Abstract
The goal of this review article is to redefine what the Mismatch Negativity (MMN) component of event-related potentials reflects in auditory scene analysis, and to provide an overview of how the MMN serves as a valuable tool in Cognitive Neuroscience research. In doing so, some of the old beliefs (five common ‘myths’) about MMN will be dispelled, such as the notion that MMN is a simple feature discriminator and that attention itself modulates MMN elicitation. A revised description of what MMN truly reflects will be provided, which includes a principal focus onto the highly context-dependent nature of MMN elicitation and new terminology to discuss MMN and attention. This revised framework will help clarify what has been a long line of seemingly contradictory results from studies in which behavioral ability to hear differences between sounds and passive elicitation of MMN have been inconsistent. Understanding what MMN is will also benefit clinical research efforts by providing a new picture of how to design appropriate paradigms suited to various clinical populations.
Keywords: mismatch negativity (MMN), attention, event-related potentials (ERPs), auditory scene analysis, context effects
Introduction
The mismatch negativity (MMN) component of event related brain potentials has become a valuable tool in Cognitive Neuroscience research for studying auditory processing in both basic and clinical research (see also Näätänen et al., this issue). MMN essentially reflects auditory change detection. This is a fundamental ability of the auditory system that is crucial for survival, such as alerting one to potential threats in the environment and directing orienting behavior (Sokolov, 1963). One of the key advantages of MMN as a neural indicator of auditory change is that it can be elicited regardless of the listener’s direction of attention. Although most transient ERP components reflect some level of ‘oddness’ detection (e.g., P3a, N2b, P3b, N400, P600), they also require a certain level of stimulus saliency to be elicited (e.g., the P3a component reflects involuntary orienting to an unexpected event) or attention directed to the sounds (e.g., the P3b component reflects aspects of voluntary target detection). MMN therefore has a wider possible range of utility. However, there are limiting factors, or parameters of use, which bear consideration to optimize its potential application to a variety of basic and clinical settings that probe passive and active listening situations.
Understanding specifically what the MMN component reflects about the auditory change detection process is crucial to maximizing its use, and for advancing our understanding about the neurobiological basis of central auditory functions. The focus of this review is on defining what MMN reflects about auditory memory and attention by dispelling five common tenets of MMN that are actually misleading (hence: ‘myths’). A refocus in thinking is essential if MMN is to be used as an effectual tool in the further study of complex auditory functions in both basic and clinical domains. There have recently been a wealth of studies focused on more complex auditory processes, such as auditory scene analysis (Atienza et al., 2003; Bendixen et al., 2010; DeSanctis et al., 2008; Dyson et al., 2005; Hung et al., 2001; Müller et al., 2005; Nager et al., 2003; Rahne & Bockmann-Bartell, 2009; Rahne et al., 2007; Rahne & Sussman, 2009; Ritter et al., 2000; Sonnadara et al., 2006; Sussman, 2005; Sussman, Bregman et al., 2005; Sussman, Horváth et al., 2007; Sussman & Steinschneider, 2006; Sussman & Steinschneider, 2009; Sussman, Ritter, & Vaughan, 1998a; Sussman, Ritter, & Vaughan, 1999; Winkler et al., 2005; Yabe et al., 2001) and language processing (Aaltonen et al., 1987, 1993; Bonte et al., 2005; Brunelliere et al., 2011; Colin et al., 2002; Cornell et al., 2011; Deguchi et al., 2010; Dehaene-Lambertz et al., 2000; Díaz et al., 2008; Froyen et al., 2008; Gao et al., 2012; Hastings et al., 2008; Hisagi et al., 2010; Jacobsen & Schröger, 2004; Jakoby et al., 2011; Koelsch et al., 2005; Kraus et al., 1995; Kujala et al., 2006; Lee et al., 2012; Lipski et al., 2012; Maiste et al., 1995; Miglietta et al., 2013; Nenonen et al., 2005; Partanen et al., 2011; Peltola et al., 2012; Pulvermüller et al., 2004, 2006; Reiche et al., 2013; Savela et al., 2003; Shafer et al., 2004; Sharma & Dorman, 1998; Sharma et al, 1993; Shtyrov & Pulvermüller, 2002; Sorokin et al., 2010; Steinberg et al., 2010; Stekelenburg & Vroomen, 2012; Sussman, Kujala et al., 2004; Syzmanski et al., 1999; van Linden et al., 2007; Wang et al., 2012; Winkler et al., 1999; Winkler et al., 2003; Xi et al., 2010; Ylinen et al., 2010). These studies have, sometimes inadvertently, revealed new information about the MMN component as a tool in research that allows us now to expand the original notion of MMN as a ‘pre-attentive’ reflection of ‘sensory discrimination’. To make progress, we need to dispel fundamental ‘myths’ about MMN, redefine some terms, and refocus our understanding about what MMN elicitation truly reflects, and what processes contribute to the auditory change detection response (for a review of the visual MMN see Czigler, this issue). The structure of the review is to first identify the ‘myth’, then provide a suggestion for how to refocus thinking onto what MMN more accurately reflects, followed by some discussion points.
Five Myths of MMN
Myth #1-MMN is a feature discriminator
Refocus: MMN is highly context dependent
Probably one of the most common myths about MMN is that it merely reflects tone feature discrimination (e.g., frequency, intensity, tone duration, spatial location). This view came about because the most widely used paradigm to elicit MMN has been the auditory oddball paradigm (Näätänen, Gaillard, & Mäntysalo, 1978; Squires et al., 1975). The auditory oddball paradigm, in which two tones are randomly presented with a frequent-to-infrequent ratio (Sams et al., 1983; Sussman, Sheridan, et al., 2003; Näätänen, 1992), has often been used as an index of the ability to discriminate between sound features to assess difficulty level (e.g., hard vs. easy) or clinical classifications (e.g., comparing frequency discrimination ability in individuals with and without dyslexia). The notion was that if MMN was elicited by infrequently presented deviants (e.g., frequency or tone duration), then one could conclude that the deviant tone feature was discriminated from the standard tone feature (Sams et al., 1985; Schröger, 1995; Tiitinen et al., 1994). Indeed, when MMN is elicited, the ability to discriminate one feature from the other contributes to its elicitation. However, when MMN is not elicited the opposite is not true. It does not necessarily mean that the two features were not discriminable on their own (e.g., answering ‘yes’ to the question ‘are the two tones different?’). Thus, MMN elicitation by itself cannot serve as an objective index of auditory feature discrimination.
One explanation is that the change detection process reflected by MMN is largely context-based (Sussman, 2007). Thus, a single tone does not always serve as the standard comparison for deviance detection. That is to say that while the processes that contribute to MMN elicitation often include discrimination of tone features, even in the simplest auditory oddball paradigm the process also include factors of the larger context of the sound sequence (Davids et al., 2009; Müller & Schröger, 2007; Rahne & Sussman, 2009; Sussman, 2007; Sussman & Steinschneider, 2006; Sussman & Winkler, 2001; Tervaniemi et al., 2009; Winkler et al., 2003). Although MMN elicitation can index the brain’s ability to distinguish tone features, the basis for MMN elicitation is regularity extraction. One could say that MMN is primarily a reflection of the standard repeating regularity, extracted from the ongoing input and structured in memory. The detected ‘standard’ forms the basis for the auditory change detection process.
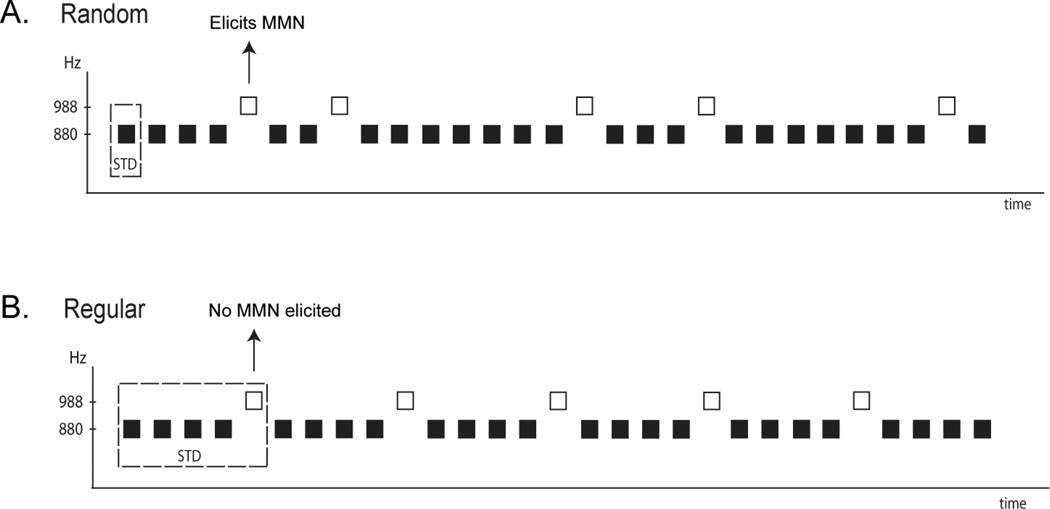
The highly context-based nature of MMN can be demonstrated with a simple, but modified auditory oddball paradigm (Sussman, Ritter, Vaughan, 1998b; Sussman et al., 2002; Sussman & Gumenyuk, 2005; Sussman, 2007; Sussman, 2013). For example, in a typical oddball paradigm, one tone is presented 80% of the time and a different frequency tone is presented randomly, 20% of the time (Fig 1A). The infrequent tone (‘deviant’) elicits MMN. If a simple modification is made so that the 20% (‘deviant’) tones occur regularly, every fifth tone (Fig 1B), then no MMN is elicited by the infrequent (‘deviant’) tones. No MMN is elicited by the infrequent tones because they are not deviant when the repeating pattern is detected. The infrequent (988 Hz) tone is part of the standard repeating pattern of tones (Fig 1B). In contrast, in the random oddball, the repeating 880 Hz tone serves as the standard and the 988 Hz tone the deviant (Fig 1A). Thus, simply having a frequent to infrequent ratio in a tone sequence is not sufficient to elicit MMN: probability alone is not sufficient for eliciting MMN. The key factor influencing MMN elicitation (deviance detection) is what is extracted and represented as the repeating standard pattern (Sussman, 2007). Standard formation can occur either by stimulus-driven factors of the sound input (Fig 2, Sussman et al., 1998b; Sussman & Gumenyuk, 2005) or by task-driven factors (Fig 3; Sussman et al., 2002; Sussman, 2013).
Figure 1. MMN is highly context-based.
An auditory oddball paradigm is displayed with random (A) and regular (B) presentation of the two tones. MMN is elicited by the 988 Hz tones in the Random presentation (A), whereas the same 988 Hz tone does not elicit MMN when presented every fifth tone, in a Regular presentation (B). The same ratio of 988/880 Hz tones are presented in both sequences. MMN elicitation is therefore dependent upon detecting the repeating regularity in passive conditions or on using the pattern to perform a task in active listening conditions. When the 988 Hz tone (the ‘probe’ tone) is detected as part of a five-tone repeating pattern, either by stimulus-driven or task-based factors, no MMN is elicited by the infrequently occurring (20%) tone (see text for further details).
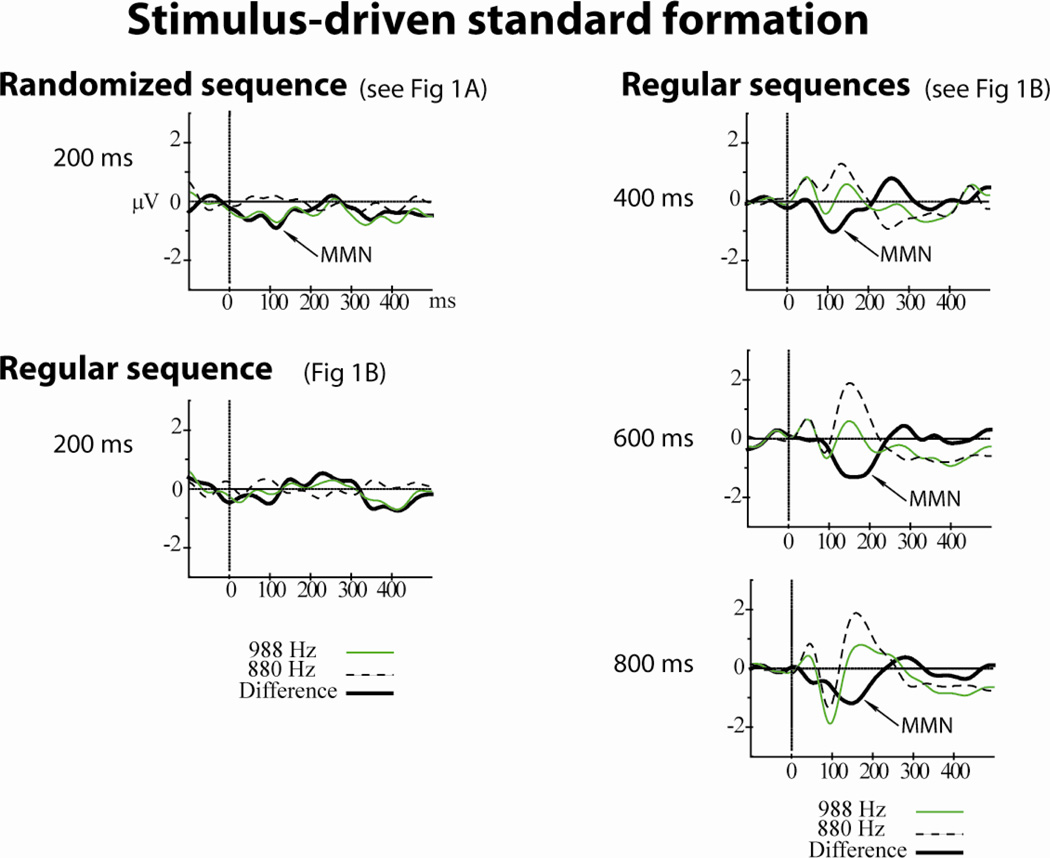
Figure 2. Stimulus-driven standard formation.
A fixed pattern of two tones (880 Hz and 988 Hz) was presented (see Fig 1B) in four conditions of stimulus rate (one tone occurring every 200, 400 ms, 600 ms, or 800 ms). ERPs to the 880 Hz tone are displayed with a dashed black line. ERPs to the 988 Hz tone are displayed with a green solid line, and the difference waveform (988 Hz-880 Hz) is displayed with a solid black line. Significant mismatch negativity (MMN) components are labeled and denoted with an arrow. Amplitude is depicted in microvolts along the y-axis, and timing is depicted in milliseconds along the x-axis. At relatively slow presentation rates (right column, 400–800 ms conditions), the MMN was elicited by the 988 Hz tones. Only at the fastest pace of the Regular sequences was there no MMN elicited by the 988 Hz tones (200 ms, left column, bottom row). The 200 ms Randomized sequence (left column, top row) shows that with the same ratio of tones as in the Regular sequence (1/5), MMN was elicited by the 988 Hz tones when the two tones were presented randomly, when there was no pattern in the sequence. Thus, the stimulus presentation rate influenced standard formation, which in turn modulated MMN elicitation. Figure adapted from Sussman & Gumenyuk (2005).
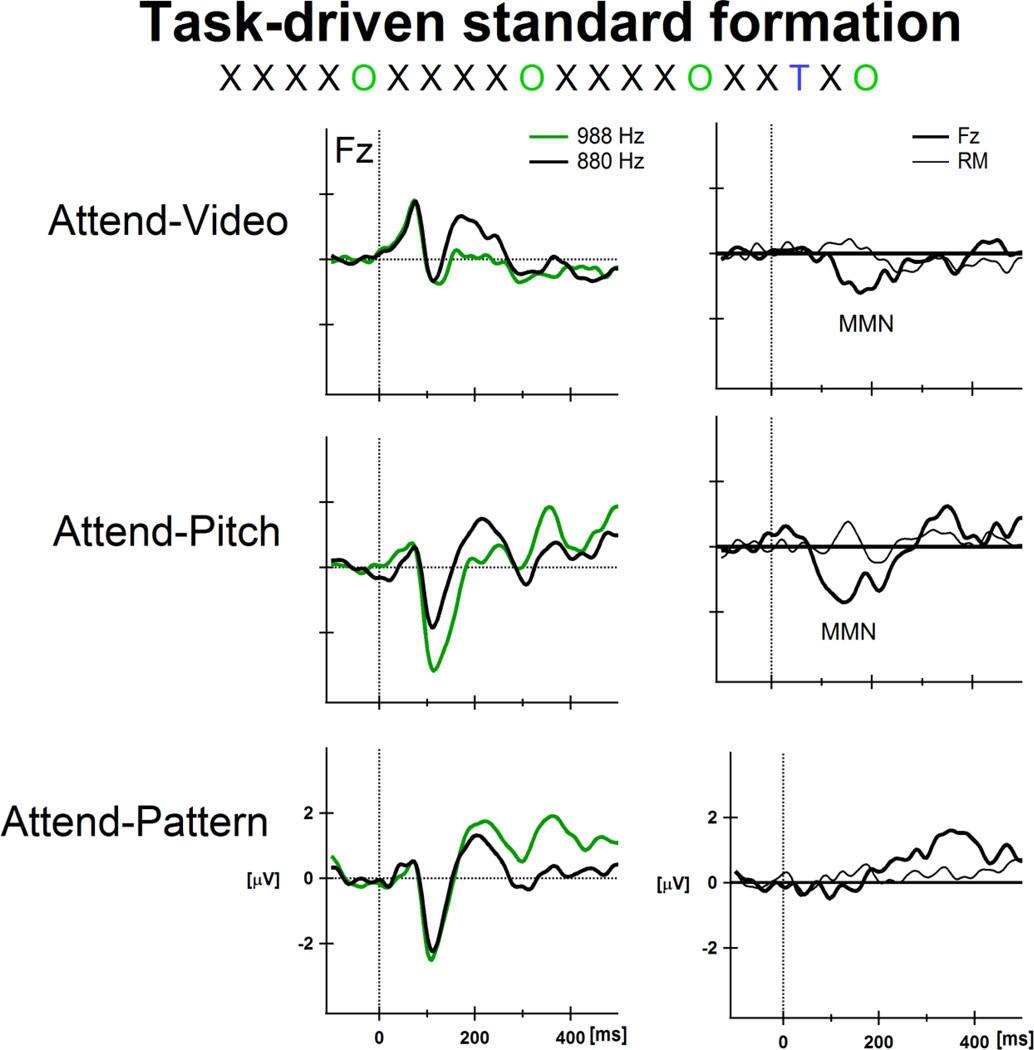
Figure 3. Task-driven standard formation.
Two tones were presented in a fixed order. The black ‘X’ represents the 880 Hz tone and the green ‘O’ represents the 988 Hz tone. The fixed order of presentation was a repeating five-tone pattern (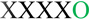 ). A lower frequency tone (784 Hz, designated by a blue ‘
). A lower frequency tone (784 Hz, designated by a blue ‘  ’) occurred rarely (∼2%) and randomly within the sequence. The patterned sequence was presented in three conditions: Attend-Video (top row), Attend-Pitch (middle row), and Attend-Pattern (bottom row; see text for description of tasks). The
’) occurred rarely (∼2%) and randomly within the sequence. The patterned sequence was presented in three conditions: Attend-Video (top row), Attend-Pitch (middle row), and Attend-Pattern (bottom row; see text for description of tasks). The  tone was the target, which required a button press in both of the active conditions. The fifth tone of the pattern (988 Hz) was the ‘probe’ tone, and was never the target. Event-related potential (ERPs) responses to the 880 Hz tone (black trace) and to the 988 Hz tone (green trace) are displayed in the left column, at the Fz electrode. The x-axis displays time in milliseconds and the y-axis displays amplitude in microvolts. Difference waveforms (ERPs to the 988 Hz-minus-ERPs to the 880 Hz) are displayed in the right column, showing the Fz electrode (thick black line) with the right mastoid (RM) overlain (thin black line). The mismatch negativity (MMN) component is labeled in the difference waveforms. The probe tones (988 Hz) elicited MMN in the Attend-Video and Attend-Pitch conditions, and not in the Attend-Pattern condition. The results show effects of task-specific standard formation because only the instruction of how to attend to the tones differed across conditions (see text for fuller explanation). ERP responses to the target (
tone was the target, which required a button press in both of the active conditions. The fifth tone of the pattern (988 Hz) was the ‘probe’ tone, and was never the target. Event-related potential (ERPs) responses to the 880 Hz tone (black trace) and to the 988 Hz tone (green trace) are displayed in the left column, at the Fz electrode. The x-axis displays time in milliseconds and the y-axis displays amplitude in microvolts. Difference waveforms (ERPs to the 988 Hz-minus-ERPs to the 880 Hz) are displayed in the right column, showing the Fz electrode (thick black line) with the right mastoid (RM) overlain (thin black line). The mismatch negativity (MMN) component is labeled in the difference waveforms. The probe tones (988 Hz) elicited MMN in the Attend-Video and Attend-Pitch conditions, and not in the Attend-Pattern condition. The results show effects of task-specific standard formation because only the instruction of how to attend to the tones differed across conditions (see text for fuller explanation). ERP responses to the target ( ) tones are not displayed (MMNs were elicited by the ‘
) tones are not displayed (MMNs were elicited by the ‘  ’ tones in all conditions). Figure adapted from Sussman et al., 2002.
’ tones in all conditions). Figure adapted from Sussman et al., 2002.
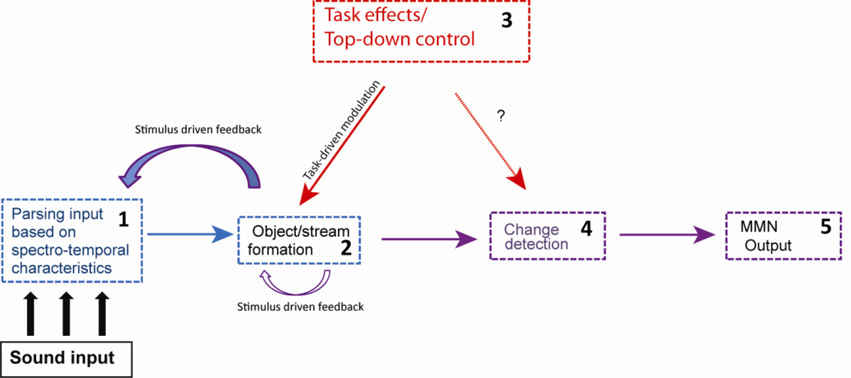
Notably, the ability to discriminate between the two tones is needed either way (no matter what the standard is) but for different purposes. In the Random condition (Fig 1A), the infrequent tone is detected as being different from the frequent tone, and in the Regular condition (Fig 1B), the infrequent tone demarcates the five-tone pattern at the terminal position. These results cannot be fully explained by stimulus-specific adaptation to a frequently occurring stimulus (i.e., the reduction of the neural response to a repeated stimulus). The difference in response to the infrequent tones indicates that the longer-term context of the temporal sequence is stored along with the values of the individual stimuli, allowing tone patterns to be stored as units as well as information about the tone features to be accessed. Therefore, the MMN cannot be thought of as reflecting simple feature detection (for a different view of MMN see May & Tiitinen, 2010). Rather, MMN is the outcome of a series of processes that precede deviance detection and is reflective of the larger auditory context (Sussman, 2005; Sussman & Winkler, 2001; Rahne & Sussman, 2009; Sussman & Steinschneider, 2006) (Fig 4).
Figure 4. Model of the MMN system.
A schematic diagram showing modulatory effects on MMN elicitation by stimulus-driven processes (blue) and task-specific goals (red). Arrows indicate a direction of influence. Input is analyzed from spectro-temporal characteristics (1), driving initial sound organization. Standard formation (2) occurs after stream segregation, and is subject to cross-stream interactions and task-driven goals. Stimulus-driven (2) and task-driven biases (3) modulate stream analysis and within-stream calculations. Change detection (4) is regulated by task-driven and stimulus-driven biases and their convergences. Competition between streams can be set up by task goals. A prediction of the model is that feature deviance detection (4) is a ‘higher-level’ process occurring on already formulated streams. MMN output (5) is the consequence of a series of processes and convergence of processes influencing change detection. Attention modulates MMN elicitation when behavioral goals influence how sounds are organized to perform a task (3, solid red line). Direct influence of attention on change detection is still unclear (3, dotted red line).
One of the processes that precede deviance detection is auditory stream segregation (Müller et al., 2005; Nager et al., 2003; Sussman et al., 1998a; Sussman et al., 1999; Sussman, 2005; Yabe et al., 2001). This has been demonstrated with MMN in that deviance detection is dependent upon regularities extracted from within-stream sound patterns that emerge after a mixture of high and low frequency sounds have been segregated (Sussman, Ritter, & Vaughan, 1998a; Sussman et al., 1999; Sussman & Steinschneider, 2006; Rahne & Sussman, 2009; Sussman, Bregman et al., 2005; Sussman-Fort & Sussman, submitted manuscript). That is, within-stream event detection occurs on the already formed streams (Sussman, 2005). Overall, the MMN reflects the longer-term stimulus characteristics, which includes temporal and spectral dynamics of the signal extracted from the stimulus history and maintained in memory. However, these longer-term contextual factors are not as readily observed in simple oddball-type paradigms as they are when more complex calculations are provoked.
Myth #2 – MMN is ‘pre-attentive’
Refocus: MMN can be elicited in passive listening situations
One of the problems with promoting the idea that MMN reflects ‘pre-attentive’ processing is that the processes generating MMN do not occur ‘before’ attention (Fig 4) or without any form of attention (note that MMN elicitation in the various states of consciousness, such as in coma, will not be addressed in this review, see Fischer et al. 2014, this issue). Stepping back, we might think about how the nomenclature ‘pre-attentive’ became attached to MMN investigations. Historically, models of selective attention have included the notion of a ‘pre-attentive’ stage of processing (Neisser, 1967). ‘Pre-attentive’ referred to the stage of processing that occurred prior to response selection (Neisser, 1967; Broadbent, 1958). That is, prior to attention selecting inputs that would then be subject to further analysis. The focus was on the degree of processing of sensory input prior to attention acting as a filter (hence, ‘pre-attentive’). However, there was also disagreement about where the ‘filter’ occurred in the processing stream (i.e., prior to or after perceptual processing) (Broadbent, 1958; Deutsch, 1963; Treisman, 1969). One of the important distinguishing factors of MMN from other prominent ERP components being researched at the time (e.g., P3, Sutton, 1965; or N2, Hillyard et al., 1973; Novak et al., 1990; 1992) was that MMN could be elicited in passive listening situations, to assess processing of unattended sounds. And, indeed, the passive listening situation was taken to mean processing before attention exerted influence. However, we now think about attention within a different framework, especially since the advent of modern brain imaging technology. The stage or “filter” model has been replaced with models that explain dynamic interactions among neural circuits conveying information in both serial and parallel processing streams. The word ‘pre-attentive’ is no longer useful.
We are always attending to something in the environment. From this perspective, there is no ‘before’ attention, and thus the word ‘pre-attentive’ does not accurately describe what we mean when we instruct participants to ‘ignore’ sounds to measure MMN. When we ask participants to ‘ignore’ the sounds, and instruct them to watch a captioned video or perform an n-back task, what we are actually asking them to do is to focus attention away from the sounds to effectuate that the sounds will be irrelevant to task goals. This allows assessment of the degree of processing for unattended sounds. In everyday environments, there are many things happening simultaneously and we choose what it is we want or need to attend to at any given time. We ‘ignore’ information because we have other goals in mind. Simply hearing sounds will not necessarily alter MMN elicitation. Attention is not one ‘thing’. The major influences on MMN generation are task demands. Specific task goals have the potential to alter neural activity to facilitate performance outcomes, and ultimately influence MMN elicitation if task performance modulates the extracted regularity (see Myth #1). To more effectively discuss about MMN, the terminology needs to be specific to what is actually being measuring. Essentially, the terminology should emphasize where or how attention is focused during presentation of the sounds. One way to refer to different conditions of attention would be to specify whether the conditions involve ‘passive’ or ‘active’ listening; thus indicating whether the participants are performing a task with the sounds or not. The word ‘passive’ provides a more direct indication that the sounds are being heard, but that there is no task being performed with them. Correspondingly, the word ‘active’ condition indicates involvement of a specific task with the sounds. This type of refocus onto where the direction of attention is, and what the specific task does to modulate unattended sounds is needed because of the potential utility of this measure for elucidating valuable information about the neural basis of attention.
Probably the most important reason why the term ‘pre-attentive’ is not a useful is that MMN can reflect processing after attention exerts its influence. To demonstrate how the direction of attention and specific task performance is central to modulating MMN elicitation, and not simply whether or not sounds are attended, refer back to the paradigm of Figure 1. Sussman and colleagues (Sussman et al., 2002; Sussman, 2013) presented the patterned sound sequence (Fig 1B) with additional rarely and randomly occurring low frequency tones (784 Hz) that served as targets in the active conditions (Fig 3, ‘T’ represents a target 784 Hz tone). The fifth tone of the pattern was the ‘probe’ tone (Fig 3, ‘O’ represents a probe 988 Hz tone), and never the target. The patterned sequence containing the rare deviants was presented in three conditions; each condition differed only in the instruction of how to focus attention to the sounds, and what task to perform (Fig 3). Participants were instructed to 1) attend to a closed-captioned video (they had no task with the sounds, Attend-Video condition); 2) attend to the pitches of the sounds (low, medium, and high) and press the response key for the rarely occurring lowest pitched tone (Attend-Pitch condition); or 3) attend to the pattern of the sounds and press the response key when a different pattern was heard (these included the lowest-pitched tones; Attend-Pattern condition). Participants pressed the response key to the same target “T” tones in both active conditions. MMN elicited by the ‘probe’ tones (the 988 Hz ‘O’ tones, Fig 3) determined how the sounds were structured in memory. That is, when the pitches of the sounds were relevant to performing the task (Attend-Pitch), MMN was elicited by the infrequently occurring probe tones (Fig 3, middle row). In contrast, when the pattern of sounds was relevant to performing the task (Attend-Pattern), no MMN was elicited by the same probe tones (the fifth tone of the repeating standard pattern, Fig 3 bottom row). Thus, the same regularly occurring tones (988 Hz) evoked different brain responses depending on the task being performed. When the task involved assessment of the pitches, MMN was elicited by the probe tones, whereas when the task involved pattern detection, no MMN was elicited by the same probe tones. MMN elicitation was thus consistent with task goals. In the passive listening condition, when participants watched a captioned video (Attend-Video) and had no task with the sounds, MMNs were elicited by the probe (988 Hz) tones (Fig 3, top row). This suggests that while watching a movie, the patterned aspect of the sequence was irrelevant, probably due to the relatively slow stimulus rate (∼2 tones per second). Even if the regular pattern of the sequence had been noticed, it was irrelevant to the task. Thus, all infrequently occurring tones elicited MMN in the passive listening condition, similar to the pitch evaluation task (Attend-Pitch condition). These results thus reveal that selective attention alters neural activity, adapting the neural response to the pertinent elements of the input required for performing a task. These studies demonstrate that task goals are fundamental to establishing what regularity (what standard) will be used in the deviance detection process generating MMN, and not the direction of attention, as in simply whether or not the sounds are attended.
Myth #3 – MMN is an early auditory process
Refocus: MMN can be elicited after attention exerts influence, or in conjunction with attentional influence
The notion that MMN is an early process is tied to Myth #2, that it occurs ‘before’ attention. However, MMN also occurs quantitatively early in time, often with a peak latency of approximately 150 ms (from stimulus onset or from onset of deviance detection), and always prior in time to the attention-dependent components. The issue of ‘early’ and ‘late’ is confusing because MMN can be elicited after attention is directed to a set of sounds (Fig 4). Moreover, MMN elicitation can be dependent upon attention, such as when attention alters the standard representation from which deviance detection occurs (Sussman, Ritter, Vaughan, 1998a; Sussman et al., 2002; Sussman, 2013). So what does early mean? Early in the sense that MMN peaks prior to non-modality-specific attention-related ERP components (e.g., N2b and P3b components), and that MMN elicitation is not dependent upon attention-related components to be elicited. However, MMN may be thought of as a relatively late occurring process in the sense that other processes precede deviance detection, relevant to establishing the standard either by stimulus-driven or top-down processing (List et al., 2007; Sussman, 2005; Sussman, 2007; Sussman et al., 2002; Panesse et al., submitted; Sussman, Ritter, Vaughan, 1998a; Sussman, Ritter, Vaughan, 1998b; Sussman, Bregman, et al., 2005; Sussman, 2005; Müller et al, 2005; Nager et al., 2003; Winkler et al., 2005; Yabe et al., 2001). MMN elicitation may also be considered a ‘late’ process for the auditory system because it has been shown to be more closely linked to task goals, to behavioral performance, than earlier elicited components or to the later P3a component (e.g., Tiitinen et al., 2004; Chen & Sussman, 2013). For example, Chen & Sussman (2013) found that the MMN amplitude was significantly correlated with the amount of behavioral distraction elicited by an irrelevant, deviant tone (i.e., larger MMN amplitude with longer reaction time) than either the N1 component or the P3a component.
Myth #4 – MMN is not modulated by attention
Refocus: MMN elicitation can be modulated by attention
Although MMN can be elicited in passive situations, without attention directed towards the sounds, MMN can also be elicited with attention focused on the sounds. But what does it mean when we say that attention modifies MMN? One way attention modifies MMN elicitation is by context-dependence. Essentially, when attention influences the information used in the deviance detection process it necessarily affects MMN elicitation. For example, when task goals (i.e., attention) alter the organization of the sounds in memory, this can alter the regularity or regularities stored in memory, which in turn can affect the information used in the deviance detection process (List et al., 2007; Rahne et al., 2007; Rahne & Sussman, 2009; Sussman, Ritter, Vaughan, 1999; Sussman, Ritter, Vaughan, 1998a; Sussman, Ritter, Vaughan, 1998b; Sussman et al., 2002; Sussman & Steinschneider, 2006). Attention modulates processes that precede deviance detection, and this influences MMN elicitation (Fig 4). In other words, attention does not directly modulate MMN, but rather indirectly through the memory structure of the auditory information. From this perspective, MMN is modulated by auditory scene analysis, by how the auditory information is structured in memory, NOT by attention itself. Neural representations of sounds in memory (that are used in the MMN process) are altered by task goals and not by simply listening to or not listening to the sounds. Primary effects of attention on the MMN process have been demonstrated on the standard formation phase (Sussman, 2007), whereas the deviance detection process per se occurs ‘automatically’, regardless of whether the sounds are attended. However, there are unexplored areas, such as effects of attention pertinent to deviance detection itself, which bear further investigation.
Most of the early studies concluding that attention modulates MMN, reported attention effects on the size of the MMN amplitude, often by comparing results between ‘attend’ and ‘ignore’ conditions. For the most part, these amplitude differences can be explained by overlap with the N2b component at frontal electrodes (Wei et al., 2002). It is difficult, if not impossible to measure the true MMN amplitude when there is overlap with the non-modality specific attention component N2b. One way to distinguish the components is at the mastoid electrode, where the peak of the MMN can be delineated by the inversion in polarity that is observed for MMN but not N2b (Novak et al., 1990; 1992). Nonetheless, our lab has found larger amplitudes for active than passive conditions at mastoid electrodes, but this has been found, so far, only when the stimuli were not simple feature changes (Sussman, Kujala et al., 2004; Chen et al., submitted). What this ‘attention effect’ is (observed at the mastoid electrodes) needs further investigation.
Moreover, if we focus on the deviant as the eliciting element in the MMN process, we can say that MMN is attention-independent, in the sense that MMN elicitation is not dependent upon whether the participant is listening to or not listening to the sounds. In the earlier days of MMN, a pertinent issue, perhaps the pertinent issue of whether attention modulated MMN amplitude was driven by the question of whether attention was focused toward the sounds or away from the sounds (Näätänen, 1991; Näätänen et al., 1993; Paavilainen et al., 1993; Woldorff et al., 1991; Woldorff et al., 1998;). This question came about following the influential studies of Woldorff and colleagues (Woldorff et al., 1991; 1998). Prior to these studies, MMN was interpreted as being ‘pre-attentive’ and unaffected by attentional manipulation. Woldorff et al. (1991) demonstrated that the MMN amplitude could be reduced by highly focused attention away from the deviant sounds. Woldorff et al. (1991) used a dichotic listening task, in which listeners performed a difficult loudness detection task with high frequency sounds presented to the right (or left) ear and ignored low frequency sounds presented to the opposite ear. The amplitude of the MMN to intensity deviants in the unattended ear was significantly reduced, but the amplitude to the frequency deviants (also presented to the unattended ear) was unaffected. The authors concluded that attention modulated the MMN component, and that the frequency dimension behaved differently than intensity. Sussman, Winkler, & Wang (2003) hypothesized that the reason the amplitude of unattended intensity deviants was reduced was due to context effects (intensity values were the same in both ears, but frequency values were segregated between the two ears) and not to the direction of attention as such. To test this, Sussman et al. reversed the paradigm so that intensity values were segregated between the two ears and the standard frequency of the sounds was the same in both ears. If highly focused attention explained the results, then intensity MMNs evoked in the unattended ear should be reduced (or abolished), and frequency MMNs should be robust, replicating Woldorff et al.’s results. On the other hand, if context explains the results, then frequency evoked MMNs should be reduced or abolished, and intensity MMNs should be robust. Results of Sussman et al. (2003) supported the context explanation: the frequency MMN was abolished, and intensity MMN was significantly elicited. These results were explained in terms of a ‘competition model’ of MMN, in which MMN elicitation can be biased by selectively attending to deviants in one ear and ignoring the same deviants in the other ear. These results also showed a limiting factor on the MMN system, where attention biased MMN elicitation to facilitate task goals. The results also support the idea that MMN elicitation is attention-independent in the sense that the direction of attention (namely, highly focused to one ear) was not the crucial influencing factor. The sound context influenced whether or not MMN was elicited. Further, there was nothing “special” about MMN and intensity being more susceptible to attentional manipulation than frequency.
It may be noted that limitations to the MMN generating system have been demonstrated now in several sets of data (Sussman et al., 1999; Sussman et al., 2003; Sussman, Bregman, et al., 2005; Panesse et al., submitted manuscript). Finding limitations on MMN generation is consistent with the postulation that MMN is not an ‘early’ component (Myth #3), it is not a protected process, and it is subject to limitations when prior processes converge or intervene on the deviance detection process (e.g., using shared attentional resources). That is, MMN can be affected by other processes occurring either in parallel or prior to deviance detection (Fig 4).
Myth #5 – MMN is not a reliable clinical research tool
Refocus: MMN can serve as a valuable clinical research tool
The wealth of influential MMN studies that have advanced our knowledge of auditory function and dysfunction are too numerous to list here. MMN has been used in a variety of clinical domains, some of which are represented in this special issue (Javitt, Salisbury, Todd, Fischer, Kraus, Escera, Trainor, Näätänen, for some examples). The MMN has been most widely used in clinical applications to provide an index of sensory discrimination through the auditory oddball paradigm and its variations. However, as described above, using MMN as a reflection of sensory discrimination ability may lead to puzzling or ambiguous results that may or may not match with sensory discrimination ability measured through behavioral performance (e.g., Bishop & Hardiman, 2010). Results of such studies have displayed a great deal of inter-subject variability, which has led to concern about its use as a reliable clinical research tool. Finding inter-subject variability is not an inherent problem, nor does the variability associated with MMN elicitation necessarily pose a problem for clinical research. However, a shift in focus is needed in designing and interpreting studies that use MMN paradigms, away from thinking of MMN as a simple sensory discrimination tool without also considering both contextual factors and attentional influence. These shifts will lead to better clarity in proper usage and understanding of MMN for setting up useful protocols and in interpreting datasets.
Consider, for example, a simple auditory oddball paradigm but with multiple deviants. The presence of multiple deviants alters the context used in the MMN-generating process. The response to a specific frequency deviant presented alone in a block compared to when it is presented amongst other deviants is altered by the context it occurs (Chen & Sussman, 2013; Grimm et al., 2004; Schröger, 1995; Schröger, 1996). Thus, if only the multi-deviant paradigm is used, the response to any particular sound may represent contextual effects, and not the simple ability to discriminate differences in one feature dimension over another. This example may explain some results, in which the ability of the experimental participant to behaviorally discriminate two sound features does not match with MMN elicitation when presented in some type of oddball paradigm (e.g., Dalebout & Stack, 1999; Gomes et al., 2000; Bishop & Hardiman, 2010). That is, some of the variability in results across different paradigms can be accounted for within this proposed framework. The stimulus history is crucial to the MMN process, often having long-range effects (>20 s) on deviance detection (Rahne & Sussman, 2009; Sussman & Winkler, 2001; Sussman & Steinschneider, 2006; Winkler et al., 2002). MMN is evoked on the basis of what is detected as the repeating standard, which can be stored in memory as a pattern of tones (e.g., Alho et al., 1993; Sussman et al., 1999; Schröger et al., 1992; Schröger et al.,1994), a conjunction of features (e.g., Gomes et al., 1997), a single repeating tone (e.g., Näätänen, Gaillard, & Mäntysalo, 1978; Hillyard et al., 1973), an abstract concept such as rising/falling (e.g., Saarinen et al., 1992; Paavilainen et al., 1999; van Zuijen et al., 2004; Wang et al., 2012), and so on, it cannot be considered a strict feature detector. Its elicitation reflects what is stored in memory as the standard perhaps more strongly than the deviance part of the equation. That is to say, the same MMN eliciting ‘deviant’ tone can be altered by the sound context (Sussman et al., 1999).
Knowledge about the context-dependent nature of the MMN system can be used to design experiments that “probe” the cortical sound representations in auditory scene analysis (Lepisto et al., 2009; Sussman & Steinschneider, 2009; Sussman, 2013). To be used as an effective clinical research tool, we need evaluate the dynamic range of features encoded into the neural trace and used in the deviance detection process that results in MMN elicitation. The MMN has the potential to be an important non-invasive tool for diagnosis and assessment of central auditory processing deficits (Perez et al., 2013). It provides one of the best tools for testing impaired populations because participants do not have to actively respond to the sounds for its elicitation (Kraus et al., 1996; Javitt et al., 1998; Kujala & Näätänen, 2001; Näätänen, 2003).
Conclusions
In understanding how to use MMN to ask questions about how the brain processes sound, it is fundamentally important to consider the sound context even in the simple auditory oddball case. Auditory context is a primary modulator of the MMN, induced either by stimulus-driven or top-down control. A second crucial issue central to the use of MMN is recognizing how task goals influence the memory representations used in the deviance detection process. Some of the things to consider in developing paradigms for basic or clinical populations are: 1) inherent patterns in the stimulus sequences; 2) the complexity of the ‘simple’ oddball paradigms; 3) influence of task goals on deviance detection; and 4) the limitations to MMN generation. To best maximize the benefits of MMN as a tool for probing central auditory functions in clinical settings, it is crucial to consider the factors that influence MMN elicitation. That is, even when using simple paradigms, there may be rather complex factors that mediate the MMN response (e.g., context, task demands). Understanding what influences MMN elicitation will also benefit clinical research efforts by providing a new picture of how to design appropriate paradigms suited to various clinical populations.
Acknowledgements
Support was provided by the National Institute on Deafness and Other Communications Disorders of the National Institutes of Health (R01DC004623, E.S.).
References
- Aaltonen O, Niemi P, Nyrke T, Tuhkanen M. Event-related brain potentials and the perception of a phonetic continuum. Biol Psychol. 1987;24(3):197–207. doi: 10.1016/0301-0511(87)90002-0. [DOI] [PubMed] [Google Scholar]
- Aaltonen O, Tuomainen J, Laine M, Niemi P. Cortical differences in tonal versus vowel processing as revealed by an ERP component called mismatch negativity (MMN) Brain Lang. 1993;44(2):139–152. doi: 10.1006/brln.1993.1009. [DOI] [PubMed] [Google Scholar]
- Alho K, Huotilainen M, Tiitinen H, Ilmoniemi RJ, Knuutila J, Näätänen R. Memory-related processing of complex sound patterns in human auditory cortex: a MEG study. Neuroreport. 1993;4(4):391–394. doi: 10.1097/00001756-199304000-00012. [DOI] [PubMed] [Google Scholar]
- Atienza M, Cantero JL, Grau C, Gomez C, Dominguez-Marin E, Escera C. Effects of temporal encoding on auditory object formation: a mismatch negativity study. Brain Res Cogn Brain Res. 2003;16(3):359–371. doi: 10.1016/s0926-6410(02)00304-x. [DOI] [PubMed] [Google Scholar]
- Bendixen A, Jones SJ, Klump G, Winkler I. Probability dependence and functional separation of the object-related and mismatch negativity event-related potential components. Neuroimage. 2010;50(1):285–290. doi: 10.1016/j.neuroimage.2009.12.037. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Hardiman MJ. Measurement of mismatch negativity in individuals: a study using single-trial analysis. 2010;47(4):697–705. doi: 10.1111/j.1469-8986.2009.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte ML, Mitterer H, Zellagui N, Poelmans H, Blomert L. Auditory cortical tuning to statistical regularities in phonology. Clin Neurophysiol. 2005;116(12):2765–2774. doi: 10.1016/j.clinph.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Perception and communication. London: Pergamon Press Ltd; 1958. [Google Scholar]
- Brunellière A, Dufour S, Nguyen N. Regional differences in the listener's phonemic inventory affect semantic processing: a mismatch negativity (MMN) study. Brain Lang. 2011;117(1):45–51. doi: 10.1016/j.bandl.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Chen S, Sussman ES. Context effects on auditory distraction. Biol Psychol. 2013 doi: 10.1016/j.biopsycho.2013.07.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin C, Radeau M, Soquet A, Demolin D, Colin F, Deltenre P. Mismatch negativity evoked by the McGurk-MacDonald effect: a phonetic representation within short-term memory. Clin Neurophysiol. 2002;113(4):495–506. doi: 10.1016/s1388-2457(02)00024-x. [DOI] [PubMed] [Google Scholar]
- Cornell SA, Lahiri A, Eulitz C. "What you encode is not necessarily what you store": evidence for sparse feature representations from mismatch negativity. Brain Res. 2011;1394:79–89. doi: 10.1016/j.brainres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Dalebout SD, Stack JW. Mismatch negativity to acoustic differences not differentiated behaviorally. J Am Acad Audiol. 1999;10(7):388–399. [PubMed] [Google Scholar]
- Davids N, vandenBrink D, vanTurennout M, Mitterer H, Verhoeven L. Towards neurophysiological assessment of phonemic discrimination: context effects of the mismatch negativity. Clin Neurophysiol. 2009;120(6):1078–1086. doi: 10.1016/j.clinph.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Deguchi C, Chobert J, Brunellière A, Nguyen N, Colombo L, Besson M. Preattentive and attentive processing of French vowels. Brain Res. 2010;1366:149–161. doi: 10.1016/j.brainres.2010.09.104. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dupoux E, Gout A. Electrophysiological correlates of phonological processing: a cross-linguistic study. J Cogn Neurosci. 2000;12(4):635–647. doi: 10.1162/089892900562390. [DOI] [PubMed] [Google Scholar]
- DeSanctis P, Ritter W, Molholm S, Kelly SP, Foxe JJ. Auditory scene analysis: the interaction of stimulation rate and frequency separation on pre-attentive grouping. Eur J Neurosci. 2008;27(5):127–1276. doi: 10.1111/j.1460-9568.2008.06080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch JA, Deutsch D. Attention: some theoretical considerations. Psychol Rev. 1963;70:80–90. doi: 10.1037/h0039515. [DOI] [PubMed] [Google Scholar]
- Díaz B, Baus C, Escera C, Costa A, Sebastián-Gallés N. Brain potentials to native phoneme discrimination reveal the origin of individual differences in learning the sounds of a second language. Proc Natl Acad Sci USA. 2008;105(42):16083–16088. doi: 10.1073/pnas.0805022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson BJ, Alain C, He Y. Effects of visual attentional load on low-level auditory scene analysis. Cogn Affect Behav Neurosci. 2005;5(3):319–338. doi: 10.3758/cabn.5.3.319. [DOI] [PubMed] [Google Scholar]
- Froyen D, Van Atteveldt N, Bonte M, Blomert L. Cross-modal enhancement of the MMN to speech-sounds indicates early and automatic integration of letters and speech-sounds. Neurosci Lett. 2008;430(1):23–28. doi: 10.1016/j.neulet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Gao S, Hu J, Gong D, Chen S, Kendrick KM, Yao D. Integration of consonant and pitch processing as revealed by the absence of additivity in mismatch negativity. PLoS One. 2012;7(5):e38289. doi: 10.1371/journal.pone.0038289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes H, Berstein R, Ritter W, Vaughan HG, Jr, Miller J. Storage of feature conjunctions in transient auditory memory. Psychophysiology. 1997;34(6):712–716. doi: 10.1111/j.1469-8986.1997.tb02146.x. [DOI] [PubMed] [Google Scholar]
- Gomes H, Molholm S, Ritter W, Kurtzberg D, Cowan N, Vaughan HG., Jr Mismatch negativity in children and adults, and effects of an attended task. Psychophysiology. 2000;37(6):807–816. [PubMed] [Google Scholar]
- Grimm S, Widmann A, Schröger E. Differential processing of duration changes within short and long sounds in humans. Neurosci Lett. 2004;356(2):83–86. doi: 10.1016/j.neulet.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Hasting AS, Winkler I, Kotz SA. Early differential processing of verbs and nouns in the human brain as indexed by event-related brain potentials. Eur J Neurosci. 2008;27(6):1561–1565. doi: 10.1111/j.1460-9568.2008.06103.x. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182(4108):177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hisagi M, Shafer VL, Strange W, Sussman ES. Perception of a Japanese vowel length contrast by Japanese and American English listeners: behavioral and electrophysiological measures. Brain Res. 2010;1360:89–105. doi: 10.1016/j.brainres.2010.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J, Jones SJ, VazPato M. Scalp potentials to pitch change in rapid tone sequences. A correlate of sequential stream segregation. Exp Brain Res. 2001;140(1):56–65. doi: 10.1007/s002210100783. [DOI] [PubMed] [Google Scholar]
- Jacobsen T, Schröger E. Input to verbal working memory: reattentive construction of the central speech representation. Exp Psychol. 2004;51(4):231–239. doi: 10.1027/1618-3169.51.4.231. [DOI] [PubMed] [Google Scholar]
- Jakoby H, Goldstein A, Faust M. Electrophysiological correlates of speech perception mechanisms and individual differences in second language attainment. Psychophysiology. 2011;48(11):1517–1531. doi: 10.1111/j.1469-8986.2011.01227.x. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalogr Clin Neurophysiol. 1998;108(2):143–153. doi: 10.1016/s0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Gunter TC, Wittfoth M, Sammler D. Interaction between syntax processing in language and in music: an ERP Study. J Cogn Neurosci. 2005;17(10):1565–1577. doi: 10.1162/089892905774597290. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Carrell TD, Sharma A. Neurophysiologic bases of speech discrimination. Ear Hear. 1995;16(1):19–37. doi: 10.1097/00003446-199502000-00003. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Kujala T, Näätänen R. The mismatch negativity in evaluating central auditory dysfunction in dyslexia. Neurosci Biobehav Rev. 2001;25:535–543. doi: 10.1016/s0149-7634(01)00032-x. [DOI] [PubMed] [Google Scholar]
- Kujala T, Halmetoja J, Näätänen R, Alku P, Lyytinen H, Sussman E. Speech- and sound-segmentation in dyslexia: evidence for a multiple-level cortical impairment. Eur J Neurosci. 2006;24(8):2420–2427. doi: 10.1111/j.1460-9568.2006.05100.x. [DOI] [PubMed] [Google Scholar]
- Lee CY, Yen HL, Yeh PW, Lin WH, Cheng YY, Tzeng YL, Wu HC. Mismatch responses to lexical tone, initial consonant, and vowel in Mandarin-speaking preschoolers. Neuropsychologia. 2012;50(14):3228–3239. doi: 10.1016/j.neuropsychologia.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Lepistö T, Kuitunen A, Sussman E, Saalasti S, Jansson-Verkasalo E, Nieminen-von Wendt T, Kujala T. Auditory stream segregation in children with Asperger syndrome. Biol Psychol. 2009;82(3):301–307. doi: 10.1016/j.biopsycho.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski SC, Escudero P, Benders T. Language experience modulates weighting of acoustic cues for vowel perception: an event-related potential study. Psychophysiology. 2012;49(5):638–650. doi: 10.1111/j.1469-8986.2011.01347.x. [DOI] [PubMed] [Google Scholar]
- List A, Justus T, Robertson LC, Bentin S. A mismatch negativity study of local-global auditory processing. Brain Res. 2007;1153:122–133. doi: 10.1016/j.brainres.2007.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiste AC, Wiens AS, Hunt MJ, Scherg M, Picton TW. Event-related potentials and the categorical perception of speech sounds. Ear Hear. 1995;16(1):68–90. doi: 10.1097/00003446-199502000-00006. [DOI] [PubMed] [Google Scholar]
- May PJ, Tiitinen H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology. 2010;47(1):66–122. doi: 10.1111/j.1469-8986.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- Miglietta S, Grimaldi M, Calabrese A. Conditioned allophony in speech perception: an ERP study. Brain Lang. 2013;126(3):285–290. doi: 10.1016/j.bandl.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Müller D, Schröger E. Temporal grouping affects the automatic processing of deviant sounds. Biol Psychol. 2007;74(3):358–364. doi: 10.1016/j.biopsycho.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Müller D, Widmann A, Schröger E. Auditory streaming affects the processing of successive deviant and standard sounds. Psychophysiology. 2005;42:668–676. doi: 10.1111/j.1469-8986.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Mismatch negativity outside strong attentional focus: a commentary on Woldorff et al. (1991) Psychophysiology. 1991;28(4):478–484. doi: 10.1111/j.1469-8986.1991.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Hillsdale, NJ: Erlbaum; 1992. Attention and brain function. [Google Scholar]
- Näätänen R. Mismatch negativity: clinical research and possible applications. Int J Psychophysiol. 2003;48:179–188. doi: 10.1016/s0167-8760(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Gaillard AWK, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Tiitinen H, Jiang D, Alho K. Attention and mismatch negativity. Psychophysiology. 1993;30(5):436–450. doi: 10.1111/j.1469-8986.1993.tb02067.x. [DOI] [PubMed] [Google Scholar]
- Nager W, Teder-Sälejärvi W, Kunze S, Münte TF. Preattentive evaluation of multiple perceptual streams in human audition. Neuroreport. 2003;14(6):871–874. doi: 10.1097/00001756-200305060-00019. [DOI] [PubMed] [Google Scholar]
- Neisser U. Cognitive psychology. New York: Appleton-Century-Crofts; 1967. [Google Scholar]
- Nenonen S, Shestakova A, Huotilainen M, Näätänen R. Speech-sound duration processing in a second language is specific to phonetic categories. Brain Lang. 2005;92(1):26–32. doi: 10.1016/j.bandl.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Novak G, Ritter W, Vaughan HG., Jr Mismatch detection and the latency of temporal judgments. Psychophysiology. 1992;29(4):398–411. doi: 10.1111/j.1469-8986.1992.tb01713.x. [DOI] [PubMed] [Google Scholar]
- Novak GP, Ritter W, Vaughan HG, Jr, Wiznitzer ML. Differentiation of negative event-related potentials in an auditory discrimination task. Electroencephalogr Clin Neurophysiol. 1990;75(4):255–275. doi: 10.1016/0013-4694(90)90105-s. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Jaramillo M, Näätänen R, Winkler I. Neuronal populations in the human brain extracting invariant relationships from acoustic variance. Neurosci Lett. 1999;265(3):179–182. doi: 10.1016/s0304-3940(99)00237-2. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Tiitinen H, Alho K, Näätänen R. Mismatch negativity to slight pitch changes outside strong attentional focus. Biol Psychol. 1993;37(1):23–41. doi: 10.1016/0301-0511(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Partanen E, Vainio M, Kujala T, Huotilainen M. Linguistic multifeature MMN paradigm for extensive recording of auditory discrimination profiles. Psychophysiology. 2011;48(10):1372–1380. doi: 10.1111/j.1469-8986.2011.01214.x. [DOI] [PubMed] [Google Scholar]
- Peltola MS, Tamminen H, Toivonen H, Kujala T, Näätänen R. Different kinds of bilinguals: different kinds of brains: the neural organization of two languages in one brain. Brain Lang. 2012;121(3):261–266. doi: 10.1016/j.bandl.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, Mathalon DH. Automatic Auditory Processing Deficits in Schizophrenia and Clinical High-Risk Patients: Forecasting Psychosis Risk with Mismatch Negativity. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Shtyrov Y. Language outside the focus of attention: the mismatch negativity as a tool for studying higher cognitive processes. Prog Neurobiol. 2006;79(1):49–71. doi: 10.1016/j.pneurobio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Shtyrov Y, Kujala T, Näätänen R. Word-specific cortical activity as revealed by the mismatch negativity. Psychophysiology. 2004;41(1):106–112. doi: 10.1111/j.1469-8986.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Rahne T, Böckmann-Barthel M. Visual cues release the temporal coherence of auditory objects in auditory scene analysis. Brain Res. 2009;1300:125–134. doi: 10.1016/j.brainres.2009.08.086. [DOI] [PubMed] [Google Scholar]
- Rahne T, Böckmann M, von Specht H, Sussman E. Visual cues can modulate integration and segregation of objects in auditory scene analysis. Brain Res. 2007;1144:127–135. doi: 10.1016/j.brainres.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahne T, Sussman E. Neural representations of auditory input accommodate to the context in a dynamically changing acoustic environment. Eur J Neurosci. 2009;29(1):205–211. doi: 10.1111/j.1460-9568.2008.06561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiche M, Hartwigsen G, Widmann A, Saur D, Schröger E, Bendixen A. Involuntary attentional capture by speech and non-speech deviations: a combined behavioral-event-related potential study. Brain Res. 2013;1490:153–160. doi: 10.1016/j.brainres.2012.10.055. [DOI] [PubMed] [Google Scholar]
- Ritter W, Sussman E, Molholm S. Evidence that the mismatch negativity system works on the basis of objects. Neuroreport. 2000;11(1):61–63. doi: 10.1097/00001756-200001170-00012. [DOI] [PubMed] [Google Scholar]
- Saarinen J, Paavilainen P, Schöger E, Tervaniemi M, Näätänen R. Representation of abstract attributes of auditory stimuli in the human brain. Neuroreport. 1992;3(12):1149–1451. doi: 10.1097/00001756-199212000-00030. [DOI] [PubMed] [Google Scholar]
- Sams M, Alho K, Näätänen R. Sequential effects on the ERP in discriminating two stimuli. Biol Psychol. 1983;17(1):41–58. doi: 10.1016/0301-0511(83)90065-0. [DOI] [PubMed] [Google Scholar]
- Sams M, Paavilainen P, Alho K, Näätänen R. Auditory frequency discrimination and event-related potentials. Electroencephalogr Clin Neurophysiol. 1985;62(6):437–448. doi: 10.1016/0168-5597(85)90054-1. [DOI] [PubMed] [Google Scholar]
- Savela J, Kujala T, Tuomainen J, Ek M, Aaltonen O, Näätänen R. The mismatch negativity and reaction time as indices of the perceptual distance between the corresponding vowels of two related languages. Brain Res Cogn Brain Res. 2003;16(2):250–256. doi: 10.1016/s0926-6410(02)00280-x. [DOI] [PubMed] [Google Scholar]
- Schöger E. Processing of auditory deviants with changes in one versus two stimulus dimensions. Psychophysiology. 1995;32(1):55–65. doi: 10.1111/j.1469-8986.1995.tb03406.x. [DOI] [PubMed] [Google Scholar]
- Schöger E. The influence of stimulus intensity and inter-stimulus interval on the detection of pitch and loudness changes. Electroencephalogr Clin Neurophysiol. 1996;100(6):517–526. [PubMed] [Google Scholar]
- Schöger E, Näätänen R, Paavilainen P. Event-related potentials reveal how non-attended complex sound patterns are represented by the human brain. Neurosci Lett. 1992;146(2):183–186. doi: 10.1016/0304-3940(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Schöger E, Paavilainen P, Näätänen R. Mismatch negativity to changes in a continuous tone with regularly varying frequencies. Electroencephalogr Clin Neurophysiol. 1994;92(2):140–147. doi: 10.1016/0168-5597(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Shafer VL, Schwartz RG, Kurtzberg D. Language-specific memory traces of consonants in the brain. Brain Res Cogn Brain Res. 2004;18(3):242–254. doi: 10.1016/j.cogbrainres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF. Exploration of the perceptual magnet effect using the mismatch negativity auditory evoked potential. J Acoust Soc Am. 1998;104(1):511–517. doi: 10.1121/1.423252. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, McGee T, Carrell T, Nicol T. Acoustic versus phonetic representation of speech as reflected by the mismatch negativity event-related potential. Electroencephalogr Clin Neurophysiol. 1993;88(1):64–71. doi: 10.1016/0168-5597(93)90029-o. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y, Pulvermüller F. Neurophysiological evidence of memory traces for words in the human brain. Neuroreport. 2002;13(4):521–525. doi: 10.1097/00001756-200203250-00033. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions: the orienting reflex. Annu Rev Phsyiol. 1963;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Sonnadara RR, Alain C, Trainor LJ. Effects of spatial separation and stimulus probability on the event-related potentials elicited by occasional changes in sound location. Brain Res. 2006;1071(1):175–185. doi: 10.1016/j.brainres.2005.11.088. [DOI] [PubMed] [Google Scholar]
- Sorokin A, Alku P, Kujala T. Change and novelty detection in speech and non-speech sound streams. Brain Res. 2010;23:77–90. doi: 10.1016/j.brainres.2010.02.052. 1327. [DOI] [PubMed] [Google Scholar]
- Squires KC, Squires NK, Hillyard SA. Vertex evoked potentials in a rating scale detection task: relation to signal probability. Behav Biol. 1975;13:21–34. doi: 10.1016/s0091-6773(75)90748-8. [DOI] [PubMed] [Google Scholar]
- Steinberg J, Truckenbrodt H, Jacobsen T. Preattentive phonotactic processing as indexed by the mismatch negativity. J Cogn Neurosci. 2010;22(10):2174–2185. doi: 10.1162/jocn.2009.21408. [DOI] [PubMed] [Google Scholar]
- Stekelenburg JJ, Vroomen J. Electrophysiological evidence for a multisensory speech-specific mode of perception. Neuropsychologia. 2012;50(7):1425–1431. doi: 10.1016/j.neuropsychologia.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Sussman E. Integration and segregation in auditory scene analysis. J Acoust Soc Am. 2005;117(3):1285–1298. doi: 10.1121/1.1854312. [DOI] [PubMed] [Google Scholar]
- Sussman E. A new view on the MMN and attention debate: auditory context effects. Journal of Psychophysiology. 2007;21(3–4):164–175. [Google Scholar]
- Sussman E. Attention matters: pitch vs. pattern processing in adolescence. Front Psychol. 2013;4(333):1–9. doi: 10.3389/fpsyg.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman E, Bregman AS, Wang WJ, Khan FJ. Attentional modulation of electrophysiological activity in auditory cortex for unattended sounds in multistream auditory environments. Cogn Affect Behav Neurosci. 2005;5(1):93–110. doi: 10.3758/cabn.5.1.93. [DOI] [PubMed] [Google Scholar]
- Sussman E, Gumenyuk V. Organization of sequential sounds in auditory memory. Neuroreport. 2005;16(13):1519–1523. doi: 10.1097/01.wnr.0000177002.35193.4c. [DOI] [PubMed] [Google Scholar]
- Sussman ES, Horváth J, Winkler I, Orr M. The role of attention in the formation of auditory streams. Percept Psychophys. 2007;69(1):136–152. doi: 10.3758/bf03194460. [DOI] [PubMed] [Google Scholar]
- Sussman E, Kujala T, Halmetoja J, Lyytinen H, Alku P, Näätänen R. Automatic and controlled processing of acoustic and phonetic contrasts. Hear Res. 2004;190(1–2):128–140. doi: 10.1016/S0378-5955(04)00016-4. [DOI] [PubMed] [Google Scholar]
- Sussman E, Ritter W, Vaughan HG., Jr Attention affects the organization of auditory input associated with the mismatch negativity system. Brain Res. 1998a;789:130–138. doi: 10.1016/s0006-8993(97)01443-1. [DOI] [PubMed] [Google Scholar]
- Sussman E, Ritter W, Vaughan HG., Jr Predictability of stimulus deviance and the mismatch negativity. Neuroreport. 1998b;9(18):4167–4170. doi: 10.1097/00001756-199812210-00031. [DOI] [PubMed] [Google Scholar]
- Sussman E, Ritter W, Vaughan HG., Jr An investigation of the auditory streaming effect using event-related brain potentials. Psychophysiology. 1999;36:22–34. doi: 10.1017/s0048577299971056. [DOI] [PubMed] [Google Scholar]
- Sussman E, Sheridan K, Kreuzer J, Winkler I. Representation of the standard: stimulus context effects on the process generating the mismatch negativity component of event-related brain potentials. Psychophysiology. 2003;40(3):465–471. doi: 10.1111/1469-8986.00048. [DOI] [PubMed] [Google Scholar]
- Sussman E, Steinschneider M. Neurophysiological evidence for context-dependent encoding of sensory input in human auditory cortex. Brain Res. 2006;1075(1):165–174. doi: 10.1016/j.brainres.2005.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman E, Steinschneider M. Attention effects on auditory scene analysis in children. Neuropsychologia v47. 2009;(3):771–785. doi: 10.1016/j.neuropsychologia.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman E, Winkler I. Dynamic sensory updating in the auditory system. Cogn Brain Res. 2001;12:431–439. doi: 10.1016/s0926-6410(01)00067-2. [DOI] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Huotilainen M, Ritter W, Näätänen R. Top-down effects on the initially stimulus-driven auditory organization. Cogn Brain Res. 2002;13:393–405. doi: 10.1016/s0926-6410(01)00131-8. [DOI] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Kreuzer J, Saher M, Näätänen R, Ritter W. Temporal integration: intentional sound discrimination does not modify stimulus-driven processes in auditory event synthesis. Clin Neurophysiol. 2002;113:909–920. doi: 10.1016/s1388-2457(02)00300-0. [DOI] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Wang W. MMN and attention: competition for deviance detection. Psychophysiology. 2003;40(3):430–435. doi: 10.1111/1469-8986.00045. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150(3700):1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Szymanski MD, Yund EW, Woods DL. Phonemes, intensity and attention: differential effects on the mismatch negativity (MMN) J Acoust Soc Am. 1999;106(6):3492–505. doi: 10.1121/1.428202. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Kruck S, DeBaene W, Schröger E, Alter K, Friederici AD. Top-down modulation of auditory processing: effects of sound context, musical expertise and attentional focus. Eur J Neurosci. 2009;30(8):1636–1642. doi: 10.1111/j.1460-9568.2009.06955.x. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, May P, Reinikainen K, Näätänen R. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature. 1994;372(6501):90–92. doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- Treisman AM. Strategies and models of selective attention. Psychol Rev. 1969;76:282–299. doi: 10.1037/h0027242. [DOI] [PubMed] [Google Scholar]
- van Linden S, Stekelenburg JJ, Tuomainen J, Vroomen J. Lexical effects on auditory speech perception: an electrophysiological study. Neurosci Lett 8. 2007;420(1):49–52. doi: 10.1016/j.neulet.2007.04.006. [DOI] [PubMed] [Google Scholar]
- van Zuijen TL, Sussman E, Winkler I, Näätänen R, Tervaniemi M. Grouping of sequential sounds—an event-related potential study comparing musicians and nonmusicians. J Cogn Neurosci. 2004;16(2):331–338. doi: 10.1162/089892904322984607. [DOI] [PubMed] [Google Scholar]
- Wang XD, Gu F, He K, Chen LH, Chen L. Preattentive extraction of abstract auditory rules in speech sound stream: a mismatch negativity study using lexical tones. PLoS One. 2012;7(1):e30027. doi: 10.1371/journal.pone.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JH, Chan TC, Luo YJ. A modified oddball paradigm "cross-modal delayed response" and the research on mismatch negativity. Brain Res Bull. 2002;57(2):221–230. doi: 10.1016/s0361-9230(01)00742-0. [DOI] [PubMed] [Google Scholar]
- Winkler I, Korzyukov O, Gumenyuk V, Cowan N, Linkenkaer-Hansen K, Ilmoniemi RJ, Alho K, Näätänen R. Temporary and longer term retention of acoustic information. 2002;39(4):530–534. doi: 10.1017/s0048577201393186. [DOI] [PubMed] [Google Scholar]
- Winkler I, Kujala T, Alku P, Näätänen R. Language context and phonetic change detection. Brain Res Cogn Brain Res. 2003;17(3):833–844. doi: 10.1016/s0926-6410(03)00205-2. [DOI] [PubMed] [Google Scholar]
- Winkler I, Lehtokoski A, Alku P, Vainio M, Czigler I, Csépe V, Aaltonen O, Raimo I, Alho K, Lang H, Iivonen A, Näätänen R. Pre-attentive detection of vowel contrasts utilizes both phonetic and auditory memory representations. Brain Res Cogn Brain Res. 1999;7(3):357–369. doi: 10.1016/s0926-6410(98)00039-1. [DOI] [PubMed] [Google Scholar]
- Winkler I, Sussman E, Tervaniemi M, Horváth J, Ritter W, Näätänen R. Preattentive auditory context effects. Cogn Affect Behav Neurosci. 2003;3(1):57–77. doi: 10.3758/cabn.3.1.57. [DOI] [PubMed] [Google Scholar]
- Winkler I, Takegata R, Sussman E. Event-related brain potentials reveal multiple stages in the perceptual organization of sound. Brain Res Cogn Brain Res. 2005;25(1):291–299. doi: 10.1016/j.cogbrainres.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Hackley SA, Hillyard SA. The effects of channel-selective attention on the mismatch negativity wave elicited by deviant tones. Psychophysiology. 1991;28(1):30–42. doi: 10.1111/j.1469-8986.1991.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Hillyard SA, Gallen CC, Hampson SR, Bloom FE. Magnetoencephalographic recordings demonstrate attentional modulation of mismatch-related neural activity in human auditory cortex. Psychophysiology. 1998;35(3):283–292. doi: 10.1017/s0048577298961601. [DOI] [PubMed] [Google Scholar]
- Xi J, Zhang L, Shu H, Zhang Y, Li P. Categorical perception of lexical tones in Chinese revealed by mismatch negativity. Neuroscience. 2010;170(1):223–231. doi: 10.1016/j.neuroscience.2010.06.077. [DOI] [PubMed] [Google Scholar]
- Yabe H, Winkler I, Czigler I, Koyama S, Kakigi R, Sutoh T, Hiruma T, Kaneko S. Organizing sound sequences in the human brain: the interplay of auditory streaming and temporal integration. Cogn Brain Res. 2001;897:222–227. doi: 10.1016/s0006-8993(01)02224-7. [DOI] [PubMed] [Google Scholar]
- Ylinen S, Uther M, Latvala A, Vepsäläinen S, Iverson P, Akahane-Yamada R, Näätänen R. Training the brain to weight speech cues differently: a study of Finnish second-language users of English. J Cogn Neurosci. 2010;22(6):1319–1332. doi: 10.1162/jocn.2009.21272. [DOI] [PubMed] [Google Scholar]