Abstract
Objective:
To assess whether faster cognitive decline in elders without dementia is associated with decreased risk of cancer mortality.
Methods:
In this population-based, prospective study of 2,627 people without dementia aged 65 years and older (Neurological Disorders in Central Spain), a 37-item version of the Mini-Mental State Examination (37-MMSE) was administered at 2 visits (baseline and follow-up, approximately 3 years later). We divided change in 37-MMSE into tertiles (lower tertile ≥2 point improvement in score, higher tertile ≥2 point decline in score). Community-dwelling elders were followed for a median of 12.9 years, after which the death certificates of those who died were examined.
Results:
A total of 1,003 (38.2%) died, including 339 (33.8%) deaths among participants who were in the higher tertile of 37-MMSE change and 664 (66.2%) deaths among those in the remaining tertiles. Cancer was reported significantly less often in those in the higher tertile of MMSE change (20.6%) than in those in the remaining tertiles (28.6%): in an unadjusted Cox model, hazard ratio for cancer mortality in participants within the higher tertile = 0.75 (p = 0.04) compared with the participants within the remaining tertiles. In a Cox model that adjusted for a variety of demographic factors and comorbidities, hazard ratio for cancer mortality in participants within the higher tertile = 0.70 (p = 0.01).
Conclusion:
In this population-based, prospective study of community-dwelling elders without dementia, faster cognitive decline was associated with a decreased risk of cancer mortality. Further studies are required to elucidate this inverse association in elders without dementia.
A series of prospective studies has shown that Alzheimer disease (AD) is associated with a reduced risk of cancer.1–3 However, the mechanisms underlying this association remain unknown. The cholinergic system deficit that occurs in patients with AD may explain, at least in part, the decreased risk of cancer.4 Because acetylcholine stimulates cell proliferation, the association suggests that the degeneration of acetylcholine-secreting cells may have a protective role on cancer onset, as this neurotransmitter would be less available to stimulate cell proliferation.5 Second, in many observational studies and animal models, inflammation is associated with AD and vascular disease,6,7 and subsequent studies have documented that inflammation driven by tumor-specific Th1 may prevent some types of cancer.8 Furthermore, a cytotoxic action has been proposed for β-amyloid (i.e., to eradicate cancer cells in an analogous manner to that performed by host defense peptides).9,10
Whereas cancer has been reported to occur less often in patients with AD, a study to determine whether there is an association between cancer and faster cognitive decline in elders without dementia has not been conducted. It has been suggested that a major problem with epidemiologic studies that have reported an inverse association between AD and cancer is the very likely underdiagnosis of cancer once dementia has been diagnosed.11 We therefore tested the hypothesis that older persons without dementia who experienced faster decline in cognition would have decreased risk of cancer mortality. To address this question, we utilized data from the Neurological Disorders in Central Spain (NEDICES) Study, in which participants were prospectively evaluated at 2 time points separated by 3 years and followed for a median of 12.9 years, after which the death certificates of those who died were examined.
METHODS
Study population.
Data for these analyses were derived from the NEDICES Study, a longitudinal, population-based survey of the prevalence, incidence, mortality, and determinants of major age-associated conditions of the elderly, including Parkinson disease, essential tremor, stroke, and dementia.12–20 Detailed accounts of the study population and sampling methods have been published.21–23
The survey area consisted of 3 communities: (1) Las Margaritas (approximately 14,800 inhabitants), a working-class neighborhood in Getafe (Greater Madrid); (2) Lista (approximately 150,000 inhabitants), a professional-class neighborhood in Salamanca district (Central Madrid), and (3) Arévalo (approximately 9,000 inhabitants), the agricultural zone of Arévalo County (125 km northwest of Madrid). Up-to-date lists of residents were generated from population registers. In each community, survey eligibility was restricted to residents aged 65 years or older who were present there on December 31, 1993, or during 6 or more months of 1993. Eligible persons who had moved away from the survey area were not traced. In Margaritas and Arévalo, every eligible subject was to be screened. However, because of the large number of elderly residents in Lista, proportionate stratified random sampling was used to select subjects for screening.
Study evaluation.
Briefly, at the time of their baseline assessment (1994–1995), 5,278 elderly subjects were interviewed using a 500-item screening questionnaire that assessed demographic factors and medical conditions. During the face-to-face interview, data were collected on demographics, current medications, and medical conditions. Subjects were asked to bring all medications taken in the past 1 week to the clinic where the interviewer recorded the name and the dose of each one. We assessed depressive symptoms by self-report, using a single screening question (“Do you suffer from depression?”). The same approach has similarly been utilized in previous population studies of depression.24,25 We also assessed the use of antidepressant medications, a marker that may be less prone to biases than a simple screening question.26
A short form of the questionnaire was mailed to subjects who declined or were unavailable for face-to-face interview, or telephone screening. This form assessed demographic characteristics, several neurologic disorders (essential tremor, stroke, dementia, and parkinsonism), current medications, and the name of their family doctor.
As described,21–23 a 37-item Mini-Mental State Examination (37-MMSE) was administered at both the baseline assessment (1994–1995) and the follow-up assessment (1997–1998).27–32 This was a Spanish adaptation of the standard MMSE.27–32 It included all of the standard MMSE items and 3 additional items: (1) an attention task, i.e., “say 1, 3, 5, 7, 9 backward,” (2) a visual order, i.e., a man raising his arms, and (3) a simple construction task, i.e., copying 2 overlapping circles.27–32
Ten percent of our sample was illiterate, although only a small proportion was completely illiterate and many could read or write a simple phrase. If the participant was completely illiterate, then the one 37-MMSE reading item and the one 37-MMSE writing item were assigned the value 0. The diagnosis of dementia was assigned using DSM-IV criteria33 and required evidence of cognitive impairment (based on a neuropsychological test battery and a clinical mental status examination) as well as impairment in social or occupational function.
During the second (i.e., follow-up) evaluation (1997–1998), the same methods were used. Follow-up data on death were collected until May 1, 2007. The date of death was obtained from the National Population Register of Spain (Instituto Nacional de Estadística). In Spain, a death certificate is completed by a doctor for all deceased individuals at the time of death. The certificate is then sent to the local authority in the municipality where the person had been living, and the information is collected in the National Register. The cause of death (using the ICD-9 for deaths occurring prior to 1999 [http://www.cdc.gov/nchs/icd/icd9.htm] and the ICD-10 [http://www.cdc.gov/nchs/icd/icd10.htm] for deaths occurring thereafter) was divided into 6 main categories: dementia, cerebrovascular disorders, cardiovascular disorders (pulmonary embolism, congestive heart failure, myocardial infarction, heart or aortic rupture, and asystole), respiratory diseases, cancer, and other causes (infections, trauma, and genitourinary or gastrointestinal disorders). In accordance with the recommendations of the World Health Organization, the classification of causes of death is based on the basic cause of death (http://www.who.int/topics/mortality/en/). This is defined as the illness or injury that started the chain of pathologic events which directly led to death (http://www.who.int/topics/mortality/en/).
Final selection of participants.
Of the 5,278 participants evaluated at baseline, we excluded 467 participants with dementia, including 306 with dementia diagnosed at baseline evaluation (1994–1995) (i.e., prevalent cases), and 161 who developed dementia by the follow-up evaluation (1997–1998) (i.e., incident cases). We further excluded 2,184 participants who were evaluated at baseline because they declined a follow-up assessment or had incomplete follow-up assessments, had died or were unreachable (n = 1,278), or had incomplete 37-MMSE examinations (n = 906) (figure e-1 on the Neurology® Web site at Neurology.org).
The final sample of 2,627 was similar to the base sample of 5,278 participants in sex (1,509 [57.4%] vs 3,040 [57.6%] women, χ2 = 0.02, p = 0.89). However, they were more educated (268 [10.2%] vs 711 [13.6%] were illiterate, χ2 = 18.71, p < 0.001) and, on average, 1.6 years younger (72.7 ± 5.9 vs 74.3 ± 7.0 years, t = 11.0, p < 0.001).
Standard protocol approvals, registrations, and patient consents.
All procedures were approved by the ethical standards committees on human experimentation at the University Hospitals 12 de Octubre (Madrid) and La Princesa (Madrid). Written (signed) informed consent was obtained from all enrollees.
Statistical analyses.
Data analyses were performed in SPSS version 20.0 (SPSS, Inc., Chicago, IL). None of the continuous variables (i.e., age, 37-MMSE score, and change in 37-MMSE score) was normally distributed (Kolmogorov-Smirnov, p < 0.001), even after log-transformation. Therefore, baseline characteristic scores were compared using Mann-Whitney test; χ2 tests were applied to determine associations between categorical variables.
Change in 37-MMSE was divided into tertiles (lower tertile ≥2-point improvement in score, higher tertile ≥2-point decline in score). For the current analyses, we dichotomized this variable into higher vs middle and lower tertiles. We determined the proportion of cases in which a diagnosis of cancer was listed on the death certificate in the higher tertile (i.e., those with faster cognitive decline) vs the proportion in the remaining tertiles.
We used Cox proportional-hazards models to estimate hazard ratios (HRs) for cancer-specific mortality; this also generated 95% confidence intervals (CIs). The time variable was the years from the date of the first evaluation (1994–1995) to the date of death in subjects who had died. The dependent (outcome) variable was presence of a cancer condition on the death certificate, with the remaining causes of death serving as the reference group. We began with an unadjusted model. Then, in adjusted models, we first considered baseline variables that in univariate analyses were associated at the p ≤ 0.30 level with both the exposure (higher tertile of 37-MMSE change vs the remaining tertiles [the reference category]) and the outcome (cancer-related mortality) (model 1 [more restrictive criteria for confounding]), and then considered baseline variables that in univariate analyses were associated at the p ≤ 0.30 level with either the exposure or the outcome (model 2 [less restrictive criteria for confounding]). A value of p ≤ 0.30 rather than p < 0.05 was conservatively chosen to allow us to more carefully include any possible source of confounding. Variables assessed at baseline that we considered included age in years, sex, educational level (illiterate, can read and write, primary studies, secondary and higher studies), geographical area (Las Margaritas, Lista, and Arévalo), living area during childhood/adolescence (rural vs urban area), self-rated health (good/very good, so-so, and bad/very bad), smoker (ever vs never), drinker (ever vs never), cancer, arterial hypertension, diabetes mellitus, heart diseases, osteoporosis, chronic obstructive pulmonary disease, stroke, depressive symptoms (“do you suffer from depression?”) or antidepressant use, and the 37-MMSE score. Finally, for completeness, we adjusted for all the potential confounders, independent of their statistical significance (i.e., even if they were not associated with either the exposure or the outcome) (model 3).
Kaplan-Meier survival curves for subjects within the higher tertile of change vs those within the middle and lower tertiles were assessed; the log-rank test was used to compare the differences between the 3 curves.
RESULTS
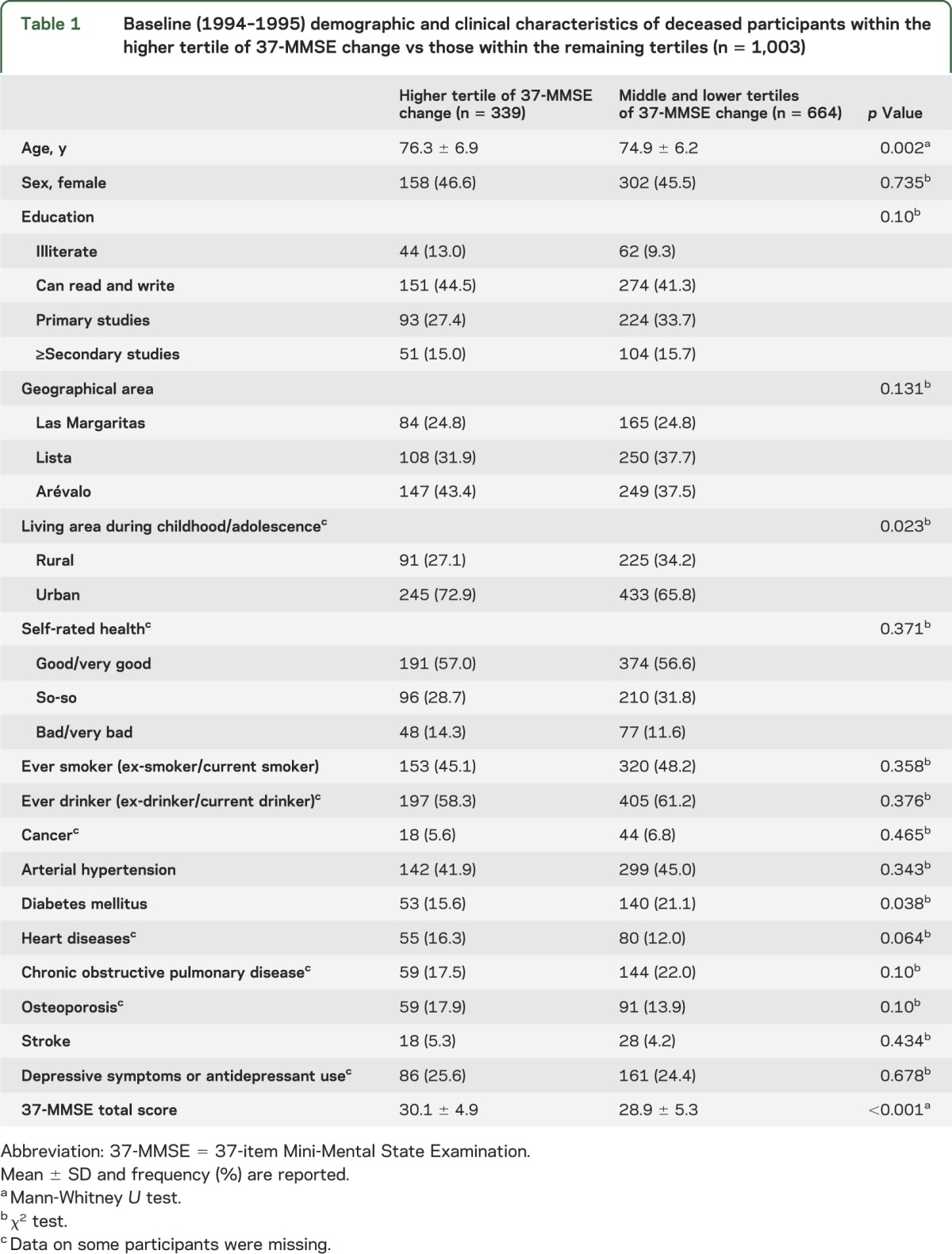
The 2,627 participants had a mean duration of follow-up of 11.2 years (median 12.9 years, range 2.7–14.1 years). One thousand three (38.2%) of 2,627 participants died over a median follow-up of 8.7 years (range 2.7–13.2 years), including 339 (33.8%) deaths among participants who were in the higher tertile of 37-MMSE change and 664 (66.2%) deaths among those in the middle and lower tertiles. There were significant differences in baseline age, diabetes mellitus, and the MMSE total score when participants within the higher tertile of 37-MMSE change and within the remaining tertiles were compared (table 1). In addition, the percentage of those living in urban areas during childhood/adolescence was significantly higher than in the remaining tertiles.
Table 1.
Baseline (1994–1995) demographic and clinical characteristics of deceased participants within the higher tertile of 37-MMSE change vs those within the remaining tertiles (n = 1,003)
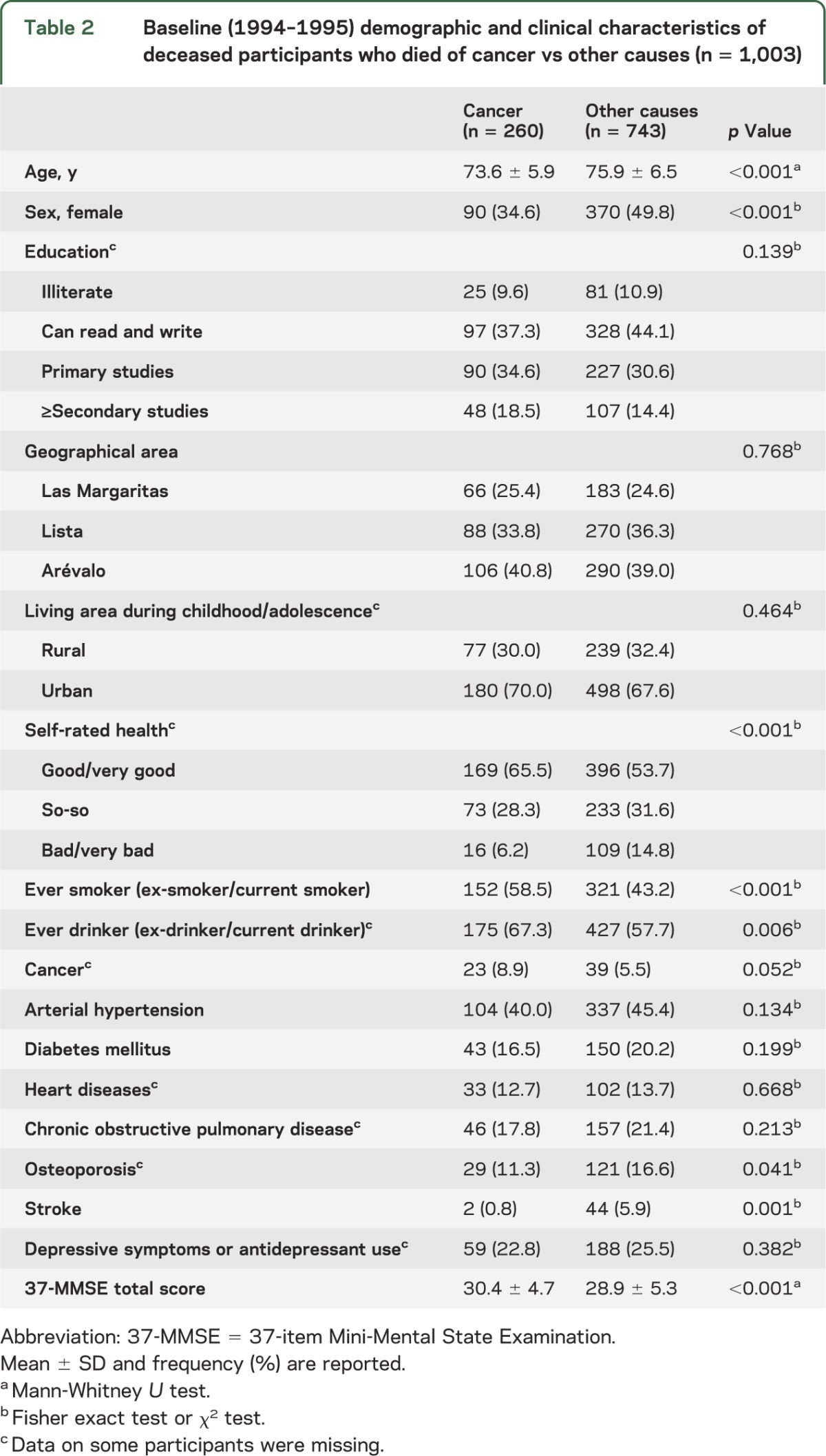
Baseline characteristics of the participants who died of cancer vs those who died of other causes are shown in table 2. At baseline, those who died of cancer were younger, scored higher in the 37-MMSE, and were more likely to be male and to have ever smoked and ever drunk, and to have rated their health as good/very good. In addition, they were less likely to have osteoporosis and stroke.
Table 2.
Baseline (1994–1995) demographic and clinical characteristics of deceased participants who died of cancer vs other causes (n = 1,003)
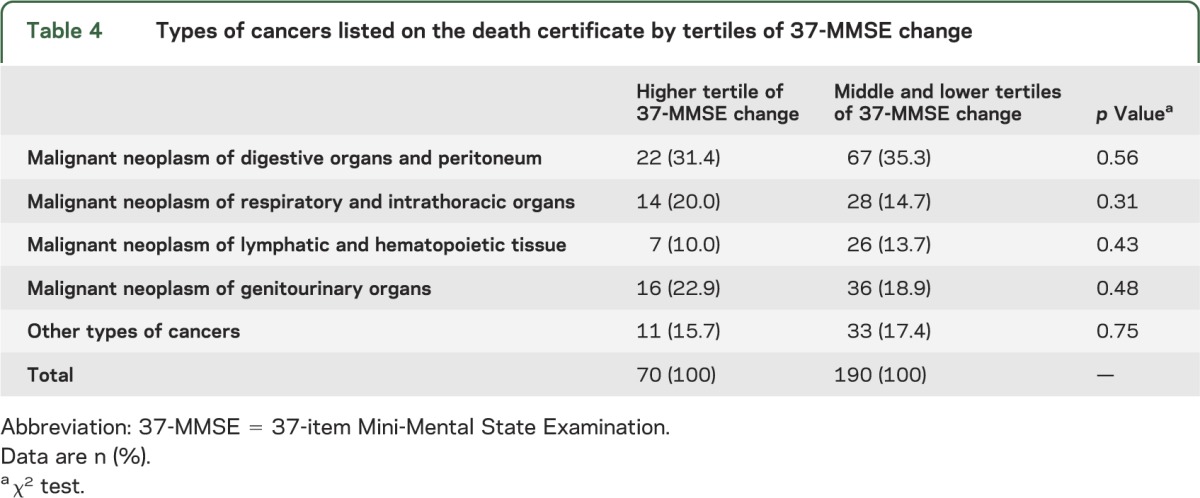
Cause of death noted on the death certificates differed significantly by tertiles of 37-MMSE change (table 3). Cancer was reported significantly less often in those in the higher tertile of MMSE change than in those in the remaining tertiles (table 3). However, as expected, dementia was reported significantly more often in those in the higher tertile of MMSE change than in those in the remaining tertiles (table 3). In addition, cardiovascular diseases were reported significantly more often in those in the higher tertile of MMSE change than in those in the remaining tertiles (table 3). Types of cancers listed on the death certificates did not differ significantly by tertiles of 37-MMSE change (table 4).
Table 3.
Primary cause of death by tertiles of 37-MMSE change
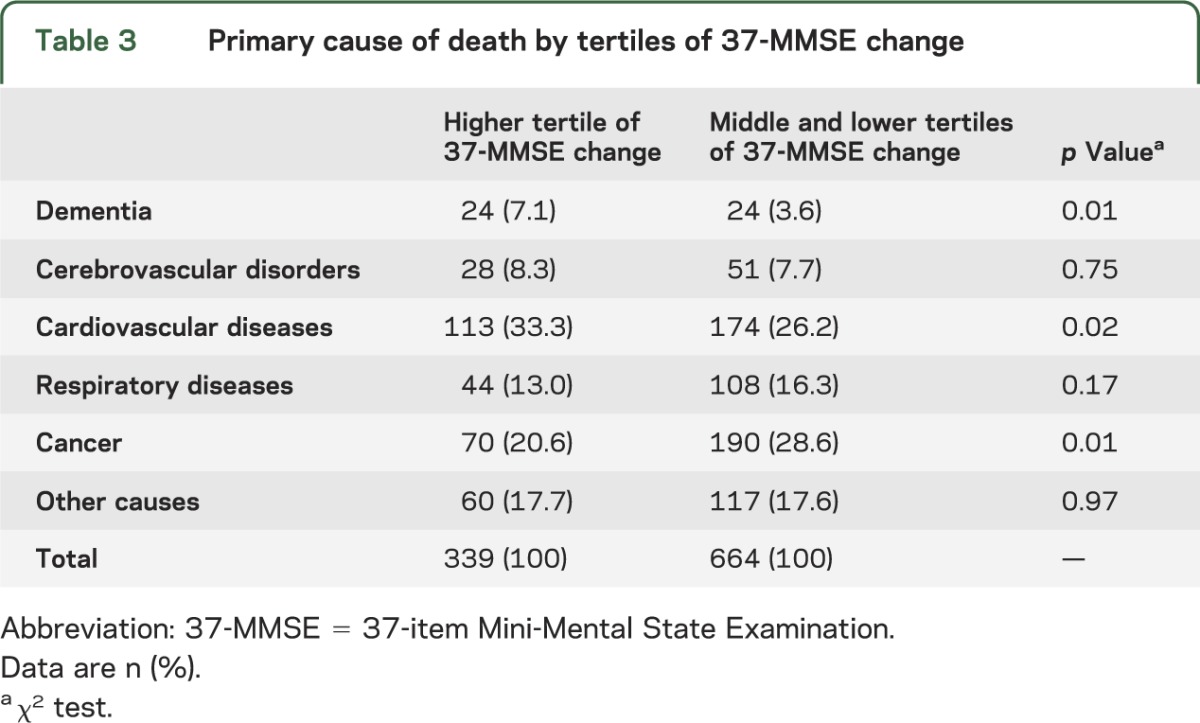
Table 4.
Types of cancers listed on the death certificate by tertiles of 37-MMSE change
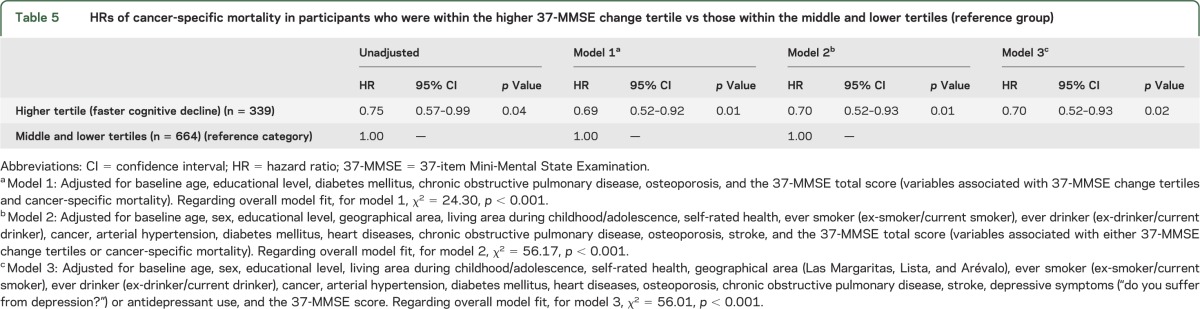
In an unadjusted Cox model, risk of cancer-specific mortality was decreased in participants within the higher tertile of 37-MMSE change vs those within the remaining tertiles (reference group) (table 5). In a Cox model that adjusted for baseline age, educational level, diabetes mellitus, chronic obstructive pulmonary disease, osteoporosis, and the 37-MMSE score (i.e., variables that were associated with both 37-MMSE change tertiles and cancer-specific mortality), the risk of mortality remained decreased in participants within the higher tertile of 37-MMSE change (model 1 in table 5). The results did not change in a Cox model that adjusted for variables that were associated with either 37-MMSE change tertile or cancer-specific mortality (i.e., baseline age, sex, educational level, geographical area, living area during childhood/adolescence, self-rated health, ever smoker [ex-smoker/current smoker], ever drinker [ex-drinker/current drinker], cancer, arterial hypertension, diabetes mellitus, heart diseases, chronic obstructive pulmonary disease, osteoporosis, stroke, and the 37-MMSE total score) (model 2 in table 5). Furthermore, in a model that adjusted for baseline age, sex, educational level, living area during childhood/adolescence, self-rated health, geographical area (Las Margaritas, Lista, and Arévalo), ever smoker (ex-smoker/current smoker), ever drinker (ex-drinker/current drinker), cancer, arterial hypertension, diabetes mellitus, heart diseases, osteoporosis, chronic obstructive pulmonary disease, stroke, depressive symptoms (“do you suffer from depression?”) or antidepressant use, and the 37-MMSE score (i.e., all potential confounders independent of their statistical significance) (model 3 in table 5), the results remained unchanged.
Table 5.
HRs of cancer-specific mortality in participants who were within the higher 37-MMSE change tertile vs those within the middle and lower tertiles (reference group)
In a final analysis, we excluded all participants (n = 21) in which a diagnosis of AD was listed on the death certificate. In these analyses, the results were similar (model 1: HR = 0.74, 95% CI = 0.56–0.99, p = 0.04; model 2: HR = 0.74, 95% CI = 0.55–0.99, p = 0.04; and model 3: HR = 0.73, 95% CI = 0.55–0.98, p = 0.04).
The Kaplan-Meier curve for overall survival (figure e-2) showed that those in the higher tertile of 37-MMSE were not at increased risk of death vs those in the middle or lower tertiles (log-rank p = 0.64).
DISCUSSION
The results of the current study suggest that elderly people without dementia with faster cognitive decline are at reduced risk of mortality from malignant neoplasm. Relative to those in the middle and lower tertiles, the HR of neoplasms as underlying cause of death was 30% lower in subjects who were within the higher tertile of 37-MMSE change (i.e., faster cognitive decline). We acknowledge that some participants with faster cognitive decline would finally developed AD. However, after excluding those who died with AD, the results were similar.
The mechanisms underlying this association remain unknown. This inverse association has also been reported in several neurodegenerative processes, including AD, Parkinson disease, and Huntington disease.1–3,34,35 As yet undiscovered mechanisms may either promote a neurodegenerative process (uncontrolled cellular destruction) or annul other conditions, namely, cancer (uncontrolled cellular proliferation). Both cancer and neurodegenerative disorders are characterized by a disarrangement of cell-regulation mechanisms, with increased cell survival and proliferation in the former and with increased cell death in the latter process.
Cognitively healthy elderly people who are experiencing subtle cognitive decline within the normal range may be undergoing a clinically silent pathologic cascade of brain changes, during this phase, with β-amyloid deposition as the primary event in this cascade.36,37 Neural cells may become vulnerable to cytotoxicity by amyloid-forming peptides, such as β-amyloid,9 which shares the same mechanism of toxicity with host defense peptides, components of innate immune response, whose mission is to eradicate a broad range of microbes and cancer cells.9 It appears that this activity is mediated by the ability of these peptides to permeabilize cell membranes via the formation of amyloid-associated structures.9,10 Augmented cell death due to oxidative stress caused by cytotoxic amyloid-forming peptides and host defense peptides is in agreement with the apparent protective effect of AD and probably age-related cognitive decline, from cancer.9,10 Furthermore, studies in subjects without dementia have suggested that low-grade peripheral systemic inflammation is associated with increased cognitive decline38 and reduced hippocampal volume.39 There is evidence that inflammation driven by tumor-specific Th1 may prevent some types of cancer.8
This study had several limitations. First, we did not collect data on comorbidity at death or data on who signed the death certificate (general physician vs neurologist vs oncologist or geriatrician). It is logical to think that the level of expertise of the physician who signed the death certificate may predict the level of accuracy of that certificate. Second, we based the diagnoses of cancer using death certificates. Nevertheless, the accuracy of cancer death certification in Spain has been shown to be high.40 Third, while the base sample comprised 5,278 participants, because of the many exclusions, the final sample comprised 2,627, and the final sample, although population-based, in some respects resembled a convenience sample. Fourth, competing mortality is an issue to consider—healthy elders who do not die of cancer are at risk of developing neurodegenerative disorders including dementia. Finally, we included participants with cancer at baseline; however, we expect that this would have made it more difficult to detect the observed inverse association because cancer-related or cancer-treatment–related issues might have resulted in more cognitive rather than fewer cognitive issues.
This study also had several strengths. First, the study was population-based, allowing us to assess a group of people without dementia who were unselected for treatment considerations. Second, the assessments were conducted prospectively in a standardized manner. Finally, we were able to adjust for the potential confounding effects of a number of important factors.
Using a prospective, population-based design, we demonstrated that faster cognitive decline in community-dwelling elders without dementia was associated with decreased risk of cancer-specific mortality. This study provides evidence of an inverse association between cancer and cognitive decline. Further studies are required to elucidate this inverse association in community-dwelling elders without dementia.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HR
hazard ratio
- ICD-9
International Classification of Diseases, ninth revision
- MMSE
Mini-Mental State Examination
- 37-MMSE
37-item Mini-Mental State Examination
- NEDICES
Neurological Disorders in Central Spain
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Benito-León: conception, organization, and execution of the research project, statistical analysis design, and writing of the manuscript first draft and review and critique of the manuscript. Dr. Romero and Dr. Louis: conception and organization of the research project, and review and critique of the manuscript. Dr. Bermejo-Pareja: conception, organization, and execution of the research project, and review and critique of the manuscript.
STUDY FUNDING
Additional information about collaborators and detailed funding of the NEDICES Study can be found on the Web (http://www.ciberned.es/estudio-nedices). The Spanish Health Research Agency and the Spanish Office of Science and Technology supported NEDICES. Dr. Benito-León is supported by the NIH, Bethesda, MD (R01 NS039422), the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR), and the Spanish Health Research Agency (grant FIS PI12/01602). Dr. Bermejo-Pareja is supported by the NIH R01 NS039422 and the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR). Dr. Elan D. Louis has received research support from the NIH: National Institute of Neurological Disorders and Stroke R01 NS042859 (principal investigator), R01 NS39422 (principal investigator), T32 NS07153-24 (principal investigator), R01 NS073872 (principal investigator), R21 NS077094 (coinvestigator), and R01 NS36630 (coinvestigator), as well as the Parkinson's Disease Foundation (principal investigator).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010;74:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driver JA, Beiser A, Au R, et al. Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study. BMJ 2012;344:e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero JP, Benito-León J, Louis ED, Bermejo-Fareja F. Alzheimer's disease is associated with decreased risk of cancer-specific mortality: a prospective study (NEDICES). J Alzheimers Dis 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry 1999;66:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavares AR, Jr, de Melo AC, Sternberg C. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010;75:1215–1216 [PubMed] [Google Scholar]
- 6.Jones RW. Inflammation and Alzheimer's disease. Lancet 2001;358:436–437 [DOI] [PubMed] [Google Scholar]
- 7.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch Neurol 2004;61:668–672 [DOI] [PubMed] [Google Scholar]
- 8.Haabeth OA, Lorvik KB, Hammarstrom C, et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun 2011;2:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinnunen PJ. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010;75:1215. [DOI] [PubMed] [Google Scholar]
- 10.Harris F, Dennison SR, Phoenix DA. Aberrant action of amyloidogenic host defense peptides: a new paradigm to investigate neurodegenerative disorders? FASEB J 2012;26:1776–1781 [DOI] [PubMed] [Google Scholar]
- 11.Bennett DA, Leurgans S. Is there a link between cancer and Alzheimer disease? Neurology 2010;74:100–101 [DOI] [PubMed] [Google Scholar]
- 12.Benito-León J, Bermejo-Pareja F, Rodríguez J, et al. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord 2003;18:267–274 [DOI] [PubMed] [Google Scholar]
- 13.Benito-León J, Bermejo-Pareja F, Morales-González JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology 2004;62:734–741 [DOI] [PubMed] [Google Scholar]
- 14.Benito-León J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord 2003;18:389–394 [DOI] [PubMed] [Google Scholar]
- 15.Benito-León J, Bermejo-Pareja F, Louis ED; Neurological Disorders in Central Spain (NEDICES) Study Group. Incidence of essential tremor in three elderly populations of central Spain. Neurology 2005;64:1721–1725 [DOI] [PubMed] [Google Scholar]
- 16.Díaz-Guzman J, Bermejo-Pareja F, Benito-León J, et al. Prevalence of stroke and transient ischemic attack in three elderly populations of central Spain. Neuroepidemiology 2008;30:247–253 [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Salio A, Benito-León J, Díaz-Guzman J, Bermejo-Pareja F. Cerebrovascular disease incidence in central Spain (NEDICES): a population-based prospective study. J Neurol Sci 2010;298:85–90 [DOI] [PubMed] [Google Scholar]
- 18.Bermejo-Pareja F, Benito-León J, Vega S, et al. Consistency of clinical diagnosis of dementia in NEDICES: a population-based longitudinal study in Spain. J Geriatr Psychiatry Neurol 2009;22:246–255 [DOI] [PubMed] [Google Scholar]
- 19.Bermejo-Pareja F, Benito-León J, Vega S, Medrano MJ, Roman GC; Neurological Disorders in Central Spain (NEDICES) Study Group. Incidence and subtypes of dementia in three elderly populations of central Spain. J Neurol Sci 2008;264:63–72 [DOI] [PubMed] [Google Scholar]
- 20.Villarejo A, Benito-León J, Trincado R, et al. Dementia-associated mortality at thirteen years in the NEDICES Cohort Study. J Alzheimers Dis 2011;26:543–551 [DOI] [PubMed] [Google Scholar]
- 21.Morales JM, Bermejo FP, Benito-León J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from Central Spain. Public Health 2004;118:426–433 [DOI] [PubMed] [Google Scholar]
- 22.Vega S, Benito-León J, Bermejo-Pareja F, et al. Several factors influenced attrition in a population-based elderly cohort: neurological disorders in Central Spain Study. J Clin Epidemiol 2010;63:215–222 [DOI] [PubMed] [Google Scholar]
- 23.Bermejo-Pareja F, Benito-León J, Vega QS, et al. The NEDICES cohort of the elderly: methodology and main neurological findings [in Spanish]. Rev Neurol 2008;46:416–423 [PubMed] [Google Scholar]
- 24.Benito-León J, Louis ED, Rivera-Navarro J, Medrano MJ, Vega S, Bermejo-Pareja F. Low morale is associated with increased risk of mortality in the elderly: a population-based prospective study (NEDICES). Age Ageing 2010;39:366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benito-León J, Louis ED, Bermejo-Pareja F; Neurological Disorders in Central Spain Study Group. Population-based case-control study of morale in Parkinson's disease. Eur J Neurol 2009;16:330–336 [DOI] [PubMed] [Google Scholar]
- 26.Louis ED, Benito-León J, Bermejo-Pareja F; Neurological Disorders in Central Spain Study Group. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol 2007;14:1138–1146 [DOI] [PubMed] [Google Scholar]
- 27.Benito-León J, Louis ED, Bermejo-Pareja F; Neurological Disorders in Central Spain Study Group. Population-based case-control study of cognitive function in essential tremor. Neurology 2006;66:69–74 [DOI] [PubMed] [Google Scholar]
- 28.Benito-León J, Louis ED, Vega S, Bermejo-Pareja F. Statins and cognitive functioning in the elderly: a population-based study. J Alzheimers Dis 2010;21:95–102 [DOI] [PubMed] [Google Scholar]
- 29.Benito-León J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. J Alzheimers Dis 2010;22:159–170 [DOI] [PubMed] [Google Scholar]
- 30.Benito-León J, Louis ED, Posada IJ, et al. Population-based case-control study of cognitive function in early Parkinson's disease (NEDICES). J Neurol Sci 2011;310:176–182 [DOI] [PubMed] [Google Scholar]
- 31.Benito-León J, Louis ED, Bermejo-Pareja F. Cognitive decline in short and long sleepers: a prospective population-based study (NEDICES). J Psychiatr Res 2013;47:1998–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benito-León J, Louis ED, Sánchez-Ferro A, Bermejo-Pareja F. Rate of cognitive decline during the premotor phase of essential tremor: a prospective study. Neurology 2013;81:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 34.Vanacore N, Spila-Alegiani S, Raschetti R, Meco G. Mortality cancer risk in parkinsonian patients: a population-based study. Neurology 1999;52:395–398 [DOI] [PubMed] [Google Scholar]
- 35.Sorensen SA, Fenger K. Causes of death in patients with Huntington's disease and in unaffected first degree relatives. J Med Genet 1992;29:911–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain 2009;132:1310–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh H, Madison C, Haight TJ, Markley C, Jagust WJ. Effects of age and beta-amyloid on cognitive changes in normal elderly people. Neurobiol Aging 2012;33:2746–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004;292:2237–2242 [DOI] [PubMed] [Google Scholar]
- 39.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry 2008;64:484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pérez-Gomez B, Aragonés N, Pollan M, et al. Accuracy of cancer death certificates in Spain: a summary of available information. Gac Sanit 2006;20(suppl 3):42–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


