Abstract
B1(dsFv)-PE33 is a recombinant immunotoxin composed of a mutant form of Pseudomonas exotoxin (PE) that does not need proteolytic activation and a disulfide-stabilized Fv fragment of the anti-Lewis(y) monoclonal antibody B1, which recognizes a carbohydrate epitope on human carcinoma cells. In this molecule, amino acids 1-279 of PE are deleted and domain Ib (amino acids 365-394) is replaced by the heavy chain variable region (VH) domain of monoclonal antibody B1. The light chain (VL) domain is connected to the VH domain by a disulfide bond. This recombinant toxin, termed B1(dsFv)-PE33, does not require proteolytic activation and it is smaller than other immunotoxins directed at Lewis(y), all of which require proteolytic activation. Furthermore, it is more cytotoxic to antigen-positive cell lines. B1(dsFv)-PE38 has the highest antitumor activity of anti-Lewis(y) immunotoxins previously constructed. B1(dsFv)-PE33 caused complete regression of tumors when given at 12 micrograms/kg (200 pmol/kg) every other day for three doses, whereas B1(dsFv)-PE38 did not cause regressions at 13 micrograms/kg (200 pmol/kg). By bypassing the need for proteolytic activation and decreasing molecular size we have enlarged the therapeutic window for the treatment of human cancers growing in mice, so that complete remissions are observed at 2.5% of the LD50.
Full text
PDF

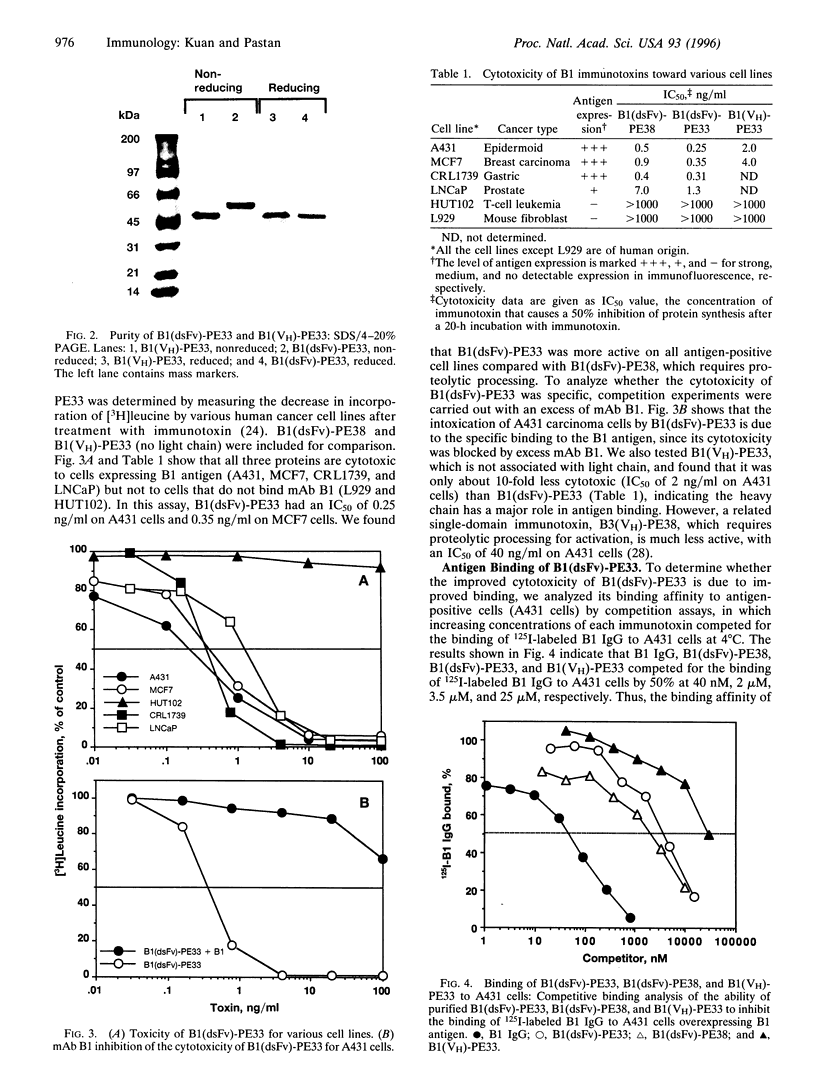
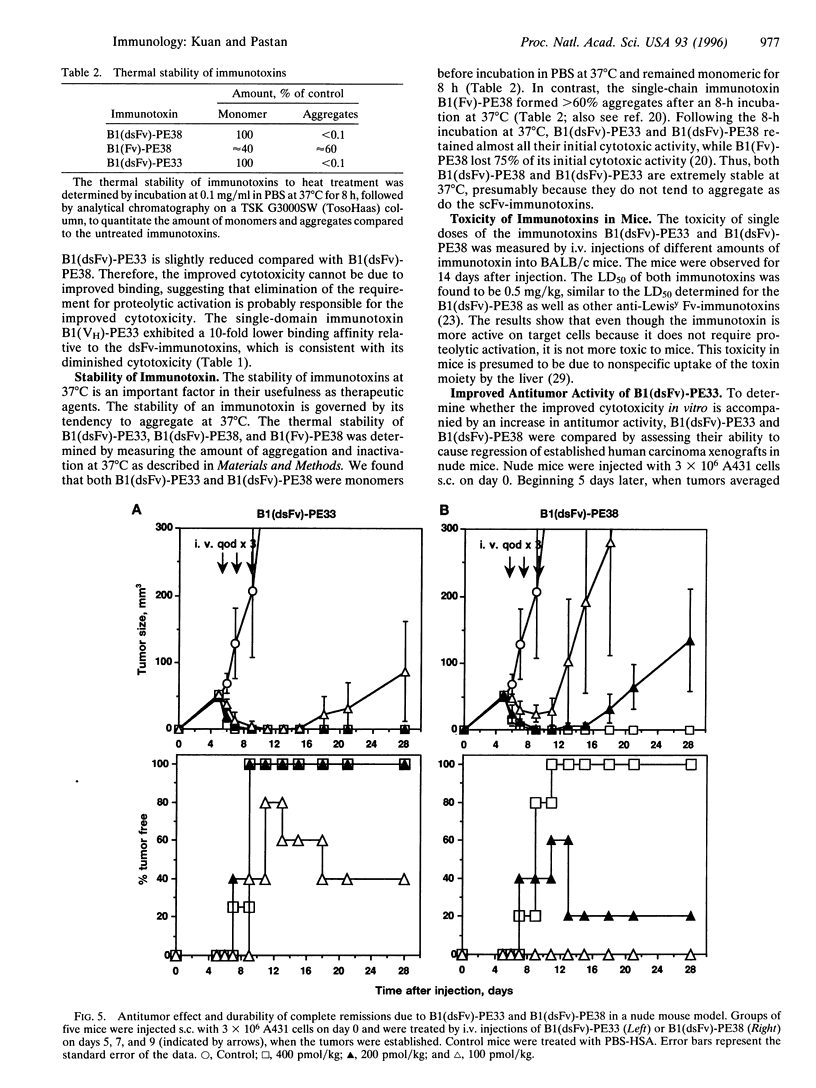

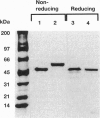
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batra J. K., Kasprzyk P. G., Bird R. E., Pastan I., King C. R. Recombinant anti-erbB2 immunotoxins containing Pseudomonas exotoxin. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5867–5871. doi: 10.1073/pnas.89.13.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhar I., Pastan I. Characterization of B1(Fv)PE38 and B1(dsFv)PE38: single-chain and disulfide-stabilized Fv immunotoxins with increased activity that cause complete remissions of established human carcinoma xenografts in nude mice. Clin Cancer Res. 1995 Sep;1(9):1023–1029. [PubMed] [Google Scholar]
- Benhar I., Pastan I. Cloning, expression and characterization of the Fv fragments of the anti-carbohydrate mAbs B1 and B5 as single-chain immunotoxins. Protein Eng. 1994 Dec;7(12):1509–1515. doi: 10.1093/protein/7.12.1509. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Brinkmann U., Buchner J., Pastan I. Independent domain folding of Pseudomonas exotoxin and single-chain immunotoxins: influence of interdomain connections. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3075–3079. doi: 10.1073/pnas.89.7.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann U., Lee B. K., Pastan I. Recombinant immunotoxins containing the VH or VL domain of monoclonal antibody B3 fused to Pseudomonas exotoxin. J Immunol. 1993 Apr 1;150(7):2774–2782. [PubMed] [Google Scholar]
- Brinkmann U., Pastan I. Immunotoxins against cancer. Biochim Biophys Acta. 1994 May 27;1198(1):27–45. doi: 10.1016/0304-419x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Brinkmann U., Reiter Y., Jung S. H., Lee B., Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7538–7542. doi: 10.1073/pnas.90.16.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron M. F., Fryling C. M., FitzGerald D. J. Cleavage of pseudomonas exotoxin and diphtheria toxin by a furin-like enzyme prepared from beef liver. J Biol Chem. 1994 Jul 8;269(27):18167–18176. [PubMed] [Google Scholar]
- Clackson T., Winter G. 'Sticky feet'-directed mutagenesis and its application to swapping antibody domains. Nucleic Acids Res. 1989 Dec 25;17(24):10163–10170. doi: 10.1093/nar/17.24.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990 Feb 1;50(3 Suppl):814s–819s. [PubMed] [Google Scholar]
- Jung S. H., Pastan I., Lee B. Design of interchain disulfide bonds in the framework region of the Fv fragment of the monoclonal antibody B3. Proteins. 1994 May;19(1):35–47. doi: 10.1002/prot.340190106. [DOI] [PubMed] [Google Scholar]
- Kihara A., Pastan I. Analysis of sequences required for the cytotoxic action of a chimeric toxin composed of Pseudomonas exotoxin and transforming growth factor alpha. Bioconjug Chem. 1994 Nov-Dec;5(6):532–538. doi: 10.1021/bc00030a008. [DOI] [PubMed] [Google Scholar]
- Kreitman R. J., Bailon P., Chaudhary V. K., FitzGerald D. J., Pastan I. Recombinant immunotoxins containing anti-Tac(Fv) and derivatives of Pseudomonas exotoxin produce complete regression in mice of an interleukin-2 receptor-expressing human carcinoma. Blood. 1994 Jan 15;83(2):426–434. [PubMed] [Google Scholar]
- Kuan C. T., Wang Q. C., Pastan I. Pseudomonas exotoxin A mutants. Replacement of surface exposed residues in domain II with cysteine residues that can be modified with polyethylene glycol in a site-specific manner. J Biol Chem. 1994 Mar 11;269(10):7610–7616. [PubMed] [Google Scholar]
- Ogata M., Fryling C. M., Pastan I., FitzGerald D. J. Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. J Biol Chem. 1992 Dec 15;267(35):25396–25401. [PubMed] [Google Scholar]
- Pastan I. H., Pai L. H., Brinkmann U., Fitzgerald D. J. Recombinant toxins: new therapeutic agents for cancer. Ann N Y Acad Sci. 1995 Jun 30;758:345–354. doi: 10.1111/j.1749-6632.1995.tb24840.x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Lovelace E. T., Gallo M. G., Rutherford A. V., Magnani J. L., Willingham M. C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991 Jul 15;51(14):3781–3787. [PubMed] [Google Scholar]
- Raag R., Whitlow M. Single-chain Fvs. FASEB J. 1995 Jan;9(1):73–80. doi: 10.1096/fasebj.9.1.7821762. [DOI] [PubMed] [Google Scholar]
- Reiter Y., Brinkmann U., Jung S. H., Lee B., Kasprzyk P. G., King C. R., Pastan I. Improved binding and antitumor activity of a recombinant anti-erbB2 immunotoxin by disulfide stabilization of the Fv fragment. J Biol Chem. 1994 Jul 15;269(28):18327–18331. [PubMed] [Google Scholar]
- Reiter Y., Brinkmann U., Webber K. O., Jung S. H., Lee B., Pastan I. Engineering interchain disulfide bonds into conserved framework regions of Fv fragments: improved biochemical characteristics of recombinant immunotoxins containing disulfide-stabilized Fv. Protein Eng. 1994 May;7(5):697–704. doi: 10.1093/protein/7.5.697. [DOI] [PubMed] [Google Scholar]
- Reiter Y., Pai L. H., Brinkmann U., Wang Q. C., Pastan I. Antitumor activity and pharmacokinetics in mice of a recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Cancer Res. 1994 May 15;54(10):2714–2718. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Theuer C. P., FitzGerald D. J., Pastan I. A recombinant form of Pseudomonas exotoxin A containing transforming growth factor alpha near its carboxyl terminus for the treatment of bladder cancer. J Urol. 1993 Jun;149(6):1626–1632. doi: 10.1016/s0022-5347(17)36464-9. [DOI] [PubMed] [Google Scholar]
- Theuer C. P., FitzGerald D., Pastan I. A recombinant form of Pseudomonas exotoxin directed at the epidermal growth factor receptor that is cytotoxic without requiring proteolytic processing. J Biol Chem. 1992 Aug 25;267(24):16872–16877. [PubMed] [Google Scholar]
- Theuer C. P., Kreitman R. J., FitzGerald D. J., Pastan I. Immunotoxins made with a recombinant form of Pseudomonas exotoxin A that do not require proteolysis for activity. Cancer Res. 1993 Jan 15;53(2):340–347. [PubMed] [Google Scholar]
- Yokota T., Milenic D. E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992 Jun 15;52(12):3402–3408. [PubMed] [Google Scholar]