Abstract
Objective:
To assess the effects of low-dose acetazolamide treatment on volumetric MRI markers and clinical outcome in idiopathic normal-pressure hydrocephalus (iNPH).
Methods:
We analyzed MRI and gait measures from 8 patients with iNPH with serial MRIs from an institutional review board–approved imaging protocol who had been treated off-label with low-dose acetazolamide (125–375 mg/day). MRI studies included fluid-attenuated inversion recovery and 3D T1-weighted high-resolution imaging. Automated analyses were employed to quantify each patient's ventricular, global white matter hyperintensities (WMH), and periventricular WMH (PVH) volumes prior to and throughout treatment. Clinical outcome was based on gait changes assessed quantitatively using the Boon scale.
Results:
Five of 8 patients responded positively to treatment, with median gait improvement of 4 points on the Boon scale. A significant decrease in PVH volume (−6.1 ± 1.9 mL, p = 0.002) was seen in these patients following treatment. One patient's gait was unchanged and 2 patients demonstrated worsened gait and were referred for shunt surgery. No reduction in PVH volume was detected in the latter 2 patients. Nonperiventricular WMH and lateral ventricle volumes remained largely unchanged in all patients.
Conclusions:
These preliminary findings provide new evidence that low-dose acetazolamide can reduce PVH and may improve gait in iNPH. PVH volume, reflecting transependymal CSF, is shown to be a potential MRI indicator of pharmacologic intervention effectiveness. Further studies of pharmacologic treatment of iNPH are needed and may be enhanced by incorporating quantitative MRI outcomes.
Classification of evidence:
This study provides Class IV evidence that low-dose acetazolamide reverses PVH volume and, in some cases, improves gait in iNPH.
Idiopathic normal-pressure hydrocephalus (iNPH) manifests as a progressive gait disorder accompanied by disturbance in urination and cognition.1 The neuroimaging hallmark of iNPH is nonobstructive enlargement of the cerebral ventricles disproportionate to brain atrophy. In many cases, periventricular white matter hyperintensities (PVH) are also noted.1 In this context, origin of PVH has been hypothesized to be transependymal movement of ventricular CSF.2
Ventricular CSF diversion by shunting is currently the standard of care and the only treatment known to reduce symptom severity.3 Shunting is associated with risk of morbidity and moderate response rate (50%–80%).4 It has been noted that CSF diversion decreases PVH in iNPH and that PVH width reduction is associated with postshunting symptom improvement, with gait demonstrating the strongest correlation.5 In contrast, ventricular size, as assessed by Evan's Index, demonstrated little to no reduction following successful shunting.6
There are currently no approved pharmacologic treatments for iNPH. The carbonic anhydrase inhibitor acetazolamide (ACZ) is commonly used in other entities such as idiopathic intracranial hypertension,7 a neurologic problem also associated with impaired CSF homeostasis,8 but only anecdotally in iNPH. In a case series of 15 patients, 10 experienced symptomatic improvement at ACZ doses of 250–500 mg/day,9 and a complete resolution of symptoms was reported in a single case.10 In the present study, quantitative volumetric MRI measures were used to examine the effect of ACZ on white matter hyperintensities (WMH) and ventricular volumes in a group of probable iNPH patients who were among the participants in an institutional review board (IRB)–approved serial MRI study and had been treated with ACZ on an off-label basis.
METHODS
Subject selection.
Standard protocol approvals, registrations, and patient consents.
Eight patients with symptoms matching guidelines for probable iNPH1 ages 72 to 90 (4 male, 4 female) and receiving off-label low-dose ACZ treatment (dose-escalated from 125 to 375 mg/day over 4- to 10-week intervals) were identified from among 100 patients who gave written informed consent for an IRB-approved investigational MRI study (ACZ is offered as elective off-label treatment in cases where shunt surgery is contraindicated or not urgently required). Patients with concurrent neurologic diseases and previous shunt surgery were excluded.
Acetazolamide dosing and course of evaluations.
All patients were initially treated with 125 mg/day oral acetazolamide. Six of the 8 patients who tolerated this dosage well were escalated to 250 mg/day at 1 month. Two patients underwent a further dose increase to 375 mg/day after 3 months. Imaging and clinical tests were obtained before treatment and at approximately 1 month post ACZ initiation. Further clinical tests and follow-up MRI scans were performed throughout treatment when clinically indicated. Outcome measures were assessed based on changes measured at the endpoint MRI scan relative to pretreatment baseline scan. Treatment duration, time from ACZ initiation to final MRI scan, ranged from 60 to 210 days. Except in 1 case (case 6), imaging and gait testing were done within 1 month of each other.
Three patients had 2 MRI scans prior to initiation of ACZ at various intervals as part of their clinical care. These scans were used to assess measurement variability and changes due to the normal evolution of the disease.
MRI acquisition and data analysis.
MRI was acquired using a GE HDx 3T scanner. Imaging protocol included axial T2 fluid-attenuated inversion recovery (FLAIR) sequence with the following parameters: repetition time (TR)/echo time (TE)/inversion time (TI) 9,600/146.5/2,250 ms, slice thickness 3–5 mm, acquisition matrix 352 × 224, and a 3D T1-weighted spoiled gradient recalled echo sequence with isotropic resolution of 1.2 mm, TR/TE/TI 8.75/3.4/450 ms, and flip angle 12°.
Global and periventricular WMH volumes are automatically segmented using the FLAIR and T1 data. The T1 volume is used to obtain a brain mask, using BET software,11 which is superimposed on the FLAIR image by linear coregistration. A threshold method is employed to identify the WMH voxels. Mean (μ) and SD intensity values of normal brain regions are calculated after intensity nonuniformity correction with FAST software.12 Then, voxels with intensity values greater than μ + 3 × SD are identified as WMH. The classification of the WMH to periventricular (PVH) and nonperiventricular (NPVH) is guided by the coregistered brain atlas. PVH voxels are defined as WMH voxels within 1 cm from the lateral ventricles' boundaries.13 This periventricular mask is projected on the WMH regions using a nonlinear registration transformation with FNIRT software.14 Lateral ventricle volumes are quantified based on 3D T1 images using Freesurfer brain parcellation software.15 Manual editing by an expert observer (A.M.B.) blinded to subject identity and clinical outcome was used when needed. A paired t test was used to evaluate significance of changes measured following ACZ.
Gait assessment.
Gait assessment utilized a quantified scale developed in Boon et al.16 Scores from this metric represent 3 summed subscores: 10-m step count, 10-m time, and the following 10 features: hesitation, wide and small steps, low clearance, impaired turning, sway, fall tendency, impaired tandem walking, and inability to walk with and without assistance. The summed score has a range from 2–40, with 2 representing unimpaired gait.
RESULTS
Five of 8 patients responded positively to the ACZ treatment, with gait improvement ranging from 4 to 8 points and a median of 4 points on the Boon scale. One patient was unchanged, and 2 patients worsened. A significant decrease in PVH volume was measured in the 5 patients who responded positively and no change was detected in the 2 patients who worsened. The patients demonstrating gait improvements had a mean PVH volume reduction of −6.1 ± 1.9 mL (p = 0.002) as compared to a mean change of −0.25 ± 0.5 mL in those who worsened. PVH and NPVH, lateral ventricles volumes at baseline, accumulated change following treatment, accumulated change in Boon scale, and final dose for each of the 8 patients are summarized in table 1. The posttreatment change in the PVH volume was the only measure that reached statistical significance. FLAIR images from a patient demonstrating reduction in the PVH volume following treatment are shown in the figure.
Table 1.
Imaging-based volumetric measures at baseline and following treatment

Figure. Fluid-attenuated inversion recovery MRI (patient 8) with segmented periventricular white matter hyperintensity (PVH) regions shows changes in PVH size following acetazolamide treatment.
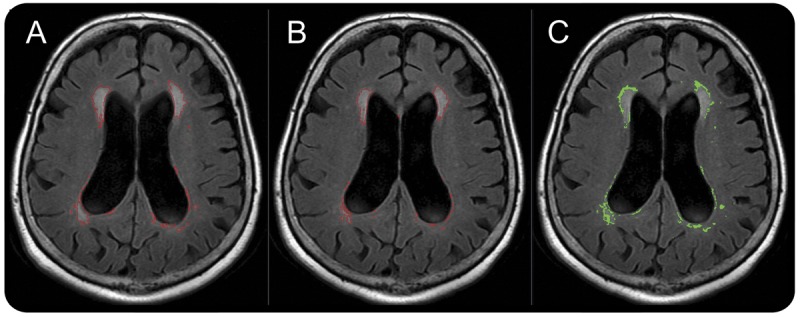
The boundary of the segmented PVH volume is shown in red. (A) Pretreatment, (B) posttreatment, (C) PVH voxels that converted to normal-appearing white matter tissue are shown in green. The corresponding reduction in PVH and ventricular size in this case were −6.2 and −1.7 mL, respectively.
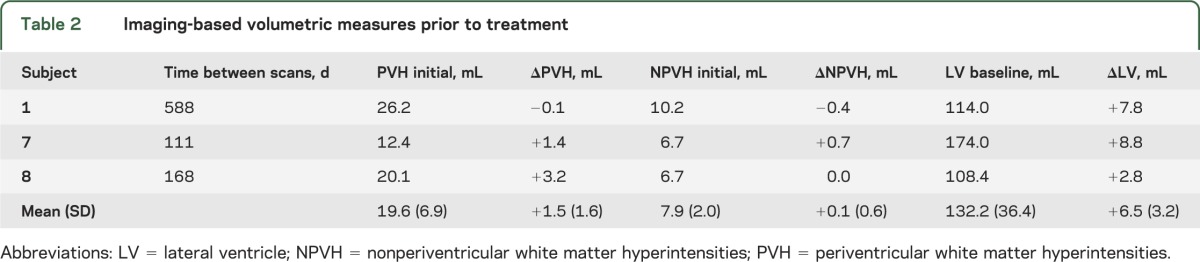
The initial WMH and ventricular volumes and changes from the first scan for the 3 patients who were scanned twice prior to ACZ are summarized in table 2. Of interest, one patient's PVH volume was stable and the other 2 demonstrated 1.4 and 3.2 mL increases, respectively. Following ACZ, these 3 patients demonstrated decreased PVH volumes of −11.7, −8.2, and −6.2 mL, respectively.
Table 2.
Imaging-based volumetric measures prior to treatment
All subjects tolerated the initial 125 mg dose and 2 patients had adverse events after dose escalation; one exhibited renal insufficiency (patient 3) that remitted after ACZ discontinuation and the other a lacunar stroke (patient 4) with uncertain relationship to ACZ treatment. Based on clinical impression, patient 4 initially seemed to improve following the initial dose, but then experienced deterioration, with gait score worsening from 8 to 13 after dose increase. A follow-up MRI demonstrated a lacunar stroke, which accounted for the increase in the NPVH volume. ACZ was discontinued and the patient was shunted. Following several shunt adjustments over a 9-month period, gait returned to the baseline value (8). A postshunting follow-up MRI scan demonstrated decreases in PVH and ventricular volumes of −10.5 and −26.9 mL, respectively. Patient 6 also initially responded well, with 6 points gait improvement, but later his gait deteriorated to 14 on a higher dose. This patient underwent shunting but did not significantly improve.
DISCUSSION
Five of the 8 patients in this case series benefited from ACZ, comparable to a previous study.9 These results add new evidence to the previously reported benefits of ACZ treatment in iNPH and also demonstrate, for the first time, that ACZ treatment can considerably reduce PVH volume similar to successful CSF diversion by shunting.5 Furthermore, in both shunting and ACZ treatment, the PVH reduction was generally associated with gait improvement.
The objective volumetric assessment employed in the current study reveals that a significant magnitude of PVH reduction can be achieved with low-dose ACZ treatment. The ACZ responders demonstrated on average 34% reduction in PVH volume relative to the baseline compared to less than 1% in the 2 patients who did not respond to ACZ. Furthermore, the bulk of the PVH reduction, 21% out of 34%, was already achieved with the initial low dose of 125 mg/day. This finding is encouraging because a lower ACZ dose reduces the chance for adverse effects such as metabolic acidosis, hypokalemia, fatigue, numbness, and kidney stones.17
The direct association between ACZ treatment and PVH reduction is further supported by the results from the repeated MRI studies prior to ACZ initiation. PVH volumes were unchanged or increased but did not decrease. The unchanged PVH volume occurred in a patient who had mild and stable symptoms. The increased PVH volume occurred in 2 patients with progressing symptoms. Ventricular volume increased in all 3 patients during this period, in contrast to the relatively unchanged ventricular volumes during the ACZ treatment.
Reduction of WMH burden occurred almost entirely within the periventricular region. This finding is consistent with a reduction of transependymal CSF movement as a possible mechanism for the WMH reversal with ACZ administration. While PVH reduction is generally associated with gait improvement, the magnitude of this reduction is not necessarily associated with the degree of gait improvement as PVH reduction may occur in brain regions not associated with gait.
Of interest, the 2 patients who worsened despite ACZ had either extremely high (123.3 mL) or very low (8.5 mL) baseline PVH volumes. The baseline PVH volumes of the patients who improved spanned a much narrower range (13.8–25.9 mL). Further studies would be needed to reveal whether atypical PVH volumes are indicative of different underlying pathophysiology.
Limitations of this study include the small number of subjects, the use of a convenience cohort of patients receiving open-label treatment, and its retrospective nature. As an open-label protocol, this study is vulnerable to placebo effects with respect to gait improvement. Thus, the observed gait improvement following ACZ treatment does not establish the efficacy of ACZ in iNPH. The safety and overall effectiveness of ACZ in iNPH can only be established by prospective double-blind placebo-controlled trials with larger numbers of patients. For this reason, this study primarily focuses on the change in PVH, which is an unbiased outcome. Furthermore, as a continuous variable, it provides increased power to discern treatment effects, as demonstrated by the statistical significance of the measured PVH reductions. The PVH volume reversals highlight the potential for development of pharmacologic therapies for iNPH.
The encouraging findings of PVH reversal and gait improvement with low-dose ACZ justify future efforts to assess safety and efficacy of ACZ or other potential pharmacologic agents for iNPH. The occurrence of PVH reversal following both ACZ and shunting suggests that PVH volume may be a useful objective marker for the physiologic efficacy of pharmacologic and surgical intervention.
Supplementary Material
GLOSSARY
- ACZ
acetazolamide
- FLAIR
fluid-attenuated inversion recovery
- iNPH
idiopathic normal-pressure hydrocephalus
- IRB
institutional review board
- NPVH
nonperiventricular white matter hyperintensities
- PVH
periventricular white matter hyperintensities
- TE
echo time
- TI
inversion time
- TR
repetition time
- WMH
white matter hyperintensities
AUTHOR CONTRIBUTIONS
N. Alperin: conceptualization and design of the study, data collection, analysis and interpretation, and drafting the manuscript. C.J. Oliu: data collection, data analyses and interpretation of the data, and drafting of the manuscript. A.M. Bagci: analyses and interpretation of the data and drafting of the manuscript. S.H. Lee: data collection and data analyses. I. Kovanlikaya: data collection and data analyses. L. Heier: MRI review and data analyses. H. Katzen: conceptualization of the study and patient selection. D. Adam: interpretation of the data and drafting of the manuscript. N. Relkin: conceptualization and design of the study, patient selection, data collection, and interpretation and drafting the manuscript. M. Ivkovic: data collection and data analyses.
STUDY FUNDING
Supported in part by NIH (R01NS052122) and the Leon Levy Foundation.
DISCLOSURE
N. Alperin is a shareholder in Alperin Noninvasive Diagnostics, Inc. C. Oliu, A. Bagci, S. Lee, I. Kovanlikaya, D. Adams, H. Katzen, M. Ivkovic, L. Heier, and N. Relkin report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S4–S16 [DOI] [PubMed] [Google Scholar]
- 2.Kimura M, Tanaka A, Yoshinaga S. Significance of periventricular hemodynamics in normal pressure hydrocephalus. Neurosurgery 1992;30:701–704 [PubMed] [Google Scholar]
- 3.Pujari S, Kharkar S, Metellus P, Shuck J, Williams MA, Rigamonti D. Normal pressure hydrocephalus: long-term outcome after shunt surgery. J Neurol Neurosurg Psychiatry 2008;79:1282–1286 [DOI] [PubMed] [Google Scholar]
- 4.Bergsneider M, Black PM, Klinge P, Marmarou A, Relkin N. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S29–S39 [DOI] [PubMed] [Google Scholar]
- 5.Tullberg M, Jensen C, Ekholm S, Wikkelsø C. Normal pressure hydrocephalus: vascular white matter changes on MR images must not exclude patients from shunt surgery. AJNR Am J Neuroradiol 2001;22:1665–1673 [PMC free article] [PubMed] [Google Scholar]
- 6.Meier U, Mutze M. Correlation between decreased ventricular size and positive clinical outcome following shunt placement in patients with normal-pressure hydrocephalus. J Neurosurg 2004;100:1036–1040 [DOI] [PubMed] [Google Scholar]
- 7.Skau M, Brennum J, Gjerris F, Jensen R. What is new about idiopathic intracranial hypertension? An updated review of mechanism and treatment. Cephalalgia 2006;26:384–399 [DOI] [PubMed] [Google Scholar]
- 8.Alperin N, Ranganathan S, Bagci AM, et al. MRI evidence of impaired CSF homeostasis in obesity-associated idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2013;34:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aimard G, Vighetto A, Gabet JY, Bret P, Henry E. Acetazolamide: an alternative to shunting in normal pressure hydrocephalus? Preliminary results. Rev Neurol 1990;146:437–439 [PubMed] [Google Scholar]
- 10.García-Gascó P, Salame GF, Tenllado DP, Chazarra TC. Complete resolution of chronic hydrocephalus of adult with acetazolamide. Med Clin 2005;124:516–517 [DOI] [PubMed] [Google Scholar]
- 11.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57 [DOI] [PubMed] [Google Scholar]
- 13.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 2005;36:50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson JL, Smith SM, Jenkinson M. FNIRT: FMRIB's non-linear image registration tool. Presented at the annual meeting of the Organization for Human Brain Mapping; 2008; Melbourne, Australia
- 15.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355 [DOI] [PubMed] [Google Scholar]
- 16.Boon AJW, Tans JTJ, Delwel EJ, et al. Dutch normal pressure hydrocephalus study: baseline characteristics with emphasis on clinical findings. Eur J Neurol 1997;4:39–47 [DOI] [PubMed] [Google Scholar]
- 17.Goodman LS. Goodman and Gilman's the Pharmacological Basis of Therapeutics. Vol 1157 New York: Pergamon Press; 1990 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


