Abstract
Several disulfide benzamides have been shown to possess wide-spectrum antiretroviral activity in cell culture at low micromolar to submicromolar concentrations, inhibiting human immunodeficiency virus (HIV) type 1 (HIV-1) clinical and drug-resistant strains along with HIV-2 and simian immunodeficiency virus [Rice, W. G., Supko, J. G., Malspeis, L., Buckheit, R. W., Jr., Clanton, D., Bu, M., Graham, L., Schaeffer, C. A., Turpin, J. A., Domagala, J., Gogliotti, R., Bader, J. P., Halliday, S. M., Coren, L., Sowder, R. C., II, Arthur, L. O. & Henderson, L. E. (1995) Science 270, 1194-1197]. Rice and coworkers have proposed that the compounds act by "attacking" the two zinc fingers of HIV nucleocapsid protein. Shown here is evidence that low micromolar concentrations of the anti-HIV disulfide benzamides eject zinc from HIV nucleocapsid protein (NCp7) in vitro, as monitored by the zinc-specific fluorescent probe N-(6-methoxy-8-quinoyl)-p-toluenesulfonamide (TSQ). Structurally similar disulfide benzamides that do not inhibit HIV-1 in culture do not eject zinc, nor do analogs of the antiviral compounds with the disulfide replaced with a methylene sulfide. The kinetics of NCp7 zinc ejection by disulfide benzamides were found to be nonsaturable and biexponential, with the rate of ejection from the C-terminal zinc finger 7-fold faster than that from the N-terminal. The antiviral compounds were found to inhibit the zinc-dependent binding of NCp7 to HIV psi RNA, as studied by gel-shift assays, and the data correlated well with the zinc ejection data. Anti-HIV disulfide benzamides specifically eject NCp7 zinc and abolish the protein's ability to bind psi RNA in vitro, providing evidence for a possible antiretroviral mechanism of action of these compounds. Congeners of this class are under advanced preclinical evaluation as a potential chemotherapy for acquired immunodeficiency syndrome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldovini A., Young R. A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990 May;64(5):1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condra J. H., Schleif W. A., Blahy O. M., Gabryelski L. J., Graham D. J., Quintero J. C., Rhodes A., Robbins H. L., Roth E., Shivaprakash M. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature. 1995 Apr 6;374(6522):569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- Coyle P., Zalewski P. D., Philcox J. C., Forbes I. J., Ward A. D., Lincoln S. F., Mahadevan I., Rofe A. M. Measurement of zinc in hepatocytes by using a fluorescent probe, zinquin: relationship to metallothionein and intracellular zinc. Biochem J. 1994 Nov 1;303(Pt 3):781–786. doi: 10.1042/bj3030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila R. T., Johnson V. A., Welles S. L., Japour A. J., Kuritzkes D. R., DeGruttola V., Reichelderfer P. S., Coombs R. W., Crumpacker C. S., Kahn J. O. Zidovudine resistance and HIV-1 disease progression during antiretroviral therapy. AIDS Clinical Trials Group Protocol 116B/117 Team and the Virology Committee Resistance Working Group. Ann Intern Med. 1995 Mar 15;122(6):401–408. doi: 10.7326/0003-4819-122-6-199503150-00001. [DOI] [PubMed] [Google Scholar]
- Dorfman T., Luban J., Goff S. P., Haseltine W. A., Göttlinger H. G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993 Oct;67(10):6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Byrnes V. W., Condra J. H., Schleif W. A., Sardana V. V. The genetic and functional basis of HIV-1 resistance to nonnucleoside reverse transcriptase inhibitors. Arch Virol Suppl. 1994;9:11–17. doi: 10.1007/978-3-7091-9326-6_2. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J., Hernandez M. D., Goik S. A., Morton J. D., McGinty J. F. Loss of zinc staining from hippocampal mossy fibers during kainic acid induced seizures: a histofluorescence study. Brain Res. 1988 Apr 19;446(2):383–386. doi: 10.1016/0006-8993(88)90899-2. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J., Kasarskis E. J., Ringo D., Frederickson R. E. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987 Jun;20(2):91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- Gorelick R. J., Nigida S. M., Jr, Bess J. W., Jr, Arthur L. O., Henderson L. E., Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990 Jul;64(7):3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. M., Berg J. M. A retroviral Cys-Xaa2-Cys-Xaa4-His-Xaa4-Cys peptide binds metal ions: spectroscopic studies and a proposed three-dimensional structure. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4047–4051. doi: 10.1073/pnas.86.11.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Bowers M. A., Sowder R. C., 2nd, Serabyn S. A., Johnson D. G., Bess J. W., Jr, Arthur L. O., Bryant D. K., Fenselau C. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processings, and complete amino acid sequences. J Virol. 1992 Apr;66(4):1856–1865. doi: 10.1128/jvi.66.4.1856-1865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler T. P., Foltin S. K., Ye Q. Z., Hupe D. J. HIV1 integrase expressed in Escherichia coli from a synthetic gene. Gene. 1993 Dec 22;136(1-2):323–328. doi: 10.1016/0378-1119(93)90488-o. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Harrigan P. R. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science. 1995 Aug 4;269(5224):696–699. doi: 10.1126/science.7542804. [DOI] [PubMed] [Google Scholar]
- Montaner J. S., Singer J., Schechter M. T., Raboud J. M., Tsoukas C., O'Shaughnessy M., Ruedy J., Nagai K., Salomon H., Spira B. Clinical correlates of in vitro HIV-1 resistance ot zidovudine. Results of the Multicentre Canadian AZT Trial. AIDS. 1993 Feb;7(2):189–196. doi: 10.1097/00002030-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Mély Y., Jullian N., Morellet N., De Rocquigny H., Dong C. Z., Piémont E., Roques B. P., Gérard D. Spatial proximity of the HIV-1 nucleocapsid protein zinc fingers investigated by time-resolved fluorescence and fluorescence resonance energy transfer. Biochemistry. 1994 Oct 11;33(40):12085–12091. doi: 10.1021/bi00206a011. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Fujita M., Sugiura Y., Ishii S., Tsutomu A., Yamamoto T., Inoue J. I. Novel zinc chelators which inhibit the binding of HIV-EP1 (HIV enhancer binding protein) to NF-kappa B recognition sequence. J Med Chem. 1994 Dec 9;37(25):4267–4269. doi: 10.1021/jm00051a002. [DOI] [PubMed] [Google Scholar]
- Rice W. G., Schaeffer C. A., Harten B., Villinger F., South T. L., Summers M. F., Henderson L. E., Bess J. W., Jr, Arthur L. O., McDougal J. S. Inhibition of HIV-1 infectivity by zinc-ejecting aromatic C-nitroso compounds. Nature. 1993 Feb 4;361(6411):473–475. doi: 10.1038/361473a0. [DOI] [PubMed] [Google Scholar]
- Rice W. G., Supko J. G., Malspeis L., Buckheit R. W., Jr, Clanton D., Bu M., Graham L., Schaeffer C. A., Turpin J. A., Domagala J. Inhibitors of HIV nucleocapsid protein zinc fingers as candidates for the treatment of AIDS. Science. 1995 Nov 17;270(5239):1194–1197. doi: 10.1126/science.270.5239.1194. [DOI] [PubMed] [Google Scholar]
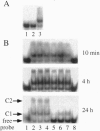
- Sakaguchi K., Zambrano N., Baldwin E. T., Shapiro B. A., Erickson J. W., Omichinski J. G., Clore G. M., Gronenborn A. M., Appella E. Identification of a binding site for the human immunodeficiency virus type 1 nucleocapsid protein. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5219–5223. doi: 10.1073/pnas.90.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. F., Henderson L. E., Chance M. R., Bess J. W., Jr, South T. L., Blake P. R., Sagi I., Perez-Alvarado G., Sowder R. C., 3rd, Hare D. R. Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci. 1992 May;1(5):563–574. doi: 10.1002/pro.5560010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surovoy A., Waidelich D., Jung G. Nucleocapsid protein of HIV-1 and its Zn2+ complex formation analysis with electrospray mass spectrometry. FEBS Lett. 1992 Oct 26;311(3):259–262. doi: 10.1016/0014-5793(92)81115-3. [DOI] [PubMed] [Google Scholar]
- You J. C., McHenry C. S. HIV nucleocapsid protein. Expression in Escherichia coli, purification, and characterization. J Biol Chem. 1993 Aug 5;268(22):16519–16527. [PubMed] [Google Scholar]
- Zalewski P. D., Forbes I. J., Betts W. H. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II). Biochem J. 1993 Dec 1;296(Pt 2):403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]