Abstract
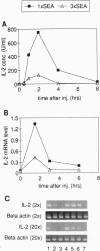
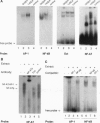
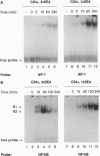
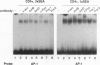
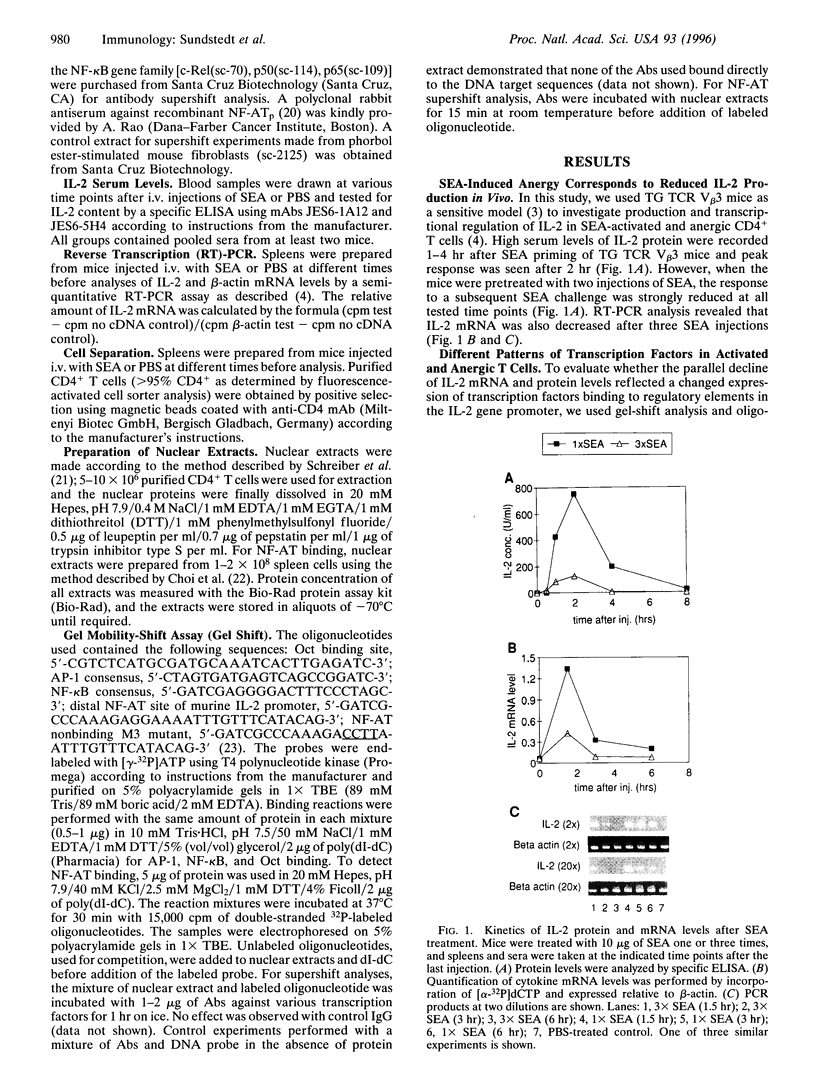
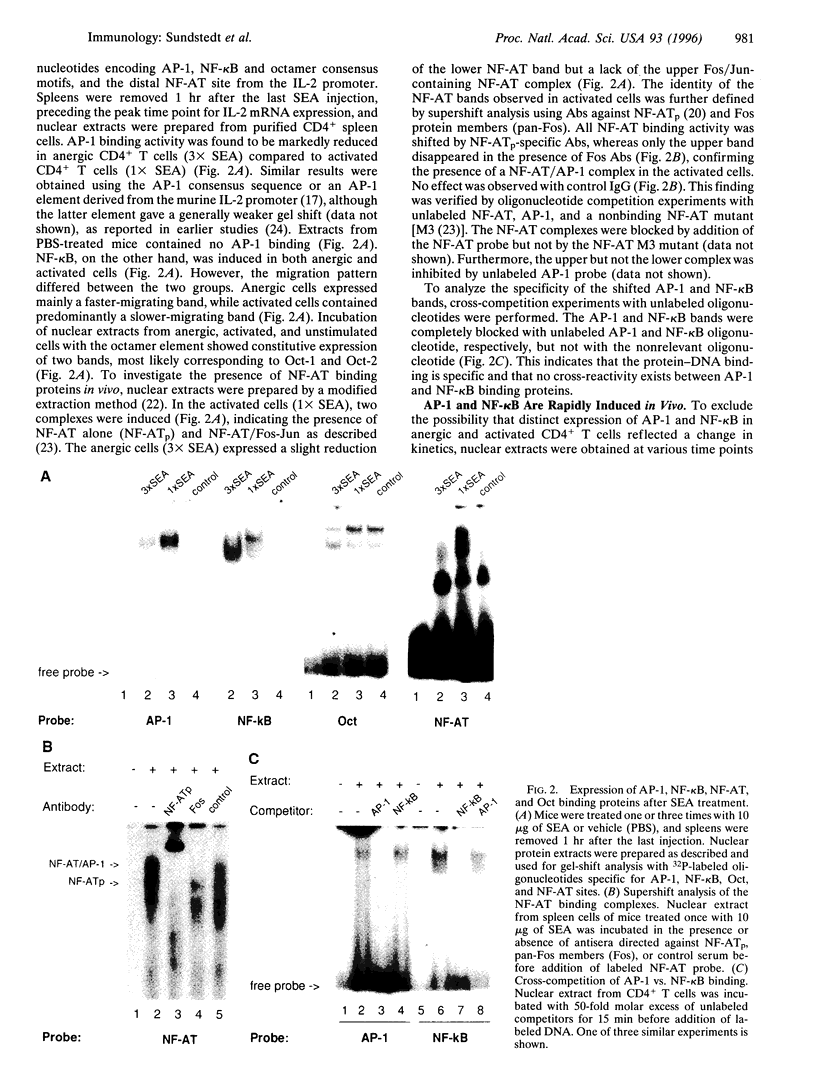
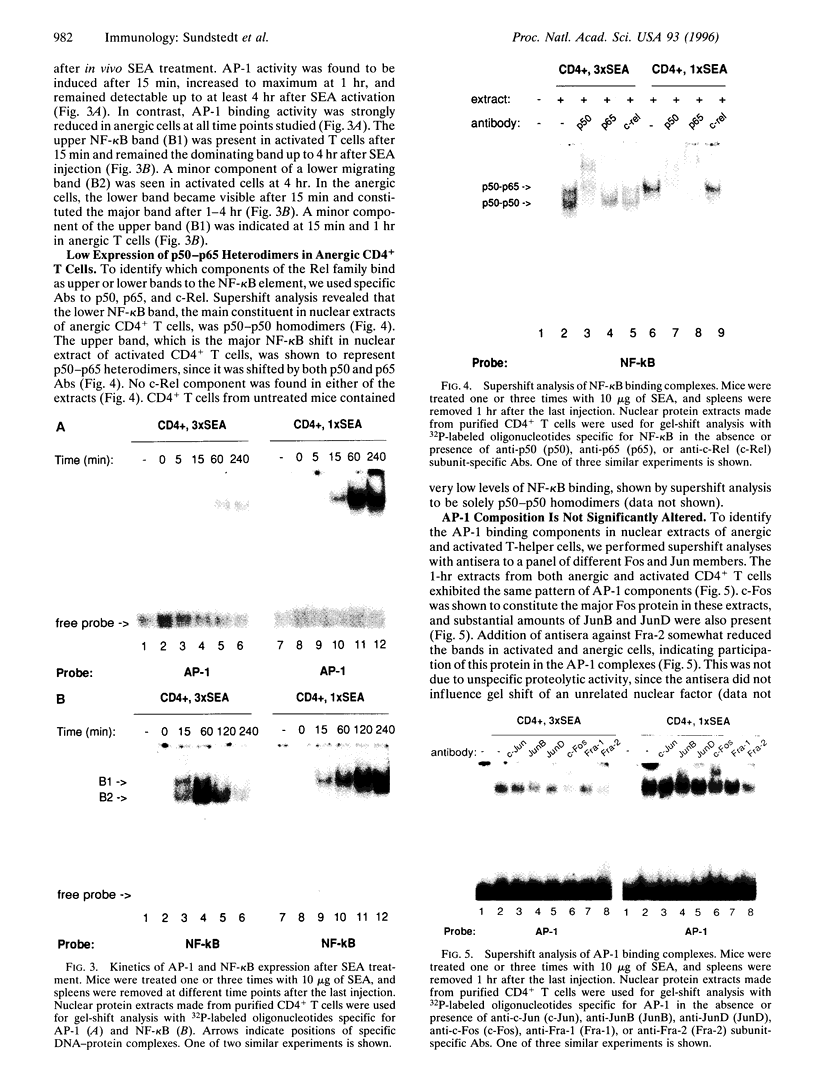
Anergy is a major mechanism to ensure antigen-specific tolerance in T lymphocytes in the adult. In vivo, anergy has mainly been studied at the cellular level. In this study, we used the T-cell-activating superantigen staphylococcal enterotoxin A (SEA) to investigate molecular mechanisms of T-lymphocyte anergy in vivo. Injection of SEA to adult mice activates CD4+ T cells expressing certain T-cell receptor (TCR) variable region beta-chain families and induces strong and rapid production of interleukin 2 (IL-2). In contrast, repeated injections of SEA cause CD4+ T-cell deletion and anergy in the remaining CD4+ T cells, characterized by reduced expression of IL-2 at mRNA and protein levels. We analyzed expression of AP-1, NF-kappa B, NF-AT, and octamer binding transcription factors, which are known to be involved in the regulation of IL-2 gene promoter activity. Large amounts of AP-1 and NF-kappa B and significant quantities of NF-AT were induced in SEA-activated CD4+ spleen T cells, whereas Oct-1 and Oct-2 DNA binding activity was similar in both resting and activated T cells. In contrast, anergic CD4+ T cells contained severely reduced levels of AP-1 and Fos/Jun-containing NF-AT complexes but expressed significant amounts of NF-kappa B and Oct binding proteins after SEA stimulation. Resolution of the NF-kappa B complex demonstrated predominant expression of p50-p65 heterodimers in activated CD4+ T cells, while anergic cells mainly expressed the transcriptionally inactive p50 homodimer. These alterations of transcription factors are likely to be responsible for repression of IL-2 in anergic T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Berg L. J., Pullen A. M., Fazekas de St Groth B., Mathis D., Benoist C., Davis M. M. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989 Sep 22;58(6):1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- Boise L. H., Petryniak B., Mao X., June C. H., Wang C. Y., Lindsten T., Bravo R., Kovary K., Leiden J. M., Thompson C. B. The NFAT-1 DNA binding complex in activated T cells contains Fra-1 and JunB. Mol Cell Biol. 1993 Mar;13(3):1911–1919. doi: 10.1128/mcb.13.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M. S., Brines R. D., Holman M. J., Klaus G. G. Induction of NF-AT in normal B lymphocytes by anti-immunoglobulin or CD40 ligand in conjunction with IL-4. Immunity. 1994 Jun;1(3):179–187. doi: 10.1016/1074-7613(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Björklund M., Sundstedt A., Hedlund G., Samson D., Kalland T. Immunopharmacology of the superantigen staphylococcal enterotoxin A in T-cell receptor V beta 3 transgenic mice. Immunology. 1993 Aug;79(4):520–527. [PMC free article] [PubMed] [Google Scholar]
- Durand D. B., Shaw J. P., Bush M. R., Replogle R. E., Belagaje R., Crabtree G. R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988 Apr;8(4):1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Tan T. H., Rice N. R., Sica A., Young H. A. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go C., Miller J. Differential induction of transcription factors that regulate the interleukin 2 gene during anergy induction and restimulation. J Exp Med. 1992 May 1;175(5):1327–1336. doi: 10.1084/jem.175.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld A. E., Strominger J. L., Doyle C. Human tumor necrosis factor alpha gene regulation in phorbol ester stimulated T and B cell lines. J Exp Med. 1991 Jul 1;174(1):73–81. doi: 10.1084/jem.174.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Nolan P. Nuclear transcription factors that bind to elements of the IL-2 promoter. Induction requirements in primary human T cells. J Immunol. 1991 Oct 15;147(8):2734–2739. [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Miner Z., Kerppola T. K., Lambert J. N., Verdine G. L., Curran T., Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993 Sep 23;365(6444):352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Valge-Archer V. E., Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992 Apr 30;356(6372):801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- Jain J., Miner Z., Rao A. Analysis of the preexisting and nuclear forms of nuclear factor of activated T cells. J Immunol. 1993 Jul 15;151(2):837–848. [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992 Feb 15;148(4):1240–1250. [PubMed] [Google Scholar]
- Kang S. M., Beverly B., Tran A. C., Brorson K., Schwartz R. H., Lenardo M. J. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992 Aug 21;257(5073):1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- Kang S. M., Tran A. C., Grilli M., Lenardo M. J. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992 Jun 5;256(5062):1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Lederer J. A., Liou J. S., Todd M. D., Glimcher L. H., Lichtman A. H. Regulation of cytokine gene expression in T helper cell subsets. J Immunol. 1994 Jan 1;152(1):77–86. [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- McCaffrey P. G., Luo C., Kerppola T. K., Jain J., Badalian T. M., Ho A. M., Burgeon E., Lane W. S., Lambert J. N., Curran T. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993 Oct 29;262(5134):750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- Ochi Y., Koizumi T., Kobayashi S., Phuchareon J., Hatano M., Takada M., Tomita Y., Tokuhisa T. Analysis of IL-2 gene regulation in c-fos transgenic mice. Evidence for an enhancement of IL-2 expression in splenic T cells stimulated via TCR/CD3 complex. J Immunol. 1994 Oct 15;153(8):3485–3490. [PubMed] [Google Scholar]
- Radler-Pohl A., Gebel S., Sachsenmaier C., König H., Krämer M., Oehler T., Streile M., Ponta H., Rapp U., Rahmsdorf H. J. The activation and activity control of AP-1 (fos/jun). Ann N Y Acad Sci. 1993 Jun 11;684:127–148. doi: 10.1111/j.1749-6632.1993.tb32277.x. [DOI] [PubMed] [Google Scholar]
- Rincón M., Flavell R. A. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 1994 Sep 15;13(18):4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Schmitz M. L., Baeuerle P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991 Dec;10(12):3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990 Feb;10(2):561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstedt A., Dohlsten M., Hedlund G., Höidén I., Björklund M., Kalland T. Superantigens anergize cytokine production but not cytotoxicity in vivo. Immunology. 1994 May;82(1):117–125. [PMC free article] [PubMed] [Google Scholar]
- Umlauf S. W., Beverly B., Kang S. M., Brorson K., Tran A. C., Schwartz R. H. Molecular regulation of the IL-2 gene: rheostatic control of the immune system. Immunol Rev. 1993 Jun;133:177–197. doi: 10.1111/j.1600-065x.1993.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Wedel A., Schraut W., Ströbel M., Wendelgass P., Sternsdorf T., Bäuerle P. A., Haas J. G., Riethmüller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994 Jun 24;269(25):17001–17004. [PubMed] [Google Scholar]
- de Grazia U., Felli M. P., Vacca A., Farina A. R., Maroder M., Cappabianca L., Meco D., Farina M., Screpanti I., Frati L. Positive and negative regulation of the composite octamer motif of the interleukin 2 enhancer by AP-1, Oct-2, and retinoic acid receptor. J Exp Med. 1994 Oct 1;180(4):1485–1497. doi: 10.1084/jem.180.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]