Abstract
Existing research has not fully explained how different types of ionizing radiation (IR) modulate the responses of cell populations or tissues. In our previous work, we showed that gap junction intercellular communication (GJIC) mediates the propagation of stressful effects among irradiated cells exposed to high linear energy transfer (LET) radiations, in which almost every cells is traversed by an IR track. In the present study, we conducted an in-depth study of the role of GJIC in modulating the repair of potentially lethal damage (PLDR) and micronuclei formation in cells exposed to low- or high-LET IR. Confluent human fibroblasts were exposed in the presence or absence of a gap junction inhibitor to 200 kV X rays (LET ∼ 1.7 keV/µm), carbon ions (LET ∼ 76 keV/µm), silicon ions (LET ∼ 113 keV/µm) or iron ions (LET ∼ 400 keV/µm) that resulted in isosurvival levels. The fibroblasts were incubated for various times at 37 °C. As expected, high-LET IR were more effective than were low-LET X rays at killing cells and damaging DNA shortly after irradiation. However, when cells were held in a confluent state for several hours, PLDR associated with a reduction in DNA damage, occurred only in cells exposed to X rays. Interestingly, inhibition of GJIC eliminated the enhancement of toxic effects, which resulted in an increase of cell survival and reduction in the level of micronucleus formation in cells exposed to high, but not in those exposed to low-LET IR. The experiment shows that gap-junction communication plays an important role in the propagation of stressful effects among irradiated cells exposed to high-LET IR while GJIC has only a minimal effect on PLDR and DNA damage following low-LET irradiation. Together, our results show that PLDR and induction of DNA damage clearly depend on gap-junction communication and radiation quality.
Keywords: Gap junction intercellular communication, Potentially lethal damage repair, Linear energy transfer, Ionizing radiation, Heavy-ion beams
1. Introduction
It is now generally accepted that ionizing radiation (IR)-induced lethal damage can be attenuated by appropriate post-irradiation treatment conditions [1,2]. Holding X-irradiated cells in the confluent state for several hours after irradiation significantly enhanced their survival [3]. This protective effect is due to the repair of potentially lethal damage (PLD) [3,4]. Most PLD repair (PLDR) studies have been performed in mammalian cells exposed to low linear energy transfer (LET) radiation such as X and γ rays [5,6]; fewer studies have observed this phenomenon in cells exposed to high-LET IR such as α particles, high-charged, high-energy (HZE) particles or heavy ions [6–8]. While much is known about factors influencing PLDR, comparatively little is known about the underlying mechanisms involved, especially in the case of cells exposed to high-LET IR. This is often explained by the hypothesis that the repair mechanisms are less effective with lesions generated by high-LET IR [4]. In general, high-LET IR induces clustered DNA damage is less easily repaired and subsequently leads to a more efficient cell killing than low-LET IR [9–11] However, the exact mechanisms occurring in cells irradiated by high-LET IR remain undefined and are likely to depend on cell-to-cell communication [12–14].
During the past decade, several mechanisms have been discovered in the propagation of IR-induced damaging effects in cells exposed to low- or high-LET IR. They include intercellular communication via the gap-junction and/or secreted cytotoxic factors from culture medium, perturbations of oxidative metabolism, and other mechanisms [15–17]. Gap junction intercellular communications (GJIC) linking adjacent cells were shown, by direct approaches, to mediate the propagation of toxic effects among irradiated cells [13,14] and between irradiated and non-irradiated bystander cells [15–18]. Whether they contribute to the propagation of stressful or protective effects among irradiated cells with low- or high-LET IR is not well understood.
Gap-junctions are dynamic structures that are critical for diverse physiological functions [19]. The intercellular channels that comprise gap-junctions are formed by connexin protein, and each of the ∼20 isoforms of connexins forms channels with distinct permeability properties [19]. By allowing direct intercellular communication between ions and low-molecular weight molecules, gap-junctions provide a powerful pathway for molecular signaling between neighboring cells. Though the properties of channels formed by each isoform differ, in general, connexin pores are considered to allow permeation of small molecules up to 1 kDa, which is well above the size of second messengers [19].
Previous studies performed at the NASA Space Radiation Laboratory (using space radiation such as high energy protons and HZE particles) and the New Jersey Medical School Cancer Center (using γ rays and α particles) showed significant PLDR in confluent normal human skin fibroblasts following exposure to low-, but not high-LET IR. However, inhibition of GJIC with a chemical inhibitor or down regulation of connexin43, a constitutive protein of junctional channels, protected against the toxic effects expressed in cells exposed to high-LET radiation during confluent holding [13,14]. The role of GJIC in PLDR and DNA damage remains unclear. Hence, the aim of our present study is to extend in vitro research specifically to the role of GJIC in the biological responses to various IR types, namely X rays, carbon ions, silicon ions or iron ions. To this end, normal NB1RGB human skin fibroblasts were plated in subconfluent or confluent monolayer, in the presence or absence of gap-junction inhibitor, exposed to different types of IR of varying LET from ∼1.7 to 400 keV/µm and assessed for clonogenic survival and micronucleus formation as biological endpoints.
2. Materials and methods
2.1. Cell culture
Low passage NB1RGB normal human skin fibroblasts obtained from the Riken BioResource in Tsukuba, Japan (Cell No. RCB0222) at passages 6–8 were grown in Eagle's minimum essential medium (MEM: NISSUE Pharmaceutical Co. Ltd., Japan) containing kanamycin (60 mg/L), supplemented with 10% fetal bovine serum (FBS: HyClone, Thermo Scientific, USA). They were maintained in 37 °C humidified incubators in an atmosphere of 5% CO2 in air. For experiments with confluent cultures (Fig. 1A), the cells were seeded at a density of ∼5 × 105 cells/dish in 25-cm2 polystyrene flasks (BD Falcon™, 353014) that allowed them to reach the density-inhibited state within 5 days. The experiments were initiated 48 h after the last feeding. Under these conditions, ∼93–94% of the cells were in the G0/G1-phase, as determined by flow cytometry (data not shown), allowing direct intercellular communication via the gap-junction. In the case of experiments with subconfluent cultures (Fig. 1B), the cells were seeded at a density of ∼1 × 105 cells/dish, 5 days prior to irradiation that allowed them to be ∼60% confluent and not in contact which each other at the time of irradiation. At 24 h before irradiation, they were incubated with MEM supplemented with 1% FBS to enrich the population with cells in G0/G1-phase. The synchronization of cells in G0/G1-phase eliminates complications in interpretation of results because radiation sensitivity changes at different phases of the cell cycle [6,13,14]. To compare the effects on confluent and subconfluent cell cultures, confluent cells were fed with MEM supplemented with 1% FBS.
Fig. 1.
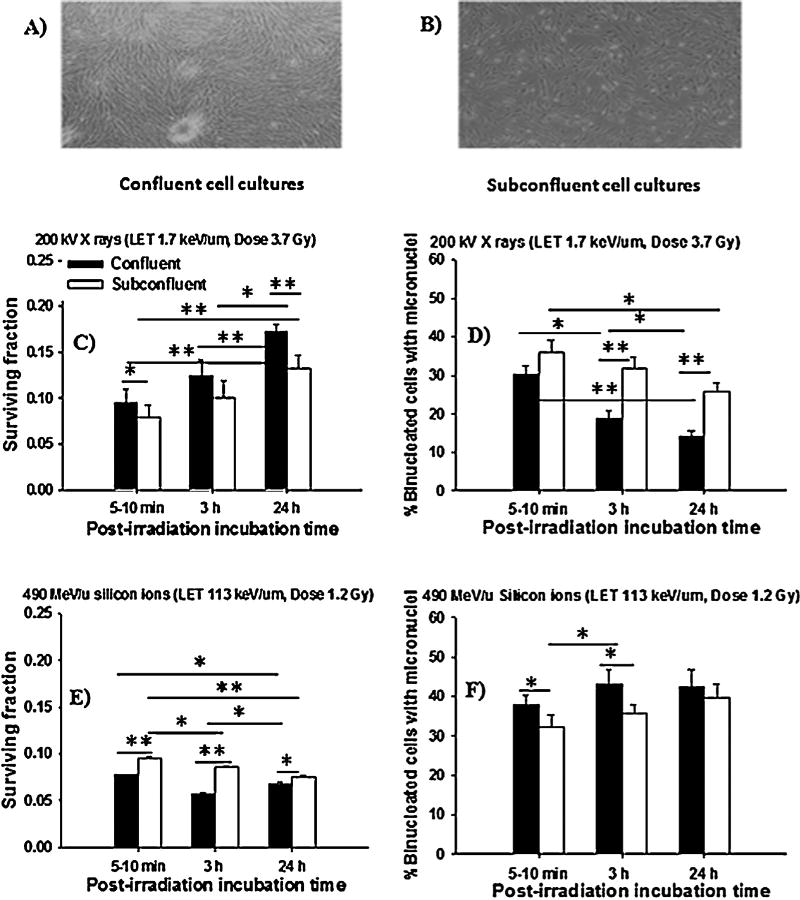
Gap junction intercellular communication in the propagation of stressful effects among NB1RGB human cells exposed to low-LET X rays and high-LET silicon ions followed by 5–10 min, 3 h or 24 h incubation at 37 °C and held in a confluent or subconfluent state. (Panel (A), confluent cell cultures; Panel (B), subconfluent cell cultures; Panel (C), clonogenic survival of confluent and subconfluent cells after exposure to X rays; Panel (D), fraction of micronucleated cells in irradiated cells held in a confluent or subconfluent state after irradiation with X rays; Panel (E), clonogenic survival of confluent and subconfluent cells exposed to silicon ions; Panel (F), fraction of micronucelated cells in irradiated cells held in a confluent or subconfluent state exposed to silicon ions) (*p < 0.05; **p < 0.01).
2.2. Irradiation
NB1RGB cell cultures were exposed to 3.7 Gy from 200 kV X rays (LET ∼1.7 keV/µm) with 0.5-mm aluminum and 0.5-mm copper filters. For high-LET radiation, they were carried out at the biology experiment port of the Heavy Ion Medical Accelerator in Chiba (HIMAC) at the National Institute of Radiological Sciences (NIRS) in Japan. The cells were irradiated with the initial energy of carbon ions (290 MeV/u, Dose 1.4 Gy, LET ∼ 76 keV/µm), silicon ions (490 MeV/u, Dose 1.2 Gy, LET ∼ 113 keV/µm) and iron ions (500 MeV/u, Dose 1.3 Gy, LET ∼ 400 keV/µm) that resulted in isosurvival levels, evaluated at D10, the dose required to reduce the survival fraction to 10% [20–22]. The radiation dose and LET at the sample position are listed in Table 1. In all cases, control cells were handled as the cells destined for irradiation. All irradiation was carried out at room temperature.
Table 1.
Dosimetry parameters for NB1RGB-irradiated cells exposure to high-LET radiations.
| Radiation | Initial energy (MeV/u) | Dose (Gy) | Fluence (particle/cm2) | LETa (keV/µm) | Unhit fraction | Hit fraction | Average hits (per cell nucleus) | Inactivation cross-section (µm2) |
|---|---|---|---|---|---|---|---|---|
| Carbon ions | 290 | 1.4 | 1.14 × 107 | 76.0 | 0 | 1 | 20 | 8.69 |
| Silicon ions | 490 | 1.2 | 6.63 × 106 | 113.0 | 0 | 1 | 10 | 15.08 |
| Iron ions | 500 | 1.3 | 2.02 × 106 | 400.0 | 0.03 | 0.97 | 3 | 49.29 |
The LETs at the sample position.
The beam characteristics, biological irradiation procedures, and dosimetry at HIMAC have been described elsewhere [23,24]. The dosimetery of heavy-ion beams was checked by an ionization chamber method, by a fluence measurement method using CR 39 track detectors, and by measurement with a silicon diode. The LET values were adjusted by changing the thickness of the absorbers, which were made of acrylic synthetic resin, in front of the sample. Then, the radiation doses at the sample position were determined by multiplying the fluence by the LET, taking fragmentation into consideration [20–24]. The fluence of each ion species was counted by using a scintillation counter and then converted to the dose using the following equation (Table 1):
The absorbed dose which is approximated from the average energy deposited by a single ion traversal through the cell nucleus of NB1RGB cells and the percentage of cells traversed in an exposed culture can be calculated [25,26], for a more detailed description of the standard calculation methods we refer to the work of Charlton and Sephton [26]. Briefly, the dose per traversal to the thin disk-shaped cell nucleus of the NB1RGB cell is d = (0.16) (LET)/A where A is the average cross-sectional area of the cell nucleus. The units for d, LET and A are Gy, keV/µm, and µm2, respectively. Considering that the LET of X rays, carbon ions, silicon ions and iron ions are ∼1.7, 76, 113 and 400 keV/µm, respectively, and the mean nuclear area of an NB1RGB cell is ∼172.3 ± 2.8 µm2 measured in confluent cultures grown under the same conditions as in this study [20], the absorbed dose per particle traversal from carbon ions, silicon ions and iron ions would be ∼0.0705, 0.1049 and 0.3714 Gy, respectively. Estimation of the fractions of nuclei traversed by low- or high-LET IR was calculated assuming Poisson statistics and is given in Table 1. The probability P that a given target area is traversed by N particle is given by P (N) = e−χ·χN/N! with χ being the product of the fluence and the nucleus cross-sectional area. Thus, in NB1RGB cells exposed to X rays, carbon ions, silicon ions and iron ions, 100, 100, 100 and ∼97% of the cells in the population would be traversed through the nucleus by IR tracks.
2.3. Cell survival
Survival fractions were generated by a standard colony formation assay. Briefly, confluent cell cultures were trypsinized within 5–10 min after irradiation or incubation periods at 37 °C for 3 h, which usually allows the repair activity and/or the commitment for permanent arrest in the cell cycle to occur [3], or 24 h, by which time most of the radiation-induced DNA damage is usually repaired and/or commitment to reproductive inactivation has happened [27]. Following dissociation, the cells were suspended in growth medium, counted, diluted, and seeded in 10-cm dishes (BD Falcon™, 353003) at numbers estimated to result in ∼100–150 clonogenic cells per dish. After incubation for 2 weeks, the plates were rinsed with phosphate buffered saline (PBS), fixed in 20% methanol, and stained with 0.2% crystal violet, and colonies consisting of 50 cells or more were counted as survivors. There was no significant difference in plating efficiency between confluent and subconfluent cell cultures in the control samples (data not shown).
2.4. Micronucleus formation
The fraction of micronucleated cells in the exposed cultures was examined using the cytokinesis block technique [28]. Briefly, after irradiation, both irradiated and non-irradiated control cells were subcultured by seeding ∼3 × 104 cells in chamber flasks (NUNC 177437, Nalgene Nunc International, Denmark) and grown in the presence of 2 µg/ml cytochalsin B (Sigma C2743, Sigma–Aldrich, USA). The cultures were maintained at 37 °C for 72 h in order to obtain an optimum frequency of binucleated cells [13,14]. Then the cells were rinsed in PBS, fixed in ethanol, stained with Hoechst 33342 solution (1 µg/ml in PBS) (Wako Pure Chemical Industries Ltd., Japan) and viewed under a fluorescence microscope (Zeiss Axioplan2 imaging microscope, Germany). At least 500–1000 cells were examined for each data point in each individual experiment, and only micronuclei in binucleated cells were considered for analysis.
2.5. Inhibition of gap-junction intercellular communication
18-α-Glycyrrhetic acid (AGA: Sigma G10105, Sigma–Aldrich, USA), a reversible inhibitor of gap junction communication, was dissolved in dimethyl sulfoxide (DMSO: Wako Pure Chemical Industries Ltd., Japan) and added to cell cultures at a concentration of 50 µM at 30 min prior to irradiation. The cells were incubated in the presence of the drug until they were trypsinized. Under this protocol, AGA did not alter the plating efficiency or micronucleus formation of non-irradiated cells but it did inhibit cell coupling (data not shown). Control cell cultures were incubated with the dissolving vehicle.
2.6. Monte Carlo simulation of heavy-ion track structure
The software Relativistic Ion Tracks (RITRACKS) uses Monte Carlo simulation code to simulate radiation tracks for heavy ions and electrons [29,30]. In principle, any ion tracks can be simulated if the energy falls in the range of which the cross sections for interactions of primary particles and secondary electrons with target molecules are known. The RITRACKS program is used to simulate the so-called physical and physicochemical stages of the radiolysis of water [31]. The ion is followed on an event-by-event basis, calculating all ionization and excitation events produced and recording the position of the generated radiolytic species and the energy and the direction of the secondary electrons. This code uses recently revisited ionization and excitation cross sections, which takes the effective charge of the ion and relativistic corrections into account [30]. Similarly, the produced electrons are followed by the electron transport part of the code, which also simulates the ionization, electronic excitation, elastic collisions and dissociative attachment events. RITRACKS has been validated by the calculations of relevant dosimetric quantities such as the stopping power and electron penetration range. For example, the radial dose distributions of ions calculated from RITRACKS are able to successfully reproduce experimental data and calculated from amorphous tracks [30]. The dose deposited by the radiation can be calculated in microvolumes and in nanovolumes [32,33]. In this study, to reproduce the effect of X rays, we use protons whose average LET value obtained in the simulations is ∼1.7 keV/µm.
2.7. Statistical analysis
The statistical significance of the clonogenic survival data was determined by using Student's t test. A statistical analysis of the fraction of micronucleated formation measurements was conducted by using χ2 analysis. Each graph presented in the results section is a representative of three separate experiments, and standard errors of the means are indicated on the figures when they are greater than the size of the points. Unless otherwise indicated, the data shown are from pooled experiments.
3. Results
To gain insight into the mechanisms underlying the enhancement of toxicity during the incubation period in human skin fibroblasts after low- or high-LET irradiation through intercellular communication mechanisms, we investigated whether direct cell-to-cell communication/physical contact via the gap-junction was involved in the propagation of stressful effects among irradiated cells. Fig. 1 shows the clonogenic survival and micronucleus formation in confluent cell cultures (>90% in contact with neighbors, Fig. 1A) and subconfluent cell cultures (<60% in contact, Fig. 1B) exposed to low-LET X rays (∼1.7 keV/µm) or high-LET silicon ions (∼113 keV/µm) at equivalent doses, evaluated at the 10% survival level [20–22]. We found that the survival fraction in confluent cell cultures exposed to low-LET X rays was significantly increased (p < 0.01) and associated with decreases in micronucleus formation (p < 0.05), as compared with subconfluent cell cultures (Fig. 1C and D). In contrast, a decrease in survival fraction and an increase in micronucleus formation (Fig. 1E and F) were observed at both cell densities exposed to high-LET silicon ions. However, only confluent cell cultures showed a higher radiosensitivity consistent with a higher level of micronucleus formation and a reduction in the survival fraction compared with that of the subconfluent cell cultures. In addition, a similar result was obtained for clonogenic survival and micronucleus formation after exposure to high-LET carbon and iron ions (data not shown). Together, these results suggest that PLDR, and micronucleus formation that is mediated by intercellular communication either by gap-junction communication or by the release of soluble factors into the culture medium from irradiated cells is significantly higher in confluent cultures than in subconfluent cultures.
We further examined the role of GJIC in the propagation of lethal effects among high-LET-irradiated cells. To this end, we exposed confluent holdings of NB1RGB cells to X rays, carbon, silicon or iron ions with LET values from ∼1.7 to 400 keV/µm in the presence or absence of gap-junction inhibitor AGA at isosurvival doses, calculated at the 10% survival level [20–22]. As expected, confluent holdings of NB1RGB cells exposed to 3.7 Gy from X rays for 5–10 min, 3 h and 24 h showed significant PLDR (p < 0.01, Fig. 2A). In contrast, the incubation of confluent cell cultures exposed to carbon ions (Fig. 2B) or iron ions (Fig. 2D), did not result in PLDR, but rather decreased survival following irradiation with silicon ions (Fig. 2C). Similar to clonogenic survival (Fig. 2), when NB1RGB cells were irradiated with X rays and held in confluence for 5–10 min, 3 h and 24 h prior to subculturing to assay for the induction of micronuclei (Fig. 3A), a significant decrease (p < 0.05) in micronucleus formation was found. In contrast, a significant increase in micronucleus formation was observed following irradiation with carbon ions, silicon ions and iron ions (p < 0.01, Fig. 3B–D). In all cases, the survival fraction and the percent of binucleated cells with micronuclei included data from at least three independent experiments, with error calculated as SD.
Fig. 2.
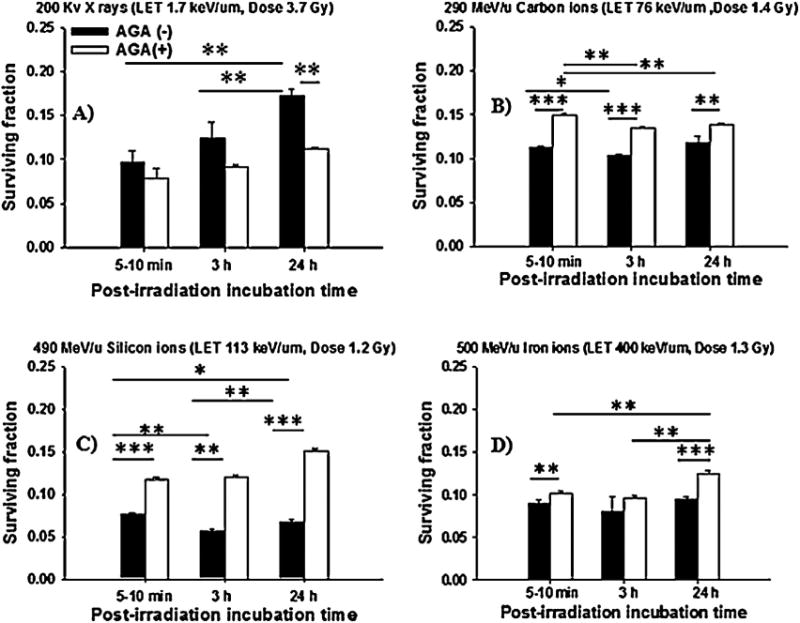
Clonogenic survival of NB1RGB cell cultures exposed to low- or high-LET radiations in the presence or absence of a gap-junction inhibitor AGA at an isosurvival dose and holding in a confluent state for 5–10 min, 3 h or 24 h after irradiation. (Panel (A), X rays; Panel (B), carbon ions; Panel (C), silicon ions; Panel (D), iron ions) (*p < 0.05; **p < 0.01; ***p < 0.001).
Fig. 3.
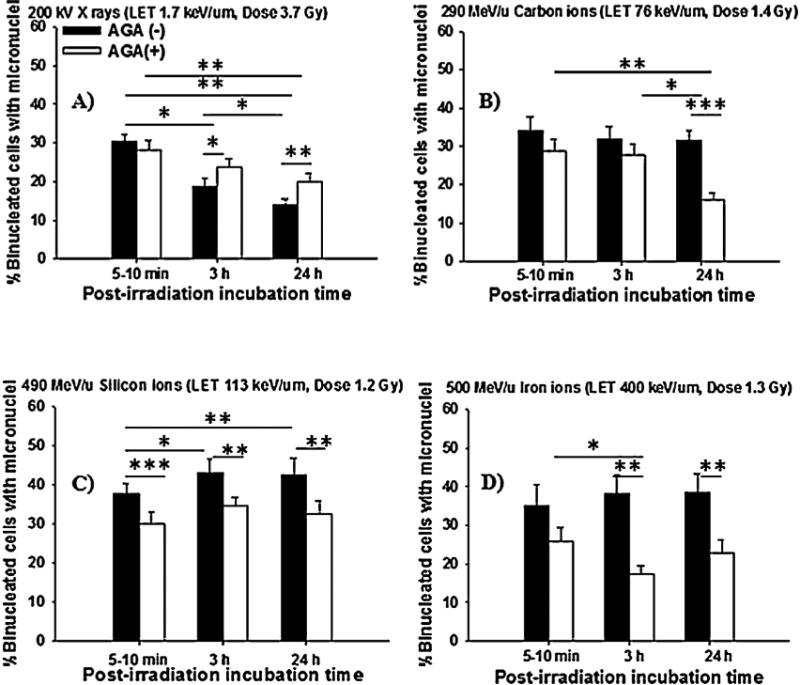
Micronucleus formation in NB1RGB cell cultures exposed to low- or high-LET radiations in the presence or absence of a gap-junction inhibitor AGA at an isosurvival dose and holding in a confluent state for 5–10 min, 3 h or 24 h after irradiation. (Panel (A), X rays; Panel (B), Carbon ions; Panel (C), Silicon ions; Panel (D), Iron ions). (*p < 0.05; **p < 0.01; ***p < 0.001).
Interestingly, Fig. 2A illustrates that treatment with AGA did not significantly affect the increase of survival of X-irradiated cells during the post-irradiation incubation time. Instead, it prevented the decrease in the survival observed in carbon ions, silicon ions and iron ions (Fig. 2B–D). Furthermore, the fraction of micronucleated cells was significantly decreased (p < 0.01) following irradiation to carbon ions, silicon ions and iron ions in the presence of AGA (Fig. 3B–D), while AGA did not alter micronucleus formation in X-irradiated cells (Fig. 3A). Overall, the data presented in Figs. 2 and 3 support the contention that involvement of GJIC in the propagation of high-LET stressful effects during confluent holding causes negligible PLDR and are consistent with the presence of increased DNA damage. In contrast, confluent holding of X-irradiated cells for several hours after irradiation promoted PLDR and decreased chromosomal damage.
4. Discussion
Currently, there are very few research works providing information on the mechanism of PLDR in human cells after high-LET irradiation, particularly in the case of irradiation with heavy ions. Such studies could have important implications for cancer treatment because heavy ions are being used increasingly in radiotherapy. Understanding the biological effects that occur shortly or a few hours after exposure to high-LET IR may help potentiate their therapeutic efficacy and clarify the associated risks to normal tissue to the tumor target. We have previously investigated the role of GJIC in the propagation of lethal effects in human cells exposed to low- or high-LET radiation and our results have shown that GJIC was the major mediator of the induced toxic effects among high-LET-irradiated cells [13,14]. Specifically, with careful attention to cell culture conditions, we studied the mechanisms involved in cellular responses of confluent or subconfluent human fibroblasts exposed to low- or high-LET radiation (Fig. 1). Clearly, we found evidence that PLDR and induction of micronuclei in cells derived from confluent cultures was significantly greater than in cells derived from subconfluent cultures in cells exposed to low-, but not high-LET radiation. These results indicated that the magnitude of the PLDR and micronucleus formation was dependent on cell density and LET of radiation. It entails the involvement of GJIC in transmitting the toxic effects among high-LET-irradiated cells. Our findings are consistent with previous studies showing greater cell killing in high-density cell cultures consisting of gap junction proficient cells following exposure to high-LET radiation [13,14,34,35].
For a better understanding of the radiobiological response of high-LET radiation, we compared the potential of cell killing and the repair of micronucleus formation to 290 MeV/u carbon ions (LET ∼ 76 keV/µm) or 490 MeV/u silicon ions (LET ∼ 113 keV/µm) or 500 MeV/u iron ions (LET ∼ 400 keV/µm) in human fibroblasts. Also, we compared the results with data obtained in confluent cell cultures, to cells exposed to X rays (LET ∼ 1.7 keV/µm) at different times after irradiation. The results demonstrate that a higher level of cell killing and its correlation with induced DNA damage was induced in confluent cell cultures exposed to high-LET heavy ions than low-LET X rays (Figs. 2 and 3). Relative to X rays, the relative biological effectiveness (RBE) of carbon, silicon and iron ions, evaluated at the 10% of survival level was ∼2.64, 3.08 and 2.84, respectively when cells were assayed for clonogenic survival within 5–10 min after irradiation. Significant PLDR occurred when cells were incubated for 3 and 24 h after exposure to X rays. In contrast, no or minimal PLDR occurred in high-LET-irradiated cells (Fig. 2). Specifically among silicon-irradiated cells, the decrease in survival by ∼10–15% following incubation periods of 3 and 24 h likely contributes to the greater biological effectiveness to those induced by carbon ions, iron ions and X rays. Thus the ability of cells to repair DNA damage can serve as an effective measure of RBE of radiation [38]. Taken together, these results show that high-LET-IR induced toxic effects were enhanced rather than attenuated during holding in the confluent state. This indicated that the damage caused by high-LET IR was being repaired poorly or was irreparable and that no or minimal PLDR led to less efficient enzymatic activities in a non-homologous end-joining (NHEJ) repair process [7–9,36–38].
In addition, one could argue that the basis of these differential responses between low- and high-LET IR may arise from the physical energy deposition properties such as the radiation track structure (Table 1 and Fig. 4) [9,10,39,40]. For example, at equivalent doses and the same cross sectional area of cell nucleus, when NB1RGB cell cultures were exposed to low-LET X rays (Fig. 4A), all of the cells would be traversed on average by multiple particle tracks. In contrast, for high-LET carbon, silicon or iron ions, on average 20, 8 and 3 particle tracks, respectively, traverse the nucleus (Table 1 and Fig. 4B–D). This may reflect the severity of the damaging effects of dense ionizations and excitations produced along the high-LET-IR track. The bursts of reactive oxygen species (ROS) in and around these high-LET-IR tracks, as well as the altered cellular redox environment in the intercellular matrix, may modify biomedical reactions, generating damage to cellular molecules and organelles that is far more extensive than the damage produced along low-LET-IR tracks [13,14]. Another issue that must be considered in interpreting these results is particle fluence. For example, the fluence of X rays was higher than that of silicon ions. This indicates that for low-LET X-rays where the dose distribution is rather homogeneous as compared to ions resultimg in a lower amount of clustered DNA damage. In contrast, for the lower fluence and high-LET silicon ions, there would be an increase in energy deposition that results in high ionization density within the track. The damages along high-LET silicon ions are close together, resulting in a large amount of clustered DNA damage lesions that are mostly irreparable. As a consequence, a greater biological effectiveness is observed for silicon ions compared to the other radiations (Figs. 1–3).
Fig. 4.
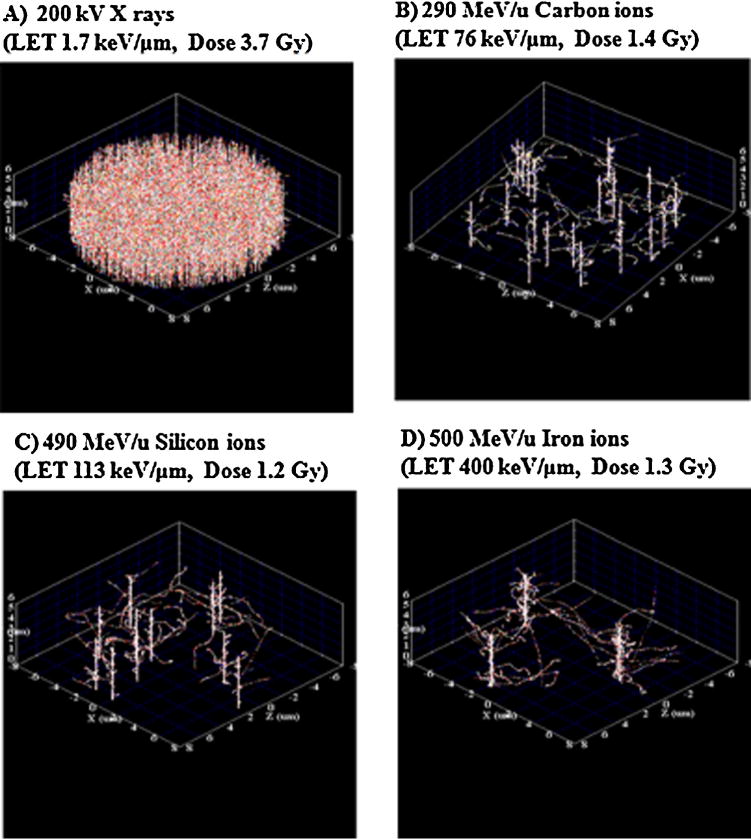
The radiation track structures of one nucleus of NB1RGB cells as simulated by RITRACKS. Each dot corresponds to reactive oxygen species (ROS). (Panel (A), X rays; Panel (B), carbon ions; Panel (C), silicon ions; Panel (D), iron ions).
Moreover, formal calculation of the dose in voxelized space and 3D visualization for the experimental condition given in this paper was performed by the study of Plante et al. [33]. These calculations show how the dose pattern varies in our different experiments. As expected, the voxels with the highest dose correspond to those with ion track cores and electron track ends. Additionally, the experiment and simulation results obtained are generally in good agreement with experimental data by Asaithamby et al. [41]. This indicated that more severe damage may be found in nuclei irradiated by high-LET radiation. As a result of the capability of cells to repair DSBs decreasing with increasing LET, thus the ability of cells to rejoin DSBs is dependent on the LET of the radiation [40]. Furthermore, consistent with the above finding, the results of the cross-sectional area of the target (cell nucleus) can then be estimated as equal to the inactivation cross section (σ) based on the survival data for each IR are shown in Table 1 [20–22,42,43].
This suggests that σ depends on the LET and σ for all IR types are smaller than the geometrical cross sections of an NB1RGB cell nucleus (∼172.3 ± 2.8 µm2) and it increased up to ∼49.29 µm2 with increasing LET (Table 1). This indicates that the sensitive site leading to cell death is smaller than the size of the cell nucleus or that single traversal of any IR does not necessarily lead to cell death [20–22,42,43]. Collectively, these data confirm that the LET of radiation or radiation qualities related to track structure are critical in the propagation of toxic effects among irradiated cells through an intercellular communication mechanism.
We also investigated the possible role of GJIC in the propagation of stressful effects among irradiated cells exposed to high-LET heavy ions, which results in an increase in DNA damage and decreased cell survival following high-LET exposure, as derived from experiments with the gap-junction inhibitor AGA (Figs. 2 and 3). The increase in DNA damage and the absence of PLDR during the incubation period following irradiation by high-LET IR was eliminated when cells were incubated with AGA. Interestingly, the lack of propagation of such stressful effects from cultures only exposed to low-LET X rays, is consistent with previous findings [13,14]. In this context, the propagation of toxic effects among high-LET-irradiated cells would be of significance in radiotherapy. For tumor cells with reduced GJIC, development of drugs that recover GJIC may provide a potent way to enhance treatment of these tumors with high-LET IR. Thus enhancement of GJIC by chemotherapeutic agents in tumor cells, coupled with high-LET radiation, would offer a therapeutic gain. In addition, these findings support the involvement of GJIC in the propagation of stressful or protective effects and suggest that the molecules with different effects, or different amounts in the same molecules, may be propagated through the gap-junction among irradiated cells [13]. In our recent studies, we have shown that human skin fibroblasts express connexins such as Cx26, Cx32, Cx43 and perhaps others [13,14,19]. It is possible that cellular responses to IR may mask differential effects mediated by individual connexin channels. Whereas Cx32 channels propagate a pro-survival effect, Cx26 or Cx43 likely propagate a toxic effect [44]. Elucidation of the nature, signaling and amount of communicated molecules through Cx26, Cx32 or Cx43 should increase our knowledge of the radiobiological response in human cells induced by low-or high-LET radiation.
5. Conclusions
This study highlights the importance of radiation quality and the role of intercellular communication via gap-junctions as a major regulator in the propagation of stressful effects among irradiated human fibroblasts during confluent holding. It illustrates the advantages of using high-LET IR in cancer treatment whenever appropriate. The results confirm that GJIC modulates PLDR and micronucleus formation in cells exposed to high- but not low-LET IR. The enhancement of the killing effect and micronuclei in high-LET-irradiated cells that would otherwise survive the toxic effect after irradiation promoted decreased survival and increased chromosomal damage. Inhibiting GJIC with AGA, and protection against such effects, are crucial mechanisms to be taken into account. In addition, the studies reported here suggest that restoring GJIC and Cx expression, using chemical treatment or by gene transfer, can be used to sensitize tumors with high-LET irradiation. Characterizing the nature of the communicated molecules among irradiated and neighboring non-irradiated cells would have translational implications in radiotherapy and the formulation of countermeasures against the toxic effects of radiation.
Acknowledgments
We highly thank the staff in HIMAC at NIRS for their excellent support. This study was supported by the Research Project with Heavy Ions at NIRS-HIMAC and in part by JSPS KAKENHI Grant Number 23-01513, 18310042, 24620014 and the Quantum Beam Technology Program from the Japan Science and Technology Agency. The authors would like to acknowledge support from the National Institutes of Health grant P01-CA 49062-21 to EIA and TKH. The authors sincerely apologize to those whose work was not cited due to space constraints.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
References
- 1.Phillips RA, Tolmach LJ. Repair of potentially lethal damages in X-irradiated HeLa cells. Radiat Res. 1966;29:413–432. [PubMed] [Google Scholar]
- 2.Elkind MM. Repair processes in radiation biology. Radiat Res. 1984;100:425–449. [PubMed] [Google Scholar]
- 3.Little JB. Repair of sub-lethal and potentially lethal radiation damage in plateau phase cultures of human cells. Nature. 1969;224:804–806. doi: 10.1038/224804a0. [DOI] [PubMed] [Google Scholar]
- 4.Little JB. Factors influencing the repair of potentially lethal radiation damage in growth-inhibited human cells. Radiat Res. 1973;56:320–333. [PubMed] [Google Scholar]
- 5.Weichselbaum RR, Withers HR, Tomkinson K, Little J. Potentially lethal damage repair (PLDR) in X-irradiated cultures of a normal human diploid fibroblasts cell strain. Int J Radiat Biol. 1983;43:313–319. doi: 10.1080/09553008314550351. [DOI] [PubMed] [Google Scholar]
- 6.Azzam EI, de Toledo SM, Waker AJ, Little JB. High and low fluence of α particles induces at G1 checkpoint in human fibroblasts. Cancer Res. 2000;60:2623–2631. [PubMed] [Google Scholar]
- 7.Raju MR, Frank JP, Brain E, Trujillo TT, Tobey RA. Repair of potentially lethal damage in Chinese hamster cells after X and alpha irradiation. Radiat Res. 1977;71:614–621. [PubMed] [Google Scholar]
- 8.Blakely EA, Chang PY, Lommel L. Cell-cycle-dependent recovery from heavyion damage in G1-phase cells. Radiat Res. 1985;(Suppl. 8):S145–S157. [PubMed] [Google Scholar]
- 9.Goodhead DT. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- 10.Nikjoo H, O'Neill P, Wilson WE, Goodhead DT. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat Res. 2001;156:577–583. doi: 10.1667/0033-7587(2001)156[0577:cafdts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Blocher D. DNA double-strand break repair determines the RBE of alphaparticles. Int Radiat Biol. 1988;54:761–771. doi: 10.1080/09553008814552201. [DOI] [PubMed] [Google Scholar]
- 12.Trosko JE. Possible role of intercellular communication in the modulation of the biological response to radiation, Yokohama Med. Bull. 1991;42:151–165. [Google Scholar]
- 13.Autsavapromporn N, de Toledo SM, Little JB, Jay-Gerin JP, Harris AL, Azzam EI. The role of gap junction communication and oxidative stress in the propagation of toxic effects among high-dose α particles-irradiated human cells. Radiat Res. 2011;175:347–357. doi: 10.1667/RR2372.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autsavapromporn N, de Toledo SM, Buonanno M, Jay-Gerin JP, Harris AL, Azzam EI. Intercellular communication amplifies stressful effects in highcharge, high-energy (HZE) particles-irradiated human cells. J Radiat Res (Tokyo) 2011;52:408–414. doi: 10.1269/jrr.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to non-irradiated cells. Proc Natl Acad Sci U S A. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azzam EI, de Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- 17.Mothersill C, Seymour CB. Medium from irradiated human epithelial cells but not human fibroblasts reduce the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Tsuruoka C. Heavy charged particles produce a bystander effect via cell-cell junctions. Biol Sci Space. 2004;18:241–246. doi: 10.2187/bss.18.241. [DOI] [PubMed] [Google Scholar]
- 19.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, Kase Y, Yamaguchi H, Kanai T, Ando K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int J Radiat Oncol Biol Phys. 2000;48:241–250. doi: 10.1016/s0360-3016(00)00568-x. [DOI] [PubMed] [Google Scholar]
- 21.Tsuruoka C, Suzuki M, Kanai T, Fujitaka K. LET and ion species dependence for cell killing in normal human skin fibroblasts. Radiat Res. 2005;163:494–500. doi: 10.1667/rr3360. [DOI] [PubMed] [Google Scholar]
- 22.Tsuruoka C, Suzuki M, Hande MP, Furusawa Y, Anzai K, Okayasu R. The difference in LET and ion species dependence for induction of initially measured and non-rejoined chromatin breaks in normal human fibroblasts. Radiat Res. 2008;170:163–171. doi: 10.1667/RR1279.1. [DOI] [PubMed] [Google Scholar]
- 23.Kanai T, Tomura H, Matsufuji N, Fukumura A, Hiraoka T, Furusawa Y, Miyahara N, Koyama-Itoh H, Endo M, Kawachi K. HIMAC beams delivery system. In: Kanai T, Takada E, editors. Proceedings of the NIRS International Seminar in the Application Therapy of Cancer in Connection with the XXI PTCOG Meeting; NIRS, Chiba. 1994. pp. 26–31. [Google Scholar]
- 24.Kanai T, Endo M, Minohara S, Miyahara N, Kayama-Itoh H, Tomura H, Matsufuji N, Futami A, Fukumura A, Kawachi K. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201–210. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- 25.Goodhead DT, Munson RJ, Thacker J, Cox R. Mutation and inactivation of cultured mammalian cells exposed to beams of accelerated heavy ions. IV: biophysical interpretation. Int J Radiat Biol. 1980;37:135–167. doi: 10.1080/09553008014550201. [DOI] [PubMed] [Google Scholar]
- 26.Charlton DE, Sephton R. A relationship between microdosimetric spectra and cell survival for high-LET irradiation. Int J Radiat Biol. 1991;59:447–457. doi: 10.1080/09553009114550401. [DOI] [PubMed] [Google Scholar]
- 27.Lobrich M, Rydberg B, Cooper PK. Repair of X-ray induced DNA double-strand breaks in specific Not I restriction fragments in human fibroblasts: joining of correct and incorrect ends. Proc Natl Acad Sci U S A. 1995;92:12050–12054. doi: 10.1073/pnas.92.26.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.French M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 29.Plante I, Cucinotta FA. Cross sections for the interactions of 1 eV–100 MeV electrons in liquid water and application to Monte-Carlo simulation of HZE radiation tracks. New J Phys. 2009;11:063047. [Google Scholar]
- 30.Plante I, Cucinotta FA. Ionization and excitation cross sections for the interaction of HZE particles and application to Monte-Carlo simulation of radiation tracks. New J Phys. 2008;10:125020. [Google Scholar]
- 31.Cobut V, Frongillo Y, Patau JP, Goulet T, Fraser MJ, Jay-Gerin JP. Monte-Carlo simulation of fast electron and proton tracks in liquid water-I. Physical and physicochemical aspects. Radiat Phys Chem. 1998;51:229–243. [Google Scholar]
- 32.Plante I, Cucinotta FA. Energy deposition and relative frequency of hits of cylindrical nanovolume in medium irradiated by ions: Monte-Carlo simulation of track structure. Radiat Environ Biophys. 2010;49:5–13. doi: 10.1007/s00411-009-0255-7. [DOI] [PubMed] [Google Scholar]
- 33.Plante I, Ponomarev AL, Cucinotta FA. 3D visualization of the irradiation dose by a Monte-Carlo simulation of the HZE particle track structure, and the application to the calculation of DSBs in cell nuclei. Radiat Prot Dosim. 2011;143:156–161. doi: 10.1093/rpd/ncq526. [DOI] [PubMed] [Google Scholar]
- 34.Jensen R, Glazer PM. Cell-interdependent cisplatin killing by ku/DNA-dependent protein kinase signaling transduced through gap junctions. Proc Natl Acad Sci U S A. 2001;101:6134–6139. doi: 10.1073/pnas.0400051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell SA, Randers-Pehrson G, Brenner DJ, Hall EJ. The bystander response in C3H 10T1/2 cells: the influence of cell-to-cell contact. Radiat Res. 2004;161:397–401. doi: 10.1667/rr3137. [DOI] [PubMed] [Google Scholar]
- 36.Kano M, Kawata T, Ito H, Shigematsu N, Liu C, Uno T, Isobe K, Kawakami H, Cucinotta F, George K, Kubo A. Repair of potentially lethal damage in normal cells and ataxia telangiectasis cells; consideration of non-homologous end-joining. J Radiat Res (Tokyo) 2007;48:31–38. doi: 10.1269/jrr.0642. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Kawata T, Shigematsu N, Cucinotta F, George K, Saito M, Uno T, Isobe K, Ito H. A comparison of chromosome repair kinetics in G0 and G1 reveals that enhanced repair fidelity under noncycling conditions accounts for increased potentially lethal damage repair. Radiat Res. 2010;174:566–573. doi: 10.1667/RR2159.1. [DOI] [PubMed] [Google Scholar]
- 38.Okayasu R. Repair of DNA damage induced by accelerated heavy ions-a mini review. Int J Cancer. 2012;130:991–1000. doi: 10.1002/ijc.26445. [DOI] [PubMed] [Google Scholar]
- 39.Cucinotta F, Nikjoo H, Goodhead DT. The effect of delta rays on the number of particle traversal per cell in laboratory and space exposures. Radiat Res. 1998;150:115–119. [PubMed] [Google Scholar]
- 40.Gonon G, Groetz JE, de Toledo SM, Howell RW, Fromm M, Azzam EI. Nontargeted stressful effects in normal human fibroblast cultures exposed to low fluences of high-charge, high-energy (HZE) particles: Kinetics of biologic responses and significance of secondary radiations. Radiat Res. 2013;179:444–457. doi: 10.1667/RR3017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asaithamby A, Uematsu N, Chatterjee A, Story MD, Burma S, Chen DJ. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat Res. 2008;169:437–446. doi: 10.1667/RR1165.1. [DOI] [PubMed] [Google Scholar]
- 42.Scholz M, Kraft G. Calculation of heavy ion inactivation probabilities based on track structure, X ray sensitivity and target size. Radiat Prot Dosim. 1994;52:29–33. [Google Scholar]
- 43.Goodhead DT. Energy deposition stochastics and track structure: what about the target? Radiat Prot Dosim. 2006;122:3–15. doi: 10.1093/rpd/ncl498. [DOI] [PubMed] [Google Scholar]
- 44.Autsavapromporn N, de Toledo SM, Jay-Gerin JP, Harris AL, Azzam EI. Human cell responses to ionizing radiation are differentially affected by the expressed connexin. J Radiat Res (Tokyo) 2013;54:251–259. doi: 10.1093/jrr/rrs099. [DOI] [PMC free article] [PubMed] [Google Scholar]


