Abstract
Pelvic floor disorders that affect stool evacuation include structural (example: rectocele) and functional disorders (example: dyssynergic defecation). Meticulous history, digital rectal examination, and physiological tests such as anorectal manometry, colonic transit study, balloon expulsion and imaging studies such as anal ultrasound, defecography, and static and dynamic MRI can facilitate an objective diagnosis and optimal treatment. Management consists of education and counseling regarding bowel function, diet, laxatives, most importantly behavioral and biofeedback therapies, and lastly surgery. Randomized clinical trials have established that biofeedback therapy is effective in treating dyssynergic defecation. Because dyssynergic defecation may co-exist with conditions such as solitary rectal ulcer syndrome (SRUS), and rectocele, before considering surgery, biofeedback therapy should be tried and an accurate assessment of the entire pelvis and its function should be performed. Several surgical approaches have been advocated for the treatment of pelvic floor disorders including open, laparoscopic and trans-abdominal approach, stapled transanal rectal resection (STARR), and robotic colon and rectal resections. However, there is lack of well controlled randomized studies and efficacy of these surgical procedures remains to be established.
Introduction
Pelvic floor disorders are common and cause significant bowel problems. The pelvic floor is a complex muscular apparatus within the pelvis and serves defecation, micturition, and sexual function. The most common pelvic floor disorders are fecal and urinary incontinence and pelvic organ prolapse. Approximately 23.7% of women have at least one pelvic floor disorder and 2.9% pelvic organ prolapse1.
Here, we focus on recent advances in the management of pelvic floor disorders affecting defecation, with a brief overview of pathophysiology and diagnosis. These disorders affect both women and men and necessitate a multidisciplinary team approach involving colorectal surgeon, gastroenterologist, pain specialist, physical therapist, radiologist, urogynecologist, and urologist.
Pelvic Floor Anatomy and Normal Physiology
The pelvic floor has superficial and deep muscle layers that interlace and envelope the rectum, bladder and uterus. The superficial layers include the internal anal sphincter (IAS) and external anal sphincter (EAS), perineal body, and transverse perinei muscles2. The deeper pelvic muscles, also known as levator ani, consist of the pubococcygeus, ileococcygeus, and puborectalis. The puborectalis maintains anorectal angulation and creates a mechanical barrier for stool flow and maintains pelvic floor integrity and its disruption/dysfunction may cause common pelvic floor disorders3,4. The pelvic floor receives nerve supply from the pudendal and perineal nerves and sympathetic and parasympathetic nerves. Branches from the sacral nerve roots of S2, S3, and S4 innervate the pelvic floor muscles. The puborectalis muscle (middle layer of pelvic floor muscle) is actually innervated by the pudendal nerve and the deep muscles (pubococcygeus, ileococcygeus, and coccygeus) are innervated by the direct branches of sacral nerve roots S3 and S4.[3] Pudendal nerve damage may cause dysfunction of puborectalis muscle and EAS muscles (both constrictor muscles) and this in turn may cause fecal incontinence. During normal defecation, the voluntary effort of bearing down increases the intra-abdominal pressure, together with the contraction of the rectum and perineal muscles. Simultaneously, the anal sphincters and puborectalis relax, the anorectal angle widens, and the perineum descends. These sequential movements facilitate the movement of stool from the rectum resulting in stool evacuation. (Figure 1).
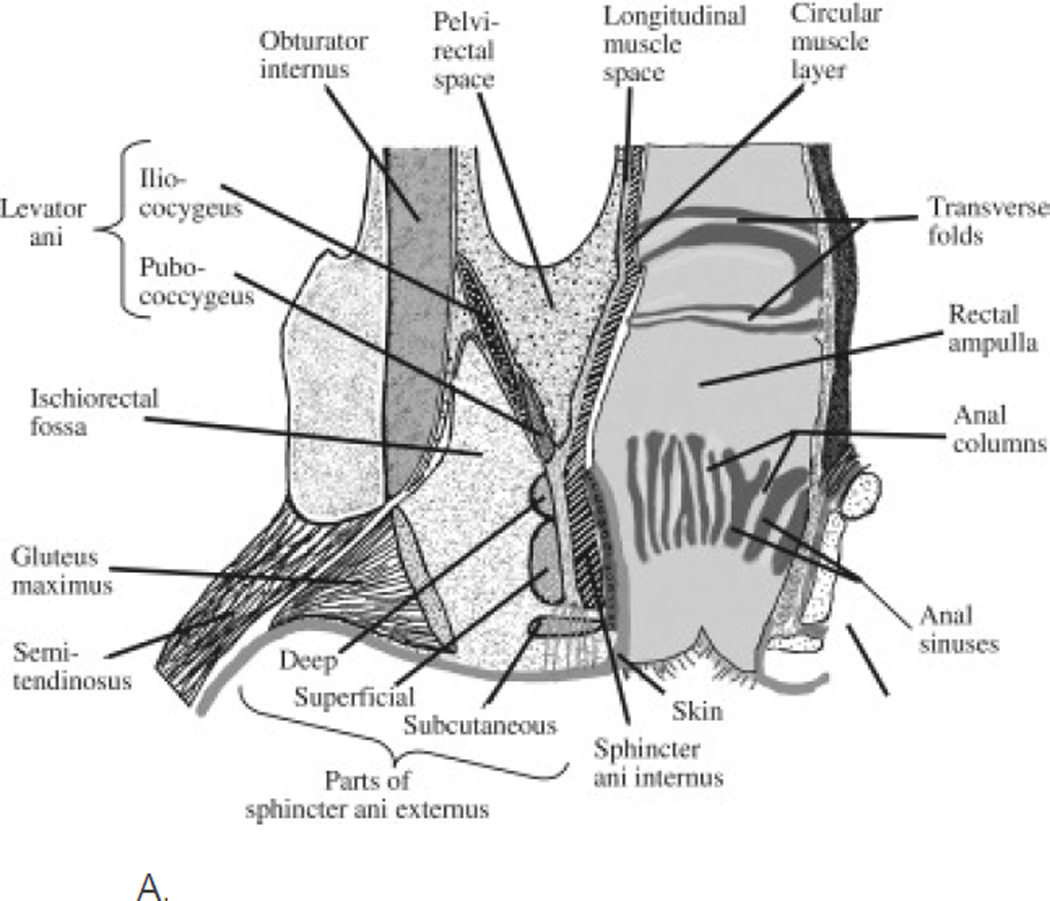
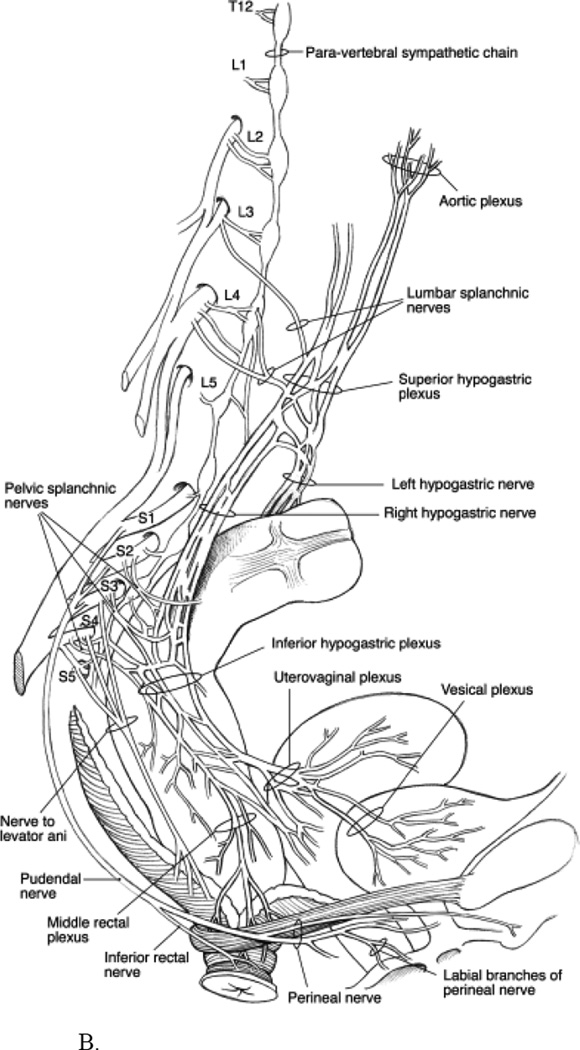
Figure 1.
A. Diagram of a coronal section of the rectum, anal canal, and adjacent structures. The pelvic barrier includes the anal sphincters and pelvic floor muscles. “Reprinted from Gastroenterology, 124, Bharucha, Fecal Incontinence, 1672-1685, 2003, with permission from Elsevier.”
B. Sympathetic, parasympathetic, and pudendal nerve supply to the anorectum. “Reprinted from Peripheral Neuropathy, 4th edition, Bharucha and Klingele, Autonomic and Somatic Systems to the Anorectum and Pelvic Floor, 279–298, 2005, with permission from Elsevier.”
Dyssynergic Defecation
Dyssynergic defecation (DD) is characterized by paradoxical anal contraction, inadequate anal relaxation, and/or impaired push effort caused by incoordination of abdominal, rectal, and anal muscles5. The most common symptoms are excessive straining (84%), feeling of incomplete evacuation (76%), abdominal bloating (74%), passage of hard stools (65%) and less than 3 bowel movements per week (62%). Digital maneuvers are frequent (~35%), although infrequently reported6.
Diagnosis
The diagnosis of DD requires fulfillment of symptomatic (Rome III) and manometric criteria, with one other quantifiable measure of abnormal defecation such as abnormal balloon expulsion test (BET), prolonged delay in colonic transit, or incomplete evacuation during defecography9.
Digital rectal exam (DRE)
Digital rectal exam (DRE) is a useful bedside screening tool. The exterior inspection can detect skin excoriation, squamous cell cancer, skin tags, anal fissures, fistulas or hemorrhoids. The perineal sensation (to exclude neuropathy) is evaluated by gently stroking the perianal skin with a cotton bud in all four quadrants.
Digital penetration may reveal a stricture, spasm, tenderness, mass, blood or stool. If there is a lack of awareness of stool in the rectum this may suggest rectal hyposensitivity. Primarily, the resting tone is evaluated, followed by asking the subject to squeeze it is possible to evaluate the anal sphincter and puborectalis muscle. The patient is asked to push and bear down as if to defecate, and during this maneuver, the examiner should perceive relaxation of the external anal sphincter and/or the puborectalis muscle, together with perineal descent. An absence of these normal findings should raise the index of suspicion for an evacuation disorder such as dyssynergic defecation. DRE has a sensitivity of 77% and specificity of 87% for detecting dyssynergia7, but is infrequently performed, even by gastroenterologists, and there is lack of training8.Thus, a concerted effort is needed to improve the training of digital rectal examination.
Anorectal manometry (ARM)
This test provides information regarding rectal and anal pressures at rest and during simulated defecation. During rectal balloon distention it provides information regarding rectal compliance and sensation, and recto-anal reflexes10. Normal subjects can exhibit dyssynergia when attempting to defecate in the left lateral position; hence manometric changes are best assessed with the patient on a commode and with a sensation of stooling11, 12. The best method to diagnose dyssynergia is to distend a balloon in the rectum and ask the subject to attempt evacuation in a sitting position13,14. Four patterns of dyssynergia have been described, and their identification helps tailor biofeedback therapy. Type I is adequate push effort with paradoxical anal contraction, type II is impaired push effort with paradoxical anal contraction, type III is impaired anal relaxation with adequate push effort, and type IV is impaired push effort with impaired anal relaxation. These patterns were established with the conventional manometry, which is very useful and still widely used in many centers. The new HRM system, (Sierra Scientific Instruments, Los Angeles, CA) allows interpolation of manometric recordings from 12 circumferential pressure sensors into a detailed topographical plot. This system can provide a greater resolution of the intraluminal pressure changes with more anatomical detail, hopefully leading to a better assessment of common pelvic floor disorders15. (Figures 2A, 2B)
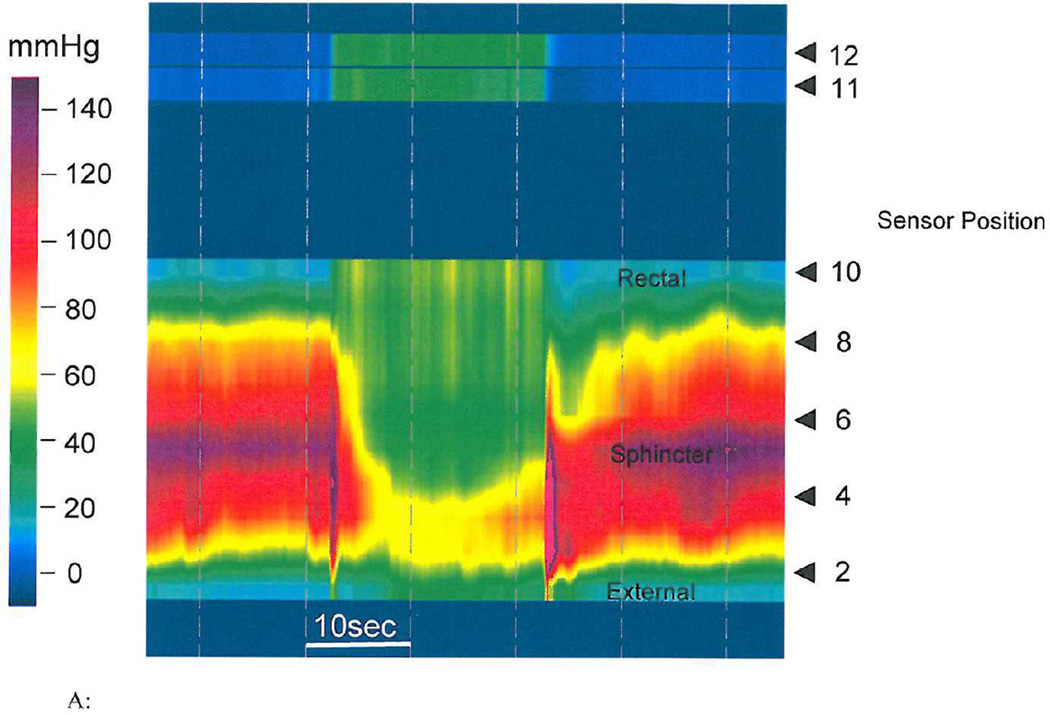
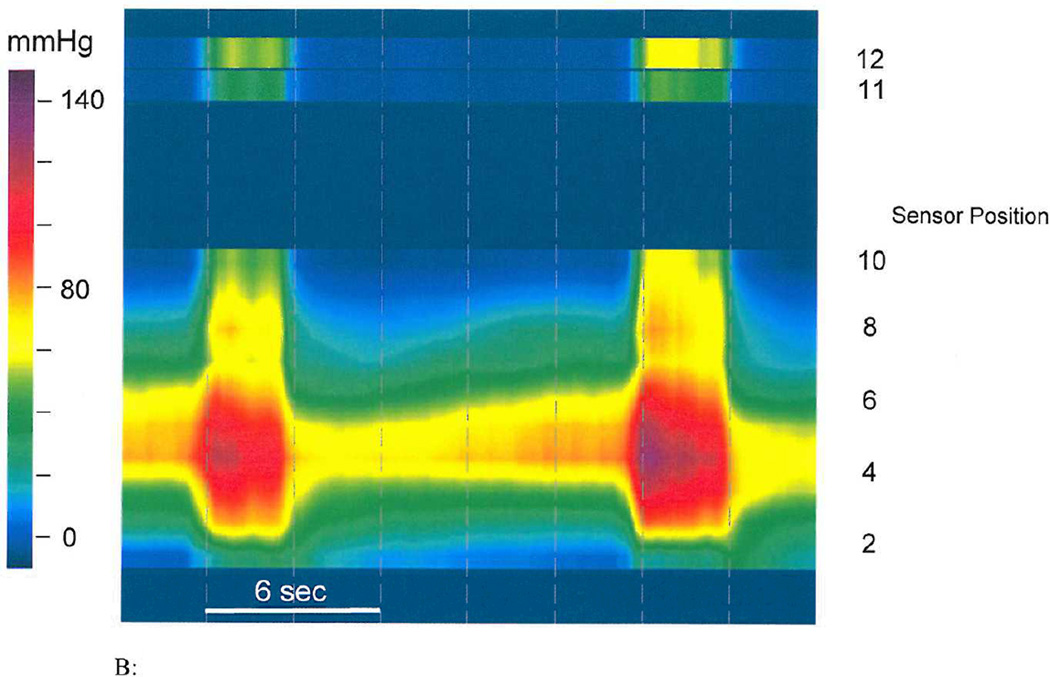
Figure 2.
A: HRM image of a healthy control showing manometric and topographic features during an attempted defecation maneuver. The upper part of the panal shows pressure changes from the rectum indicating that subject generated a good push effort. The lower part of the panel shows topographic images from the entire anal canal and puborectalis showing that both the anal sphincter and puborectalis relaxed normally with a significant drop in pressure. The location of sensors from the anal verge and the pressure gauge as represented by the various colors are also shown.
B: HRM image of a patient with constipation showing manometric and topographic features during two attempted defecation maneuvers. The upper part of the panal shows pressure changes from the rectum indicating that patient generated a good push effort. The lower part of the panel shows topographic images from the entire anal canal and puborectalis showing that both the anal sphincter and puborectalis exhibited paradoxical anal contraction, typical of dyssynergic defection. The location of sensors from the anal verge and the pressure gauge as represented by the various colors are also shown.
Balloon Expulsion Test
This provides information regarding the ability to expel a 50-ml water-filled balloon placed in the rectum. Normal expulsion time is one minute. It has 80–90% specificity and 97% negative predictive value for identifying dyssynergia. Although it has a sensitivity of only 50%, it is a simple and useful screening procedure to identify constipated patients who do not have dyssynergia16.
Colonic Transit Study
Slow transit constipation (STC) can coexist in 2/3rd of patients with DD, and it is imperative to differentiate between patients with isolated DD or mixed with STC. Colon transit study is considered abnormal if more than five markers (20%) are present on a plain abdominal film 5 days after ingestion of a Sitzmarker® capsule containing 24 radio-opaque markers17.
Colonic Transit Scintigraphy
Colonic transit scintigraphy is indicated in patients with suspected colonic motility disorders or more diffuse disorders involving the stomach or small intestine. It quantifies slow colonic transit in patients with constipation and can influence patient management. Two methods have been described: 1. Colon transit of 111In DTPA-labeled water consumed in a standard solid-liquid meal for gastric scintigraphy 2. A capsule (containing 111In adsorbed on activated charcoal) coated with the pH-sensitive polymer methacrylate that dissolves in the alkaline terminal ileum, releasing the radioisotope into the lumen. The clinical utility of scintigraphic testing has been demonstrated in previous studies. The colonic transit scintigraphy is recommended for assessing colonic transit in patients with constipation or diarrhea but is available in a limited number of centers18.
Wireless Motility Capsule Test (SmartPill®)
The Wireless Motility Capsule (WMC) (Smart Pill Corporation, Buffalo, NY), is a wireless pH, temperature and pressure recording capsule. This novel, valuable ambulatory technique of assessing regional (gastric, small bowel, colonic) and whole-gut transit time without radiation offers a standardized method of identifying normal and slow colonic transit18–20 and recommended as useful by the American Neurogastroenterology & Motility Society. Currently the FDA has approved the SmartPill GI Monitoring System, version 2.0, for the evaluation of colonic transit time in patients with suspected chronic constipation, and for evaluating patients with suspected gastroparesis.
3D Anal Ultrasonography
Three-dimensional endoanal ultrasonography (3D-EAUS) provides excellent anatomical details of the anal sphincter complex, including coronal and segmental sections. It is well-tolerated and inexpensive. The sphincter anatomy can be evaluated spatially, and "damaged" sphincter integrity and volume, as well as fistulous track(s) or potential fluid collections can be assessed21.
MR (magnetic resonance) Defecography
MR Defecography (MRD) visualizes the pelvic viscera and supporting soft-tissue structures without radiation but not widely available. It can be performed with a closed or open system. Open MRI acquires images in a sitting position, simulating true defecation. In a closed-configuration MRI system, images are acquired in the supine position22. A recent study demonstrated that Dynamic MRD with an open-configuration and low-field tilting MR system is feasible23.
Management General and supportive therapies
Medical treatment consists of avoiding constipating medications, increasing fluid and dietary fiber intake, regular exercise, and practicing timed toilet training24. The American College of Gastroenterology (ACG) task force has defined levels of evidence and graded most treatments. Level I evidence was defined: Randomized Control Trials (RCTs) with P<0.05, adequate sampling and appropriate methodology. Level II: RCTs with P<0.05, with inadequate sample size and/or inappropriate methodology. Level III: Non randomized trials with contemporaneous controls. Level IV: Non randomized trials with historical controls. Level V: Case series. Grade A recommendations are supported by two or more level I trials without conflicting evidence from other level I trials. Grade B recommendations are based on evidence from a single level I trial OR recommendations based on evidence from two or more level I trials with conflicting evidence from other level I trials OR supported by evidence from two or more level II trials. Grade C recommendations are based on level III-V evidence25. A fiber intake of 20–25 grams per day is recommended, and if required supplemented with psyllium (grade B recommendation). A recent study had shown that dried plums, 50gm BID, are more effective than equivalent dose of psyllium for mild to moderate constipation26. However, this study was published recently and dried plums were not graded by the ACG task force. Although medications that promote bowel movement such as stool softeners, stimulant laxatives, and osmotic laxatives can be useful in clinical practice, the ACG task force states that there is insufficient evidence for these treatments25. The ACG gave polyethylene glycol (PEG) a grade A. A recent 6 month study reported adequate relief of constipation in 52% of patients treated with PEG compared to 11% treated with placebo27.
Lubiprostone, a chloride channel-2 activator, at a dose of 24ug BID was more effective than placebo in improving stool frequency and symptoms of chronic constipation (CC)25. Prucalopride, a 5-hydroxytryptamine-4 receptor agonist at a dose of 2–4mg QD was also effective in the treatment of CC, and although not FDA approved, it is approved for use in Europe28. Linaclotide, a luminally acting agonist of the guanylate cyclase-C receptor, produces rapid and sustained improvement of bowel symptoms, global relief, and improved quality of life29. It is currently under FDA review for the treatment of irritable bowel syndrome with constipation (IBS-C) and chronic constipation (CC). Thus far, their efficacy in dyssynergic defecation has not been validated.
Biofeedback Therapy
This is the most effective treatment for DD. The main purpose is to restore a normal pattern of defecation using “operant conditioning” techniques30. The primary goals: (i) correct the underlying dyssynergia; and (ii) improve rectal sensory perception. The goals are to improve abdominal push effort, facilitate pelvic floor relaxation, and expel artifical stool. The procedure involves placing a manometric probe into the rectum which in turn provides instant feedback to the patient regarding their performance and how the rectal and anal muscles are behaving. About 10–15 maneuvers are usually attempted in a single session, and the number of sessions and duration of each session are customized. Typically 4–6 sessions, one hour each are performed.
Several randomized controlled trials have demonstrated that biofeedback therapy is superior to sham feedback, standard therapy, or laxatives in the management of patients with DD31,32. It was not beneficial for patients with isolated slow transit constipation33. Recently biofeedback has been shown to provide sustained improvement of bowel symptoms and anorectal function for up to one year, whereas standard therapy with laxatives was ineffective34. Also, home biofeedback therapy was as effective as office biofeedback therapy and more cost effective35,36. Home biofeedback therapy comprised of home training. The patients were instructed to insert a disposable 2 sensor probe into the rectum. The probe is attached to an LCD display box and provides visual input to the subject regarding their performance. The patients practiced at home twice a day for 20 minutes per session. When home biofeedback therapy was compared with the standard treatment of office biofeedback, there was no difference between the two treatments with both treatments showing significant improvement in the number of complete spontaneous bowel movements per week, dyssynergia pattern, balloon expulsion time, and bowel satisfaction score. Currently this treatment is not covered by insurance and could cost up to $400/month which may limit its application. (Table 1).
Table 1.
Summary of the randomized controlled trials of biofeedback therapy for dyssynergic defecation
| Rao et al (35) | Chiarioni et al (32) | Rao et al (86) | Chiarioni et al (34) |
Heymen et al (33) | |
|---|---|---|---|---|---|
| Trial Design | Biofeedback versus standard | Biofeedback versus polyethylene glycol, 14.6 g | Biofeedback versus standard versus sham biofeedback | Biofeedback for slow transit versus dyssynergia | Biofeedback versus diazepam, 5 mg, versus placebo |
| Subjects and randomization | 26 (23 women) 13 Biofeedback 13 standard |
104 women 54 biofeedback 55 polyethylene glycol |
77 (69 women) 1:1:1 distribution |
52 (49 women) 34 dyssynergia 12 slow transit 6 mixed |
84 (71 women) 30 biofeedback 30 diazepam 24 placebo |
| Duration and number of biofeedback sessions | 3 months 6,9,12, months reinforcement sessions |
3 months and 1 year, 5 weekly, 30-minute training sessions performed by physician investigator | 3 months, biweekly, 1 hour, maximum of six sessions over 3 months, performed by biofeedback nurse therapist | Five weekly 30-minute training sessions, performed by physician investigator | Six biweekly, 1-hour sessions |
| Primary outcomes | Number of complete spontaneous bowel movements (CSBM) | Global improvement of symptoms Worse=0 No improvement=1 Mild=2 Fair=3 Major improvement=4 |
1. Presence of dyssynergia 2. Balloon expulsion time 3. Number of complete spontaneous bowel movements (CSBM) 4. Global satisfaction |
Symptom improvement None=1 Mild=2 Fair=3 Major=4 |
Global symptom relief |
| Dyssynergia corrected or symptoms improved | The number of CSBMs per week increased significantly (P<0.001) in the biofeedback group | 79.6% reported major improvement at 6 and 12 months, 81.5% reported major improvement at 24 months |
Dyssynergia corrected at 3 months in 79% with biofeedback versus 4% sham and 6% in standard group; CSBM=biofeedback group versus sham or standard, p<0.05 | 71% with dyssynergia and 8% with slow transit alone reported fair improvement in symptoms | 70% improved with biofeedback compared with 38% with placebo and 30% with diazepam |
| Conclusions | Biofeedback provided sustained improvement compared to standard therapy | Biofeedback was superior to laxatives | Biofeedback was superior to sham feedback and standard therapy | Biofeedback benefits dyssynergia and not slow transit constipation | Biofeedback is superior to placebo and diazepam |
Rectal Prolapse
Rectal prolapse is defined as an abnormal protrusion of all layers of the rectal wall through the anus. The incidence is 2.5/100,000, with the highest incidence among elderly women. In younger people, the gender ratio is equal37.
The primary mechanism is excessive straining, that over many years gradually weakens the pelvic floor and its support structures. The repeated injury to the pudendal nerve and other nerves weakens the IAS and EAS and the puborectalis. The lax anal sphincters offer very little counter-acting resistive force during defecation, thereby leading to an abnormal protrusion of the rectal wall through the anus. Other causes for prolapse include pudendal neuropathy from either aging or obstetric injury.
Most patients present with anal protrusion and/or passage of blood, or symptoms of obstructed defecation or fecal incontinence38. Pre-existing dysmotility, DD, or intussusception also predispose. Anal inspection may reveal normal anal area but when the patient bears down the prolapsed rectum is visible, often edematous and sometimes with a friable and ulcerated mucosa. Rectal prolapse is graded into four types: grade 1=up to anal verge; grade 2=prolapse outside the anus but reduces spontaneously; grade 3=prolapses outside the anus but can be manually reduced; and grade 4=prolapse cannot be reduced manually.
Diagnosis
The diagnosis is made through careful perineal examination and during attempted defecation. A patulous anus with full thickness rectal protrusion is usually diagnostic. If not seen at bedside, a Valsalva maneuver on commode should bring on the prolapse; a prone jackknife position is not helpful. Endoscopic evaluation to identify other causes associated with rectal prolapse may be useful. Defecography usually demonstrates the prolapse, and may reveal an obtuse anorectal angle. MRD may reveal other anatomical defects that can aid in assessment of surgical intervention. 3D-EAUS may show asymmetry and thickening of the IAS and submucosa. Demonstration of anal sphincter defect is useful when considering sphincter reconstruction39. Anorectal manometry may reveal sphincter weakness especially in patients with coexisting fecal incontinence. Rectal sensation and compliance may be either normal or impaired40.
Management
Grade 1–2 prolapse that is asymptomatic does not require surgery, and could be managed with high-fiber diet and/or laxatives, followed by biofeedback therapy to correct underlying dyssynergia. However, symptomatic grade 3–4 prolapse requires surgery. Both abdominal and perineal approaches are available. The abdominal approach allows the surgeon to deal with factors that are associated with rectal prolapse including a deep cul-de-sac of the pouch of Douglas, lack of sacral fixation, and redundancy of the sigmoid colon41. The method used to mobilize the rectum is controversial due to evidence suggesting that this may cause or worsen constipation in 1/3rd of patients while decreasing the risk of prolapse recurrence. Sacral fixation of the rectum is accomplished by either posterior suture or mesh rectopexy. Resection of sigmoid colon is preferably performed in patients with significant redundancy, but is avoided in patients with the combination of diarrhea and/or incontinence as these symptoms may worsen with resection42.
Perineal repairs are currently the most common surgical treatment for rectal prolapse in the US43. The perineal approach has a lower perioperative morbidity and higher recurrence rate and less satisfactory functional results44. In general, elderly patients, those with significant medical comorbidities, or those with contraindications for abdominal surgery are often the best candidates. Most commonly a perineal proctosigmoidectomy (Altemeir procedure) is performed45. This may be combined with transperineal levatoroplasty which may help to reduce the risk of recurrence46. Recent studies have shown that robotic colorectal resection is a safe and feasible option; however, more studies are required47.
Recurrence rates for transabdominal rectopexy are low (0–8%); however, after posterior rectopexy 50% of patients complain of severe constipation48. Perineal procedures have a recurrence rate of 5–21% with similar incidence of constipation.
Rectocele
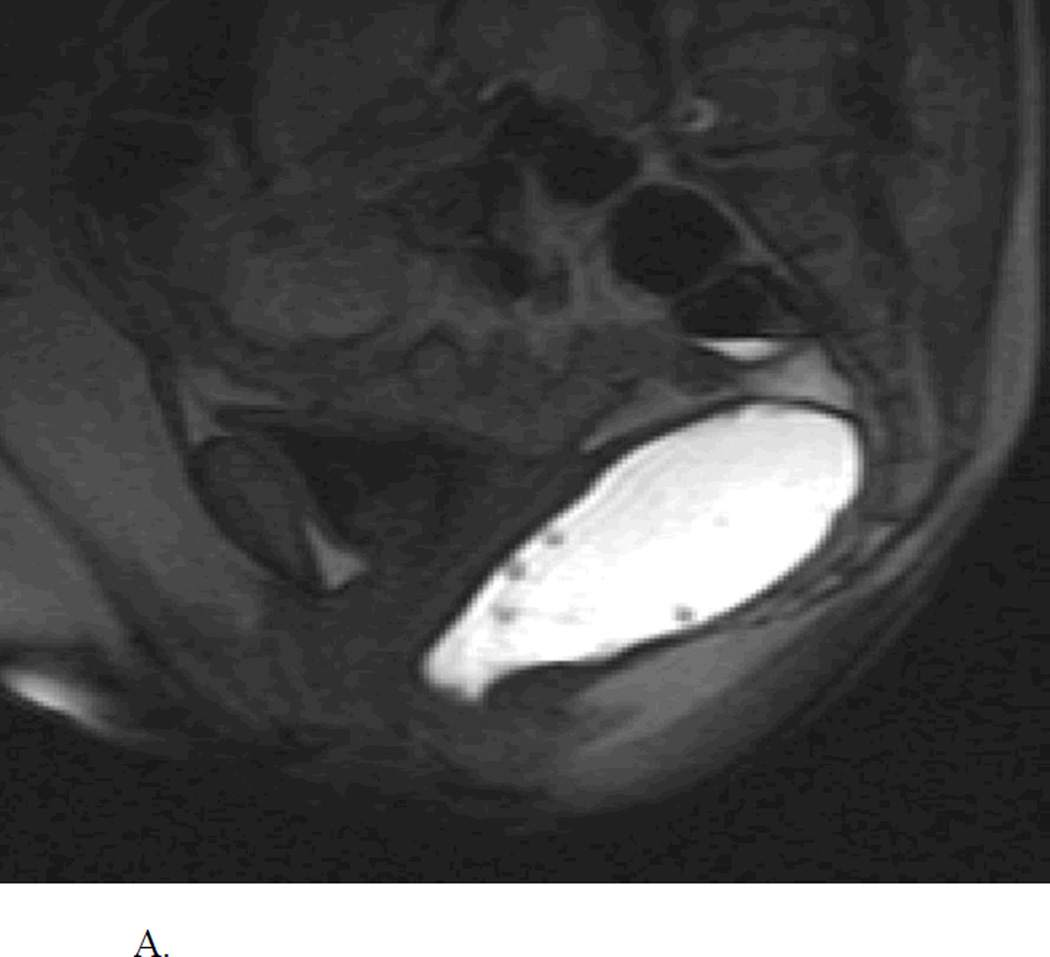
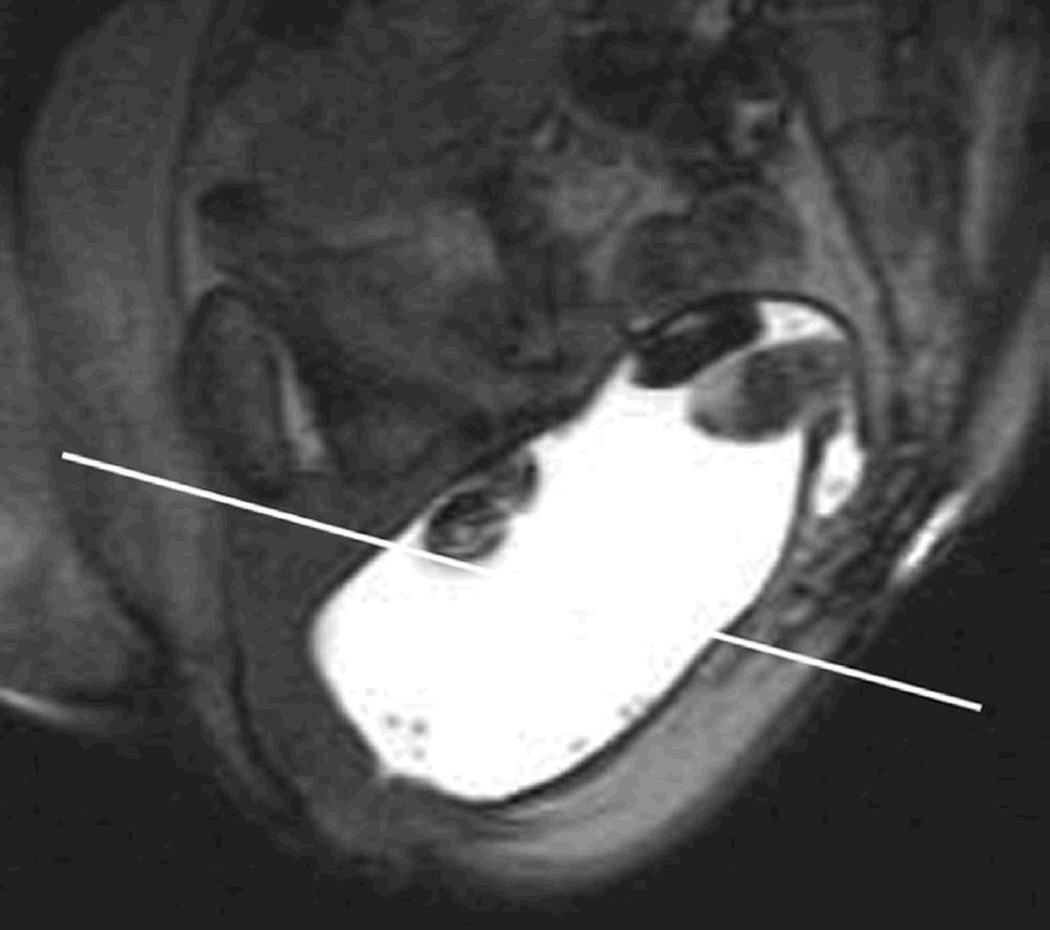
Rectocele is an abnormal sac-like protrusion of the rectal wall either towards the vagina (anterior) and rarely towards the sacrum (posterior) that often becomes apparent during defecation (Figure 3A, 3B). It is common in adult women (20%), majority are small (<2 cm), asymptomatic, and require no treatment49,50.
Figure 3.
A. Normal MR Defecogram showing normal sized rectum filled with contrast and adjacent pelvic muscular and bony structures. Reproduced, with permission, from Roos J, Weishaput D, Wildermuth S, Willmann J, Marincek B, Hilfiker P, Experience of 4 Years with Open MR Defecography: Pictorial Review of Anorectal Anatomy and Disease, Radiographics, 2002, 22, 819.
B. Abnormal MR Defecogram during an attempted defecation showing a large anterior rectocele (>4 cm). Reproduced, with permission, from Roos J, Weishaput D, Wildermuth S, Willmann J, Marincek B, Hilfiker P, Experience of 4 Years with Open MR Defecography: Pictorial Review of Anorectal Anatomy and Disease, Radiographics, 2002, 22, 825.
Rectocele is believed to be caused by weakness of the rectovaginal septum and/or pelvic floor; however, the exact etiology is not known. Obstetric injury and multiple vaginal deliveries have been proposed as precipitating factors. However, rectocele is also seen in nulliparous women37,51. There are no specific anorectal physiological findings for a rectocele, a previous study had reported that dyssynergic pattern of defecation was seen in 60% of patients with rectocele when compared to 24% without a rectocele52. However, a recent study showed similar prevalence53. Rectocele can be a cause or consequence of CC with excessive straining, and may be associated with DD or rectal mucosal intussusception, or excessive perineal descent. Whether it is a cause or an effect of these changes is unclear3,52. Excessive straining or childbirth may weaken the vaginal septum. Consequently, during defecation a pressure gradient is created between the higher intrarectal pressure and lower vaginal pressure producing a rectocele. Rectocele is usually asymptomatic, and is diagnosed by examination or defecography. Patients may report symptoms such as a feeling of incomplete evacuation, prolonged straining, or vaginal splinting. Some report a vaginal or perineal bulge and others describe a sensation of blockage or inability to evacuate37. Patients may complain of dyspareunia, anorectal/vaginal pain, fecal soiling, and urologic symptoms54.
Diagnosis
DRE may reveal an anterior out pouching of the rectal wall, particularly during straining. Bimanual rectal and vaginal examination can further help to confirm or facilitate diagnosis. A posterior rectocele is difficult to detect clinically.
Defecography is considered the gold standard for diagnosis of anterior or posterior rectocele, measure its size, and quantify stool retention. It also provides information regarding coexisting conditions such as rectal mucosal intussusception or excessive perineal descent55. Clinical correlation is important before labeling a rectocele as clinically significant. Rectoceles >3 cm and those with retention of barium or stool are considered to be clinically significant. Although widely used for identification and staging of rectoceles, defecography cannot predict the outcome of rectocele repair (REF). Anal sphincter pressures, rectal sensation, and rectal compliance are usually normal but one study found dyssynergia in about 50% of patients with rectocele <2.5 cm56.
MRI provides good visualization of the rectocele, and dynamic MRI can correlate with pelvic floor movements. It is recommended that real-time continuous imaging with a dynamic true fast imaging with steady-state precession sequence should be included in MR protocols to evaluate pelvic floor dysfunction in addition to dynamic multiplanar sequences57. A recent study showed that images during the defecation phase can identify abnormal bladder, vaginal, and rectal descent and previously undetected rectoceles that were ≥2 cm58.
Management
The first approach is to treat any underlying defecation disorder. Fiber supplements, laxatives, timed-toilet training, and behavioral approaches can be effective but has not been systematically evaluated. If symptoms persist and DD is identified, biofeedback may help. It is important to identify and treat co-existing psychological disorders and other organic disorders (urogynecological)59,60. Surgical treatments carry a high risk of recurrence for both rectocele and rectal mucosal intussusception. However, surgery is appropriate for patients with large rectocele (>3 or 4 cm) or those with coexisting vaginal prolapse, but after failure of medical therapy. In these patients, rectocele repair improves anorectal function by improving rectal sensitivity61.
Surgical repair of a rectocele must be tailored to each patients needs, keeping in mind that uterovaginal and rectal prolapse may co-exist, and generally a transperineal repair is superior to transanal repair for both structural and functional outcome62. In patients with both rectocele and DD, the transanal approach is ideal, although it may compromise anal sphincter pressures. The stapled transanal rectal resection approach uses sequential deployments of a gastrointestinal stapling device to resect the redundant anterior and posterior rectal walls, thereby restoring normal rectal anatomy and reducing rectal volume. However, a recent long-term study demonstrated rapid deterioration in outcomes after 18 months63.
Descending perineum syndrome
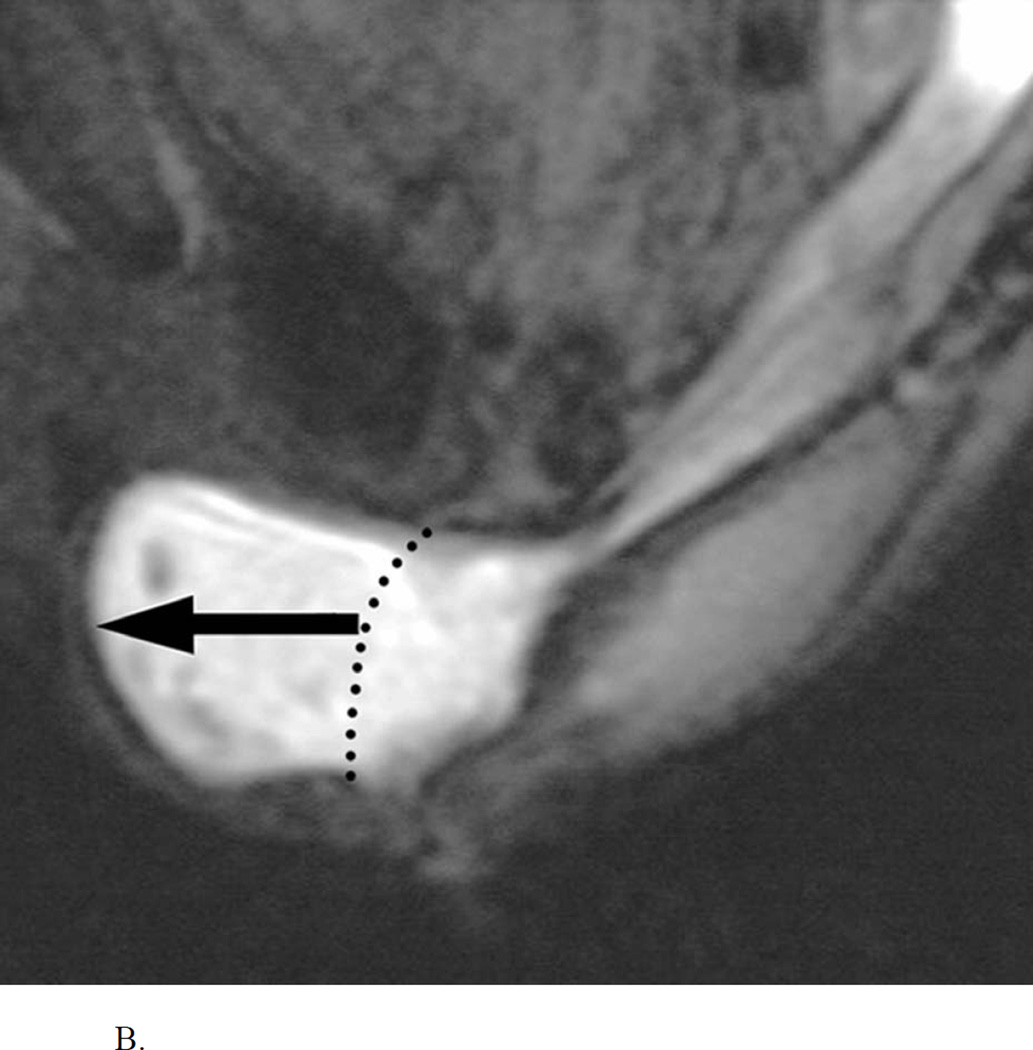
Descending perineum syndrome (DPS) is characterized by ballooning and excessive descent of the perineum, often several centimeters below the bony outlet of the pelvis, during straining64 (Figure 4).
Figure 4.
MR defecogram study showing excessive (>4 cm) descent of the perineum during straining, typical of the Descending Perineum Syndrome (White line= Pubococcygeal line). Reproduced, with permission, from Roos J, Weishaput D, Wildermuth S, Willmann J, Marincek B, Hilfiker P, Experience of 4 Years with Open MR Defecography: Pictorial Review of Anorectal Anatomy and Disease, Radiographics, 2002, 22, 828.
Typically, patients present with a long history of painful defecation, excessive straining, and sense of incomplete evacuation or fecal incontinence65. One study showed a link between DPS, fecal incontinence, and abdominal hysterectomy66.
DPS can be diagnosed on physical examination, or defecography. The most common abnormality is >4 cm perineal descent at rest or ≥3 cm perineal descent during a maximal push effort67. A perineometer, which measures the strength of voluntary contractions of the pelvic floor muscles, may be useful68.
Dynamic MR imaging demonstrates simple or complex organ descent in all pelvic compartments and may become standard preoperative evaluation for pelvic floor abnormalities. The MR images facilitate planning of surgery and can increase rate of successful outcome, but is expensive and not widely available69.
Management
Treatment consists of mainly correcting the excessive straining, use of an artificial device-defecom - a polycarbonate plate with two separate holes for passing urine and stool and a built in hump which supports the perineum when sitting on a commode. The defecom together with biofeedback therapy may improve symptoms in ~50%70. Pelvic floor retraining may also be useful but there is no information and the extent of perineal descent appears to be a useful predictor of response to retraining. The defecom and a similar device “Colorec”, are unavailable in the USA and not FDA approved.
Until recently, there was no surgical option for isolated DPS. However, recently a retrospective case series of nine women who underwent isolated retro-anal levator plate myorrhaphy for symptomatic DPS71. The mean reduction of perineal descent was 1.08 cm (0–1.5) reported after 9 months. There was improvement in stress urinary incontinence (100%), frequency, nocturia, urgency (66%), dysuria (75%), fecal incontinence (100%), dyschezia (87%), dyspareunia (80%), cystocele and rectocele (75%). A prospective controlled study is needed to validate this result.
Solitary rectal ulcer syndrome
Solitary rectal ulcer syndrome (SRUS) is characterized by erythema and single/few ulcers. Its etiology remains obscure but is often associated with evacuation disorders. The annual incidence of SRUS is 1–3.6/100,000; 80% of patients are less than 50 years of age72 with slightly higher prevalence in females. Rectal intussusception is often present and evacuation is delayed73,74. Ulceration is thought to occur during repeated forceful straining against an immobile pelvic floor or DD together with trauma from digital manipulations and ischemic necrosis of the prolapsing rectal mucosa75. Patients present with rectal bleeding and/or pain, mucus discharge, straining and tenesmus and a feeling of incomplete evacuation. Majority use digital maneuvers but rarely admit. About 55% present with constipation, 20–40% with diarrhea, and 25% are asymptomatic, and 25% are misdiagnosed or treated as inflammatory bowel disease. In some patients, an underlying psychologic disorder, such as obsessive-compulsive disorder may be present74.
Diagnosis
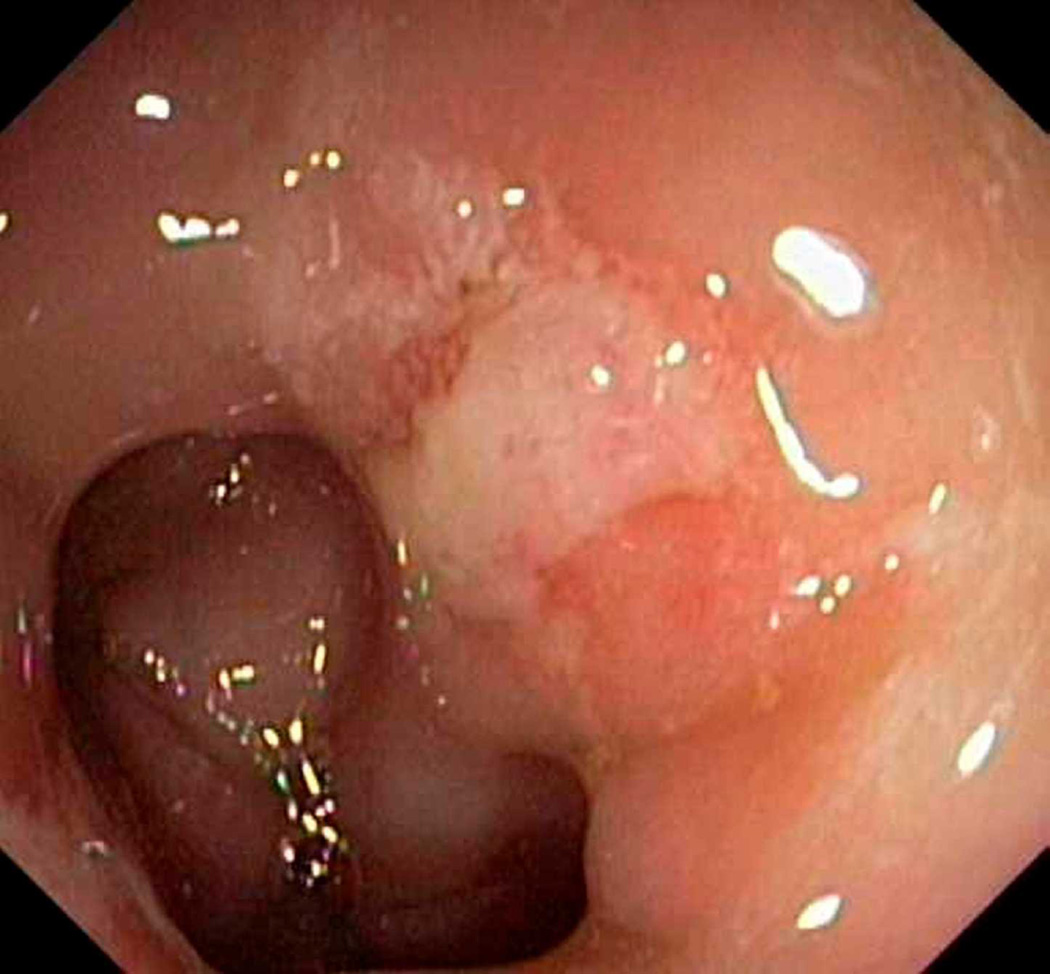
Sigmoidoscopy may reveal a small, shallow ulcer with a white slough or hyperemic mucosa on the anterior wall of the rectum (Figure 5). The lesions can be multiple (30%), ulcerated (57%), polypoid (25%) or with patches of hyperemic mucosa (18%). SRUS is usually found on the anterior or anterolateral wall of the rectum, over a rectal fold, about 5–10cm from anus76. Histologically, the mucosa appears elongated with distorted glands at the base, with an edematous fibroblast-rich lamina propria and thickened inner circular muscular layer77. When the glands migrate down to the submucosa, bleeding may occur. Pathognomonic features in SRUS include: decussation of the two muscularis layers, nodular induration of the inner layer, and grouping of outer longitudinal layers into bundles78. Biopsy is needed to differentiate SRUS from ulcers due to other etiologies (NSAIDs, malignancy, endometriosis)79,80. Defecography may show other abnormalities such as rectal mucosal intussusception in 45–80% of subjects. Barium enema is unreliable81. Anorectal manometry does not help in establishing the diagnosis or predicting therapeutic response; however, it may reveal a number of physiological abnormalities such as dyssynergia in 80% of patients or a hypersensitive rectum, and prolonged BET82. Ultrasonography may show marked thickening of the IAS, submucosa, and EAS, as well as rectal wall and muscularis propria78.
Figure 5.
Solitary rectal ulcer syndrome. Endoscope view of an ulcer (white area towards upper right) in a patient’s rectum. Photo Researchers Picture Number: C013/0462. Credit: Gastrolab/Photo Researchers, Inc. License: Rights Managed.
Management
Behavioral therapy remains the mainstay of treatment and includes reducing excessive straining, spending less than 5 minutes during evacuation, and discontinuing the use of digital maneuvers. These recommendations, together with biofeedback therapy improved symptoms in 67% of patients with sigmoidoscopic improvement in 30%76. A high-fiber diet showed a variable response rate of 19–70%, suggesting that although diet helps by itself, it is insufficient. Local treatment with topical steroids and sulphasalazine is generally ineffective. Although there is limited data, sucralfate enemas and topical human fibrin sealant have been tried83. A recent study suggests that Argon plasma coagulation (APC) may be useful in controlling bleeding and improving healing of ulcers, but controlled studies are lacking84.
Biofeedback therapy appears to be effective, although RCTs are scarce. One study showed that 75% (12/16) of patients had symptomatic improvement with biofeedback therapy and 31% (5/16) had ulcer resolution on sigmoidoscopy85. Mucosal flow improved in patients who felt subjectively better after biofeedback. Another prospective study of 11 patients with refractory SRUS showed that biofeedback therapy improved straining effort and stool frequency, digital maneuvers were discontinued in 45% and bleeding ceased in 56%. Ulcer healing was reported in 10 patients: 4 had complete healing, 2 had >50%, and 4 had <50%75.
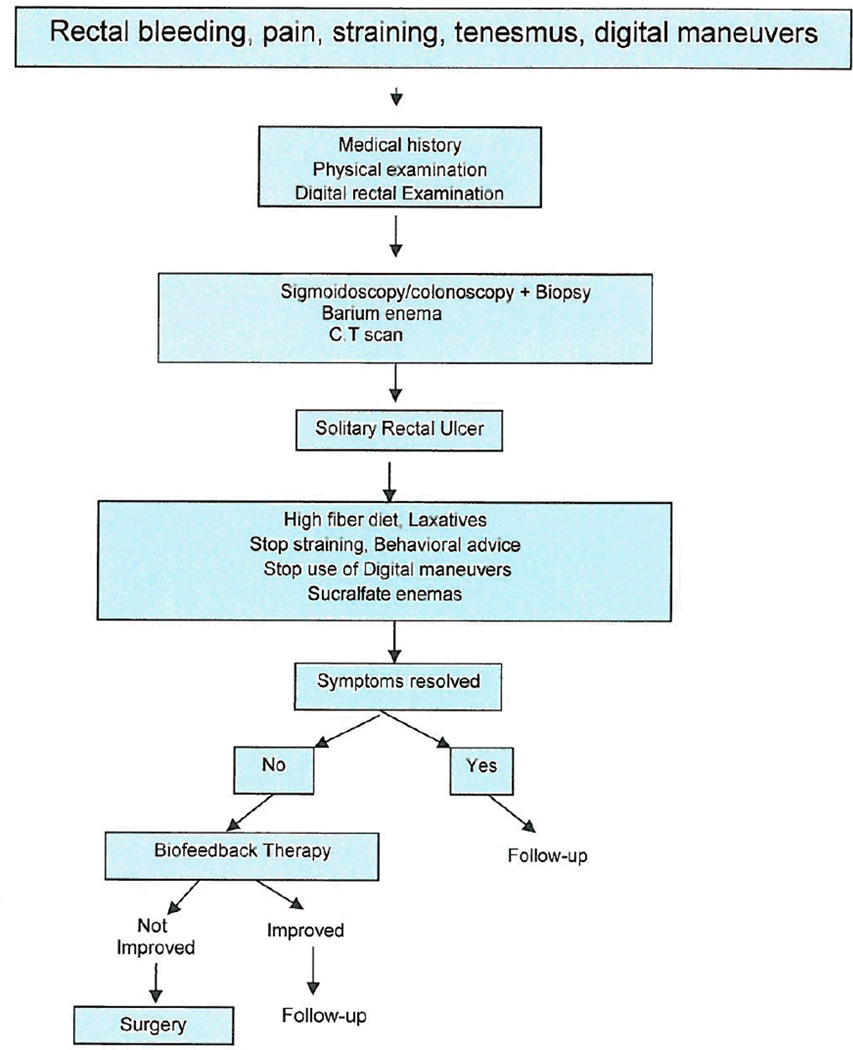
Rectopexy with or without anterior resection should be performed in highly selected cases. Outcomes of surgery are often disappointing, because of either persistent symptoms, postoperative bleeding or sexual dysfunction86,87. (Figure 6).
Figure 6.
Algorithm for investigation and treatment of Solitary Rectal Ulcer syndrome (SRUS)
Conclusions
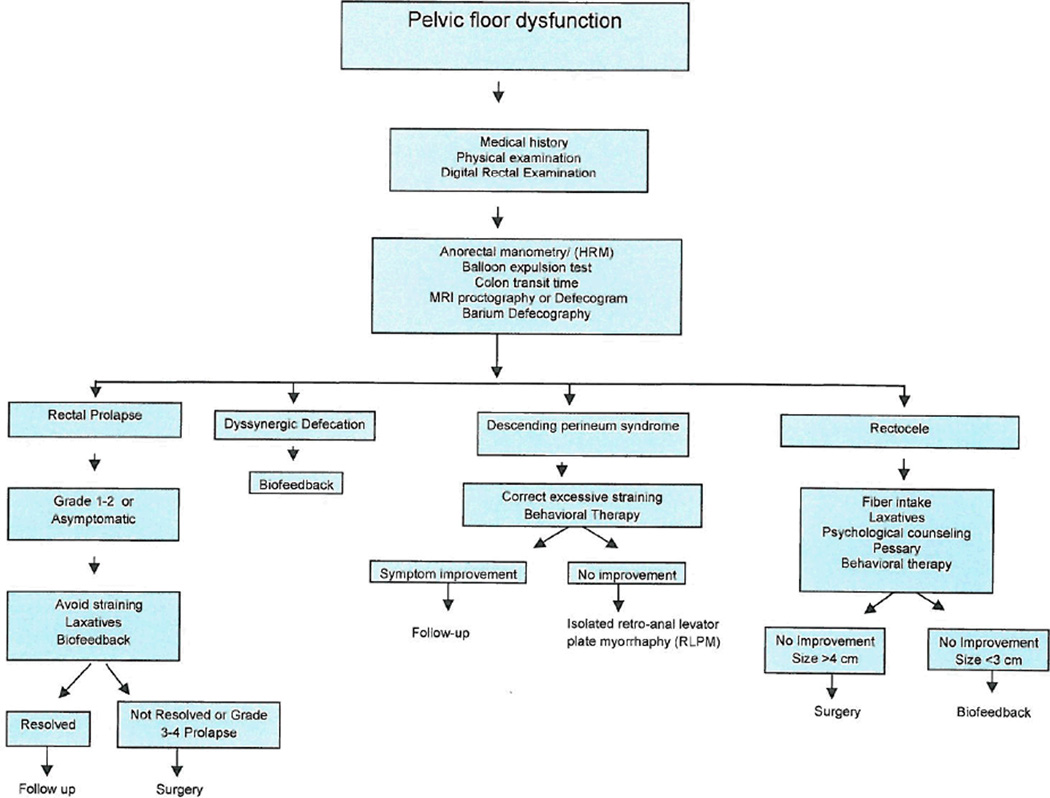
Pelvic floor disorders that cause difficulty with defecation are very common and predominantly affect women. A gastroenterologist or colorectal surgeon is best suited to evaluate and manage these problems but there is lack of experience and working knowledge of these conditions. Physiologic tests such as ARM, balloon expulsion test, and imaging such as defecography and MRI play a key role in objective diagnosis. Biofeedback therapy is an established treatment not only for patients with DD but also for others such as SRUS. Dyssynergia may also co-exist with other structural disorders such as SRUS or rectocele. Hence, before considering surgery, biofeedback therapy should be considered. Correcting the underlying pathophysiological dysfunction offers patients a better control of their symptoms. (Figure 7).
Figure 7.
Suggested algorithm for evaluation and management of common pelvic floor disorders
Several surgical approaches including open, laparoscopic, trans-abdominal approach, stapled transanal rectal resection, and robotic colon and rectal resections have been advocated, and may prove useful in selected cases, but lack randomized controlled trials and rigorous outcome measures.
Acknowledgments
Grant Support: Dr. Satish Rao is supported by NIH grant RO1 DK 57100-05
Footnotes
Disclosures: none
Conflict of Interest
- Which author is the guarantor of the manuscript? Dr. Satish Rao; however, Dr. Ron Schey will be the corresponding author.
- What was each author’s contribution to the paper? Dr. Ron Schey – collecting all the data and writing the manuscript. Dr. Satish Rao – initiating the manuscript and critical reviewing of the manuscript. Dr. John Cromwell – wrote the surgical aspect and treatments of all the disorders.
- What financial support was given to the project? – none
- What potential competing interests exist? - none
References
- 1.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J, Brody DJ. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raizada V, Mittal RK. Pelvic floor anatomy and applied physiology. Gastroenterol Clin North Am. 2008 Sep;37(3):493–509. vii. doi: 10.1016/j.gtc.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azpiroz F, Fernandez-Fraga X, Merletti R, et al. The puborectalis muscle. Neurogastroenterol Motil. 2005 Jun;17(Suppl 1):68–72. doi: 10.1111/j.1365-2982.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Guaderrama N, Nager CW, Pretorius DH, Master S, Mittal RK. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol. 2006 May;101(5):1092–1097. doi: 10.1111/j.1572-0241.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 5.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: A failure of rectoanal coordination. Am J Gastroenterol. 1998 Jul;93(7):1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS, Tuteja AK, Vellema T, et al. Dyssynergic defecation: Demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2004 Sep;38(8):680–685. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 7.Tantiphlachiva K, Rao P, Attaluri A, Rao SS. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010 Nov;8(11):955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Wong R, Drossman D, Bharucha A, Rao SSC, Wald A, Morris C, Oxentenko A, Van Handel D, Edwards H, Hu Y, Bangdiwala S. The Utility of the Digital Rectal Exam (DRE) Amongst Physicians and Students: A Multi-Center Study. Gastroenterology. 2010;138:S-472. [Google Scholar]
- 9.Bharucha AE. Update of tests of colon and rectal structure and function. J Clin Gastroenterol. 2006 Feb;40(2):96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 10.Faccioli N, Comai A, Mainardi P, Perandini S, Moore F, Pozz-Mucelli RP. Defecography: a practical approach. Diagn Interv Radiol. 2010 Sep;16(3):209–216. doi: 10.4261/1305-3825.DIR.2584-09.1. [DOI] [PubMed] [Google Scholar]
- 11.Rao SS, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999 Mar;94(3):773–783. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 12.Rao SS, Azpiroz F, Diamant N, et al. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002 Oct;14(5):553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 13.Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol. 2006 Dec;101(12):2790–2796. doi: 10.1111/j.1572-0241.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 14.Rao SS. Dyssynergic defecation and biofeedback therapy. Gastroenterol Clin North Am. 2008 Sep;37(3):569–586. doi: 10.1016/j.gtc.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol. 2007 Apr;102(4):850–855. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 16.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004 Jan;126(1):57–62. doi: 10.1053/j.gastro.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 17.Hinton JM, Lennard-Jones JE, Young AC. A new method for studying gut transit times using radioopaque markers. Gut. 1969;10(10):842–847. doi: 10.1136/gut.10.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, Scott MS, Simren M, Soffer E, Szarka L. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterology and motility. 23.1(2011):8–23. doi: 10.1111/j.1365-2982.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Thorne NK, Ringel Y, Hasler WL, Kuo B, Esfandyari T, Gupta A, Scott SM, McCallum RW, Parkman HP, Soffer E, Wilding GE, Semler JR, Rao SS. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil. 2010 Aug;22(8):874–882. doi: 10.1111/j.1365-2982.2010.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao SS, Kuo B, McCallum RW, Chey WD, DiBaise JK, Hasler WL, Koch KL, Lackner JM, Miller C, Saad R, Semler JR, Sitrin MD, Wilding GE, Parkman HP. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009 May;7(5):537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Etienney I, de Parades V. Three-dimensional endoanal ultrasonography in daily proctological practice. Clin Res Hepatol Gastroenterol. 2011 Apr;35(4):260–270. doi: 10.1016/j.clinre.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Flusberg M, Sahni VA, Erturk SM, Mortele KJ. Dynamic MR defecography: assessment of the usefulness of the defecation phase. AJR Am J Roentgenol. 2011 Apr;196(4):W394–W399. doi: 10.2214/AJR.10.4445. [DOI] [PubMed] [Google Scholar]
- 23.Fiaschetti V, Squillaci E, Pastorelli D, Rascioni M, Funel V, Salimbeni C, Fanucci E, Simonetti G, Dynamic MR. defecography with an open-configuration, low-field, tilting MR system in patients with pelvic floor disorders. Radiol Med. 2011 Jun;116(4):620–633. doi: 10.1007/s11547-011-0660-2. [DOI] [PubMed] [Google Scholar]
- 24.Leung FW, Rao SS. Fecal incontinence in the elderly. Gastroenterol Clin North Am. 2009 Sep;38(3):503–511. doi: 10.1016/j.gtc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 25.An evidence - based approach to the management of chronic constipation in north America. Am J Gastroenterol. 2005;100(suppl 1):S1–S4. doi: 10.1111/j.1572-0241.2005.50613_1.x. [DOI] [PubMed] [Google Scholar]
- 26.Attaluri A, Donahoe R, Valestin J, Brown K, Rao SS. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011 Apr;33(7):822–828. doi: 10.1111/j.1365-2036.2011.04594.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramkumar D, Rao S. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. The American Journal of Gastroenterology. 2005;100:936–971. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]
- 28.Lembo AJ, Johanson JF, Parkman HP, Rao SS, Miner PB, Jr, Ueno R. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig Dis Sci. 2011 Sep;56(9):2639–2645. doi: 10.1007/s10620-011-1801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tack J. Prucalopride: a new drug for the treatment of chronic constipation. Expert Rev Gastroenterol Hepatol. 2009 Aug;3(4):337–343. doi: 10.1586/egh.09.38. [DOI] [PubMed] [Google Scholar]
- 30.Lembo AJ, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, Jeglinski BI, Johnston JM. Efficacy of linaclotide for patients with chronic constipation. Gastroenterology. 2010 Mar;138(3):886–895. doi: 10.1053/j.gastro.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 31.Rao SS, Welcher KD, Pelsang RE. Effects of biofeedback therapy on anorectal function in obstructive defecation. Dig Dis Sci. 1997 Nov;42(11):2197–2205. doi: 10.1023/a:1018846113210. [DOI] [PubMed] [Google Scholar]
- 32.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–664. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum. 2007;50:428–441. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]
- 34.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul;129(1):86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Rao SS, Valestin J, Brown CK, Zimmerman B, Schulze K. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. Am J Gastroenterol. 2010 Apr;105(4):890–896. doi: 10.1038/ajg.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao SS, Valestin J, Brown CK, Hamdy S, Bradley C, Schulze KS, Zimmerman B. Home or office Biofeedback Therapy for Dyssynergic Defecation- Randomized Controlled Trial. 2011 doi: 10.1038/ajg.2010.53. Abstract DDW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Go J, Valestin J, Brown CK, Bradley C, Schulze KS, Hamdy S, Rao SS. Is Biofeedback Therapy Effective in improving quality of life in Dyssynergic Defecation? A Randomized Clinical trial. 2011 Abstract DDW. [Google Scholar]
- 38.Felt-Bersma RJ, Stella MT, Cuesta MA. Rectal prolapse, rectal intussusception, rectocele, solitary rectal ulcer syndrome, and enterocele. Gastroenterol Clin North Am. 2008 Sep;37(3):645–668. ix. doi: 10.1016/j.gtc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Madoff RD, Mellgren A. One hundred years of rectal prolapse surgery. Dis Colon Rectum. 1999 Apr;42(4):441–450. doi: 10.1007/BF02234164. [DOI] [PubMed] [Google Scholar]
- 40.Bharucha AE. Update of tests of colon and rectal structure and function. J Clin Gastroenterol. 2006 Feb;40(2):96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 41.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol. 2007 Apr;102(4):850–855. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 42.Tou S, Brown SR, Malik AI, et al. Surgery for complete rectal prolapse in adults. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD001758.pub2. CD001758. [DOI] [PubMed] [Google Scholar]
- 43.Deen KI, Grant E, Billingham C, et al. Abdominal resection rectopexy with pelvic floor repair versus perineal rectosigmoidectomy with pelvic floor repair for full thickness rectal prolapse. Br J Surg. 1994;81(2):302–304. doi: 10.1002/bjs.1800810253. [DOI] [PubMed] [Google Scholar]
- 44.HCUP Nationwide Inpatient Sample (NIS) Rockville, MD: Agency for Healthcare Research and Quality; 2007–2009. Healthcare Cost and Utilization Project (HCUP) www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 45.Hoel AT, Skarstein A, Ovrebo KK. Prolapse of the rectum, long-term results of surgical treatment. Int J Colorectal Dis. 2009 Feb;24(2):201–207. doi: 10.1007/s00384-008-0581-2. [DOI] [PubMed] [Google Scholar]
- 46.Agachan F, Reisman P, Pfeifer J, et al. Comparison of three perineal procedures for treatment of rectal prolapse. South Med J. 1997;90(9):925–932. doi: 10.1097/00007611-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Chun SW, Pikarsky AJ, You SY, Gervaz P, Efron J, Weiss E, Nogueras JJ, Wexner SD. Perineal rectosigmoidectomy for rectal prolapse: role of levatorplasty. Tech Coloproctol. 2004 Mar;8(1):3–8. doi: 10.1007/s10151-004-0042-z. [DOI] [PubMed] [Google Scholar]
- 48.Zimmern A, Prasad L, Desouza A, Marecik S, Park J, Abcarian H. Robotic Colon and Rectal Surgery: A Series of 131 Cases. World J Surg. 2010 Aug;34(8):1954–1958. doi: 10.1007/s00268-010-0591-4. [DOI] [PubMed] [Google Scholar]
- 49.Boons P, Collinson R, Cunningham C, Lindsey I. Laparoscopic ventral rectopexy for external rectal prolapse improves constipation and avoids de novo constipation. Colorectal Dis. 2010 Jun;12(6):526–532. doi: 10.1111/j.1463-1318.2009.01859.x. [DOI] [PubMed] [Google Scholar]
- 50.Dietz HP, Clarke B. Prevalence of rectocele in young nulliparous women. Aust N Z J Obstet Gynaecol. 2005 Oct;45(5):391–394. doi: 10.1111/j.1479-828X.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 51.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J, Brody DJ. Prevalence of symptomatic pelvic floor disorders in US women. Pelvic Floor Disorders Network. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beevors MA, Lubowski DZ, King DW, et al. Pudendal nerve function in women with symptomatic utero-vaginal prolapse. Int J Colorectal Dis. 1991 Feb;6(1):24–28. doi: 10.1007/BF00703956. [DOI] [PubMed] [Google Scholar]
- 53.Mellgren A, Lopez A, Schultz I, et al. Rectocele is associated with paradoxical anal sphincter reaction. Int J Colorectal Dis. 1998;13(1):13–16. doi: 10.1007/s003840050124. [DOI] [PubMed] [Google Scholar]
- 54.Go JT, Balawi T, Schneider M, Valestin J, Rao SSC. Is Dyssynergia Associated with Rectoceles? Neurogastroenterol Mot. 2011;19:63. [Google Scholar]
- 55.Ben Amna M, Grise P, Michot F, Sibert L. Impact of rectal prolapse and rectocele on TVT results. Prog Urol. 2003 Jun;13(3):453–458. [PubMed] [Google Scholar]
- 56.Van Dam JH, Ginai AZ, Gosselink MJ, et al. Role of defecography in predicting clinical outcome of rectocele repair. Dis Colon Rectum. 1997;40:201–207. doi: 10.1007/BF02054989. [DOI] [PubMed] [Google Scholar]
- 57.Marrufo-García CA, Sánchez-Avila MT, Morales-Garza LA, Carrillo-Martínez MA, Aguirre-Mar D, Sánchez-Avila JF. Manometry and defecography in constipated patients with dyschezia. Rev Gastroenterol Mex. 2005 Oct-Dec;70(4):424–429. [PubMed] [Google Scholar]
- 58.Hecht EM, Lee VS, Tanpitukpongse TP, Babb JS, Taouli B, Wong S, Rosenblum N, Kanofsky JA, Bennett GL. MRI of pelvic floor dysfunction: dynamic true fast imaging with steady-state precession versus HASTE. AJR Am J Roentgenol. 2008 Aug;191(2):352–358. doi: 10.2214/AJR.07.3403. [DOI] [PubMed] [Google Scholar]
- 59.Flusberg M, Sahni VA, Erturk SM, Mortele KJ, Dynamic MR. defecography: assessment of the usefulness of the defecation phase. AJR Am J Roentgenol. 2011 Apr;196(4):W394–W399. doi: 10.2214/AJR.10.4445. [DOI] [PubMed] [Google Scholar]
- 60.Pescatori M, Spyrou M, Pulvirenti d'Urso A. A prospective evaluation of occult disorders in obstructed defecation using the 'iceberg diagram'. Colorectal Dis. 2007 Jun;9(5):452–456. doi: 10.1111/j.1463-1318.2006.01094.x. [DOI] [PubMed] [Google Scholar]
- 61.Kudish BI, Iglesia CB. Posterior wall prolapse and repair. Clin Obstet Gynecol. 2010 Mar;53(1):59–71. doi: 10.1097/GRF.0b013e3181cd41e3. [DOI] [PubMed] [Google Scholar]
- 62.Farid M, Madbouly KM, Hussein A, Mahdy T, Moneim HA, Omar W. Randomized controlled trial between perineal and anal repairs of rectocele in obstructed defecation. World J Surg. 2010 Apr;34(4):822–829. doi: 10.1007/s00268-010-0390-y. [DOI] [PubMed] [Google Scholar]
- 63.Nieminen K, Hiltunen KM, Laitinen J, Oksala J, Heinonen PK. Transanal or vaginal approach to rectocele repair: a prospective, randomized pilot study. Dis Colon Rectum. 2004 Oct;47(10):1636–1642. doi: 10.1007/s10350-004-0656-2. [DOI] [PubMed] [Google Scholar]
- 64.Madbouly KM, Abbas KS, Hussein AM. Disappointing long-term outcomes after stapled transanal rectal resection for obstructed defecation. World J Surg. 2010 Sep;34(9):2191–2196. doi: 10.1007/s00268-010-0638-6. [DOI] [PubMed] [Google Scholar]
- 65.Zhang B, Ding JH, Yin SH, Zhang M, Zhao K. Stapled transanal rectal resection for obstructed defecation syndrome associated with rectocele and rectal intussusception. World J Gastroenterol. 2010 May 28;16(20):2542–2548. doi: 10.3748/wjg.v16.i20.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harewood GC, Coulie B, Camilleri M, Rath-Harvey D, Pemberton JH. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999 Jan;94(1):126–130. doi: 10.1111/j.1572-0241.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- 67.Pucciani F, Boni D, Perna F, et al. Descending perineum syndrome: Are abdominal hysterectomy and bowel habits linked? Dis Colon Rectum. 2005 Nov;48(11):2094–2099. doi: 10.1007/s10350-005-0163-0. [DOI] [PubMed] [Google Scholar]
- 68.Wexner SD, Stollman N. Diseases of the Colon. CRC Press; 2006. Perineal descent syndrome; p. 127. [Google Scholar]
- 69.Karulf RE, Coller JA, Bartolo DC, Bowden DO, Roberts PL, Murray JJ, Schoetz DJ, Jr, Veidenheimer MC. Anorectal physiology testing. A survey of availability and use Dis Colon Rectum. 1991 Jun;34(6):464–468. doi: 10.1007/BF02049930. [DOI] [PubMed] [Google Scholar]
- 70.Chi TW, Chen SH. Dynamic magnetic resonance imaging used in evaluation of female pelvic prolapse: experience from nine cases. Kaohsiung J Med Sci. 2007 Jun;23(6):302–308. doi: 10.1016/S1607-551X(09)70413-9. [DOI] [PubMed] [Google Scholar]
- 71.Lesaffer L, Milo R. Descending perineum syndrome: control defecogram with a "perineum device", perspective in prevention and conservative therapy. J Belge Radiol. 1988;71(6):709–712. [PubMed] [Google Scholar]
- 72.Beco J. Interest of retro-anal levator plate myorrhaphy in selected cases of descending perineum syndrome with positive anti-sagging test. BMC Surg. 2008 Jul 30;8:13. doi: 10.1186/1471-2482-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haray PN, Morris-Stiff GJ, Foster ME. Solitary rectal ulcer syndrome: an underdiagnosed condition. Int J Colorectal Dis. 1997;12:313–315. doi: 10.1007/s003840050113. [DOI] [PubMed] [Google Scholar]
- 74.Tjandra JJ, Fazio VW, Church JM, et al. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum. 1992;35:227–234. doi: 10.1007/BF02051012. [DOI] [PubMed] [Google Scholar]
- 75.Womack NR, Williams NS, Mist JH, et al. Anorectal function in the solitary rectal ulcer syndrome. Dis Colon Rectum. 1987;30:319–323. doi: 10.1007/BF02555447. [DOI] [PubMed] [Google Scholar]
- 76.Rao SS, Ozturk R, De Ocampo S, et al. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol. 2006;101:613–618. doi: 10.1111/j.1572-0241.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 77.Ho YH, Ho JM, Parry BR, et al. Solitary rectal ulcer syndrome: the clinical entity and anorectal physiological findings in Singapore. Aust N Z J Surg. 1995;65:93–97. doi: 10.1111/j.1445-2197.1995.tb07268.x. [DOI] [PubMed] [Google Scholar]
- 78.Kang YS, Kamm MA, Engel AF, et al. Pathology of the rectal wall in solitary rectal ulcer syndrome and complete rectal prolapse. Gut. 1996;38:587–590. doi: 10.1136/gut.38.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halligan S, Sultan A, Rottenberg G, Bartram CI. Endosonography of the anal sphincters in solitary rectal ulcer syndrome. Int J Colorectal Dis. 1995;10(2):79–82. doi: 10.1007/BF00341201. [DOI] [PubMed] [Google Scholar]
- 80.Tsuchida K, Okayama N, Miyata M, et al. Solitary rectal ulcer syndrome accompanied by submucosal invasive carcinoma. Am J Gastroenterol. 1998;93:2235–2238. doi: 10.1111/j.1572-0241.1998.00622.x. [DOI] [PubMed] [Google Scholar]
- 81.Daya D, O'Connell G, DeNardi F. Rectal endometriosis mimicking solitary rectal ulcer syndrome. Mod Pathol. 1995;8:599–602. [PubMed] [Google Scholar]
- 82.Halligan S, Nicholls RJ, Bartram CI. Evacuation proctography in patients with solitary rectal ulcer syndrome: anatomic abnormalities and frequency of impaired emptying and prolapse. AJR Am J Roentgenol. 1995;164:91–95. doi: 10.2214/ajr.164.1.7998576. [DOI] [PubMed] [Google Scholar]
- 83.Keighley MR, Shouler P. Clinical and manometric features of the solitary rectal ulcer syndrome. Dis Colon Rectum. 1984;27:507–512. doi: 10.1007/BF02555506. [DOI] [PubMed] [Google Scholar]
- 84.Ederle A, Bulighin G, Orlandi PG, et al. Endoscopic application of human fibrin sealant in the treatment of solitary rectal ulcer syndrome. Endoscopy. 1992;24:736–737. doi: 10.1055/s-2007-1010574. [DOI] [PubMed] [Google Scholar]
- 85.Somani SK, Ghosh A, Avasthi G, Goyal R, Gupta P. Healing of solitary rectal ulcers with multiple sessions of argon plasma coagulation. Dig Endosc. 2010 Apr;22(2):107–111. doi: 10.1111/j.1443-1661.2010.00941.x. [DOI] [PubMed] [Google Scholar]
- 86.Jarrett ME, Emmanuel AV, Vaizey CJ, et al. Behavioural therapy (biofeedback) for solitary rectal ulcer syndrome improves symptoms and mucosal blood flow. Gut. 2004;53:368–370. doi: 10.1136/gut.2003.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sitzler PJ, Kamm MA, Nicholls RJ, et al. Long-term clinical outcome of surgery for solitary rectal ulcer syndrome. Br J Surg. 1998;85:1246–1250. doi: 10.1046/j.1365-2168.1998.00854.x. [DOI] [PubMed] [Google Scholar]
- 88.Torres C, Khaikin M, Bracho J, et al. Solitary rectal ulcer syndrome: clinical findings, surgical treatment, and outcomes. Int J Colorectal Dis. 2007;22:1389–1393. doi: 10.1007/s00384-007-0344-5. [DOI] [PubMed] [Google Scholar]