator, which is needed to overcome target gene repression by Groucho/TLE proteins and to permit promoter activation as the final consequence of Wnt signaling (157). The vertebrate transcription factors T cell factor (TCF) and lymphocyte enhancer binding factor (LEF) interact with β-catenin and mediate Wnt signaling (158). XTcf-3, also known as transcription factor 7-like 1 (T-cell specific, HMG-box), is a maternally expressed Xenopus homolog of the mammalian (high-mobility-group) HMG box factors Tcf-1 and Lef-1. N-terminal deletion of XTcf-3 (delta N) abrogates the interaction with β-catenin, as well as the consequent transcription activation (159). Tcf1 is one of the two Tcf genes expressed in osteoblasts. Mice lacking Tcf1 exhibit a low bone mass phenotype that is caused by a secondary increase in bone resorption, as indicated by the increased number of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated osteoclasts (153) Mice lacking Tcf1 also exhibit dwarfism caused by inhibition of chondrocytes differentiation (160). Constitutive expressing β-catenin binding and cooperation element, Lef1ΔN, in osteoblasts increases trabecular bone mass (161). In Lef−/−Tcf1−/− double knockout mice, embryos have defects in limb bud development and paraxial mesoderm differentiation (162). Opg expression is decreased in Tcf1−/− osteoblasts, indicating that TCF1 regulates osteoclast differentiation through Opg (153).
Runx2 (runt homology domain transcription factor 2) is the major transcription factor for osteogenesis (163). It determines the osteoblastic differentiation at the early stage and inhibits it at the late stage (164). Wnt-dependent gene expression increases during the early phase of osteoblast differentiation in vitro, is enhanced by prostaglandin activation of the transcription factor Runx2, and is specifically suppressed in Runx2 antisense-depleted osteoblasts (165). Runx2 can form a complex with Lef or TCF, which then binds the composite binding site in the fgf18 promoter, a direct target of Wnt canonical signaling and an essential regulator of bone development (166).
4. NONCANONICAL WNT SIGNALING PATHWAY PROMOTES BONE FORMATION
For a long time it was thought that all Wnt signaling was mediated through β-catenin. However, research now proves that Wnt also signals through β-catenin-independent mechanisms, known as the noncanonical pathway, to regulate vertebrate development (167). Like the canonical Wnt pathway, which plays an important role in bone development and diseases, the noncanonical Wnt pathway also participates in bone formation. Noncanonical pathways can be divided in two major subpathways (Figure 1E): the Wnt-planar cell polarity pathway (Wnt-PCP pathway)(12) and the Wnt-calcium pathway (Wnt-Ca2+ pathway)(13). In the Wnt-PCP pathway, Wnt5a regulates limb morphogenesis (168), chondrogenesis (169–171) and osteoblastogenesis (172) with receptor tyrosine kinase-like orphan receptor (Ror) proteins. Moreover, the Wnt-PCP pathway also regulates osteoclastogenesis. Wnt5a-Ror2 signals activates JNK and recruits c-Jun on the promoter of the gene encoding RANK (Figure 1F) (173, 174). In the Wnt-Ca2+ pathway, Wnt5a binds to the Frizzled receptor, which leads to a short-lived increase of 1,4,5-triphosphate(IP3), 1,2 diacylglycerol (DAC), and Ca2+ with PLC, triggers NFκB and NFAT activation, and regulates osteoclastogenesis (175). In a recent study, investigators found that Wnt-Lrp5 signaling may induce mTORC2-AKT signaling activity and trigger glycolytic enzymes in bone cells to promote bone formation (176). This finding indicates that Wnt signaling may regulate bone homeostasis cooperate with glucose metabolism.
5. NETWORK BETWEEN WNT AND OTHER BONE DEVELOPMENT PATHWAYS
5.1. Crosstalk between the Parathyroid hormone (PTH) pathway and Wnt signaling
Parathyroid hormone (PTH) is an 84-amino-acid polypeptide hormone functioning as a major mediator of bone remodeling and as an essential regulator of calcium homeostasis (177). However, the mechanisms of PTH’s anabolic effect on bone are not fully studied.
5.1.1. PTH pathway induces osteoblast differentiation through Wnt/β-catenin signaling
Substantial data suggests that PTH can influence Wnt signaling in different phases and then bone development (Figure 1D). Also, PTH treatment can increase the expression of the Wnt protein, wnt4 (178). PTH also can decrease the expression of Wnt inhibitors such as Sost by directly inhibiting Sost transcription, which leads to an increase in Wnt signaling (178–180). A recent study showed that in osteoblastic MC3T3-E1 cells, the up-regulation of expression levels of osteoblast differentiation markers when treated with hPTH(1–34) were blocked by knocking down β-catenin expression (181). Transgenic mice expressing a constitutively active PTH receptor exclusively in osteocytes exhibit increased bone mass and bone remodeling, as well as reduced expression of the osteocyte-derived Wnt antagonist SOST, increased Wnt signaling, increased osteoclast and osteoblast numbers, and decreased apoptosis (182). In postmenopausal woman, intermittent PTH can reduce the circulating sclerostin levels (183). Moreover, the effects of PTH on the canonical Wnt signaling pathway can up-regulate the receptor complex proteins (FZD-1or LRP6) and decrease the antagonist (Dkk-1)(184). Although PTH treatment reduces Dkk1 expression, the over-expression of Dkk1 does not attenuate the anabolic response to PTH in vivo (185, 186). In addition, in vitro and in vivo evidence suggests direct crosstalk of PTH1R and Wnt signaling pathway (187). Binding of PTH to PTH1R induces association of the PTH–PTH1R complex with the extracellular domain of the Lrp6 Wnt co-receptor in absence of the Wnt ligand binding. This results in rapid phosphorylation of Lrp6 by PKA, which is activated by the cAMP signaling pathway downstream of PTH–PTH1R. Phosphorylated Lrp6 recruits Axin and thereby targets the β-catenin degradation complex to the cell membrane. A recent study identified a Dvl-binding motif in the PTH receptor (PTH1R), which activates the β-catenin pathway by directly recruiting Dvl independent of Wnt or LRP5/6(188). These studies suggest that PTH-induces osteoblast differentiation mainly through activation of the Wnt canonical pathway.
5.1.2. Wnt/β-catenin signaling controls chondrocyte hypertrophy and maturation through the PTH pathway
Wnt/β-catenin signaling regulates initiation of chondrocyte hypertrophy by inhibiting parathyroid hormone-related protein (PTHrP) signaling activity, but it does not regulate PTHrP expression (189). In addition, Wnt/β-catenin signaling regulates chondrocyte hypertrophy in a non-cell autonomous manner and growth differentiation factor 5 (Gdf5)/Bmp signaling may be part of the downstream pathway (189). Furthermore, Wnt/β-catenin signaling also controls final maturation of hypertrophic chondrocytes, but such regulation is PTHrP signaling-independent (189). In long bone development, Wnt5a is required for longitudinal skeletal outgrowth and both Wnt5a and Wnt5b regulate the transition between different chondrocyte zones independently of the Indian hedgehog(Ihh)/PTHrP negative feedback loop(30).
5.2. Crosstalk between the Indian hedgehog pathway and Wnt signaling
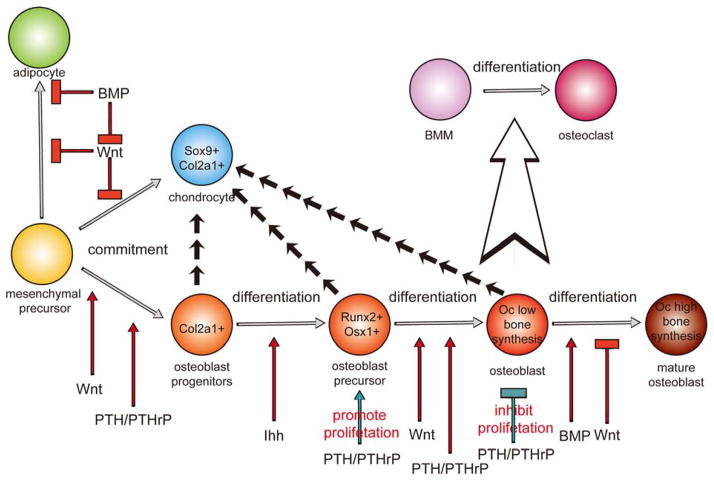
During normal development, Indian hedgehog (Ihh) signaling appears to act as a switch within a specific population of inner perichondral mesenchyme to initiate a program of bone formation (190, 191). Ihh is the only member of the hedgehog family of secreted molecules that is expressed in chondrocytes during endochondral bone formation. Ihh is synthesized by prehypertrophic chondrocytes and by early hypertrophic chondrocytes. Ihh−/− mice have normally shaped skeletal elements at the condensation stage, but subsequently dramatic abnormalities of bone development appear. Investigators indicate that the proliferative effect of Ihh is likely due to a direct action of this molecule on non-hypertrophic chondrocytes (192). Failure to activate this switch results in cells adopting an alternative chondrocyte pathway of development. Ihh is involved in the differentiation of osteoblast progenitors into runt-related transcription factor 2 (Runx2)-positive osteoblast precursors (Figure 2).
Figure 2.
The role of the canonical Wnt, Ihh, Bmp and PTH/PTHrP signaling pathways in regulating the differentiation of mesenchymal precursors. The Wnt canonical pathway and Ihh, Bmp, PTH/PTHrP pathways control the commitment of mesenchymal precursors and also the differentiation of osteoblasts/osteocytes. Though these processes, they regulate the osteoblastogenesis and bone remodeling.
Because the expression of Wnt7b and Tcf1 in the perichondrium is lost in the Ihh mutant, it was proposed that Ihh may signal upstream of Wnt signaling(42). Loss of function of Wnt9 could temporally and spatially downregulate Ihh signaling in the appendicular skeleton and ultimately lead to a delay by 1 day in chondrocyte and osteoblast maturation, as well as shorten the proximal long bone(28). Wnt5a cooperates with Ihh to trigger degradation of NK3 homeobox 2 (Nkx3.2), an early-stage chondrogenic factor, and represses chondrogenesis (193). Along with the fact that β-catenin and Lef1 associate with the Ihh promoter in vivo, this data suggests that Wnt9a-dependent regulation of Ihh is probably mediated via the canonical/β-catenin pathway. This is further supported by the observation that Ihh expression levels in humeri of Wnt9a;β-catenin double heterozygous animals were slightly reduced and that, depending on the cre-deleter line, Ihh expression varies from downregulation to temporary loss or delayed expression in the skeletal elements of mice lacking β-catenin activity (28, 42, 147). Besides Indian hedgehog, Wnt signaling interacts with sonic hedgehog to regulate tooth spatial patterning (194, 195). Wnt and sonic hedgehog (SHH) signaling antagonize each other to regulate patterning through Shh antagonist Gli3 expression (19, 196–199) and Wnt antagonist Sfrp1 and Sfrp2(200–202).
5.3. Crosstalk between the TGF-β/BMP pathway and Wnt signaling
BMPs, members of the transforming growth factor beta (TGF-β) superfamily, are potent osteogenic agents that stimulate maturation of mesenchymal osteoprogenitor cells to osteoblasts (203). BMPs transduce signals by binding to heteromeric complex of type 1 and type 2 serine/threonine kinase receptors (type 1 receptors are divided into three kinds, BMPR1A, BMPR1B and ActR1) (204). Smads are the major signal transducers for the serine/threonine kinase receptors (205). There are three classes of Smads: receptor-regulated Smads (R-Smads) that can be TGF-β/BMP activated, common partner TGF-β/BMP mediator Smads (Co-Smads), such as Smad 4; and inhibitory Smads (I-Smads). Upon ligand stimulation and activation by type II receptors, type I receptors phosphorylate R-Smads, which in turn form complexes with Co-Smads (206). The R-Smad/Co-Smad complexes then translocate into the nucleus and regulate transcription of target genes by interacting with various transcription factors and transcriptional co-activators or co-repressors. The third class of Smads, I-Smads, negatively regulates signaling by the R-Smads and Co-Smads. Runx2 and R-Smads physically interact with each other upon activation of BMP signaling, and cooperatively regulate the transcription of target genes, leading to the osteoblast differentiation of mesenchymal progenitor cells (207–209). BMP induces Runx2 expression in mesenchymal progenitor cells through the action of R-Smads (210), and R-Smads in turn interact with Runx2 to further induce osteoblastic differentiation.
There are several ways that Wnt and BMP signaling pathways interact with each other and influence bone development. First, BMPR1A signaling upregulates the expression of sclerostin, which is the SOST gene product and acts as a downstream effector of BMPR1A, leading to an inhibition of canonical Wnt signaling and a decrease in bone mass by upregulating osteoclastogenesis through the RANKL-OPG pathway (211). Moreover, GSK3/Wnt regulates BMP/Smad1 signal termination (212). Smad1, an R-Smad, contains mitogen-activated protein kinase (MAPK) and GSK3 phosphorylation sites in its linker region (213). GSK3 phosphorylation is required for the polyubiquitination of Smad1 (213). BMP signaling triggers sequential Smad1 phosphorylation by BMPR, MAPK, and GSK3 and then polyubiquitination (213). Once Smad1 is targeted for degradation, it is transported to the centrosome where the triply phosphorylated and polyubiquitinated Smad1 is degraded by proteasomes (213). This process may be regulated by Wnt signaling. Wnt3a protein inhibits Smad1 phosphorylation by GSK3 and stabilizes pSmad1Cter, which is a Smad1 C-terminal phosphorylated by BMPR(213). Thus, the inhibitory phosphorylation of the MAPK and GSK3 sites regulate the duration of the Smad1/5/8 signal (212). In this way, BMP determines the intensity of the Smad1/5/8 response, while FGF decreases and Wnt increases its duration (Figure 1A) (212). Furthermore, BMP-2 antagonizes Wnt signaling in osteoblast progenitors by promoting an interaction between Smad1 and Dvl-1 that restricts β-catenin activation (214). Treatment with Wnt3a (but not BMP-2) stimulated Lef1-mediated transcriptional activity, whereas co-stimulation with both Wnt3a and BMP-2 markedly reduced Wnt3a-induced reporter activity (214). Without stimulation, Dvl-1 and Smad1 are co-immunoprecipitated and form a complex through the linker region of Smad1(214). Wnt3a treatment transiently disrupted the Dvl-1/Smad1 interaction coincident with nuclear accumulation of β-catenin (214). In contrast, when cells were exposed to both Wnt3a and BMP-2, there was an enhanced accumulation of the Dvl-1-Smad1 complex and a decreased nuclear accumulation of β-catenin (Figure 1B) (214). In addition, canonical Wnt signaling can be activated by BMP-2 during osteoblast differentiation (215). When primary calvarial osteoblast cells were treated with BMP-2, there was an increase in the expression of Wnt Ligands (i.e. Wnt7a, Wnt10b, Wnt11, and Wnt13) and Wnt Receptors (e.g. Fz3, Fz10, and Lrp6)(215). Additionally, Axin regulates TGF-β signaling by promoting the degradation of Smad7 (216) and regulating the stability and transcriptional activity of the Smad3 co-response with GSK3β (217, 218). Axin, Arkadia, and Smad7 formed a ternary complex with their protein-protein interactions (216). Then Axin acts as a scaffold to facilitate Arkadia-mediated polyubiquitination of Smad (an I-Smad), regardless of TGF-β signaling, and leads to Smad7 degradation (216). A study in 2001 showed that Axin physically interacted with Smad3 through its C-terminal region located between the β-catenin binding site and the Dishevelled-homologous domain (218). Axin colocalized with Smad3 in the cytoplasm in vivo and the transcriptional activity of TGF-β was enhanced by Axin (218). Recent research draws an inverse conclusion about the role that Axin plays in TFG-β signaling. It was shown that Axin facilitates GSK3β-mediated phosphorylation of Smad3 at Thr66, which triggers Smad3 ubiquitination and degradation, while reduction in the expression or activity of Axin/GSK3β leads to increased Smad3 stability and transcriptional activity without affecting TGF-β receptors (217). Since the physiological level of Axin protein is usually extremely low and this study relies on loss-of-function assays, the role of Axin in Smad7 degradation remains debatable (217). Axin may negatively regulate TGF-β signaling by ubiquitination and degradation of Smad3 with GSK3β (217).
The abovementioned findings indicate a complicated crosstalk between Wnt and TGF-β/BMP signaling. In skeletal bone formation, activation of Wnt signaling determined osteoblast progenitor commitment, otherwise mesenchymal precursors differentiate into chondrocytes or adipocytes (146, 148, 219). BMP signaling indirectly promotes chondrogenesis by blocking Wnt signaling (220). The proliferation of osteoprogenitors is promoted by Wnt signaling and the maintenance of their precursor status (220). TGF-β/BMP signals stimulate those cells to become mature osteoblasts (220–222). Hence, TGF-β/BMP and Wnt signals have opposing effects on osteoprogenitors and cooperative effects in osteoblasts since both the BMP and Wnt pathways promote further osteoblast differentiation as indicated by expression of alkaline phosphatase (ALP) and mineralization (Figure 2) (220).
6. WNT INVOLVEMENT IN SKELETAL DISEASES
Given the important and diverse biological functions of Wnt signaling, it is not surprising that defects or deregulation of Wnt signaling leads to various human skeletal diseases. Table 2 provides a list of human diseases that are caused by Wnt signaling disorders. WNT3 is required at the early stages of human limb formation. Tetra-amelia, a rare human genetic disorder characterized by complete absence of all four limbs and other anomalies, is reportedly caused by Wnt3 loss-of-function mutations (25). A WNT5A mutation has been found in patients with Robinow syndrome, which is characterized by short-limbed dwarfism and abnormalities in the head, face, and external genitalia (223). Mutations of WNT7A cause Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome (AARRS), which is a rare autosomal recessive disorder characterized by severe malformations of upper and lower limbs with severely hypoplastic pelvis and abnormal genitalia, and Fuhrmann syndrome, which is a syndrome consisting of bowed femurs, aplasia or hypoplasia of the fibula, and poly-, syn-, and oligodactyly (37). Studies of two families with distinct limb malformation disorders indicate that the R292C mutation of WNT7A causes AARRS while the A109T mutation of WNT7A causes Fuhrmann syndrome in humans (37). In 2011, Balwi et al. determined that the G204A mutation of WNT7A also causes AARRS (224). Mutations in WNT10A cause odontoonychodermal dysplasia (OODD), which is described as an ectodermal dysplasia with dystrophic nails, misshapen teeth (e.g. peg-shaped incisors), erythematous lesions of the face, and the thickening of palms and soles which showed hyperhidrosis (225–228). A study of 44 human osteosarcoma samples indicated that WNT10B expression correlated with survival rate (229). Split-hand/split-foot malformation (SHFM), which is a rare limb development characterized by variable degrees of median clefts of hands and feet, can result from a homozygous missense mutation of WNT10B (230–232).
Table 2.
Human genetic skeletal disease and Wnt signaling
| Gene | Nature of miscues | Diseases | References |
|---|---|---|---|
| WNT3 | Loss of function | Tetra-amelia | (25) |
| WNT5A/ROR2 | Mutations | Robinow syndrome | (223) |
| WNT7A | Mutations | Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome(AARRS) | (37, 224) |
| Fuhrmann syndrome | (37) | ||
| WNT10A | Mutations | Odontoonychodermal dysplasia(OODD) | (225–228) |
| WNT10B | Mutations | Split-hand/foot malformation(SHFM) | (230–232) |
| Expression correlates with survival rate | Osteosarcoma | (229) | |
| SOST | Mutations | Sclerosteosis | (244) |
| deletion Sost-specific regulatory element | Van Buchem disease | (114, 245, 246) | |
| Mutations | Craniodiaphyseal dysplasia(CCD) | (247) | |
| DKK1 | Expression is higher | Paget’s disease | (292) |
| LRP4 | Mutations | Cenani-Lenz syndactyly syndrome | (251, 252) |
| Mutations | Sclerosteosis2 | (112) | |
| LRP5 | Mutations | Osteoporosis prseudoglioma syndrome(OPPG) | (77, 256–260) |
| Mutations | High bone mass syndrome | (80, 261–264) | |
| Mutations affect binding affinity of Wnt antagonists and LRPs | High bone mass | (265, 266) | |
| Axin2 | Loss of function | Family tooth agenesis | (269) |
| FRP3 | Polymorphic SNPs | Higher incidence of osteoarthritis in females | (250) |
For the production and secretion of Wnt ligands, both Porcupine (Porcn) and Wntless (Wls) have crucial and non-redundant roles as indicated by the severe phenotypes in Porcn and Wls mouse models that are similar to several Wnt knockout mice (6, 233–239). It was recently discovered that mutations in PORCN drive the X-linked dominant syndrome known as focal dermal hypoplasia (FDH) or Goltz syndrome (OMIM: 305600)(6, 234, 240–242).
Wnt agonists, R-spondins, are newly recognized factors in osteoarthritis. A recent study showed that R-spondin1 acts as an anabolic agent for preservation of joint architecture in arthritis by antagonizing DKK1(50). The expression level of R-spondin2 is reduced in osteoarthritis osteoblasts and is at least partially responsible for their reduced Wnt signaling and abnormal mineralization (243). Wnt antagonist Sclerostin is related with several bone diseases. Sclerosteosis, an autosomal recessive sclerosing bone dysplasia is due to the loss of the SOST expression (108, 244). Van Buchem disease, an inherited skeletal dysplasia characterized by enlargement of the lower jaw and a thickening of the long bones and the top of the skull, is also caused by the deletion of SOST-specific regulatory element in the patients’ genome (114, 245, 246). Craniodiaphyseal dysplasia (CCD), which results in facial distortion, is the most severe form of sclerotic bone diseases caused by mutations in SOST (247). Plenty of studies demonstrate that DKK1 is attributed to cancer bone metastases, osteolytic lesions, osteopenia, and multiple myeloma (89, 91, 248). SNPs in the sFRP1 intron and 3′-untranslated region were significantly associated with the BMD value of Japanese women (249). Functional polymorphisms within the frizzled-related protein 3 gene (FZP3) confer susceptibility for hip osteoarthritis in females (250). Mutations of LRP4 cause Cenani-Lenz syndrome, a rare autosomal recessive disorder characterized by syndactyly and oligodactyly of fingers and toes as well as disorganization and fusion of metacarpals (251, 252). Sclerosteosis2, which presents cortical hyperostosis, syndactyly of fingers, and the shortening and radial deviation of several distal phalanges, is less severe than Sclerosteosis (253, 254). Kneissel et al. identified two mutations of LRP4 (i.e. R1170W and W1186S) in patients suffering from Sclerosteosis2 (112). Osteoporosis pseudoglioma syndrome (OPPG) is an autosomal recessive disorder characterized by severe juvenile-onset osteoporosis and congenital or juvenile-onset blindness (255). Several mutations of LRP5 were observed in OPPG patients (77, 256–260). Mutations in LRP5 will not only cause osteoporosis, but also cause high bone mass syndrome (80, 261–264). The binding affinity of Wnt antagonists and LRPs was decreased by LRP5/6 mutations and results in a high-bone-mass phenotype in humans (265–268). Ringelschwanz is caused by a point mutation in Lrp6 in mice, and it leads to delayed ossification at birth and osteoporosis in adults (82). Mice lacking Axin2 exhibit malformation of skull structures, a phenotype resembling intramembranous ossification in humans (133). Axin2 is also linked to normal tooth development since the loss-of-function of Axin2 may cause family tooth agenesis (269). Repression of Wnt canonical signaling in osteocytes contributes to a bone pathology characterized by bone mineralization deficiency and known as renal osteodystrophy or chronic kidney disease-mineral and bone disorder (CKD-MBD) (270).
7. TARGETING WNT SIGNALING TO TREAT BONE DISEASES
Historically, diseases of bone loss have been treated with agents that block bone resorption. However, this type of therapy stimulates only a modest increase in bone mineral density, and osteoporotic patients retain an elevated risk for fracture (271). Wnt signaling has emerged as a key regulator of skeletogenesis. In most cases, Wnt ligands promote bone growth, which leads to the expectation that Wnt signaling factors could be used to treat bone diseases. Wnt canonical signaling offers multiple steps that may be considered as potential drug targets.
Osteosclerosis, an elevation in bone density, is normally detected on an X-ray as an area of whiteness. The pathogenesis of osteosclerosis involves an inactivating mutation in the SOST gene. The SOST gene encodes a protein Sclerostin that is expressed in various tissues, but is found chiefly on bone cells (osteocytes)(272). In the Wnt signaling pathway, sclerostin acts as an inhibitor by inactivating LRP5. As aforementioned, SOST−/− mice showed high bone mass and transgenic mice overexpressing SOST exhibit low bone mass and decreased bone strength (108, 113, 114). These findings indicate that sclerostin inhibits bone anabolic effects and may be a therapeutic target for osteoporosis. Osteoporosis is a silent disease that makes bone fragile and increases the risk of fracture. Osteoporosis is considered a major public health threat for 44 million Americans, including approximately 30 million women. In a recent first-in-human study, administration of sclerostin monoclonal antibody (AMG 785) to healthy men and postmenopausal women inhibited sclerostin and showed promise for further clinical studies for stimulating bone formation in bone diseases such as osteoporosis (273). A recent study in mice demonstrated that the sclerostin antibody improves skeletal parameters in the osteogenesis imperfecta mouse model (274). This finding provides a new therapy to increase bone mass and reduce fractures in pediatric OI.
Dickkopf-1 (Dkk1) is a soluble inhibitor of Wnt, which disrupts osteoblast differentiation and action (275). In a femoral fractures repair study, the anti-Dkk1 antibody (Dkk1 Ab) influences fracture repair, with prompt activation enhancing repair and inactivation impairing it (276). Femoral fractures were generated in C57BL/6 mice. The mice were treated twice a week with vehicle or Dkk1 Ab initiated immediately postoperatively (Day 0). Day 0 initiation enhanced repair, with significant gains seen for callus area, BMC, BMD, and biomechanical properties. These data suggest that Dkk Ab may have clinical utility in facilitating fracture repair. Multiple myeloma (MM) is associated with the development of osteolytic bone disease, mediated by increased osteoclastic bone resorption and impaired osteoblastic bone formation. In the study of the effect of Dkk1 on the development of osteolytic lesions in the 5T2MM murine model of myeloma, inhibiting Dkk1 prevented the suppression of bone formation and prevented the development of osteolytic bone disease in myeloma (277). Dkk1 is expressed by murine 5T2MM myeloma cells. After injection of 5T2MM cells into C57BL/KaLwRij mice, anti-Dkk1 treatment prevented 5T2MM-induced suppression of osteoblast numbers and surface. Treatment increased the mineralizing surface by 28%, increased the bone formation rate by 25%, significantly protected against 5T2MM-induced trabecular bone loss, and reduced the development of osteolytic bone lesions. By evaluating the bone anabolic effects of a Dkk1 neutralizing antibody (BHQ880) in MM, we know that Dkk1 inhibits osteoblast activity (278). In vitro BHQ880 increased OB differentiation, neutralized the negative effect of MM cells on osteoblastogenesis, and reduced IL-6 secretion. In a severe combined immunodeficiency (SCID)-hu murine model of human MM, BHQ880 treatment led to a significant increase in OB number, serum human osteocalcin level, and trabecular bone. Also, in vivo BHQ880 treatment inhibits MM cell growth in the SCID-hu murine model. These studies provide evidence that confirm Dkk1 as an important therapeutic target in myeloma and provide the rationale for clinical evaluation of the Dkk1 antibody to improve bone disease and to inhibit MM growth.
GSK-3β is a crucial regulator of the Wnt canonical pathway and lithium is an inhibitor of GSK-3β (279). Lithium enhances bone formation and improves bone mass in mice, perhaps via activation of the canonical Wnt pathway (279). Activation of β-catenin by lithium treatment has the potential to improve fracture healing, but only when utilized in later phases of repair after mesenchymal cells have become committed to the osteoblast lineage (280). Furthermore, lithium chloride (LiCl) treatment inhibited myeloma bone disease and decreased the tumor burden in bone (281). As a potential clinical treatment to bone diseases, lithium also has the advantage that it has been used safely and effectively for over half a century to treat bipolar illness (282).
In the design of therapeutic drugs for Wnt signaling related bone diseases, there are several advantages in targeting sclerostin, dkk, and GSK-3β. Sclerostin and dkk are characterized as extracellular targets that are suitable for the use of biologics. In addition, the inhibition of GSK-3β or the absence of sclerostin or dkk results in increased bone mass. Sclerostin has the additional advantage of being selectively expressed in bone, which is better than Dkk and GSK-3β (283). Dkk is also highly expressed in bone. Notably, Wnt agonists and R-spondins are extracellular ligands which modulate the Wnt pathway through LRP5 and have great potential as Wnt signaling targets for the design of drugs for osteoarthritis.
8. SUMMARY AND FUTURE DIRECTIONS
The relationship between Wnt signaling components and human bone diseases or skeletal abnormalities observed in mutant mice revealed the importance of Wnt signaling in bone development. Defects in Wnt ligands and its agonists have resulted in bone development disorders, joint formation deficiency, or osteoporosis (284). Mutation in Wnt cell surface receptor LRP5/6 leads to various kinds of bone diseases. The extracellular antagonist sclerostin is related to several bone diseases, including sclerosteosis, van Buchem disease, and CCD. The mutation of other antagonists (e.g. Dkk and WIF) results in altered bone density. Furthermore, the Wnt signaling pathway has networks with other bone development signaling pathways such as the PTH pathway, the Indian hedgehog pathway, and the TGF-β/BMP-Smad pathway. Through this network, Wnt signaling regulates bone remodeling and mesenchymal stem cell fate determination. After decades of studying Wnt signaling, a picture is formed of how Wnt ligands bind to cell surface receptors and trigger intracellular responses and the transcription of downstream genes. However, many important questions regarding this pathway remain unresolved (e.g. molecular structure of Wnt pathway components and their mechanism of interaction, the complicated network between the canonical Wnt pathway, noncanonical Wnt pathway, and other pathways in bone).
Because of the important role of Wnt signaling in bone development and diseases, researchers have designed several drugs based on this pathway. Preclinical studies with agents designed to inhibit SOST, Dkk1, and GSK-3β hold promises in treating bone diseases. However, potential problems exist with the long-term use of GSK-3 inhibitors since GSK-3 inhibitors would be expected to mimic the overexpression of Wnt signaling and, therefore, may become oncogenic (285). Another approach to the Wnt pathway has been to focus on extracellular mediators such as Sclerostin, which is selectively and highly expressed in bone. Targeting Sclerostin has great promise for treating osteoporosis and for fracture repair, but the kinetics of bone formation changes over time remain to be studied (283). The Wnt pathway has many ligands, antagonists, and intracellular proteins that influence bone development and diseases. Thus, there are many potential drug targets in Wnt signaling that may be useful in treating bone diseases. Future research will include determining which target is the best to use in clinical therapy. In any drug discovery program, issues of safety are paramount, especially in the treatment of chronic bone diseases that will likely involve long-term therapy (271). When considering how best to direct drug discovery in the Wnt canonical pathway, identification and screening upstream in the pathway is more promising than targeting β-catenin and downstream events (271). For instance, Wnt 10b, LRP5, and sFRP1 all have no negative side effects such as familial exudative vitreoretinopathy (286) and irregular skin thickness (287). R-spondins are potential drug targets as well.
Acknowledgments
We apologize to the many researchers whose work could not be cited due to space limitations. Work in our laboratory is supported by grants by National Institutes of Health (NIH) Grants AR-44741 (Y.-P.L.), and AR-055307 (Y.-P. L.).
Abbreviations
- GSK
glycogen synthase kinase-3β
- SOST
sclerostin
- DKK1
Dickkopf-1
- Tbx5b
T-box 5b
- fgf10
fibroblast growth factor 10
- LRP
low-density lipoprotein receptor-related protein
- Dsh
Dishevelled
- APC
Adenomatous Polyposis Coli
- sFRPs
Frizzled protein and its antagonists
- Fz
Frizzled
- aa
amino acids
- CRD
cysteine residues
- TCF
factors T cell factor
- LEF
lymphocyte enhancer binding factor
- EGF
epidermal growth factor
- BMD
bone mineral density
- rs
ringelschwanz
- OPPG
osteoporosis-pseudoglioma syndrome
- BMP
Bone morphogenetic proteins
- HBM
high bone mass
- Krm1
Kremen1
- Krm2
Kremen2
- RSpo
R-spondin
- WIF-1
Wnt-inhibitory factor-1
- Apc
adenomatous polyposis coli
- CKI
Casein kinase I
- RGS
Regulators of G protein signaling
- S
serine
- T
threonine
- PI-3K
phosphatidylinositol-3 kinase
- JNK2
c-Jun NH2-terminal kinase 2
- HMG
high-mobility-group
- Runx2
(runt homology domain transcription factor 2
- DAC
diacylglycerol
- PTHrP
parathyroid hormone-related protein
- PTH
Parathyroid hormone
- Ihh
Indian hedgehog
- R-Smads
receptor-regulated Smads
- Co-Smads
common partner Smads
- I-Smads
inhibitory Smads
- ALP
alkaline phosphatase
- AARRS
Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome
- OODD
odontoonychodermal dysplasia
- SHFM
Split-hand/split-foot malformation
- CCD
Craniodiaphyseal dysplasia
- FZP3
frizzled-related protein 3 gene
- CKD-MBD
chronic kidney disease-mineral and bone disorder
- MM
Multiple myeloma
- SCID
severe combined immunodeficiency
- Sox9
SRY-box containing gene 9
- β-Trcp
beta-transducin repeat containing protein
- Skp1
S-phase kinase-associated protein 1
- OPG
osteoprotegerin
- AER
apical ectodermal ridge
- RANKL
receptor activator of NF-kappaB ligand
- XTcf-3
transcription factor 7-like 1 (T-cell specific, HMG-box)
- TRAP
Tartrate-resistant acid phosphatase
- Gdf5
Growth/differentiation factor 5
- Nkx3.2
NK3 homeobox 2
- SHH
sonic hedgehog
- TGF-β
transforming growth factor beta
- MAPK
Mitogen-activated protein kinases
- Gli
Gliotactin
- Col1A1
collagen, type I, alpha 1
- Dkk1-AS
antisense oligonucleotide
Footnotes
This is an un-copyedited author manuscript that has been accepted for publication in the Frontiers in Bioscience. Cite this article as it appears in the Journal of Frontiers in Bioscience. Full citation can be found by searching the Frontiers in Bioscience (Search for articles) following publication and at PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed) following indexing. This article may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright holder, the Frontiers in Bioscience. From the time of acceptance following peer review, the full final copy edited article of this manuscript will be made available at http://www.bioscience.org. The Frontiers in Bioscience disclaims any responsibility or liability for errors or omissions in this version of the un-copyedited manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Soltanoff CS, Yang S, Chen W, Li YP. Signaling networks that control the lineage commitment and differentiation of bone cells. Crit Rev Eukaryot Gene Expr. 2009;19(1):1–46. doi: 10.1615/critreveukargeneexpr.v19.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu M, Deng L, Zhu G, Li YP. G Protein and its signaling pathway in bone development and disease. Front Biosci. 2010;15:957–85. doi: 10.2741/3656. [DOI] [PubMed] [Google Scholar]
- 3.Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50(4):649–57. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 4.Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nat Rev Mol Cell Biol. 2007;8(4):331–6. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- 5.Herr P, Hausmann G, Basler K. WNT secretion and signalling in human disease. Trends Mol Med. 2012;18(8):483–93. doi: 10.1016/j.molmed.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Maupin KA, Droscha CJ, Williams BO. A Comprehensive Overview of Skeletal Phenotypes Associated with Alterations in Wnt/β-catenin Signaling in Humans and Mice. Bone Research. 2013;1:27–71. doi: 10.4248/BR201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najdi R, Proffitt K, Sprowl S, Kaur S, Yu J, Covey TM, Virshup DM, Waterman ML. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation. 2012;84(2):203–13. doi: 10.1016/j.diff.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11(6):791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Tang X, Wu Y, Belenkaya TY, Huang Q, Ray L, Qu J, Lin X. Roles of N-glycosylation and lipidation in Wg secretion and signaling. Dev Biol. 2012;364(1):32–41. doi: 10.1016/j.ydbio.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125(3):509–22. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 12.Jenny A. Planar cell polarity signaling in the Drosophila eye. Curr Top Dev Biol. 2010;93:189–227. doi: 10.1016/B978-0-12-385044-7.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–46. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahiminiya S, Majewski J, Mort J, Moffatt P, Glorieux FH, Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet. 2013 doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 16.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–85. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 17.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346(6287):847–50. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 18.McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62(6):1073–85. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Marti E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135(2):237–47. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- 20.Capdevila J, Tabin C, Johnson RL. Control of dorsoventral somite patterning by Wnt-1 and beta-catenin. Dev Biol. 1998;193(2):182–94. doi: 10.1006/dbio.1997.8806. [DOI] [PubMed] [Google Scholar]
- 21.Ng JK, Kawakami Y, Buscher D, Raya A, Itoh T, Koth CM, Rodriguez Esteban C, Rodriguez-Leon J, Garrity DM, Fishman MC, Izpisua Belmonte JC. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development. 2002;129(22):5161–70. doi: 10.1242/dev.129.22.5161. [DOI] [PubMed] [Google Scholar]
- 22.Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8(2):174–89. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- 23.Kengaku M, Capdevila J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Izpisua Belmonte JC, Tabin CJ. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280(5367):1274–7. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- 24.Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17(3):394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, Weber JL, Muller U. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet. 2004;74(3):558–63. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18(19):2404–17. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127(14):3141–59. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 28.Spater D, Hill TP, O’Sullivan JR, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133(15):3039–49. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 29.Lee HH, Behringer RR. Conditional expression of Wnt4 during chondrogenesis leads to dwarfism in mice. PLoS One. 2007;2(5):e450. doi: 10.1371/journal.pone.0000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130(5):1003–15. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126(6):1211–23. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 32.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9(11):1273–85. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 33.Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibanez G, MacDougald OA. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50(2):477–89. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–9. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM, Lane TF, Krishnan V, Hankenson KD, MacDougald OA. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22(12):1924–32. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- 36.Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res. 2010;25(10):2138–47. doi: 10.1002/jbmr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods CG, Stricker S, Seemann P, Stern R, Cox J, Sherridan E, Roberts E, Springell K, Scott S, Karbani G, Sharif SM, Toomes C, Bond J, Kumar D, Al-Gazali L, Mundlos S. Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. Am J Hum Genet. 2006;79(2):402–8. doi: 10.1086/506332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loomis CA, Harris E, Michaud J, Wurst W, Hanks M, Joyner AL. The mouse Engrailed-1 gene and ventral limb patterning. Nature. 1996;382(6589):360–3. doi: 10.1038/382360a0. [DOI] [PubMed] [Google Scholar]
- 39.Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C. Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell. 1995;83(4):631–40. doi: 10.1016/0092-8674(95)90103-5. [DOI] [PubMed] [Google Scholar]
- 40.Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995;374(6520):350–3. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Niswander L. Interaction between the signaling molecules WNT7a and SHH during vertebrate limb development: dorsal signals regulate anteroposterior patterning. Cell. 1995;80(6):939–47. doi: 10.1016/0092-8674(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 42.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132(1):49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 43.Friedman MS, Oyserman SM, Hankenson KD. Wnt11 promotes osteoblast maturation and mineralization through R-spondin 2. J Biol Chem. 2009;284(21):14117–25. doi: 10.1074/jbc.M808337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12(1):113–27. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng HF, Tobias JH, Duncan E, Evans DM, Eriksson J, Paternoster L, Yerges-Armstrong LM, Lehtimaki T, Bergstrom U, Kahonen M, Leo PJ, Raitakari O, Laaksonen M, Nicholson GC, Viikari J, Ladouceur M, Lyytikainen LP, Medina-Gomez C, Rivadeneira F, Prince RL, Sievanen H, Leslie WD, Mellstrom D, Eisman JA, Moverare-Skrtic S, Goltzman D, Hanley DA, Jones G, St Pourcain B, Xiao Y, Timpson NJ, Smith GD, Reid IR, Ring SM, Sambrook PN, Karlsson M, Dennison EM, Kemp JP, Danoy P, Sayers A, Wilson SG, Nethander M, McCloskey E, Vandenput L, Eastell R, Liu J, Spector T, Mitchell BD, Streeten EA, Brommage R, Pettersson-Kymmer U, Brown MA, Ohlsson C, Richards JB, Lorentzon M. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012;8(7):e1002745. doi: 10.1371/journal.pgen.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. Mutational analysis of Norrin-Frizzled4 recognition. J Biol Chem. 2007;282(6):4057–68. doi: 10.1074/jbc.M609618200. [DOI] [PubMed] [Google Scholar]
- 47.Ai M, Heeger S, Bartels CF, Schelling DK. Clinical and molecular findings in osteoporosis-pseudoglioma syndrome. Am J Hum Genet. 2005;77(5):741–53. doi: 10.1086/497706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7(4):525–34. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 49.Hankenson KD, Sweetwyne MT, Shitaye H, Posey KL. Thrombospondins and novel TSR-containing proteins, R-spondins, regulate bone formation and remodeling. Curr Osteoporos Rep. 2010;8(2):68–76. doi: 10.1007/s11914-010-0017-0. [DOI] [PubMed] [Google Scholar]
- 50.Kronke G, Uderhardt S, Kim KA, Stock M, Scholtysek C, Zaiss MM, Surmann-Schmitt C, Luther J, Katzenbeisser J, David JP, Abdollahi-Roodsaz S, Tran K, Bright JM, Binnerts ME, Akhmetshina A, Bohm C, Distler JH, Joosten LA, Schett G, Abo A. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum. 2010;62(8):2303–12. doi: 10.1002/art.27496. [DOI] [PubMed] [Google Scholar]
- 51.Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell. 2011;20(3):303–14. doi: 10.1016/j.devcel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 52.de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13(3):242. doi: 10.1186/gb-2012-13-3-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 54.Rawadi G, Roman-Roman S. Wnt signalling pathway: a new target for the treatment of osteoporosis. Expert Opin Ther Targets. 2005;9(5):1063–77. doi: 10.1517/14728222.9.5.1063. [DOI] [PubMed] [Google Scholar]
- 55.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5(9):691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 56.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382(6588):225–30. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 57.Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338(6212):263–4. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J Biol Chem. 1996;271(8):4468–76. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- 59.Albers J, Keller J, Baranowsky A, Beil FT, Catala-Lehnen P, Schulze J, Amling M, Schinke T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol. 2013;200(4):537–49. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albers J, Schulze J, Beil FT, Gebauer M, Baranowsky A, Keller J, Marshall RP, Wintges K, Friedrich FW, Priemel M, Schilling AF, Rueger JM, Cornils K, Fehse B, Streichert T, Sauter G, Jakob F, Insogna KL, Pober B, Knobeloch KP, Francke U, Amling M, Schinke T. Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol. 2011;192(6):1057–72. doi: 10.1083/jcb.201008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dennis S, Aikawa M, Szeto W, d’Amore PA, Papkoff J. A secreted frizzled related protein, FrzA, selectively associates with Wnt-1 protein and regulates wnt-1 signaling. J Cell Sci. 1999;112(Pt 21):3815–20. doi: 10.1242/jcs.112.21.3815. [DOI] [PubMed] [Google Scholar]
- 62.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88(6):747–56. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18(5):1222–37. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 64.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–40. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 65.Gaur T, Wixted JJ, Hussain S, O’Connell SL, Morgan EF, Ayers DC, Komm BS, Bodine PV, Stein GS, Lian JB. Secreted frizzled related protein 1 is a target to improve fracture healing. J Cell Physiol. 2009;220(1):174–81. doi: 10.1002/jcp.21747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao W, Cheng Z, Shahnazari M, Dai W, Johnson ML, Lane NE. Overexpression of Secreted Frizzled-Related Protein 1 Inhibits Bone Formation and Attenuates PTH Bone Anabolic Effects. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hausler KD, Horwood NJ, Chuman Y, Fisher JL, Ellis J, Martin TJ, Rubin JS, Gillespie MT. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res. 2004;19(11):1873–81. doi: 10.1359/JBMR.040807. [DOI] [PubMed] [Google Scholar]
- 68.Morello R, Bertin TK, Schlaubitz S, Shaw CA, Kakuru S, Munivez E, Hermanns P, Chen Y, Zabel B, Lee B. Brachy-syndactyly caused by loss of Sfrp2 function. J Cell Physiol. 2008;217(1):127–37. doi: 10.1002/jcp.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106(9):3160–5. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 70.Nakanishi R, Akiyama H, Kimura H, Otsuki B, Shimizu M, Tsuboyama T, Nakamura T. Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass. J Bone Miner Res. 2008;23(2):271–7. doi: 10.1359/jbmr.071007. [DOI] [PubMed] [Google Scholar]
- 71.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 72.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 73.Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407(6803):527–30. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 74.Hussain MM. Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front Biosci. 2001;6:D417–28. doi: 10.2741/hussain1. [DOI] [PubMed] [Google Scholar]
- 75.Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19(12):2033–40. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 76.Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem. 2002;277(44):41762–9. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- 77.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 78.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7(4):801–9. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 79.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157(2):303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–9. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18(6):960–74. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 82.Kokubu C, Heinzmann U, Kokubu T, Sakai N, Kubota T, Kawai M, Wahl MB, Galceran J, Grosschedl R, Ozono K, Imai K. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development. 2004;131(21):5469–80. doi: 10.1242/dev.01405. [DOI] [PubMed] [Google Scholar]
- 83.Mani A, Radhakrishnan J, Wang H, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315(5816):1278–82. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–37. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hay E, Buczkowski T, Marty C, Da Nascimento S, Sonnet P, Marie PJ. Peptide-based mediated disruption of N-cadherin-LRP5/6 interaction promotes Wnt signaling and bone formation. J Bone Miner Res. 2012;27(9):1852–63. doi: 10.1002/jbmr.1656. [DOI] [PubMed] [Google Scholar]
- 86.Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One. 2009;4(11):e7930. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J, Zhang X, Zhang L, Zhou F, van Dinther M, Ten Dijke P. LRP8 mediates Wnt/beta-catenin signaling and controls osteoblast differentiation. J Bone Miner Res. 2012;27(10):2065–74. doi: 10.1002/jbmr.1661. [DOI] [PubMed] [Google Scholar]
- 88.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Izpisua Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1(3):423–34. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 89.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–66. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 90.Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–45. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 91.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JD., Jr The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113(3):517–25. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96(4):646–53. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 94.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109(5):2106–11. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang FS, Ko JY, Yeh DW, Ke HC, Wu HL. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology. 2008;149(4):1793–801. doi: 10.1210/en.2007-0910. [DOI] [PubMed] [Google Scholar]
- 96.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3(7):683–6. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 97.Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, Wu D. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37(9):945–52. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 98.Oh H, Ryu JH, Jeon J, Yang S, Chun CH, Park H, Kim HJ, Kim WS, Kim HH, Kwon YG, Chun JS. Misexpression of Dickkopf-1 in endothelial cells, but not in chondrocytes or hypertrophic chondrocytes, causes defects in endochondral ossification. J Bone Miner Res. 2012;27(6):1335–44. doi: 10.1002/jbmr.1583. [DOI] [PubMed] [Google Scholar]
- 99.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 100.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302(1–2):179–83. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- 101.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417(6889):664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 102.Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, 3rd, Dixon M, Hazell SA, Wagle M, Nie WS, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, Abo A. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A. 2007;104(37):14700–5. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schulze J, Seitz S, Saito H, Schneebauer M, Marshall RP, Baranowsky A, Busse B, Schilling AF, Friedrich FW, Albers J, Spiro AS, Zustin J, Streichert T, Ellwanger K, Niehrs C, Amling M, Baron R, Schinke T. Negative regulation of bone formation by the transmembrane Wnt antagonist Kremen-2. PLoS One. 2010;5(4):e10309. doi: 10.1371/journal.pone.0010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hurson CJ, Butler JS, Keating DT, Murray DW, Sadlier DM, O’Byrne JM, Doran PP. Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Musculoskelet Disord. 2007;8:12. doi: 10.1186/1471-2474-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 106.Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19(13):1842–4. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 107.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–14. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–5. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 110.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–7. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 111.Holdsworth G, Slocombe P, Doyle C, Sweeney B, Veverka V, Le Riche K, Franklin RJ, Compson J, Brookings D, Turner J, Kennedy J, Garlish R, Shi J, Newnham L, McMillan D, Muzylak M, Carr MD, Henry AJ, Ceska T, Robinson MK. Characterization of the interaction of sclerostin with the low density lipoprotein receptor-related protein (LRP) family of Wnt co-receptors. J Biol Chem. 2012;287(32):26464–77. doi: 10.1074/jbc.M112.350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leupin O, Piters E, Halleux C, Hu S, Kramer I, Morvan F, Bouwmeester T, Schirle M, Bueno-Lozano M, Fuentes FJ, Itin PH, Boudin E, de Freitas F, Jennes K, Brannetti B, Charara N, Ebersbach H, Geisse S, Lu CX, Bauer A, Van Hul W, Kneissel M. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem. 2011;286(22):19489–500. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohyama Y, Nifuji A, Maeda Y, Amagasa T, Noda M. Spaciotemporal association and bone morphogenetic protein regulation of sclerostin and osterix expression during embryonic osteogenesis. Endocrinology. 2004;145(10):4685–92. doi: 10.1210/en.2003-1492. [DOI] [PubMed] [Google Scholar]
- 114.Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15(7):928–35. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130(18):4295–305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 116.Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398(6726):431–6. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 117.Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol. 2001;3(7):628–36. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- 118.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301(5638):1391–4. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 119.Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 2000;19(18):4944–54. doi: 10.1093/emboj/19.18.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13(1):149–56. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 121.Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4(3):407–18. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 122.Choi SH, Choi KM, Ahn HJ. Coexpression and protein-protein complexing of DIX domains of human Dvl1 and Axin1 protein. BMB Rep. 2010;43(9):609–13. doi: 10.5483/BMBRep.2010.43.9.609. [DOI] [PubMed] [Google Scholar]
- 123.Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19(6):4414–22. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16(9):1066–76. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol. 2005;5(1):21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 126.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1(1):55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 127.Miclea RL, Karperien M, Bosch CA, van der Horst G, van der Valk MA, Kobayashi T, Kronenberg HM, Rawadi G, Akcakaya P, Lowik CW, Fodde R, Wit JM, Robanus-Maandag EC. Adenomatous polyposis coli-mediated control of beta-catenin is essential for both chondrogenic and osteogenic differentiation of skeletal precursors. BMC Dev Biol. 2009;9:26. doi: 10.1186/1471-213X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280(22):21162–8. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 129.Cliffe A, Hamada F, Bienz M. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13(11):960–6. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- 130.Yavropoulou MP, Yovos JG. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones (Athens) 2007;6(4):279–94. doi: 10.14310/horm.2002.1111024. [DOI] [PubMed] [Google Scholar]
- 131.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90(1):181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 133.Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132(8):1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dao DY, Yang X, Flick LM, Chen D, Hilton MJ, O’Keefe RJ. Axin2 regulates chondrocyte maturation and axial skeletal development. J Orthop Res. 2010;28(1):89–95. doi: 10.1002/jor.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Embi N, Rylatt DB. Cohen Glycogen synthase kinase-3 from rabbit skeletal muscle P. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107(2):519–27. [PubMed] [Google Scholar]
- 136.Ahmed Y, Nouri A, Wieschaus E. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development. 2002;129(7):1751–62. doi: 10.1242/dev.129.7.1751. [DOI] [PubMed] [Google Scholar]
- 137.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35(3):161–8. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 139.Itoh S, Saito T, Hirata M, Ushita M, Ikeda T, Woodgett JR, Algul H, Schmid RM, Chung UI, Kawaguchi H. GSK-3alpha and GSK-3beta proteins are involved in early stages of chondrocyte differentiation with functional redundancy through RelA protein phosphorylation. J Biol Chem. 2012;287(35):29227–36. doi: 10.1074/jbc.M112.372086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 141.Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5(3):523–32. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 142.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133(2):340–53. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Topol L, Chen W, Song H, Day TF, Yang Y. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J Biol Chem. 2009;284(5):3323–33. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A. 1999;96(11):6273–8. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995;7(5):634–40. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 146.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 147.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–87. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 149.Dao DY, Jonason JH, Zhang Y, Hsu W, Chen D, Hilton MJ, O’Keefe RJ. Cartilage-specific beta-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J Bone Miner Res. 2012;27(8):1680–94. doi: 10.1002/jbmr.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen J, Long F. beta-catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. J Bone Miner Res. 2013;28(5):1160–9. doi: 10.1002/jbmr.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18(10):1842–53. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 153.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 154.Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan Y. Biphasic and dosage-dependent regulation of osteoclastogenesis by beta-catenin. Mol Cell Biol. 2011;31(23):4706–19. doi: 10.1128/MCB.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jackson A, Vayssiere B, Garcia T, Newell W, Baron R, Roman-Roman S, Rawadi G. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36(4):585–98. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 156.Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development. 2006;133(7):1219–29. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- 157.Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19(8):1839–50. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88(6):789–99. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 159.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86(3):391–9. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 160.Mikasa M, Rokutanda S, Komori H, Ito K, Tsang YS, Date Y, Yoshida CA, Komori T. Regulation of Tcf7 by Runx2 in chondrocyte maturation and proliferation. J Bone Miner Metab. 2011;29(3):291–9. doi: 10.1007/s00774-010-0222-z. [DOI] [PubMed] [Google Scholar]
- 161.Hoeppner LH, Secreto FJ, Razidlo DF, Whitney TJ, Westendorf JJ. Lef1DeltaN binds beta-catenin and increases osteoblast activity and trabecular bone mass. J Biol Chem. 2011;286(13):10950–9. doi: 10.1074/jbc.M110.165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13(6):709–17. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cheng A, Genever PG. SOX9 determines RUNX2 transactivity by directing intracellular degradation. J Bone Miner Res. 2010 doi: 10.1002/jbmr.174. [DOI] [PubMed] [Google Scholar]
- 164.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349–55. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 165.McCarthy TL, Centrella M. Novel links among Wnt and TGF-beta signaling and Runx2. Mol Endocrinol. 2010;24(3):587–97. doi: 10.1210/me.2009-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reinhold MI, Naski MC. Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. J Biol Chem. 2007;282(6):3653–63. doi: 10.1074/jbc.M608995200. [DOI] [PubMed] [Google Scholar]
- 167.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 168.Wang B, Sinha T, Jiao K, Serra R, Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Hum Mol Genet. 2011;20(2):271–85. doi: 10.1093/hmg/ddq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Randall RM, Shao YY, Wang L, Ballock RT. Activation of Wnt Planar Cell Polarity (PCP) signaling promotes growth plate column formation in vitro. J Orthop Res. 2012;30(12):1906–14. doi: 10.1002/jor.22152. [DOI] [PubMed] [Google Scholar]
- 170.DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, Yancopoulos GD. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24(3):271–4. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- 171.Witte F, Chan D, Economides AN, Mundlos S, Stricker S. Receptor tyrosine kinase-like orphan receptor 2 (ROR2) and Indian hedgehog regulate digit outgrowth mediated by the phalanx-forming region. Proc Natl Acad Sci U S A. 2010;107(32):14211–6. doi: 10.1073/pnas.1009314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Y, Bhat RA, Seestaller-Wehr LM, Fukayama S, Mangine A, Moran RA, Komm BS, Bodine PV, Billiard J. The orphan receptor tyrosine kinase Ror2 promotes osteoblast differentiation and enhances ex vivo bone formation. Mol Endocrinol. 2007;21(2):376–87. doi: 10.1210/me.2006-0342. [DOI] [PubMed] [Google Scholar]
- 173.Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, Takahashi N. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18(3):405–12. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 174.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8(7):645–54. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 175.De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai) 2011;43(10):745–56. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 176.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 Signaling Induces Warburg Effect through mTORC2 Activation during Osteoblast Differentiation. Cell Metab. 2013;17(5):745–55. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Swarthout JT, D’Alonzo RC, Selvamurugan N, Partridge NC. Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002;282(1–2):1–17. doi: 10.1016/s0378-1119(01)00798-3. [DOI] [PubMed] [Google Scholar]
- 178.Lee M, Partridge NC. Parathyroid hormone signaling in bone and kidney. Curr Opin Nephrol Hypertens. 2009;18(4):298–302. doi: 10.1097/MNH.0b013e32832c2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37(2):148–58. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 180.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–83. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 181.Tian Y, Xu Y, Fu Q, He M. Parathyroid hormone regulates osteoblast differentiation in a Wnt/beta-catenin-dependent manner. Mol Cell Biochem. 2011 doi: 10.1007/s11010-011-0856-8. [DOI] [PubMed] [Google Scholar]
- 182.O’Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3(8):e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. Effects of Parathyroid Hormone Treatment on Circulating Sclerostin Levels in Postmenopausal Women. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95(6):1178–90. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- 185.Yao GQ, Wu JJ, Troiano N, Insogna K. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab. 2011 doi: 10.1007/s00774-010-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R, Bringhurst FR, Kronenberg HM. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11(2):161–71. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab. 2010;21(4):237–44. doi: 10.1016/j.tem.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 188.Romero G, Sneddon WB, Yang Y, Wheeler D, Blair HC, Friedman PA. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. J Biol Chem. 2010;285(19):14756–63. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo X, Mak KK, Taketo MM, Yang Y. The Wnt/beta-catenin pathway interacts differentially with PTHrP signaling to control chondrocyte hypertrophy and final maturation. PLoS One. 2009;4(6):e6067. doi: 10.1371/journal.pone.0006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 191.Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, McMahon AP. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131(6):1309–18. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- 192.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13(16):2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Choi SW, Jeong DU, Kim JA, Lee B, Joeng KS, Long F, Kim DW. Indian Hedgehog signalling triggers Nkx3.2 protein degradation during chondrocyte maturation. Biochem J. 2012;443(3):789–98. doi: 10.1042/BJ20112062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cho SW, Kwak S, Woolley TE, Lee MJ, Kim EJ, Baker RE, Kim HJ, Shin JS, Tickle C, Maini PK, Jung HS. Interactions between Shh, Sostdc1 and Wnt signaling and a new feedback loop for spatial patterning of the teeth. Development. 2011;138(9):1807–16. doi: 10.1242/dev.056051. [DOI] [PubMed] [Google Scholar]
- 195.Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci U S A. 2000;97(9):4520–4. doi: 10.1073/pnas.97.9.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Borycki A, Brown AM, Emerson CP., Jr Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development. 2000;127(10):2075–87. doi: 10.1242/dev.127.10.2075. [DOI] [PubMed] [Google Scholar]
- 197.Yu W, McDonnell K, Taketo MM, Bai CB. Wnt signaling determines ventral spinal cord cell fates in a time-dependent manner. Development. 2008;135(22):3687–96. doi: 10.1242/dev.021899. [DOI] [PubMed] [Google Scholar]
- 198.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125(12):2315–25. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 199.Hilton MJ, Tu X, Cook J, Hu H, Long F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development. 2005;132(19):4339–51. doi: 10.1242/dev.02025. [DOI] [PubMed] [Google Scholar]
- 200.Lee CS, Buttitta LA, May NR, Kispert A, Fan CM. SHH-N upregulates Sfrp2 to mediate its competitive interaction with WNT1 and WNT4 in the somitic mesoderm. Development. 2000;127(1):109–18. doi: 10.1242/dev.127.1.109. [DOI] [PubMed] [Google Scholar]
- 201.He J, Sheng T, Stelter AA, Li C, Zhang X, Sinha M, Luxon BA, Xie J. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J Biol Chem. 2006;281(47):35598–602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 202.Katoh Y, Katoh M. WNT antagonist, SFRP1, is Hedgehog signaling target. Int J Mol Med. 2006;17(1):171–5. [PubMed] [Google Scholar]
- 203.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21(4):637–46. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102(14):5062–7. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16(3):251–63. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 206.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 207.Zhang YW, Yasui N, Ito K, Huang G, Fujii M, Hanai J, Nogami H, Ochi T, Miyazono K, Ito Y. A RUNX2/PEBP2alpha A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc Natl Acad Sci U S A. 2000;97(19):10549–54. doi: 10.1073/pnas.180309597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene. 2004;23(24):4232–7. doi: 10.1038/sj.onc.1207131. [DOI] [PubMed] [Google Scholar]
- 209.Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev. 2003;13(1):43–7. doi: 10.1016/s0959-437x(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 210.Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004;23(3):552–63. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135(22):3801–11. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Eivers E, Demagny H, De Robertis EM. Integration of BMP and Wnt signaling via vertebrate Smad1/5/8 and Drosophila Mad. Cytokine Growth Factor Rev. 2009;20(5–6):357–65. doi: 10.1016/j.cytogfr.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131(5):980–93. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu Z, Tang Y, Qiu T, Cao X, Clemens TL. A dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. J Biol Chem. 2006;281(25):17156–63. doi: 10.1074/jbc.M513812200. [DOI] [PubMed] [Google Scholar]
- 215.Chen Y, Whetstone HC, Youn A, Nadesan P, Chow EC, Lin AC, Alman BA. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem. 2007;282(1):526–33. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- 216.Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan SC, Chen YG, Han J, Lin SC. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 2006;25(8):1646–58. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3- control Smad3 protein stability and modulate TGF- signaling. Genes Dev. 2008;22(1):106–20. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, Kikuchi A, Miyazono K, Kato M. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol. 2001;21(15):5132–41. doi: 10.1128/MCB.21.15.5132-5141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27(11):2344–58. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Itasaki N, Hoppler S. Crosstalk between Wnt and bone morphogenic protein signaling: a turbulent relationship. Dev Dyn. 2010;239(1):16–33. doi: 10.1002/dvdy.22009. [DOI] [PubMed] [Google Scholar]
- 221.Amedee J, Bareille R, Rouais F, Cunningham N, Reddi H, Harmand MF. Osteogenin (bone morphogenic protein 3) inhibits proliferation and stimulates differentiation of osteoprogenitors in human bone marrow. Differentiation. 1994;58(2):157–64. [PubMed] [Google Scholar]
- 222.Hughes FJ, Collyer J, Stanfield M, Goodman SA. The effects of bone morphogenetic protein-2, -4, and -6 on differentiation of rat osteoblast cells in vitro. Endocrinology. 1995;136(6):2671–7. doi: 10.1210/endo.136.6.7750491. [DOI] [PubMed] [Google Scholar]
- 223.Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, Schleiffarth JR, Billington CJ, Jr, van Bokhoven H, Hoogeboom JM, Mazzeu JF, Petryk A, Schimmenti LA, Brunner HG, Ekker SC, Lohr JL. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn. 2010;239(1):327–37. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Eyaid W, Al-Qattan MM, Al Abdulkareem I, Fetaini N, Al Balwi M. A novel homozygous missense mutation (c.610G>A, p.Gly204Ser) in the WNT7A gene causes tetra-amelia in two Saudi families. Am J Med Genet A. 2011;155A(3):599–604. doi: 10.1002/ajmg.a.33717. [DOI] [PubMed] [Google Scholar]
- 225.van den Boogaard MJ, Creton M, Bronkhorst Y, van der Hout A, Hennekam E, Lindhout D, Cune M, Ploos van Amstel HK. Mutations in WNT10A are present in more than half of isolated hypodontia cases. J Med Genet. 2012;49(5):327–31. doi: 10.1136/jmedgenet-2012-100750. [DOI] [PubMed] [Google Scholar]
- 226.Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, Belguith H, de Mazancourt P, Megarbane A. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet. 2007;81(4):821–8. doi: 10.1086/520064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bohring A, Stamm T, Spaich C, Haase C, Spree K, Hehr U, Hoffmann M, Ledig S, Sel S, Wieacker P, Ropke A. WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am J Hum Genet. 2009;85(1):97–105. doi: 10.1016/j.ajhg.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kantaputra P, Sripathomsawat W. WNT10A and isolated hypodontia. Am J Med Genet A. 2011;155A(5):1119–22. doi: 10.1002/ajmg.a.33840. [DOI] [PubMed] [Google Scholar]
- 229.Chen K, Fallen S, Abaan HO, Hayran M, Gonzalez C, Wodajo F, MacDonald T, Toretsky JA, Uren A. Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr Blood Cancer. 2008;51(3):349–55. doi: 10.1002/pbc.21595. [DOI] [PubMed] [Google Scholar]
- 230.Khan S, Basit S, Zimri FK, Ali N, Ali G, Ansar M, Ahmad W. A novel homozygous missense mutation in WNT10B in familial split-hand/foot malformation. Clin Genet. 2012;82(1):48–55. doi: 10.1111/j.1399-0004.2011.01698.x. [DOI] [PubMed] [Google Scholar]
- 231.Ugur SA, Tolun A. Homozygous WNT10b mutation and complex inheritance in Split-Hand/Foot Malformation. Hum Mol Genet. 2008;17(17):2644–53. doi: 10.1093/hmg/ddn164. [DOI] [PubMed] [Google Scholar]
- 232.Blattner A, Huber AR, Rothlisberger B. Homozygous nonsense mutation in WNT10B and sporadic split-hand/foot malformation (SHFM) with autosomal recessive inheritance. Am J Med Genet A. 2010;152A(8):2053–6. doi: 10.1002/ajmg.a.33504. [DOI] [PubMed] [Google Scholar]
- 233.Liu W, Shaver TM, Balasa A, Ljungberg MC, Wang X, Wen S, Nguyen H, Van den Veyver IB. Deletion of Porcn in mice leads to multiple developmental defects and models human focal dermal hypoplasia (Goltz syndrome) PLoS One. 2012;7(3):e32331. doi: 10.1371/journal.pone.0032331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A. 2011;108(31):12752–7. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, Yao W, Guan M, Helms JA, Lane NE, Lang RA, Williams BO. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A. 2012;109(33):E2197–204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhu X, Zhu H, Zhang L, Huang S, Cao J, Ma G, Feng G, He L, Yang Y, Guo X. Wls-mediated Wnts differentially regulate distal limb patterning and tissue morphogenesis. Dev Biol. 2012;365(2):328–38. doi: 10.1016/j.ydbio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 237.Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48(9):554–8. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 2011;240(2):365–71. doi: 10.1002/dvdy.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci U S A. 2009;106(44):18598–603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang X, Reid Sutton V, Omar Peraza-Llanes J, Yu Z, Rosetta R, Kou YC, Eble TN, Patel A, Thaller C, Fang P, Van den Veyver IB. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. 2007;39(7):836–8. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- 241.Grzeschik KH, Bornholdt D, Oeffner F, Konig A, del Carmen Boente M, Enders H, Fritz B, Hertl M, Grasshoff U, Hofling K, Oji V, Paradisi M, Schuchardt C, Szalai Z, Tadini G, Traupe H, Happle R. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet. 2007;39(7):833–5. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- 242.Leoyklang P, Suphapeetiporn K, Wananukul S, Shotelersuk V. Three novel mutations in the PORCN gene underlying focal dermal hypoplasia. Clin Genet. 2008;73(4):373–9. doi: 10.1111/j.1399-0004.2008.00975.x. [DOI] [PubMed] [Google Scholar]
- 243.Abed E, Chan TF, Delalandre A, Martel-Pelletier J, Pelletier JP, Lajeunesse D. R-spondins are newly recognized players in osteoarthritis that regulate Wnt signaling in osteoblasts. Arthritis Rheum. 2011;63(12):3865–75. doi: 10.1002/art.30625. [DOI] [PubMed] [Google Scholar]
- 244.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–89. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–7. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Balemans W, Cleiren E, Siebers U, Horst J, Van Hul W. A generalized skeletal hyperostosis in two siblings caused by a novel mutation in the SOST gene. Bone. 2005;36(6):943–7. doi: 10.1016/j.bone.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 247.Kim SJ, Bieganski T, Sohn YB, Kozlowski K, Semenov M, Okamoto N, Kim CH, Ko AR, Ahn GH, Choi YL, Park SW, Ki CS, Kim OH, Nishimura G, Unger S, Superti-Furga A, Jin DK. Identification of signal peptide domain SOST mutations in autosomal dominant craniodiaphyseal dysplasia. Hum Genet. 2011;129(5):497–502. doi: 10.1007/s00439-011-0947-3. [DOI] [PubMed] [Google Scholar]
- 248.Hall CL, Kang S, MacDougald OA, Keller ET. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97(4):661–72. doi: 10.1002/jcb.20735. [DOI] [PubMed] [Google Scholar]
- 249.Ohnaka K, Yamamoto K, Nakamura K, Adachi M, Kawate H, Kono S, Takayanagi R. Association of single nucleotide polymorphisms in secreted frizzled-related protein 1 gene with bone mineral density in Japanese women. Geriatr Gerontol Int. 2009;9(3):304–9. doi: 10.1111/j.1447-0594.2009.00540.x. [DOI] [PubMed] [Google Scholar]
- 250.Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, Ferreira A, Ciesielski C, Carson DA, Corr M. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004;101(26):9757–62. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kariminejad A, Stollfuss B, Li Y, Bogershausen N, Boss K, Hennekam RC, Wollnik B. Severe Cenani-Lenz syndrome caused by loss of LRP4 function. Am J Med Genet A. 2013;161(6):1475–9. doi: 10.1002/ajmg.a.35920. [DOI] [PubMed] [Google Scholar]
- 252.Li Y, Pawlik B, Elcioglu N, Aglan M, Kayserili H, Yigit G, Percin F, Goodman F, Nurnberg G, Cenani A, Urquhart J, Chung BD, Ismail S, Amr K, Aslanger AD, Becker C, Netzer C, Scambler P, Eyaid W, Hamamy H, Clayton-Smith J, Hennekam R, Nurnberg P, Herz J, Temtamy SA, Wollnik B. LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani-Lenz syndrome. Am J Hum Genet. 2010;86(5):696–706. doi: 10.1016/j.ajhg.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Itin PH, Keseru B, Hauser V. Syndactyly/brachyphalangy and nail dysplasias as marker lesions for sclerosteosis. Dermatology. 2001;202(3):259–60. doi: 10.1159/000051649. [DOI] [PubMed] [Google Scholar]
- 254.Bueno M, Olivan G, Jimenez A, Garagorri JM, Sarria A, Bueno AL, Bueno M, Jr, Ramos FJ. Sclerosteosis in a Spanish male: first report in a person of Mediterranean origin. J Med Genet. 1994;31(12):976–7. doi: 10.1136/jmg.31.12.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gong Y, Vikkula M, Boon L, Liu J, Beighton P, Ramesar R, Peltonen L, Somer H, Hirose T, Dallapiccola B, De Paepe A, Swoboda W, Zabel B, Superti-Furga A, Steinmann B, Brunner HG, Jans A, Boles RG, Adkins W, van den Boogaard MJ, Olsen BR, Warman ML. Osteoporosis-pseudoglioma syndrome, a disorder affecting skeletal strength and vision, is assigned to chromosome region 11q12–13. Am J Hum Genet. 1996;59(1):146–51. [PMC free article] [PubMed] [Google Scholar]
- 256.Cheung WM, Jin LY, Smith DK, Cheung PT, Kwan EY, Low L, Kung AW. A family with osteoporosis pseudoglioma syndrome due to compound heterozygosity of two novel mutations in the LRP5 gene. Bone. 2006;39(3):470–6. doi: 10.1016/j.bone.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 257.Barros ER, Dias da Silva MR, Kunii IS, Lazaretti-Castro M. Three years follow-up of pamidronate therapy in two brothers with osteoporosis-pseudoglioma syndrome (OPPG) carrying an LRP5 mutation. J Pediatr Endocrinol Metab. 2008;21(8):811–8. doi: 10.1515/jpem.2008.21.8.811. [DOI] [PubMed] [Google Scholar]
- 258.Streeten EA, McBride D, Puffenberger E, Hoffman ME, Pollin TI, Donnelly P, Sack P, Morton H. Osteoporosis-pseudoglioma syndrome: description of 9 new cases and beneficial response to bisphosphonates. Bone. 2008;43(3):584–90. doi: 10.1016/j.bone.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Laine CM, Chung BD, Susic M, Prescott T, Semler O, Fiskerstrand T, D’Eufemia P, Castori M, Pekkinen M, Sochett E, Cole WG, Netzer C, Makitie O. Novel mutations affecting LRP5 splicing in patients with osteoporosis-pseudoglioma syndrome (OPPG) Eur J Hum Genet. 2011;19(8):875–81. doi: 10.1038/ejhg.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Korvala J, Juppner H, Makitie O, Sochett E, Schnabel D, Mora S, Bartels CF, Warman ML, Deraska D, Cole WG, Hartikka H, Ala-Kokko L, Mannikko M. Mutations in LRP5 cause primary osteoporosis without features of OI by reducing Wnt signaling activity. BMC Med Genet. 2012;13:26. doi: 10.1186/1471-2350-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 262.Pangrazio A, Boudin E, Piters E, Damante G, Lo Iacono N, D’Elia AV, Vezzoni P, Van Hul W, Villa A, Sobacchi C. Identification of the first deletion in the LRP5 gene in a patient with autosomal dominant osteopetrosis type I. Bone. 2011;49(3):568–71. doi: 10.1016/j.bone.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rickels MR, Zhang X, Mumm S, Whyte MP. Oropharyngeal skeletal disease accompanying high bone mass and novel LRP5 mutation. J Bone Miner Res. 2005;20(5):878–85. doi: 10.1359/JBMR.041223. [DOI] [PubMed] [Google Scholar]
- 264.Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, Warman ML, De Vernejoul MC, Bollerslev J, Van Hul W. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003;72(3):763–71. doi: 10.1086/368277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol. 2005;25(12):4946–55. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Balemans W, Piters E, Cleiren E, Ai M, Van Wesenbeeck L, Warman ML, Van Hul W. The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif Tissue Int. 2008;82(6):445–53. doi: 10.1007/s00223-008-9130-9. [DOI] [PubMed] [Google Scholar]
- 267.Bourhis E, Wang W, Tam C, Hwang J, Zhang Y, Spittler D, Huang OW, Gong Y, Estevez A, Zilberleyb I, Rouge L, Chiu C, Wu Y, Costa M, Hannoush RN, Franke Y, Cochran AG. Wnt antagonists bind through a short peptide to the first beta-propeller domain of LRP5/6. Structure. 2011;19(10):1433–42. doi: 10.1016/j.str.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 268.Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006;21(11):1738–49. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- 269.Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74(5):1043–50. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sabbagh Y, Graciolli FG, O’Brien S, Tang W, dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani ME, Carvalho AB, Jorgetti V, Moyses RM, Schiavi SC. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012;27(8):1757–72. doi: 10.1002/jbmr.1630. [DOI] [PubMed] [Google Scholar]
- 271.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116(5):1202–9. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Roux S. New treatment targets in osteoporosis. Joint Bone Spine. 2010;77(3):222–8. doi: 10.1016/j.jbspin.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 273.Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 274.Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res. 2013;28(1):73–80. doi: 10.1002/jbmr.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gavriatopoulou M, Dimopoulos MA, Christoulas D, Migkou M, Iakovaki M, Gkotzamanidou M, Terpos E. Dickkopf-1: a suitable target for the management of myeloma bone disease. Expert Opin Ther Targets. 2009;13(7):839–48. doi: 10.1517/14728220903025770. [DOI] [PubMed] [Google Scholar]
- 276.Komatsu DE, Mary MN, Schroeder RJ, Robling AG, Turner CH, Warden SJ. Modulation of Wnt signaling influences fracture repair. J Orthop Res. 2010;28(7):928–36. doi: 10.1002/jor.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD, Jr, Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;24(3):425–36. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 278.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–9. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Clement-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssiere B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406–11. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4(7):e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111(5):2833–42. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Livingstone C, Rampes H. Lithium: a review of its metabolic adverse effects. J Psychopharmacol. 2006;20(3):347–55. doi: 10.1177/0269881105057515. [DOI] [PubMed] [Google Scholar]
- 283.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 284.Rawadi G. Wnt signaling and potential applications in bone diseases. Curr Drug Targets. 2008;9(7):581–90. doi: 10.2174/138945008784911778. [DOI] [PubMed] [Google Scholar]
- 285.Rey JP, Ellies DL. Wnt modulators in the biotech pipeline. Dev Dyn. 2010;239(1):102–14. doi: 10.1002/dvdy.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, Craig JE, Jiang L, Yang Z, Trembath R, Woodruff G, Gregory-Evans CY, Gregory-Evans K, Parker MJ, Black GC, Downey LM, Zhang K, Inglehearn CF. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet. 2004;74(4):721–30. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, Opp MR, MacDougald OA. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279(34):35503–9. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 288.Johnson EB, Hammer RE, Herz J. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum Mol Genet. 2005;14(22):3523–38. doi: 10.1093/hmg/ddi381. [DOI] [PubMed] [Google Scholar]
- 289.Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–91. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Niziolek PJ, Farmer TL, Cui Y, Turner CH, Warman ML, Robling AG. High-bone-mass-producing mutations in the Wnt signaling pathway result in distinct skeletal phenotypes. Bone. 2011;49(5):1010–9. doi: 10.1016/j.bone.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kugimiya F, Kawaguchi H, Ohba S, Kawamura N, Hirata M, Chikuda H, Azuma Y, Woodgett JR, Nakamura K, Chung UI. GSK-3beta controls osteogenesis through regulating Runx2 activity. PLoS One. 2007;2(9):e837. doi: 10.1371/journal.pone.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Marshall MJ, Evans SF, Sharp CA, Powell DE, McCarthy HS, Davie MW. Increased circulating Dickkopf-1 in Paget’s disease of bone. Clin Biochem. 2009;42(10–11):965–9. doi: 10.1016/j.clinbiochem.2009.04.007. [DOI] [PubMed] [Google Scholar]