Abstract
Background & Aims
The liver has one of the highest rates of heme synthesis of any organ. More than 50% of the heme synthesized in the liver is used for synthesis of P450 enzymes, which metabolize exogenous and endogenous compounds that include natural products, hormones, drugs, and carcinogens. Feline leukemia virus subgroup C cellular receptor 1a (FLVCR1a) is plasma membrane heme exporter that is ubiquitously expressed and controls intracellular heme content in hematopoietic lineages. We investigated the role of Flvcr1a in liver function in mice.
Methods
We created mice with conditional disruption of Mfsd7b, which encodes Flvcr1a, in hepatocytes (Flvcr1afl/fl;alb-cre mice). Mice were analyzed under basal conditions, after phenylhydrazine-induced hemolysis, and after induction of cytochromes P450 synthesis. Livers were collected and analyzed by histologic, quantitative real-time polymerase chain reaction, and immunoblot analyses. Hepatic P450 enzymatic activities were measured.
Results
Flvcr1afl/fl;alb-cre mice accumulated heme and iron in liver despite up-regulation of heme oxygenase 1, ferroportin, and ferritins. Hepatic heme export activity of Flvcr1a was closely associated with heme biosynthesis, which is required to sustain cytochrome induction. Upon cytochromes P450 stimulation, Flvcr1afl/fl;alb-cre mice had reduced cytochrome activity, associated with accumulation of heme in hepatocytes. The expansion of the cytosolic heme pool in these mice was likely responsible for the early inhibition of heme synthesis and increased degradation of heme, which reduced expression and activity of cytochromes P450.
Conclusions
In livers of mice, Flvcr1a maintains a free heme pool that regulates heme synthesis and degradation as well as cytochromes P450 expression and activity. These findings have important implications for drug metabolism.
Keywords: ALAS1, CYP, Flvcr, HO-1, Flvcr1
Abbreviations used in this paper: ALA, 5-aminolevulinic acid; ALAS, 5-aminolevulinic acid synthase; Be(a)P, benzo(a)pyrene; CYP, cytochrome P450; FLVCR1a, feline leukemia virus subgroup C cellular receptor 1a; Fpn, ferroportin; HO, heme oxygenase; mRNA, messenger RNA
Aerobic cells require heme as the prosthetic moiety of several hemoproteins, including hemoglobin, cytochromes, myoglobin, catalases, and peroxidases.1 In addition, heme plays important regulatory roles in cell signaling and in control of gene expression.2 Heme biosynthesis occurs partially in the mitochondria and partially in the cytoplasm by a multistep pathway involving 8 enzymatic reactions. 5-Aminolevulinic acid synthase (ALAS), which catalyzes the condensation of glycine and succinyl-CoA to form ALA (5-aminolevulinic acid) in the mitochondrion, is the first and rate-controlling enzyme of heme biosynthesis.1
The rate of heme synthesis is balanced by the rate of its degradation through heme oxygenases (HO-1 and HO-2) to ensure that heme supply is adequate to physiological needs, without a significant accumulation in excess.3 The tight control of heme synthesis vs heme degradation is essential because free heme is a pro-oxidant and toxic molecule.4, 5 Both heme synthesis and heme degradation are tunely regulated by heme itself. Heme controls Alas1 transcription, the stability of Alas1 messenger RNA (mRNA) and the accumulation of the mature protein in the mitochondrion.6, 7, 8 On the opposite side, heme controls Ho-1 gene expression by removing the transcriptional repressor BACH1 from its promoter.9 The pool of heme that exerts this control, the so-called “free” or “regulatory” heme pool, is determined by a balance between heme synthesis and degradation and because of its small size, dynamic properties, and ability to readily exchange with heme-containing proteins, reflects the overall status of cellular heme content.10
Recently, heme export through the cell-surface transporter feline leukemia virus subgroup C cellular receptor 1a (Flvcr1a) was proposed as an additional control step to prevent the intracellular accumulation of heme.11, 12 Flvcr1 gene is essential for erythropoiesis and systemic iron homeostasis.12 It encodes for 2 proteins, FLVCR1a and FLVCR1b, expressed at the plasma membrane and on the mitochondrion, respectively. FLVCR1a belongs to the SLC49 family of the major facilitator superfamily of transporters with 12 hydrophobic transmembrane domains.12, 13 FLVCR1b is a shorter protein with only 6 transmembrane domains, supposed to homodimerize to form a functional transporter.13 We recently demonstrated a crucial role for FLVCR1b in the last step of heme biosynthetic pathway, ie, heme export from mitochondria.13 On the other hand, FLVCR1a exerts its heme export activity at the plasma membrane and avoids intracellular heme loading. Previous studies showed that FLVCR1a-mediated heme export in macrophages prevents heme-derived iron accumulation after erythrophagocytosis.14 Consistently, silencing of Flvcr1a in HeLa cells results in cytosolic heme loading, HO-1 induction, and oxidative stress. Finally, Flvcr1a deletion in mice causes embryo lethality due to extended hemorrhages.13
The liver is one of the body compartments with the highest heme rate synthesis. More than 50% of the heme synthesized in the liver is committed to the synthesis of cytochromes P450 (CYPs),15 the major enzymes involved in drug metabolism.16 As the prosthetic moiety of all CYPs, heme is responsible for the catalytic activity of these enzymes. In addition, the free heme pool also regulates CYP protein synthesis and disposal.10
Here we show that Flvcr1a function in hepatocytes is critical for the maintenance of a heme pool that controls CYP expression and activity.
Methods
Mice and Treatment
Mice used in these studies were 2/3-month-old and 6-month-old littermates, maintained on a standard chow diet and kept with free access to food and water. All experiments were approved by the animal ethical committee of the University of Torino (Italy).
Heme and Iron Content
Heme content in tissues and bile was quantified by the oxalic acid method. Tissue nonheme iron content was determined by a colorimetric method using 4,7-diphenyl-1, 10-phenantroline disulfonic acid (Sigma, St Louis, MO) as chromogen.
HO Activity
HO activity was measured by spectrophotometric determination of bilirubin produced from hemin added as substrate.
Lipid Peroxidation
Lipid peroxidation from tissue extracts was measured using the colorimetric assay kit Bioxytech LPO-586 from Oxis International (Portland, OR).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted using Pure Link RNA Mini Kit (Ambion, Life Technologies Italia, Torino, Italy). One microgram total RNA was reverse transcribed using M-MLV reverse transcriptase and random primers (Life Technologies Italia). Quantitative real-time polymerase chain reaction was performed on a 7300 Real Time PCR System (Applied Biosystems, Life Technologies Italia). Primers and probes were designed using the ProbeFinder software (www.roche-applied-science.com).
Protein Extraction and Western Blotting
Tissue and cell proteins were extracted as reported previously17 and concentration was determined using the Bio-Rad protein assay system (Bio-Rad, Munich, Germany). Fifty micrograms total protein extracts were separated on 8%−12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and analyzed by Western blotting using antibodies against HO-1 (Stressgen, Victoria, Canada), L- and H-ferritin (kindly provided by Sonia Levi), ferroportin (Fpn; Alpha Diagnostic Intl. Inc, San Antonio, TX), CYP1A1, CYP3A, CYP2E1, and actin (Santa Cruz Biotechnology, Inc., Dallas, TX).
Histology
Tissues were fixed in 10% formalin overnight at room temperature and embedded in paraffin. Microtome sections, 5-μm thick, were stained with Perl's reaction followed by methanol 3,3-diaminobenzidine (Boehringer Mannheim, Germany) development.
ALAS Activity
ALAS activity was assayed by measuring ALA formation in liver homogenates after glycine addition.
CYP Activity
CYP1A1 activity was assessed by measuring ethoxyresorufin-O-deethylase activity in liver microsomes using 7-ethoxyresorufin as a substrate. CYP3A activity was assessed by measuring conversion of the substrate proluciferin-PFBE to luciferin (V8901 P450-Glo CYP3A4 Assay; Promega, Madison, WI). CYP2E1 activity was determined by assaying the hydroxylation of p-nitrophenol to 4-nitrocatechol in the liver microsomal fraction.
Statistical Analysis
Results were expressed as mean ± SEM. Comparisons between 2 groups were performed with 2-sided Welch t tests and among more than 2 groups with 1- or 2-way analysis of variance followed by Bonferroni post test. P values <.05 were regarded as significant (∗P <. 05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
See also Supplementary Material.
Results
Generation of Liver-Specific Flvcr1a Knockout Mice
To study the function of the heme exporter FLVCR1a in the liver, we generated a liver-specific Flvcr1a knockout mouse (Supplementary Figure 1A). Liver-specific Flvcr1a knockout (Flvcr1afl/fl;alb-cre) mice were born at the expected Mendelian ratio and were viable and fertile.
Flvcr1afl/fl;alb-cre mice showed the recombinant allele only in the liver (Supplementary Figure 1B) and a strong reduction of hepatic Flvcr1a expression (Supplementary Figure 1C and D). As expected, Flvcr1a mRNA could not be detected in primary hepatocytes isolated from Flvcr1afl/fl;alb-cre mice (Supplementary Figure 1E), demonstrating that this mouse is a liver-specific knockout model for Flvcr1a.
Flvcr1afl/fl;alb-cre mice showed no gross liver abnormalities (Supplementary Figure 1F). Blood analysis did not reveal any difference between Flvcr1afl/fl;alb-cre and Flvcr1afl/fl mice (Supplementary Table 1).
Flvcr1a Deletion in the Liver Results in Altered Hepatic Heme/Iron Homeostasis
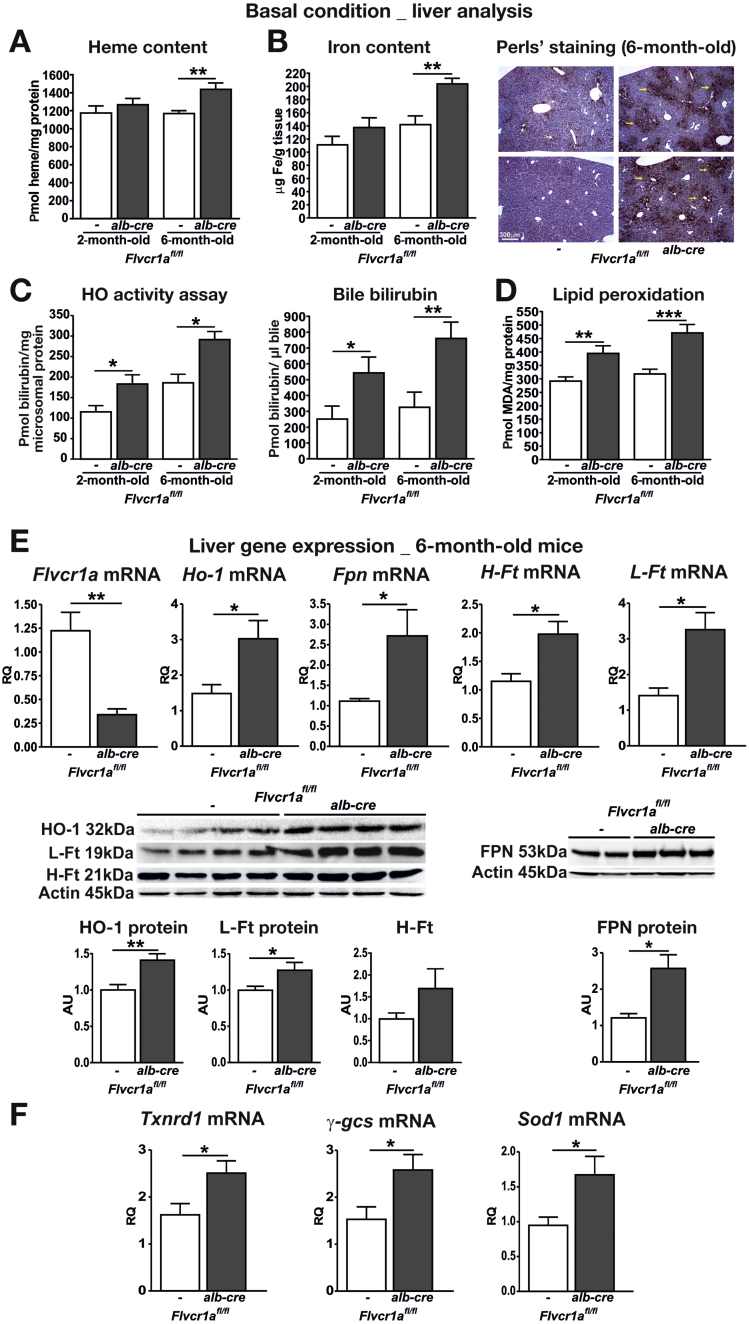
To evaluate if the deletion of Flvcr1a alters hepatic heme homeostasis, we analyzed the livers of 2- and 6-month-old Flvcr1afl/fl;alb-cre compared with those of an Flvcr1afl/fl counterpart. Hepatic heme and iron content were comparable at 2 months of age, but were significantly higher in 6-month-old Flvcr1afl/fl;alb-cre than in Flvcr1afl/fl mice (Figure 1A and B). Iron accumulation in 6-month-old Flvcr1afl/fl;alb-cre mice was further confirmed by Perl's staining on liver sections (Figure 1B). Consistently, Flvcr1afl/fl;alb-cre mice showed an enhanced HO activity as well as an increased bilirubin excretion in the bile compared with Flvcr1afl/fl mice (Figure 1C). In addition, Flvcr1afl/fl;alb-cre mice showed increased lipid peroxidation in the liver (Figure 1D). The analysis of hepatic gene expression revealed that Flvcr1afl/fl;alb-cre mice up-regulated genes that encode for proteins involved in heme metabolism (Ho-1),18, 19 iron export (Fpn)20, 21 and storage (H- and L-Ferritin),22 and antioxidant response (Txnrd1, γ-gcs, Sod1),23 compared with Flvcr1afl/fl mice (Figure 1E and F; Supplementary Figure 2 for gene expression analysis of 2-month-old mice). On the other hand, expression of the other known heme exporter Abcg2 was not increased in the liver of Flvcr1afl/fl;alb-cre mice (Supplementary Figure 3), indicating that no other heme exporter was able to compensate for the lack of Flvcr1a.
Figure 1.
Flvcr1a deletion in the liver alters hepatic heme/iron homeostasis. Data on the livers of 2- (A–D) and 6-month-old (A–F) Flvcr1afl/fl and Flvcr1afl/fl;alb-cre mice are shown. Heme (A) and iron (B) content. (A) n = 10. (B) n = 10. Liver sections of 6-month-old mice stained with Perl's reaction are shown on the right. Scale bar = 300 μm. (C) HO activity and bile bilirubin content, n = 5. (D) malondialdehyde (MDA) content, n = 14. (E) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of Flvcr1a, Ho-1, Fpn, H- and L-ferritin (Ft) mRNA level (n = 6) and representative Western blots of HO-1, L- and H-Ft and FPN protein, n = 10. (F) qRT-PCR analysis of Txnrd1, γ-gcs, and Sod1 mRNA level (n = 5). Unpaired T test analysis with Welch's correction was performed. AU, arbitrary units; RQ, relative quantity. *P < .05; **P < .01; ***P < .001.
FLVCR1a Is Required to Export Heme on Heme Accumulation Inside Hepatocytes
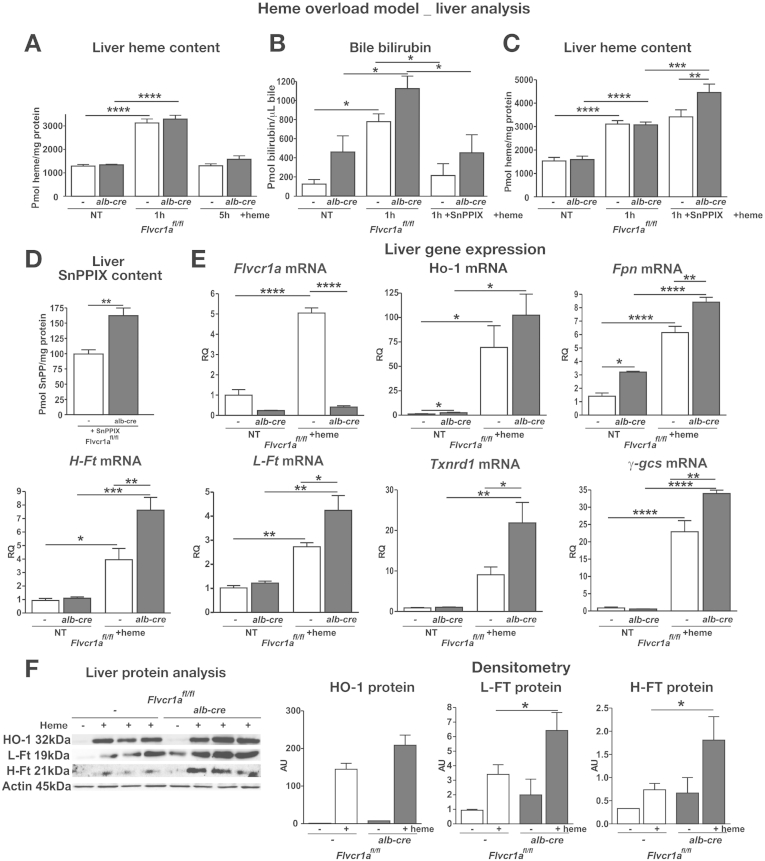
The phenotype of liver-specific Flvcr1a knockout mice suggests that FLVCR1a-mediated heme export prevents hepatic heme accumulation. To further address this point, mice were injected with hemin, the substrate of FLVCR1a. One hour after heme injection, heme accumulated in the liver of both Flvcr1afl/fl;alb-cre and Flvcr1afl/fl mice at the same extent, but bilirubin production was significantly higher in Flvcr1afl/fl;alb-cre mice than in Flvcr1afl/fl mice, likely because of the enhanced HO activity (Figure 2A and B). Consistently, if animals were pretreated with the heme analog Tin-Protoporphyrin IX that inhibits HO, before heme injection, Flvcr1afl/fl;alb-cre mice showed a significantly higher hepatic heme content 1 hour after heme infusion compared with control mice (Figure 2C). When we injected mice with Tin-Protoporphyrin IX alone, we found it accumulated more in the liver of Flvcr1afl/fl;alb-cre mice than in that of Flvcr1afl/fl controls (Figure 2D), strengthening the FLVCR1a export function. Measurement of heme content in the bile at 1 hour after heme injection demonstrated that it was excreted at the same extent in both Flvcr1afl/fl;alb-cre and Flvcr1afl/fl mice (Supplementary Figure 4A), suggesting that Flvcr1a did not export heme in the bile but likely vs the bloodstream. Accordingly, the analysis of a human hepatocarcinoma cell line, HepG2, overexpressing Flvcr1a-myc, showed that FLVCR1a localized at the plasma cell membrane, along the sinusoidal surface (Supplementary Figure 4B).
Figure 2.
FLVCR1a is required to export heme on heme accumulation inside hepatocytes. Data on the liver of hemin-treated Flvcr1afl/fl and Flvcr1afl/fl;alb-cre mice are shown. Heme content in the liver (A, C) and bilirubin content in the bile (B) at different time points after 30 μmol/kg heme injection. In (B, C) mice were injected with 15 μmol/kg Tin-Protoporphyrin IX (SnPP) 30 minutes before heme treatment (n = 5). (D) SnPP content in the liver 4 hours after SnPP injection (n = 4). (E) Quantitative real-time polymerase chain reaction analysis (qRT-PCR) of Flvcr1a, Ho-1, Fpn, H- and L-ferritin (Ft), Txnrd1, and γ-gcs mRNA level (n = 6). (F) Representative Western blots of HO-1, L- and H-Ft protein (n = 4). Two-way analysis of variance with Bonferroni post-test analysis and unpaired T test analysis with Welch's were performed on data in (A), (B), (C), (F), and in (D), respectively. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Data shown in Figure 2C indicate that the enhanced HO activity was able to compensate for the lack of FLVCR1a to maintain heme content in the normal range on transient heme accumulation. This was further demonstrated by the analysis of gene expression. On heme treatment, Flvcr1afl/fl mice showed a strong induction of Flvcr1a in the liver, as well as an up-regulation of Ho-1, Fpn, H- and L-ferritin. Flvcr1afl/fl;alb-cre mice that were unable to induce Flvcr1a, showed a stronger induction of the heme degradation and iron storage/export pathways, as an attempt to compensate for the lack of heme export (Figure 2E and F). This was not sufficient to control oxidative stress, as demonstrated by the significantly higher induction of the antioxidant genes in the liver of Flvcr1a-deleted mice after heme injection (Figure 2E).
These data demonstrate that FLVCR1a is a heme exporter in hepatocytes that works in close association with the heme degradation pathway to maintain heme/iron homeostasis.
FLVCR1a-Mediated Heme Export Function Is Strictly Associated With Heme Biosynthesis
The liver is, at the same time, one of the organs with the highest rate of heme synthesis and the main body site deputed to the detoxification of heme coming from the bloodstream. We asked in which of these processes is FLVCR1a mainly involved. To address this point, we treated mice with the heme precursor ALA or with the hemolytic agent phenylhydrazine, to promote heme synthesis or heme recovery from the bloodstream, respectively.
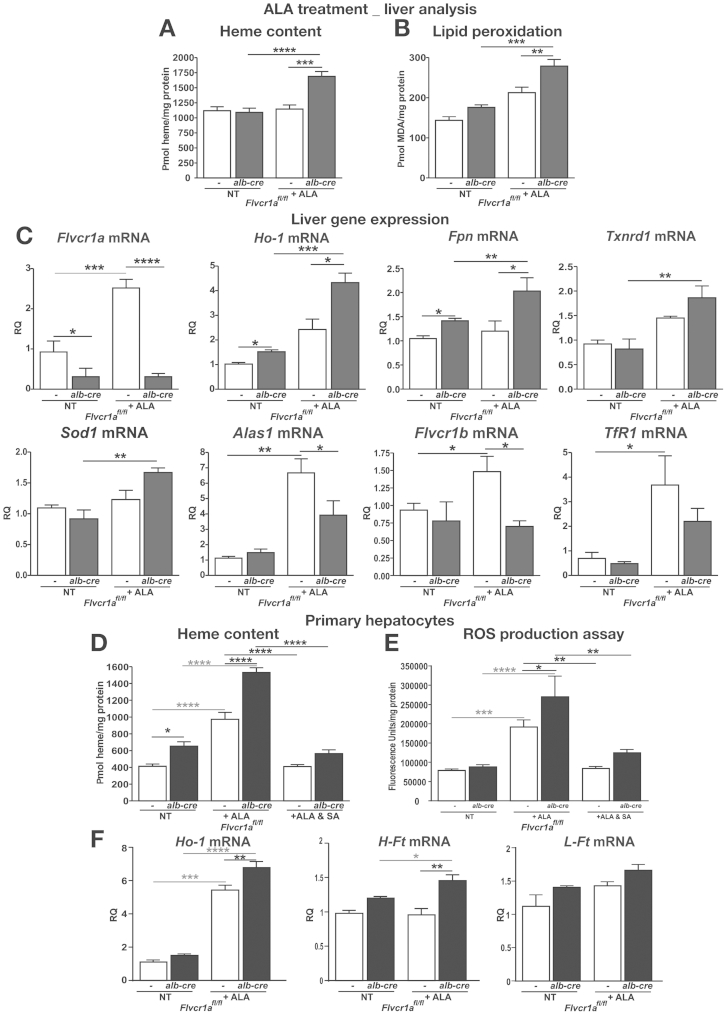
Although we did not observe any difference after phenylhydrazine treatment (Supplementary Results, Supplementary Figure 5), increased heme content was found in the liver of Flvcr1afl/fl;alb-cre mice compared with Flvcr1afl/fl mice after ALA treatment, suggesting that on de novo synthesis, heme accumulated in the liver when FLVCR1a was absent (Figure 3A). This resulted in a marked increase in the hepatic lipid peroxidation index (Figure 3B). Interestingly, Flvcr1a was strongly induced by ALA treatment in the liver of Flvcr1afl/fl mice (Figure 3C). On the other hand, the genes involved in heme and iron metabolism, such as Ho-1 and Fpn, were up-regulated to an higher extent in the liver of Flvcr1afl/fl;alb-cre mice than in that of Flvcr1afl/fl mice, and this was associated with a higher induction of the genes of the antioxidant response (Figure 3C). Conversely, we observed a reduced expression of Alas1, Flvcr1b, and Tfr1 in the liver of Flvcr1afl/fl;alb-cre compared with Flvcr1afl/fl mice (Figure 3C), suggesting an attenuation of the heme biosynthetic pathway in these animals. These results were confirmed in vitro on primary hepatocytes treated with ALA (Figure 3D−F; Supplementary Material).
Figure 3.
FLVCR1a-mediated heme export function is strictly associated with heme biosynthesis. Data on ALA-treated Flvcr1afl/fl and Flvcr1afl/fl;alb-cre mice are shown. Heme (A) and malondialdehyde (MDA) (B) content in the liver (n = 4). (C) Quantitative real-time polymerase chain reaction analysis of Flvcr1a, Ho-1, Fpn, Txnrd1, Sod1, Alas1, Flvcr1b, and TfR1 mRNA level in the liver (n = 4). (D, E) Heme uptake and reactive oxygen species (ROS) production on primary hepatocytes isolated from the liver of Flvcr1afl/fl and Flvcr1afl/fl;alb-cre mice, untreated or treated with 5 mM ALA or 5mM ALA and 0.5 mM succinylacetone (SA) (n = 4). (F) qRT-PCR analysis of Ho-1, H-, and L-Ft mRNA level in ALA-treated primary hepatocytes (n = 6). Two-way analysis of variance with Bonferroni post-test analysis was performed. *P < .05; **P < .01; ***P < .001; ****P < .0001.
These data indicate that FLVCR1a-mediated heme export function is strictly associated with heme synthesis.
FLVCR1a-Mediated Heme Export Function Is Associated With CYP Induction
In the liver, most of the newly synthesized heme is committed to CYP synthesis. To test whether FLVCR1a function is linked to heme synthesis stimulation on cytochromes induction, we treated our mice with inducers of 3 distinct classes of CYPs.
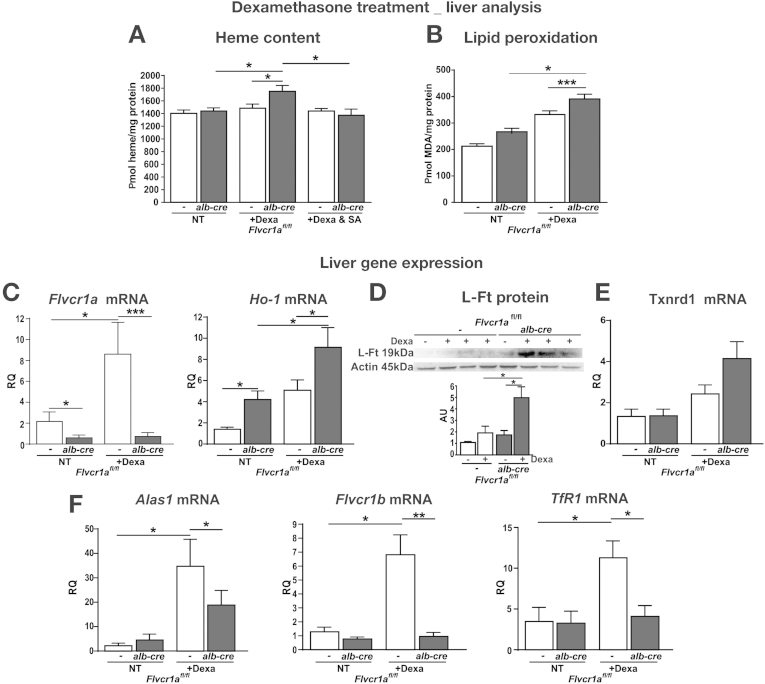
Firstly, we injected mice with dexamethasone, an inducer of CYP3A. Dexamethasone treatment caused an increase in heme content in the liver of Flvcr1afl/fl;alb-cre mice, that was almost negligible in Flvcr1afl/fl counterpart (Figure 4A). This effect was abrogated by co-treatment with the inhibitor of heme biosynthesis, succinylacetone (Figure 4A). As a consequence of heme accumulation, a higher amount of lipid peroxides was generated on dexamethasone treatment in the liver of Flvcr1afl/fl;alb-cre mice compared with Flvcr1afl/fl mice (Figure 4B). The analysis of gene expression demonstrated that Flvcr1a was induced in the liver of Flvcr1afl/fl mice after dexamethasone treatment, as occurred on ALA treatment (Figure 4C). On the other hand, the heme-, iron-, and stress-related genes were induced to a higher extent in the liver of dexamethasone-treated Flvcr1afl/fl;alb-cre mice compared with the Flvcr1afl/fl counterpart (Figure 4C–E), suggesting that the higher induction of these genes compensated for the lack of Flvcr1a. In addition, genes involved in heme biosynthesis, such as Alas-1, Flvcr1b, and Tfr1, were found to be significantly less expressed in Flvcr1afl/fl;alb-cre mice compared with Flvcr1afl/fl mice after dexamethasone treatment (Figure 4F).
Figure 4.
Flvcr1a deficiency affects liver homeostasis after dexamethasone-induced cytochrome synthesis. Data on the liver of dexamethasone-treated Flvcr1afl/fl and Flvcr1afl/fl;alb-cre mice are shown. Heme (A) and malondialdehyde (MDA) (B) content. (A) n = 5. (B) n = 5. (C, E, F) Quantitative real-time polymerase chain reaction analysis of Flvcr1a and Ho-1, Txnrd1, Alas1, Flvcr1b, and TfR1 mRNA level (n = 5). (D) Representative Western blot of L-Ft protein (n = 4). Two-way analysis of variance with Bonferroni post-test analysis was performed. *P < .05; **P < .01; ***P < .001.
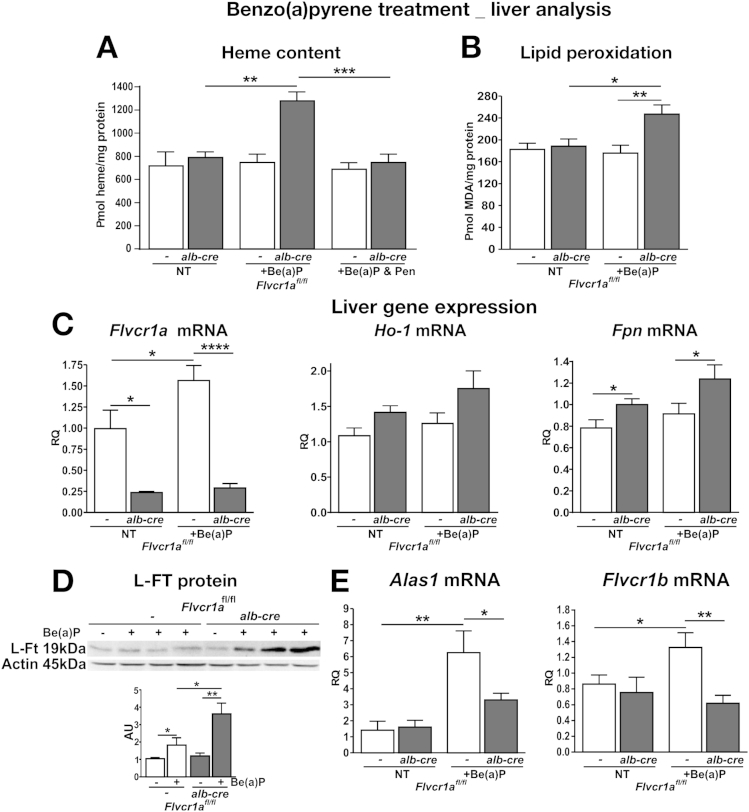
Similar results were obtained when mice were treated with benzo(a)pyrene (Be[a]P), an inducer of CYP1A1 and CYP1A2 (Figure 5, Supplementary Figure 6), and imidazole, an inducer of CYP2E1 (Supplementary Results; Supplementary Figure 7). Because the induction of Alas1 8h after Be(a)P injection was comparable in Flvcr1afl/fl;alb-cre and Flvcr1afl/fl (Supplementary Figure 8), the difference found at 16 hours post injection likely indicates that the heme biosynthetic pathway was switched off earlier in Flvcr1a-deleted mice than in its wild-type counterparts, as an attempt to compensate for the excess of heme accumulated in the liver.
Figure 5.
Flvcr1a deficiency affects liver homeostasis after Be(a)P-induced cytochrome synthesis. Data on the liver of Be(a)P-treated Flvcr1afl/fl and Flvcr1afl/fl;alb-cre mice are shown. Heme (A) and malondialdehyde (MDA) (B) content (n = 6). (C, E) Quantitative real-time polymerase chain reaction analysis of Flvcr1a, Ho-1, and Fpn and of Alas1 and Flvcr1b mRNA level (n = 8). (D) Representative Western blot of L-Ft protein (n = 4). Two-way analysis of variance with Bonferroni post-test analysis was performed. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Collectively, these data indicate that FLVCR1a-mediated heme export is associated with CYP induction.
FLVCR1a Controls CYP Activity by Regulating Heme Synthesis and Degradation
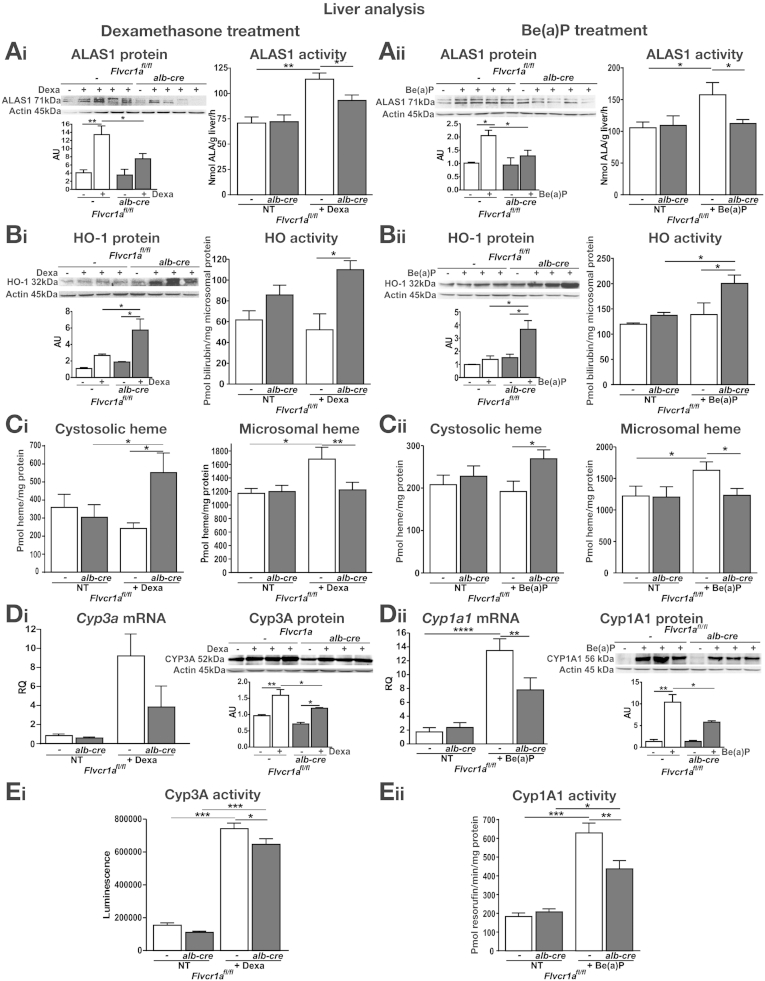
In the previous section, we showed that Ho-1 and Alas1 mRNA levels were higher and lower, respectively, in the liver of dexamethasone-, Be(a)P-, or imidazole- treated Flvcr1afl/fl;alb-cre compared with Flvcr1afl/fl mice, suggesting that heme degradation is increased and heme synthesis is inhibited when FLVCR1a-mediated heme export is blocked. Consistently, Ho-1 was found up-regulated and Alas1, as well as Flvcr1b, down-regulated in the liver of sickle cell anemia and β-thalassemia mice, in which Flvcr1a levels were strongly decreased (Supplementary Figure 9). This observation strengthens the idea that Flvcr1a deletion/down-regulation leads to the coordinated induction of heme degradation and down-regulation of the heme biosynthetic pathway. To evaluate this point, we analyzed HO-1 as well as ALAS1 protein and activity in the liver of Flvcr1afl/fl;alb-cre and Flvcr1afl/fl mice, treated with dexamethasone or Be(a)P. After the stimulation of CYP synthesis, HO-1 and ALAS1 expression were induced in the liver of both Flvcr1afl/fl;alb-cre and Flvcr1afl/fl mice (Figure 6A and B). HO-1 induction was significantly higher and ALAS1 expression was markedly reduced in the liver of Flvcr1afl/fl;alb-cre mice compared with Flvcr1afl/fl counterparts. This correlated with the enzymatic activities of HO-1 and ALAS1, which were respectively higher and lower in the liver of Flvcr1afl/fl;alb-cre mice than in that of Flvcr1afl/fl animals (Figure 6A and B). HO-1 induction as well as ALAS1 inhibition were likely mediated by heme overload occurring in Flvcr1afl/fl;alb-cre mice. Consistently, after the stimulation of CYP synthesis, heme accumulated to a higher extent in the cytosolic fraction of Flvcr1afl/fl;alb-cre mice compared with Flvcr1afl/fl controls. On the other hand, heme content was significantly lower in the microsomal fraction of Flvcr1afl/fl;alb-cre mice than in that of Flvcr1afl/fl animals (Figure 6C). As microsomal heme reflects the heme fraction contained in CYPs, we measured mRNA and protein expression and enzymatic activity of CYP3A and CYP1A1 in the livers of our mice. In agreement with heme levels, CYP3A and CYP1A1 mRNA, protein levels and activities were significantly lower in the livers of dexamethasone- and Be(a)P-treated Flvcr1afl/fl;alb-cre mice than in those of treated-Flvcr1afl/fl animals (Figure 6D and E). Similar results were obtained when mice were treated with imidazole (Supplementary Results; Supplementary Figure 10).
Figure 6.
The increase of cytosolic heme pool size due to Flvcr1a deficiency inhibits ALAS1 and induces HO-1, impairing CYP activity. Data on the liver of Flvcr1afl/fl and Flvcr1afl/fl;alb-cre mice after dexamethasone or B(a)P treatment are shown on the left and right, respectively. (A) ALAS1 expression and ALAS activity (n = 8). (B) HO-1 expression and HO activity (n = 8). (C) Cytosolic and microsomal heme content (n = 5). (D) Quantitative real-time polymerase chain reaction analysis of Cyp3a11/Cyp1a1 mRNA level and representative Western blot of CYP3A/CYP1A1 protein. (E) CYP3A and CYP1A1 activity (n = 7). Two-way analysis of variance with Bonferroni post-test analysis was performed. *P < .05; **P < .01; ***P < .001; ****P < .0001.
On the enhancement of heme demand, Flvcr1a deletion resulted in an expansion of the cytosolic heme pool that stimulates heme degradation and inhibits heme and CYP synthesis.
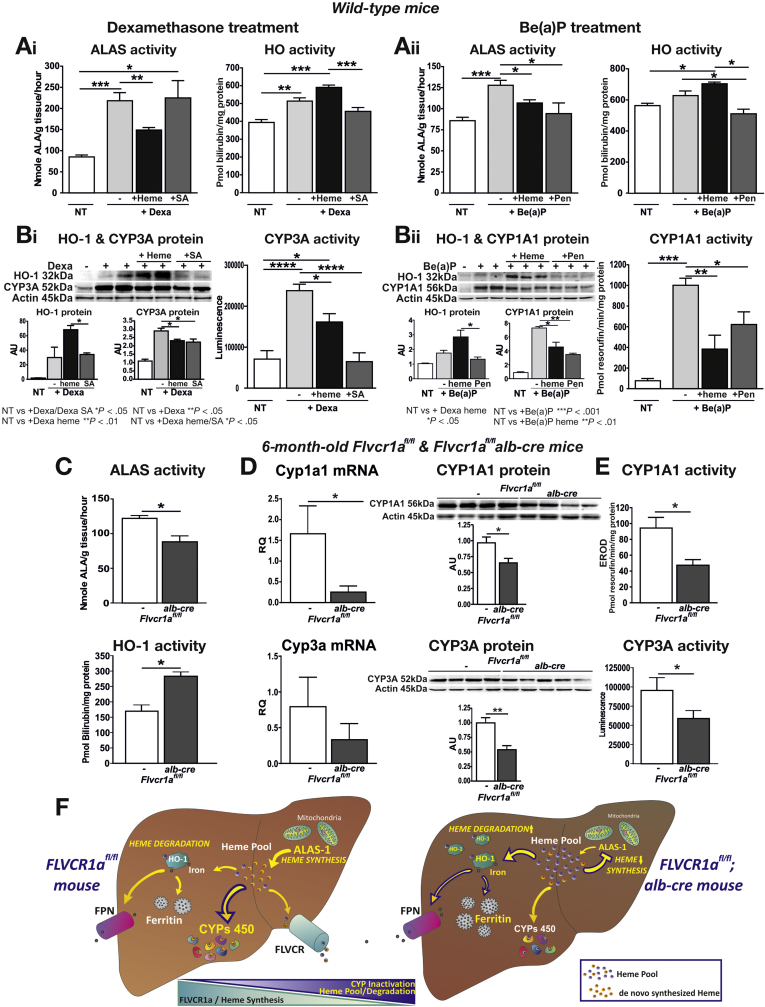
To test whether the main determinant for CYP expression/function was the size of heme pool or the rate of heme synthesis, both impaired in Flvcr1a-deleted liver, we treated wild-type mice with dexamethasone or Be(a)P alone or together with hemin, to mimic heme overload occurring in Flvcr1afl/fl;alb-cre mice, or with succinylacetone or DL-penicillamine, 2 inhibitors of heme biosynthesis. As expected, dexamethasone and Be(a)P treatment caused a marked increase in ALAS1 activity as well as in CYP expression/activity, and HO-1 expression/activity was only slightly induced (Figure 7A and B). Hemin co-treatment significantly reduced hepatic ALAS1 activity, while increasing HO-1 expression/activity, compared with mice treated with dexamethasone or Be(a)P only (Figure 7A and B). This correlated nicely with a reduced expression and activity of CYPs (Figure 7B). Similarly, we observed that co-treatment with Be(a)P and the ALAS-inhibitor DL-penicillamine decreased ALAS activity as well as the expression and activity of CYP1A1 (Figure 7A and B, right). Administration of succinylacetone, a heme synthesis inhibitor acting on 5-aminolevulinic acid dehydratase downstream of ALAS1, caused a feedback up-regulation of ALAS1 activity, as expected, but a decrease in CYP3A activity, as a consequence of reduced heme availability (Figure 7A and B, left). We can conclude that the effect of heme overload on cytochrome function parallels that of heme synthesis inhibition, fostering the concept that cytochrome function is strictly associated to de novo heme production rather than to heme pool size itself.
Figure 7.
Heme overload as well as heme synthesis inhibition decrease CYP expression and activity. Data on the liver of wild-type mice after dexamethasone or B(a)P treatment are shown on the upper left and upper right panels, respectively. (A) ALAS activity and HO activity (n = 5). (B) Representative Western blots of HO-1 and CYP3A/CYP1A protein, and CYP3A/CYP1A1 activity (n = 6). Data on the liver of 6-month-old Flvcr1afl/fl and Flvcr1afl/fl;alb-cre mice are shown on bottom panel. (C) ALAS activity and HO activity (n = 5). (D) Quantitative real-time polymerase chain reaction analysis of Cyp1a1/Cyp3a11 mRNA level and representative Western blot of CYP1A1/CYP3A protein (n = 5). (E) CYP1A1 and CYP3A activity (n = 5). One-way analysis of variance with Bonferroni post-test analysis and unpaired T test analysis with Welch's correction was performed on data in the upper and bottom panel, respectively. (F) Illustration showing FLVCR1a mood of action in the liver (see also Supplementary Material). *P < .05; **P < .01; ***P < .001; ****P < .0001.
As further confirmation, we observed that 6-month-old Flvcr1afl/fl;alb-cre mice showed a reduction in ALAS1 activity as well as an increase in HO activity (Figure 7C). This misbalance in heme synthesis/degradation resulted in a reduced CYP expression at both mRNA and protein level (CYP1A1 and CYP3A, Figure 7D; CYP2E1, Supplementary Figure 11) and reduced CYP activity (Figure 7E).
These data indicate that FLVCR1a-mediated heme export in hepatocytes controls the expansion of the heme pool, which in turns determines the balance between heme synthesis and degradation and CYP activity.
Discussion
Here we showed that FLVCR1a is essential for the maintenance of heme and iron homeostasis in the liver and that its function is strictly associated with the heme biosynthetic process that is crucial for the control of CYP activity.
Previous studies demonstrated that FLVCR1a exerts a detoxifying function in macrophages and erythroid cells, by exporting heme excess.11, 13, 14 Our results indicate that FLVCR1a is similarly important in the liver, as its deletion leads to progressive heme and iron loading and to the compensatory up-regulation of the genes responsible for heme degradation and iron storage. Consistently with our finding in mice, Flvcr1 was found mutated in human subjects with mild hepatic iron overload.24
Our data show that FLVCR1a export function is associated with heme biosynthesis in agreement with data showing that ALA treatment causes heme accumulation in Flvcr1a-silenced HeLa cells.13 In addition, we observed a concerted up-regulation of Flvcr1a and Flvcr1b, Alas1, and TfR1 in the liver of ALA-treated wild-type mice that strengthens the link between FLVCR1a function and heme biosynthesis.
More than half of the hepatic production of heme is used for the formation of CYPs,25, 26 which are engaged in steroid metabolism and in the oxidative metabolism of foreign compounds, including pharmaceutical drugs.10, 15, 27 Our data showed that Flvcr1a is up-regulated after CYP induction, suggesting that its function is strictly associated with enhanced heme demand to support cytochrome induction. Similarly, Alas1 as well as Flvcr1b and TfR1 are up-regulated to sustain newly heme synthesis in such condition.
Because either a deficiency or an excess of heme is toxic to the cell, hepatic heme production has to be tightly controlled. Previous works showed that in primary cultures of adult rat hepatocytes, 20% of newly formed heme is converted to bile pigments, and 80% is used for the formation of hemoproteins, mainly CYPs.28 Our data indicate that not only heme degradation, but also FLVCR1a-mediated heme export, is critical to ensure that the amount of available heme matches cell requirements. The alteration of one of these pathways, heme synthesis, degradation or export, in hepatocytes leads to an imbalance in heme homeostasis. In particular, FLVCR1a deletion causes an increase in the cytosolic heme fraction, when heme demand is increased to support CYP induction.
The cytosolic heme fraction contains a pool of newly synthesized heme that serves both precursor and regulatory functions.10 The free heme pool controls heme biosynthesis, through the regulation of ALAS1. If increased, the regulatory heme pool may repress ALAS1,7 and its depletion causes ALAS1 induction.10 Our results indicate that ALAS1 induction occurs in wild-type as well as in Flvcr1a-null mice shortly after cytochrome stimulation, to sustain heme synthesis for cytochrome formation. Then, Alas1 down-regulation occurs earlier in Flvcr1a-null mice than in wild-type animals because of the negative feedback exerted by the expanded cytosolic free heme pool. This is in agreement with many observations, according to which the addition of heme in hepatocyte cultures inhibits the drug-induced synthesis of ALAS.29, 30, 31, 32, 33 Although xenobiotics might have some primary inducing effect on hepatic ALAS1,34, 35 many chemical inducers are believed to increase ALAS1 by depleting the free heme pool in hepatocytes.10 This is in agreement with our observation in wild-type mice in which ALAS1 expression, CYP activity, and microsomal heme are increased, and cytosolic heme levels are reduced after drug treatment. Conversely, liver-specific Flvcr1a-null mice showed an expansion of the cytosolic heme pool, suggesting that Flvcr1a deletion promotes intracellular heme accumulation, preventing the depletion of the free heme pool as a stimulus for ALAS1 induction and on the contrary, promoting its inhibition.
In liver-specific Flvcr1a -null mice, the decreased heme synthesis well correlates with a reduction of CYP expression and activity, in line with the previous observation that the enhancement in heme synthesis is required to sustain the induction/activity of CYPs.26, 36, 37, 38 Conversely, when a bolus of hemin is administered to experimental animals, the induction/activity of CYPs is greatly suppressed and this effect is considered to be the result of inhibition of heme biosynthesis by ALAS1.39, 40 Short-term hemin administration is known to both increase HO1 expression41 and interfere with the formation of CYP.40 Consistently, drug-treated Flvcr1a-null mice showed a significantly higher induction of HO1 and reduction in the expression and activity of ALAS1 and CYPs compared with wild-type animals, indicating that heme accumulation resulting from Flvcr1a deletion resembles what occurs after hemin administration.
In conclusion, the block of heme export due to Flvcr1a deletion promotes the expansion of the cytosolic heme pool, thus leading to ALAS inhibition and HO induction. We propose that the lack of FLVCR1a causes a reduction in the newly synthesized heme, impairing both CYP expression and activity (Figure 7F). It appears that in the hepatocytes, heme is formed in slight excess over its metabolic needs28 and its levels are maintained adequate by a combination of synthetic, degradative, and export mechanisms, suggesting that they are equipped with a “sensing” system to monitor changes in the size of “uncommitted” heme pool.
We can speculate that FLVCR1a is part of this sensing system and that, by sensing heme levels and exporting heme excess out of the cell, it controls the size of the cytosolic heme pool, playing a crucial regulatory role in cell metabolism and in the maintenance of a proper oxidative status. We expect that mutations in Flvcr1a and/or pathologic situations that affect its expression can result in a reduced CYP activity, altering drug metabolism, in particular in individuals that routinely assume drugs for therapeutic purposes.
Acknowledgments
The authors thank Ligia Goncalves and Laura Braccini for hepatocyte culture, Paolo Provero for statistical analysis, Sonia Levi for the gift of anti-ferritin antibodies, and Rolf Sprengel for mice carrying the FLP recombinase under the control of the actin promoter.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by Telethon Grant GGP12082 to Emanuela Tolosano.
Author names in bold designate shared co-first authors.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2014.01.053.
Supplementary Material
References
- 1.Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Furuyama K., Kaneko K., Vargas P.D. Heme as a magnificent molecule with multiple missions: heme determines its own fate and governs cellular homeostasis. Tohoku J Exp Med. 2007;213:1–16. doi: 10.1620/tjem.213.1. [DOI] [PubMed] [Google Scholar]
- 3.Abraham N.G., Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 4.Tolosano E., Fagoonee S., Morello N. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12:305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- 5.Vinchi F., Tolosano E. Therapeutic approaches to limit hemolysis-driven endothelial dysfunction: scavenging free heme to preserve vasculature homeostasis. Oxid Med Cell Longev. 2013:396527. doi: 10.1155/2013/396527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoh S., Nakamura T., Kataoka T. Egr-1 regulates the transcriptional repression of mouse δ-aminolevulinic acid synthas8e 1 by heme. Gene. 2011;472:28–36. doi: 10.1016/j.gene.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Kolluri S., Sadlon T.J., May B.K., Bonkovsky H.L. Haem repression of the housekeeping 5-aminolaevulinic acid synthase gene in the hepatoma cell line LMH. Biochem J. 2005;392:173–180. doi: 10.1042/BJ20050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J., Shan Y., Lambrecht R.W. Differential regulation of human ALAS1 mRNA and protein levels by heme and cobalt protoporphyrin. Mol Cell Biochem. 2008;319:153–161. doi: 10.1007/s11010-008-9888-0. [DOI] [PubMed] [Google Scholar]
- 9.Sun J., Hoshino H., Takaku K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correia M.A., Sinclair P.R., De Matteis F. Cytochrome P450 regulation: the interplay between its heme and apoprotein moieties in synthesis, assembly, repair, and disposal. Drug Metab Rev. 2011;43:1–26. doi: 10.3109/03602532.2010.515222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quigley J.G., Yang Z., Worthington M.T. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Khan A.A., Quigley J.G. Control of intracellular heme levels: heme transporters and heme oxygenases. Biochim Biophys Acta. 2011;1813:668–682. doi: 10.1016/j.bbamcr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiabrando D., Marro S., Mercurio S. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J Clin Invest. 2012;122:4569–4579. doi: 10.1172/JCI62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keel S.B., Doty R.T., Yang Z. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 15.Guengerich F.P. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 2006;8:E101–E111. doi: 10.1208/aapsj080112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guengerich F.P. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 17.Vinchi F., Gastaldi S., Silengo L. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol. 2008;173:289–299. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gozzelino R., Jeney V., Soares M.P. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 19.Fraser S.T., Midwinter R.G., Berger B.S., Stocker R. Heme oxygenase-1: a critical link between iron metabolism, erythropoiesis, and development. Adv Hematol. 2011:473709. doi: 10.1155/2011/473709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKie A.T., Marciani P., Rolfs A. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 21.Marro S., Chiabrando D., Messana E. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95:1261–1268. doi: 10.3324/haematol.2009.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arosio P., Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002;33:457–463. doi: 10.1016/s0891-5849(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 23.Vinchi F., De Franceschi L., Ghigo A. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013;127:1317–1329. doi: 10.1161/CIRCULATIONAHA.112.130179. [DOI] [PubMed] [Google Scholar]
- 24.Lee P.L., Gaasterland T., Barton J.C. Mild iron overload in an African American man with SLC40A1 D270V. Acta Haematol. 2012;128:28–32. doi: 10.1159/000337034. [DOI] [PubMed] [Google Scholar]
- 25.Buzaleh A.M., del Camen Martinez M., del Carmen Batlle A.M. Relevance of cytochrome P450 levels in the actions of enflurane and isoflurane in mice: studies on the haem pathway. Clin Exp Pharmacol Physiol. 2000;27:796–800. doi: 10.1046/j.1440-1681.2000.03346.x. [DOI] [PubMed] [Google Scholar]
- 26.Baron J., Tephly T.R. The role of heme synthesis during the induction of hepatic microsomal cytochrome P-450 and drug metabolism produced by benzpyrene. Biochem Biophys Res Commun. 1969;36:526–532. doi: 10.1016/0006-291x(69)90336-2. [DOI] [PubMed] [Google Scholar]
- 27.Wrighton S.A., Stevens J.C. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 28.Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- 29.Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970;67:517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granick S., Kappas A. Steroid control of porphyrin and heme biosynthesis: a new biological function of steroid hormone metabolites. Proc Natl Acad Sci U S A. 1967;57:1463–1467. doi: 10.1073/pnas.57.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966;241:1359–1375. [PubMed] [Google Scholar]
- 32.Marver H.S., Collins A., Tschudy D.P., Rechcigl M. Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966;241:4323–4329. [PubMed] [Google Scholar]
- 33.Marver H.S., Tschudy D.P., Perlroth M.G., Collins A. Delta-aminolevulinic acid synthetase. I. Studies in liver homogenates. J Biol Chem. 1966;241:2803–2809. [PubMed] [Google Scholar]
- 34.Baron J., Tephly T.R. Further studies on the relationship of the stimulatory effects of phenobarbital and 3,4-benzpyrene on hepatic heme synthesis to their effects on hepatic microsomal drug oxidations. Arch Biochem Biophys. 1970;139:410–420. doi: 10.1016/0003-9861(70)90494-7. [DOI] [PubMed] [Google Scholar]
- 35.Marver H.S., Schmid R., Schützel H. Heme and methemoglobin: naturally occurring repressors of microsomal cytochrome. Biochem Biophys Res Commun. 1968;33:969–974. doi: 10.1016/0006-291x(68)90408-7. [DOI] [PubMed] [Google Scholar]
- 36.Dwarki V.J., Francis V.N., Bhat G.J., Padmanaban G. Regulation of cytochrome P-450 messenger RNA and apoprotein levels by heme. J Biol Chem. 1987;262:16958–16962. [PubMed] [Google Scholar]
- 37.Tephly T.R., Hasegawa E., Baron J. Effect of drugs on heme synthesis in the liver. Metabolism. 1971;20:200–214. doi: 10.1016/0026-0495(71)90092-8. [DOI] [PubMed] [Google Scholar]
- 38.Baron J., Tephly T.R. Effect of 3-amino-1,2,4-triazole on the stimulation of hepatic microsomal heme synthesis and induction of hepatic microsomal oxidases produced by phenobarbital. Mol Pharmacol. 1969;5:10–20. [PubMed] [Google Scholar]
- 39.Schacter B.A., Nelson E.B., Marver H.S., Masters B.S. Immunochemical evidence for an association of heme oxygenase with the microsomal electron transport system. J Biol Chem. 1972;247:3601–3607. [PubMed] [Google Scholar]
- 40.Marver H.S. In: Microsomes and drug oxidation. Gillette J.R., Conney A.H., Cosmides G.J., editors. Academic Press; New York: 1969. The role of heme in the synthesis and repression of microsomal protein; pp. 495–511. [Google Scholar]
- 41.Maines M.D., Kappas A. Metals as regulators of heme metabolism. Science. 1977;198:1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.