Abstract
The cell wall peptidoglycan (PG) of Burkholderia cenocepacia, an opportunistic pathogen, has not yet been characterized. However, the B. cenocepacia genome contains homologs of genes encoding PG biosynthetic functions in other bacteria. PG biosynthesis involves the formation of the undecaprenyl-pyrophosphate-linked N-acetyl glucosamine-N-acetyl muramic acid-pentapeptide, known as lipid II, which is built on the cytosolic face of the cell membrane. Lipid II is then translocated across the membrane and its glycopeptide moiety becomes incorporated into the growing cell wall mesh; this translocation step is critical to PG synthesis. We have investigated candidate flippase homologs of the MurJ family in B. cenocepacia. Our results show that BCAL2764, herein referred to as murJBc, is indispensable for viability. Viable B. cenocepacia could only be obtained through a conditional mutagenesis strategy by placing murJBc under the control of a rhamnose-inducible promoter. Under rhamnose depletion, the conditional strain stopped growing and individual cells displayed morphological abnormalities consistent with a defect in PG synthesis. Bacterial cells unable to express MurJBc underwent cell lysis, while partial MurJBc depletion sensitized the mutant to the action of β-lactam antibiotics. Depletion of MurJBc caused accumulation of PG precursors consistent with the notion that this protein plays a role in lipid II flipping to the periplasmic compartment. Reciprocal complementation experiments of conditional murJ mutants in B. cenocepacia and Escherichia coli with plasmids expressing MurJ from each strain indicated that MurJBc and MurJEc are functional homologs. Together, our results are consistent with the notion that MurJBc is a PG lipid II flippase in B. cenocepacia.
Keywords: cell wall, cystic fibrosis, essential gene, flippase, undecaprenylphosphate
Introduction
Burkholderia cenocepacia, a metabolically diverse Gram-negative β-proteobacterium, is widespread in the rhizosphere. This bacterium often causes opportunistic, chronic lung infections in patients with cystic fibrosis (Vandamme et al. 1997). Environmental bacteria are usually exposed to dramatic changes in growth conditions, which include variations in temperature, pH, concentration of nutrients and salinity, exposure to toxic metals and biocides and encounters with predator and host organisms. We speculate that the ability of these bacteria to adapt to and withstand these changes relies in part on the synthesis and remodeling of the cell wall peptidoglycan (PG), which is key to cell division, bacterial shape and survival under high internal turgor pressure. PG surrounds bacterial cells forming a mesh-like glycopeptide polymer made of repeating units of N-acetyl glucosamine (GlcNAc) and N-acetyl muramic acid (MurNAc). The carboxy group of the MurNAc residues is substituted by a pentapeptide that consists of l-alanine, l-glutamic acid, meso-diaminopimelic acid (or l-lysine) and d-alanyl-d-alanine. The neighboring glycan chains are cross-linked by peptide linkages, resulting in rigid mesh-like macromolecular structure (van Heijenoort 2001).
To our knowledge, the biosynthesis of PG in the Burkholderia genus has not yet been studied. However, the B. cenocepacia genome contains homologs of the genes encoding all the enzymes required for the synthesis of PG precursors in other bacteria, suggesting that the PG biosynthesis pathway is conserved. PG biosynthesis starts in the cytoplasm with the synthesis of the UDP-MurNAc-pentapeptide, which is catalyzed by the Mur ligases (Barreteau et al. 2008) MurC (BCAL3641), MurD (BCAL3464), MurE (BCAL3467) and MurF (BCAL3466). This molecule is the substrate of the membrane protein MraY (BCAL3465) that catalyzes the formation of a phosphoanhydride bond with the lipid carrier, undecaprenyl phosphate (Und-P), and gives rise to Und-PP-MurNAc-pentapeptide also known as lipid I. The GlcNAc moiety from UDP-GlcNAc is transferred to lipid I, resulting in lipid II, a reaction catalyzed by the inner membrane-associated transferase MurG (BCAL3462) (Bouhss et al. 2008). Lipid II is translocated across the cytoplasmic membrane to incorporate the PG subunits into the growing cell wall mesh. At the periplasmic side of the inner membrane, various penicillin-binding proteins catalyze the maturation of the growing PG chain. Glycosyltransferases assemble lipid II to the existing PG releasing Und-PP, which is recycled by an as-yet-unclear mechanism (Valvano 2008). The peptide chains are cross-linked by transpeptidases and terminal d-Ala is removed from the pentapeptide by d-d-carboxypeptidases (van Dam et al. 2007).
The transport of lipid II across the inner membrane is a key step in the synthesis of PG, but its mechanism is not completely elucidated. Studies using a fluorescence lipid II analog have revealed that the transbilayer movement of lipid II is independent of ATP hydrolysis or proton motive force, but it appears to be coupled to transglycosylation activity on the periplasmic side of the inner membrane (van Dam et al. 2007). The Escherichia coli MurJ (Ruiz 2008), also known as MviN (Inoue et al. 2008), is an integral membrane protein with multiple predicted transmembrane helices that belongs to the multi-antimicrobial extrusion-like superfamily. Members of this family include proteins like Wzx, which are implicated in the membrane flipping of lipid-linked oligosaccharide and polysaccharide precursors of lipopolysaccharide O-antigen and capsules (Islam and Lam 2013; Valvano and Hanuszkiewicz 2012). Studies in E. coli demonstrated that MviN is essential for viability. Cells from conditional mutants under non-permissive conditions burst and accumulate lipid II (Inoue et al. 2008; Ruiz 2008), suggesting that this protein is involved in PG biosynthesis and functions as the lipid II flippase, being renamed MurJ (Ruiz 2008). Other studies have shown that MurJ (MviN) is essential in Burkholderia pseudomallei (Ling et al. 2006) and Sinorhizobium meliloti (Rudnick et al. 2001). In contrast, the simultaneous inactivation of four murJ homologs in Bacillus subtilis did not compromise bacterial viability, casting doubts on the functional assignment of this protein as a flippase (Fay and Dworkin 2009). It has been proposed that in E. coli, other membrane proteins such as RodA and FtsW function as lipid II flippases (Ehlert and Holtje 1996; Mohammadi et al. 2011). RodA is required for the synthesis of lateral PG, while FtsW is involved in septal PG synthesis (Ishino et al. 1986, 1989; Ikeda et al. 1989; Khattar et al. 1994; Henriques et al. 1998; Mercer and Weiss 2002; Tamaki et al. 1980). A biochemical study using E. coli membrane vesicles demonstrated that transport of lipid II requires FtsW, but not MurJ, to mediate the transbilayer movement of lipid II in model membranes (Mohammadi et al. 2011). However, this study did not rule out nonspecific flipping, a common occurrence in isolated vesicles and reconstituted in vitro systems (Kol et al. 2001, 2003). More recently, a three-dimensional model of the E. coli MurJ, together with topological studies and functional analyses of specific charged residues, strongly suggest that MurJ is a member of a large family of flippase proteins involved in O-antigen and capsule biosynthesis (Butler et al. 2013).
In this study, we investigated candidate homologs of the MurJ (MviN) family in B. cenocepacia. We show that BCAL2764, herein designated murJBc, encodes a membrane protein that is indispensable for the viability of B. cenocepacia and also required for PG synthesis, while the other flippase homologs were not required for bacterial survival under laboratory conditions. Furthermore, MurJBc shared predicted structural features with its E. coli counterpart, and complementation experiments of conditional murJ mutants in B. cenocepacia and E. coli with plasmids expressing MurJ from each strain indicated that MurJBc and MurJEc are functional homologs.
Results and discussion
B. cenocepacia MurJ is an essential gene and a functional homolog of the E. coli murJ
The initial aim of this study was to identify and elucidate the function of predicted flippases in B. cenocepacia. The annotated genome of B. cenocepacia J2315 was manually inspected for genes encoding proteins with 12 or more transmembrane helices, which were further examined by HHPRED using the Bioinformatics Toolkit from the Max-Planck Institute for Developmental Biology (http://toolkit.tuebingen.mpg.de/hhpred; Remmert et al. 2011) to identify hypothetical proteins with predicted flippase function based on homologies with domains commonly found in these proteins (MVIN, RfbX and Rft1 domains). This analysis identified the following genes: BCAL1907, BCAL2764 (herein designated murJBc) and BCAL3114 in chromosome 1; BCAM0204 and BCAM1007 in chromosome 2. No flippase homologs were noted in chromosome 3 genes. The gene context was also taken into account. BCAL1907 is part of a potential two-gene operon that includes BCAL1906, a predicted glycosyltransferase. murJBc is also part of a potential two-gene operon including BCAL2763, encoding a protein of unknown function. BCAL3114 was previously identified as wzx and is located on a transcriptional region immediately adjacent to the O-antigen gene cluster (Ortega et al. 2005). Deletion of this gene does not affect O-antigen production in B. cenocepacia K56-2 (X. Ortega and M. A. Valvano, unpublished), since the O-antigen in this strain is exported by an ABC transporter encoded by wzt and wzm, both of which are located within the O-antigen gene cluster (Ortega et al. 2005). BCAM0204 and BCAM1007 are part of the capsule polysaccharide cluster. Therefore, BCAL3114, BCAM0204 and BCAM1007 were not investigated further. B. cenocepacia has RodA and FtsW homologs (BCAL0478 and BCAL3463, respectively), which were also not investigated in this study.
To elucidate the possible function of BCAL1907 and murJBc, we constructed unmarked deletion mutants as described in Materials and Methods. Whereas BCAL1907 was readily deleted without any apparent functional consequences to the bacterial cells, attempts to delete murJBc were consistently unsuccessful, as colonies carrying the deleted gene were never obtained. We suspected from these results that murJBc could be essential for B. cenocepacia viability. Therefore, we constructed a conditional mutant derivative of strain K56-2 by placing murJBc under the control of the rhamnose-inducible promoter (Prha). The resulting Prha::murJBc strain (YFM1, Table I) grew on solid medium in the presence of rhamnose (permissive conditions), but failed to grow in the presence of glucose (non-permissive conditions), as expected for a mutant with an essential gene under the control of Prha (Figure. 1A). Loss of viability in the absence of rhamnose was comparable with that found with the positive control strain XOA11, which has the essential gene arnT gene under the control of rhamnose (Prha::arnT) (Ortega et al. 2007) (Figure. 1A). In contrast, the negative control strain XOA10 (Prha::BCAL1928) grew at the same rate with either rhamnose or glucose, as expected (Ortega et al. 2007). Growth without added rhamnose was restored upon complementation of the Prha::murJBc conditional mutant by introduction of the replicative plasmid pDA12 expressing murJBc from a constitutive promoter (Figure. 1B). This confirmed that the growth defect was due to lack of murJBc expression and demonstrated that this gene encodes an essential function supporting bacterial viability.
Table I.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | F− 80 lacZΔM15 endA1 recA1 hsdR17(r−Km+K) supE44 thi-1 ΔgyrA96 (ΔlacZYA-argF)U169 relA1 | Invitrogen |
| GT115 | F− mcrA Δ(mrr-hsdRMS-mcrBC) 80lacZΔM15 ΔlacX74 recA1 rpsL endA1 Δdcm uidA(ΔMluI)::pir-116 ΔsbcC-sbcD | Lab stock |
| NR1152 | NR754; araD+ revertant of MC4100; murJΩ (-14::bla araC PBAD (Ruiz 2008) | |
| W3110 | IN (rrnD-rrnE)1 rph-1 | Lab stock |
| B. cenocepacia | ||
| K56-2 | Clinical isolate, ET12 clone related to J2315 | BCRRC |
| YFM1 | K56-2; Prha::murJBc (BCAL2764); TpR | This study |
| YFM2 | YFM11; Prha::murJBc (BCAL2764); TpR | This study |
| YFM11 | K56-2; ΔlysA (BCAM2076) | This study |
| XOA10 | K56-2; Prha::BCAL1928 | Ortega et al. (2007) |
| XOA11 | K56-2; Prha::arnT (BCAL1929) | Ortega et al. (2007) |
| Plasmids | ||
| pDA12 | Cloning vector, oripBBR1, TetR, mob+, Pdhfr | Aubert et al. (2008) |
| pDAI-SceI-SacB | oripBBR1, TetR, Pdhfr, mob+, sacB+ | Hamad et al. (2010) |
| pEXT21 | Low copy number cloning vector, oriIncW−pSF6, SpR, Ptac | Dykxhoorn et al. (1996) |
| pGPISce-I | oriR6K, mob+, ΩTpR, ISce-I restriction site | Flannagan et al. (2008) |
| pRK2013 | oricolE1, RK2 derivative, KanR, mob+, tra+ | Figurski and Helinski (1979) |
| pSC200 | Cloning vector, oripBBR1 rhaR, rhaS, PrhaB, TpR | Cardona and Valvano (2005) |
| pYM1 | pSC200-murJBc | This study |
| pYM3 | pGPI-SceI with fragments flanking BCAM2076 | This study |
| pYM21 | pDA12-murJEc, TetR | This study |
| pYM28 | pDA12-murJBc, TetR | This study |
| pYM29 | pDA12-FLAGmurJBc, N-terminal FLAG-Tag, TetR | This study |
| pYM30 | pEXT21-FLAGmurJBc, N-terminal FLAG-Tag, SpR | This study |
| pYM31 | pDA12-FLAGmurJBc-R18A, N-terminal FLAG-Tag, TetR | This study |
| pYM32 | pDA12-FLAGmurJBc-R24A, N-terminal FLAG-Tag, TetR | This study |
| pYM33 | pDA12-FLAGmurJBc-D39A, N-terminal FLAG-Tag, TetR | This study |
| pYM34 | pDA12-FLAGmurJBc-R52A, N-terminal FLAG-Tag, TetR | This study |
| pYM35 | pDA12-FLAGmurJBc-R274A, N-terminal FLAG-Tag, TetR | This study |
aTpR, trimethoprim resistance; TetR, tetracycline resistance; KanR, kanamycin resistance; SpR, spectinomycin resistance.
bBCRRC, B. cepacia Research and Referral Repository for Canadian CF Clinics.
Fig. 1.
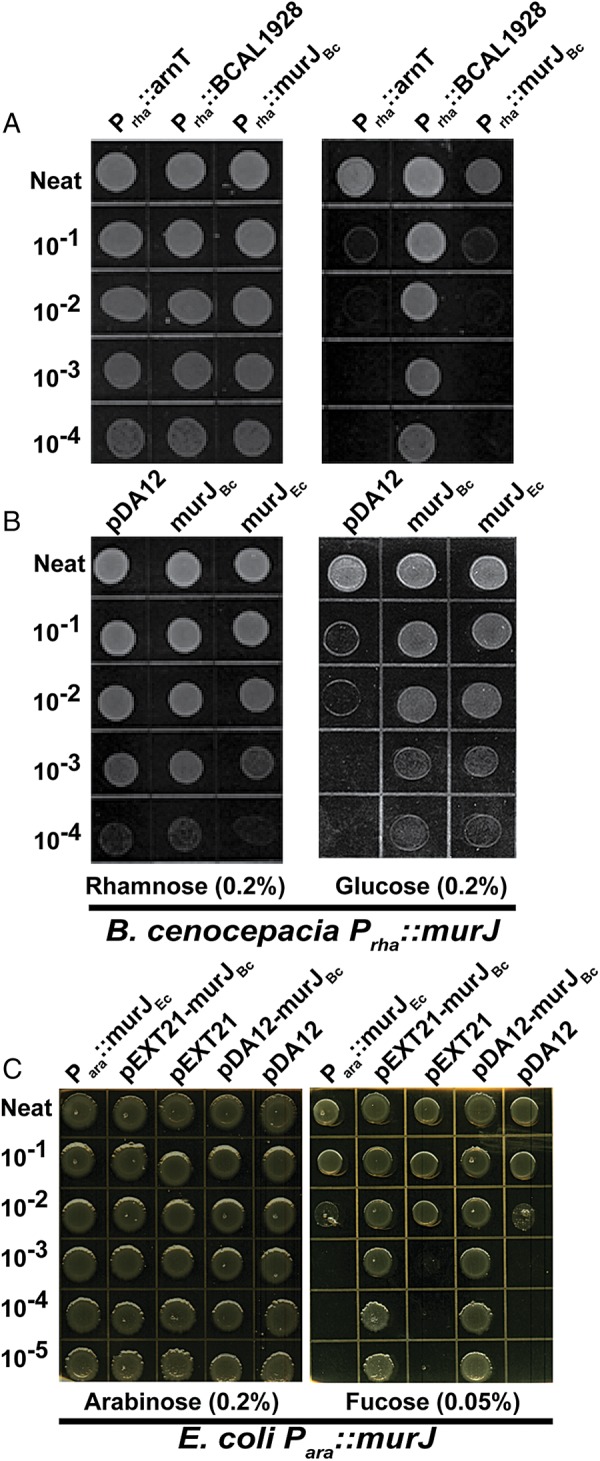
(A) Conditional lethal phenotype of strain YFM1 (Prha::murJBc) on LB agar plates supplemented with 0.2% rhamnose or 0.2% glucose. XOA10 (Prha::BCAL1928) and XOA11 (Prha::arnT) are rhamnose-independent and -dependent control mutants (Ortega et al. 2007). (B) Complementation of YFM1 (Prha::murJBc) with pDA12-BCAL2764 (murJBc) and pDA12-murJEc compared with vector control in the presence and absence of rhamnose. (C) Complementation of E. coli NR1152 (Para::murJEc) with pEXT21-murJBc and pDA12-murJBc compared with the corresponding vector controls in the presence and absence of arabinose.
We also assessed whether murJBc is functionally analogous to its E. coli ortholog. For these experiments, we employed the E. coli strain NR1152, in which murJEc expression is under the control of the arabinose inducible promoter (Para::murJEc) (Ruiz 2008). Introduction of either the constitutive pDA12 or IPTG-inducible pEXT21 plasmids encoding murJBc into NR1152 supported growth of this strain in the absence of arabinose, while the strain containing the empty vectors was nonviable (Figure. 1C). In the converse experiment, pDA12-murJEc was able to support viability of the strain carrying the Prha::murJBc construct (Figure. 1B). Together, these reciprocal complementation experiments demonstrated that the MurJ proteins of B. cenocepacia and E. coli are functionally interchangeable.
Suppression of MurJBc expression causes growth arrest, dramatic morphological changes and cell lysis
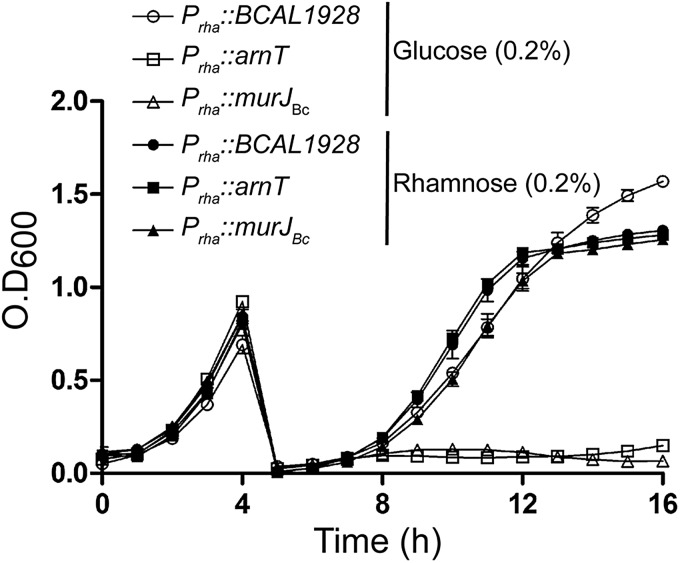
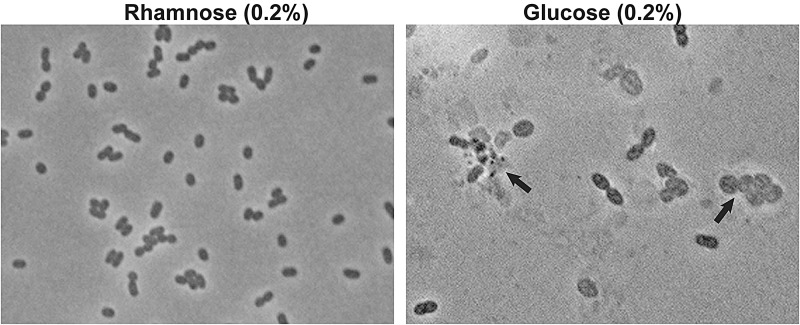
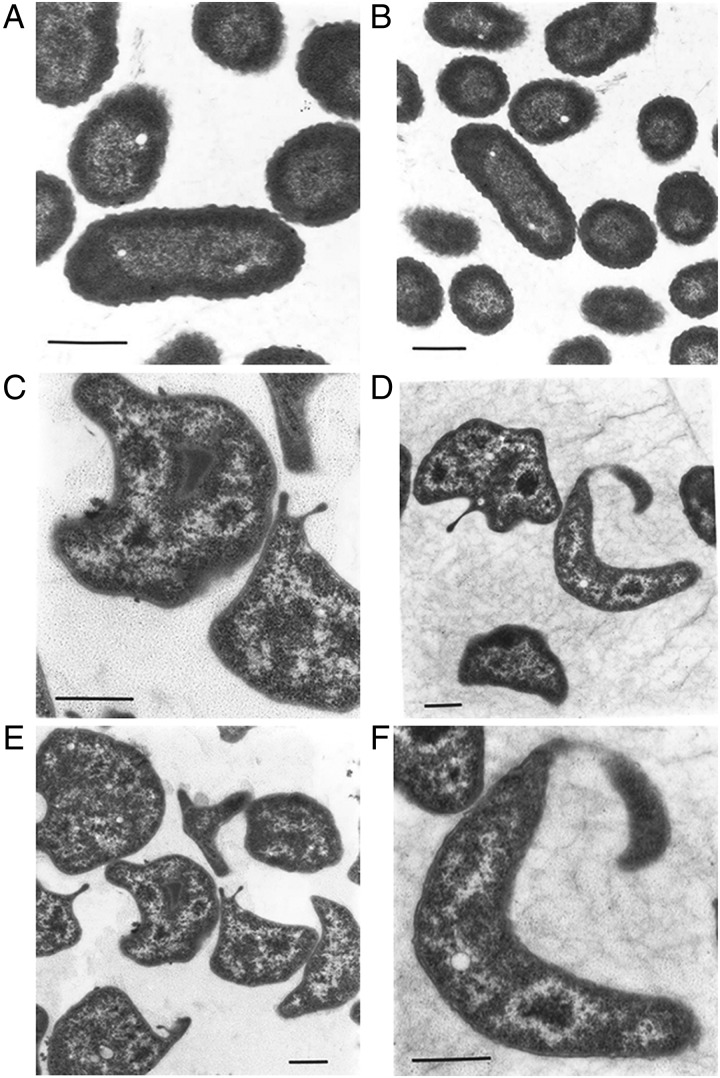
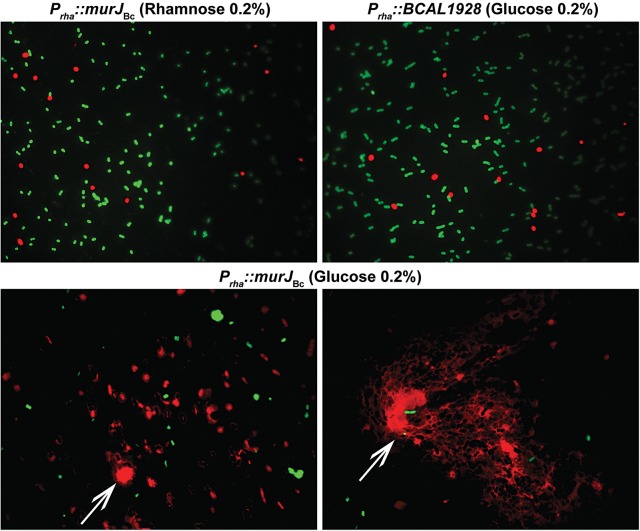
To better characterize the phenotype of the Prha::murJBc strain under non-permissive conditions, we performed rhamnose depletion experiments in liquid medium (Ortega et al. 2007). Figure. 2 shows that after serial passage in medium with glucose, the turbidity of the Prha::murJBc strain culture rapidly reached a plateau compared with wild-type strain and strain Prha::murJBc grown in the presence of rhamnose. Bacterial cells at 6-h post-depletion were examined for morphological changes by phase-contrast microscopy. The majority of the Prha::murJBc cells appeared swollen, forming blebs, and also many cells had burst forming “ghosts” (Figure. 3). Ultrastructural analysis by transmission electron microscopy revealed that cells had extremely irregular shapes, suggesting a dramatic defect in the rigidity of cell wall (Figure. 4). Using fluorescent microscopy and the LIVE/DEAD kit, we noticed that under rhamnose depletion bacterial cells became permeable to propidium iodide (Figure. 5). Also, red fluorescent mesh-like aggregates appeared suggesting binding of propidium iodide to released DNA upon cell lysis (Figure. 5). None of these changes in morphology or viability was apparent in Prha::murJBc cells that were not subjected to rhamnose depletion. Together, these results demonstrate that under non-permissive conditions, Prha::murJBc bacteria were not only unable to grow, but also lost their normal shape, becoming osmotically fragile and bursting, which suggests a major defect in cell wall PG synthesis.
Fig. 2.
Depletion experiment in liquid medium using YFM1 (Prha::murJBc), and the control strains XOA10 (Prha::BCAL1928) and XOA11 (Prha::arnT) (Ortega et al. 2007). The solid symbols indicate permissive conditions, whereas the empty ones correspond to non-permissive conditions. Growth was monitored every hour using a Bioscreen system. The figure is representative of three independent biological repeats with similar results. Bars indicate the standard errors of triplicate observations at each time point.
Fig. 3.
Phase-contrast microscopy of YFM1 (Prha::murJBc) bacteria at 6-h following growth under permissive (0.2% rhamnose) and non-permissive (0.2% glucose) conditions using oil-immersion objective lens in a Zeiss Axioscope II microscope (magnification: ×1000). Arrows indicate ghost cells.
Fig. 4.
Transmission electron microscopy of YFM1 (Prha::murJBc) bacteria under permissive (A and B) and non-permissive (C–F) conditions. Bars: 0.5 μm.
Fig. 5.
LIVE/DEAD staining of YFM1 (Prha::murJBc) under permissive (0.2% rhamnose) and non-permissive (0.2% glucose) conditions compared with rhamnose-independent control strain XOA10 (Prha::BCAL1928). Live bacteria appear fluorescent green, while dead bacteria and bacteria with compromised membranes appear fluorescent red. Arrow indicates a red fluorescent mesh consistent with extracellular DNA.
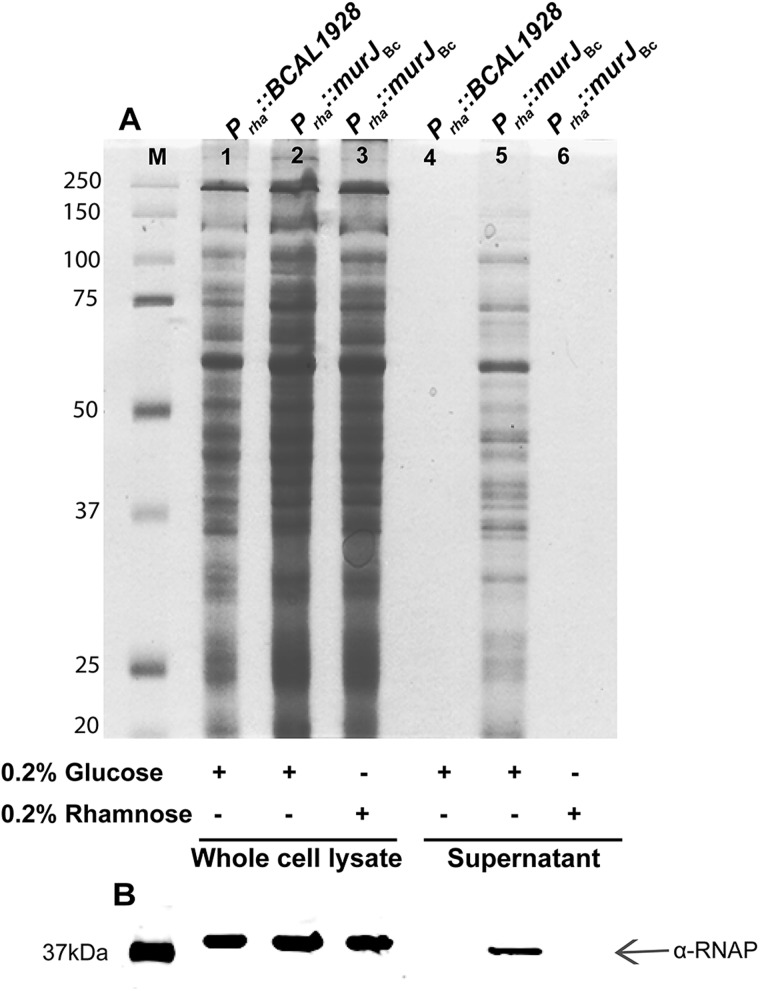
More conclusive evidence of cell lysis was obtained by examining leakage of intracellular components into the culture supernatants of Prha::murJBc cells placed under permissive and non-permissive conditions. The results show that the supernatant from bacteria exposed to non-permissive conditions has much more protein content than that of control cells (Figure. 6A). An immunoblot of the same gel using anti-RNA-polymerase α-subunit antibody showed the leakage of this large protein into the supernatant (Figure. 6B). RNA polymerase is normally localized in the cytoplasmic compartment and cannot be released by active secretion in cells containing an intact cell envelope. This experiment confirms that the supernatant of cells under non-permissive conditions contains released cytoplasmic content, supporting the conclusion that cells unable to express murJBc undergo cell lysis.
Fig. 6.
Loss of cell membrane integrity by determining release of cytoplasmic content to the culture supernatant. Whole-cell lysates and culture supernatants of YFM1 (Prha::murJBc) and the control strain XOA10 (Prha::BCAL1928) were prepared as described in Material and methods after 6 h of growth in non-permissive (Lanes 1, 2, 4 and 5) and -permissive (Lanes 3 and 6) conditions (Lanes 3 and 6). Ten to Fifteen micrograms of protein were loaded in each lane. (A) Coomassie blue staining. (B) Western blot developed using anti-RNA polymerase antibodies. Only the section of the blot with the detected protein bands is shown. The arrow indicates the presence of α subunit of the RNA polymerase (α-RNAP). M, Precision Plus™ Dual Color Protein Standards (a mixture of 10 recombinant proteins of 250, 150, 100, 75, 50, 37, 25, 20, 15 and 10 kDa).
Partial suppression of MurJBc expression sensitizes cells to the action of β-lactam antibiotics
We reasoned that if MurJBc functions in PG synthesis, bacterial growth of the conditional mutant under limiting amounts of rhamnose would be further reduced in the presence of cell wall-acting antibiotics. We used ceftazidime and imipenem for these experiments. Ceftazidime is a semisynthetic aminothiazolyl cephalosporin that inhibits penicillin-binding proteins, mainly penicillin-binding protein-3, and induces rapid cell lysis (Hayes and Orr 1983). Imipenem is a carbapenem antibiotic that like all other β-lactams inhibits bacterial cell wall synthesis by binding to and inactivating penicillin-binding proteins (Neu 1983) and also induces formation of spherical cells and subsequent cell lysis (Rodloff et al. 2006). Bacteria were grown in either 0.2 or 0.05% rhamnose. The high-rhamnose concentration is the normal inducing concentration for optimal gene expression, as empirically determined by the absence of any growth and morphology phenotypes, as described above. The lower concentration was selected to reach a state of partial depletion. At this concentration, the growth rate of the conditional mutant was not affected, as minimal amounts of rhamnose can induce the expression of MurJBc and rescue the mutant from cell death (Supplementary data, Figure S1). However, under this minimal amount of rhamnose, defects in PG start to appear which are still not enough to compromise viability. Under the rhamnose-rich concentration, Prha::murJBc had the same MIC values for ceftazidime and imipenem as the XOA10 (Prha::BCAL1928) control strain (Table II). However, the Prha::murJBc bacteria grown under rhamnose-limiting conditions had 6-fold and more than 32-fold decrease in the MIC to ceftazidime and imipenem, respectively. In contrast, the MIC value for chloramphenicol, a non-cell wall-acting antibiotic, did not change under the same conditions (Table II). In liquid growth experiments, a pronounced killing effect of ceftazidime and imipenem was also observed in cells grown under rhamnose-limiting conditions (Supplementary data, Figure S1), while growth rate was comparable under rhamnose-rich and -limiting concentrations in the absence of the antibiotics. Furthermore, growth rate did not vary in the presence of chloramphenicol (Supplementary data, Figure S1). We conclude from these experiments that conditions leading to murJBc reduced expression result in higher bacterial susceptibility to cell wall-acting antibiotics, supporting the notion of a defect in PG synthesis.
Table II.
MIC of ceftazidime and imipenem
| Antibiotic | MIC (µg/ml) |
|||
|---|---|---|---|---|
| Rhamnose 0.2% |
Rhamnose 0.05% |
|||
| YFM1 | XOA10 | YFM1 | XOA10 | |
| Ceftazidime | 0.75 | 0.75 | 0.125 | 0.75 |
| Imipenem | >32 | >32 | 1 | >32 |
| Chloramphenicol | 32 | 32 | 32 | 32 |
Depletion of MurJBc causes accumulation of [3H]-DAP
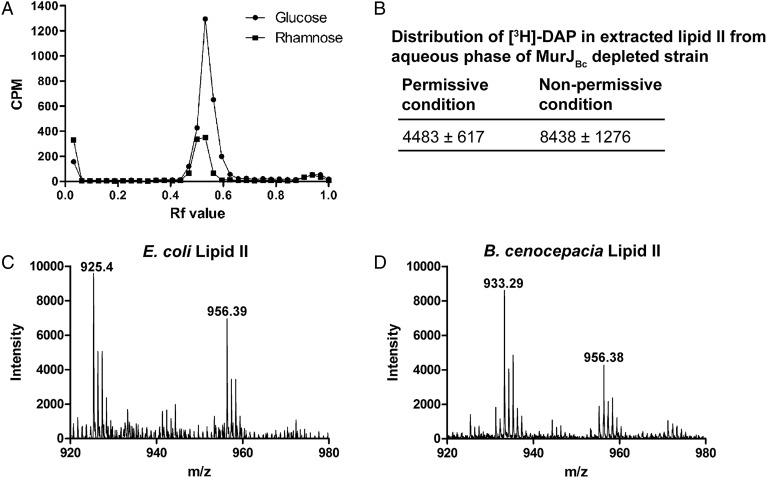
The E. coli murJ has been proposed to encode the lipid II flippase required for PG synthesis (Ruiz 2008). Therefore, to further understand the function of murJBc in B. cenocepacia PG synthesis, we determined the level of accumulation of PG cell wall precursors under rhamnose depletion in a Prha::murJBc derivative carrying a lysA (BCAM 2076) deletion (strain YFM2, Table III). To achieve this, we radiolabeled lipid II with [3H]-DAP, which is incorporated into the MurNAc-pentapeptide chain. We tested the cellular distribution of [3H]-DAP into the mature PG from an equivalent amount of cells under permissive and non-permissive conditions. Using ascending paper chromatography, we observed that rhamnose-depleted bacterial cells labeled with [3H]-DAP accumulated a radioactive species with an average Rf value of 0.47 (Figure. 7A). Also, the amount of incorporation of the radioactive label under non-permissive conditions decreased to half as compared with permissive conditions. Lipids were extracted from the conditional mutant cells after labeling under permissive and non-permissive conditions with a modified Bligh and Dyer two-phase system (Guan et al. 2005). Depending on the selective solubility of lipid II at different pH values, this molecule partitions in the aqueous phase at neutral pH and in the organic phase at acidic pH (Guan et al. 2005). Our results showed a higher level of [3H]-DAP accumulation in the aqueous phase at neutral pH for the cell extracts under non-permissive conditions in comparison with the level of [3H]-DAP in the presence of rhamnose (2.25 ± 0.45 ratio) as shown in Figure. 7B. Separation of the aqueous phase by ascending chromatography revealed peaks of [3H]-DAP accumulation at comparable Rf value as obtained before from ascending chromatography of the cell pellets (Figure. 7A). The PG is expected to remain at the origin in the paper chromatography and the lipid precursors migrate to a further distance by the effect of the lipophilic solvent. Ruiz showed that the nucleotide and lipid precursors accumulated in MurJ-depleted mutant in E. coli at Rf values of 0.25–0.35 and 0.8–0.9, respectively (Ruiz 2008). However, it was reported that a mixture of lipid I and lipid II could migrate to an Rf value of 0.6 in E. coli (El Ghachi et al. 2006). We confirmed the identity of lipid II by MS analysis using MALDI-TOF in negative ion mode and comparing the Mass spectra of lipid II from E. coli W3110 and B. `cenocepacia K56-2 (Figure. 7C and D). The lipid II from E. coli showed two prominent peaks at m/z 956.39 and 925.4 (monoisotopic peaks) (Figure. 7C), which are very similar to those previously reported (Guan et al. 2005). These peaks correspond to molecular weights of 1919 and 1851, which match the expected masses of lipid II with 11 and 10 isoprene units, respectively (Guan et al. 2005). The B. cenocepacia lipid II preparation also revealed two peaks in the region of the spectrum expected for lipid II (Figure. 7D). The 956.39 peak of the B. cenocepacia lipid II was identical to the corresponding peak of the E. coli lipid II (Figure. 7C). However, the B. cenocepacia lipid II had a novel peak at an m/z value of 933.29 (Figure. 7D). Compared with the corresponding peak in E. coli lipid II, the difference in m/z value indicates a difference in molecular weight of ∼15.78, which could correspond to either an extra methyl or amino group in lipid II of B. cenocepacia. The nature of this difference will be investigated in a separate study, but it can account for a change that can also be reflected as a difference in the lipid II migration by paper chromatography and hence an Rf value different from that reported for E. coli (Ruiz 2008).
Table III.
Primers
| Primer number | Oligonucleotide sequence, 5′–3′ | Restriction enzyme |
|---|---|---|
| 6122 | AAAAAACATATGAATCTATTCCGAGCCCTGCT | NdeI |
| 6123 | AAAAAATCTAGATCTTGAACTCGGCGAGGAT | XbaI |
| 6290 | AAAACTCGAGGACGTTCGAATTCGCCTTCT | XhoI |
| 6291 | AAAACTCGAGGCGTCGATGTCGTCGAACTA | XhoI |
| 6347 | AAAATCTAGACGTTCGAGCCCCACATAC | XbaI |
| 6348 | AAAAGAATTCGTTCTTCGGCCTCCTGCT | EcoRI |
| 6491 | TTTTTTCATATGGCGCAACGTCCGTATAATAA | NdeI |
| 6388 | TTTTTCTAGATCACTTGGCGCGCCTT | XbaI |
| 6578 | GATCGATCATATGAATTTATTAAAATCGCTGGCCGCCGTCAGCTCG | NdeI |
| 6579 | CCGCACTCTAGATTACACCGTCCGGCGGGCAAATTCTTTAACTTTG | XbaI |
| Q294 | TTTTTTCATATGgactacaaggacgacgacgacgacaagGCGCAACGTCCGTATAATAA | NdeI |
| Q369 | TTTTTTGAATTCgactacaaggacgacgacgacgacaagGCGCAACGTCCGTATAATAA | EcoRI |
| Q295 | TTCACGCTGCTGTCGGCGGTGACCGGACTGGCC | |
| Q296 | GGCCAGTCCGGTCACCGCCGACAGCAGCGTGAA | |
| Q297 | GTGACCGGACTGGCCGCGGAGACGCTGATCGCC | |
| Q298 | GGCGATCAGCGTCTCCGCGGCCAGTCCGGTCAC | |
| Q299 | CCAGTCAATACACCGCGGCGTTCTACGTCGCC | |
| Q300 | GGCGACGTAGAACGCCGCGGTGTATTGACTGG | |
| Q301 | ATTCCGAACCTGCTGGCGCGCCTGTCTGCCGAA | |
| Q302 | TTCGGCAGACAGGCGCGCCAGCAGGTTCGGAAT | |
| Q303 | ATCAATTACGCCGACGCGCTGATGGAGTTCCCG | |
| Q304 | CGGGAACTCCATCAGCGCGTCGGCGTAATTGAT |
Fig. 7.
Characterization of lipid II in B. cenocepacia. (A) Ascending paper chromatography of cell pellets of the strain YFM2 (Prha::murJBc, ΔlysA) under permissive (rhamnose) and non-permissive (glucose) conditions showing accumulation of cell wall precursors under non-permissive conditions. This experiment is representative of three independent experiments showing accumulation of lipid II at average Rf value of 0.47. (B) Accumulation of extracted lipid II from aqueous phase of the strain YFM2 under non-permissive conditions. Data correspond to the average of CPM ± SD of three independent experiments. n = 3. P-value = 0.0247. (C) MALDI-TOF MS of lipid II of E. coli W3110. D. MALDI-TOF MS of lipid II of B. cenocepacia K56-2. (C) and (D) are representative spectra of three independent preparations of lipid II.
Murj proteins from B. cenocepacia and E. coli share a similar predicted fold and criticszal amino acid residues
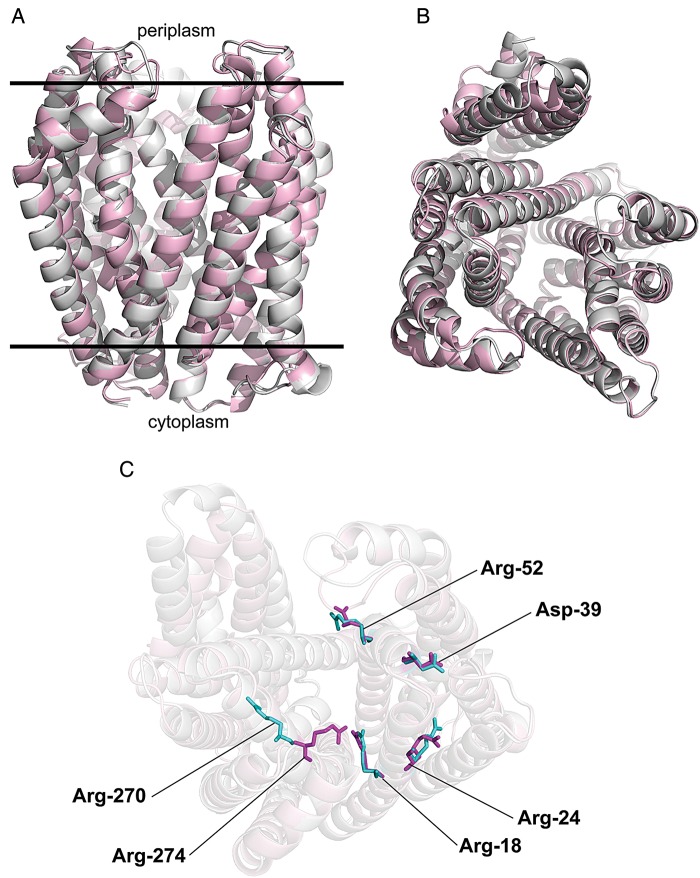
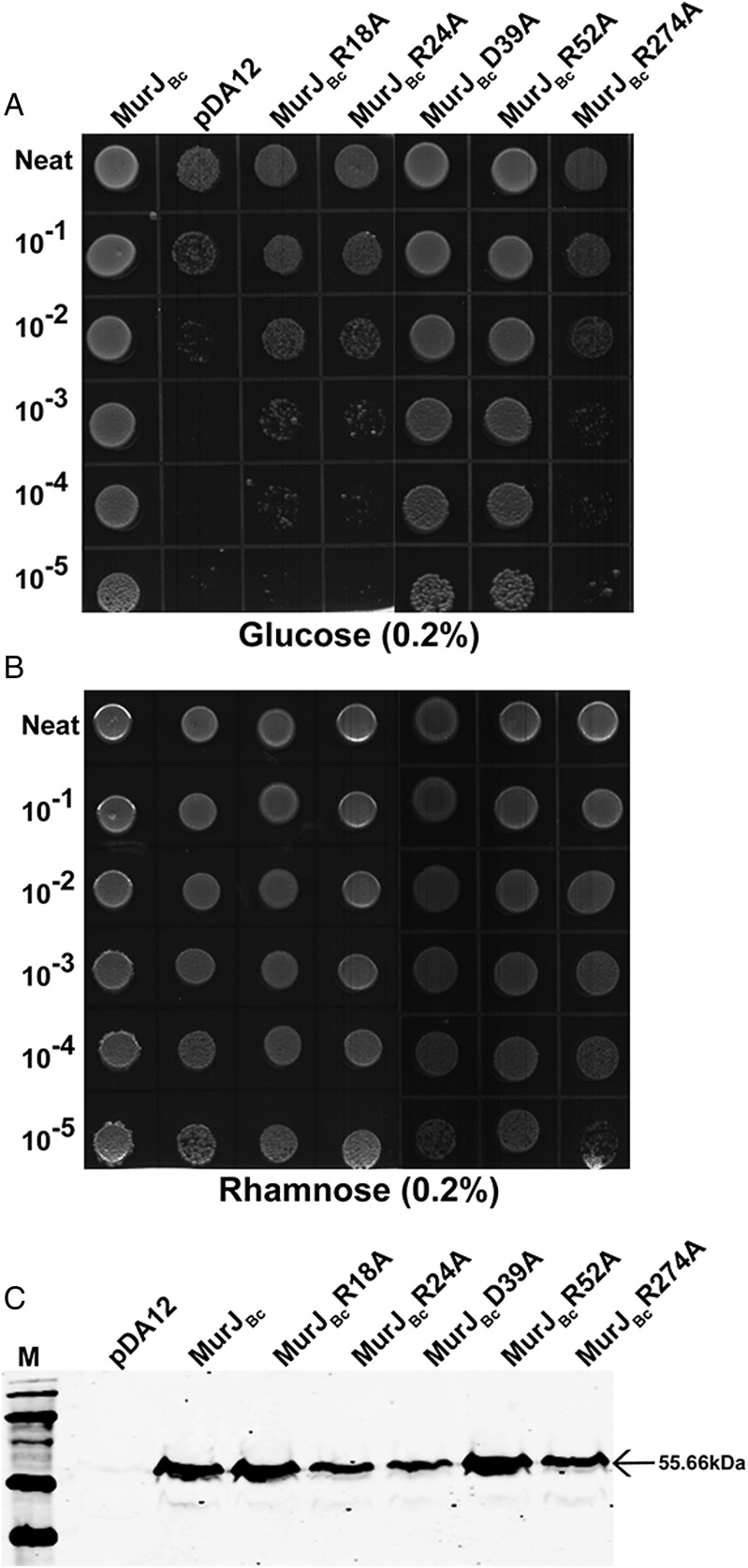
In a recent study (Butler et al. 2013), a structural model of MurJEc was proposed predicting a central, solvent-exposed cavity containing charged residues that are essential for function. Essential amino acid residues in the central cavity of MurJEc were identified by a combination of cysteine and alanine replacements (Butler et al. 2013). This work demonstrated that the charged residues located in trans-membrane helix 1 (R18 and R24), periplasmic loop 1 (D39), trans-membrane helix 2 (R52) and trans-membrane helix (R270) of MurJEc are accessible from the periplasmic space and required for function, as determined by complementation (Butler et al. 2013). By CLUSTAL analysis, MurJ from E. coli and B. cenocepacia share 50% similarity (data not shown). As with the E. coli homolog, an in silico structural model of MurJBc was generated by I-Tasser (Zhang 2008; Roy et al. 2010), and the resulting 3D structure was aligned to that of MurJEc. Both structures were nearly superimposable at most positions (Figure. 8A) and display a characteristic central cavity exposed to the periplasmic space (Figure. 8B). The alignment of the 3D predicted models for the E. coli and B. cenocepacia MurJ proteins revealed that the MurJBc residues R18, R24, D39 and R52 are localized in the same regions as the corresponding residues in MurJEc, while MurJBc R274 corresponds to MurJEc R270 (Figure. 8C). MurJBc derivatives containing alanine replacements of these residues were constructed and tested for their ability to restore the viability of the Prha::murJBc mutant under non-permissive conditions. Alanine replacement of R18, R24, and R274 in MurJBc rendered the protein not functional, while the replacements in D39 and R52 did not compromise function (Figure. 9A and B). These results were not due to lack of protein expression, as all the mutant proteins were expressed at levels similar to that of the parental MurJBc (Figure. 9C). In MurJEc, the D39 residue was also not required for function, although it might be required only for structural stability (Butler et al. 2013), which could explain why the alanine replacement of the MurJBc D39 did not impair function. Another difference between the E. coli and B. cenocepacia MurJ proteins is that MurJEc completely loses function when R52 is replaced by cysteine, and has a partial defect when replaced by alanine (Butler et al. 2013). Remarkably, the structural alignment of the predicted MurJBc and MurJEc structures also revealed that the side chains of the residues investigated by alanine replacement mutagenesis were also superimposable spatially, except for R270 and R274. These differences could be attributed to the nature of the modeling approach that is not based on raw structural data but rather from an in silico approach. Regardless, both R270 and R274 were located at the same site and face of the predicted α-helix in both models and were also required for function. Together, these results show that despite some differences, MurJBc and MurJEc belong to the same family of putative lipid II flippases.
Fig. 8.
Structural model of MurJ proteins from B. cenocepacia (gray) and E. coli (pink). (A) Front view, (B) top view from the periplasmic side and (C) position of conserved functional residues.
Fig. 9.
Growth of YFM1 (Prha::murJBc) using MurJBc and its derivatives carrying alanine replacements in the following charged residues: arginine-18 (R18A), arginine-24 (R24A), aspartic acid-39 (D39A), arginine-52 (R52A) and arginine-274 (R274A). Bacteria were grown under non-permissive (A) and permissive conditions (B). (C). Western blot demonstrating that all MurJBc parental and mutant derivatives (expressed as N-terminal FLAG fusions) are correctly expressed (arrow) in the (Prha::murJBc) strain background. M, molecular mass ladder.
Concluding remarks
The role of MurJ as the lipid II flippase has been disputed in part due to the reported absence of a lethal phenotype of a B. subtilis quadruple mutant in the murJ homologs ytgP, spoVB, ykvU and yabM (Fay and Dworkin 2009; Vasudevan et al. 2009). However, B. subtilis has two other genes, epsK and tuaB, which encode membrane proteins that share homologies with MurJ/MVIN/Wzx/Rft1 PFAM motifs (data not shown). Although EpsK and TuaB are respectively required for the synthesis of extracellular matrix and teichuronic acid, it may also be possible that any of these proteins provides functional redundancy involving lipid II flipping. This is conceivable, taking into account that the related Wzx family flippases, which are involved in the export of lipid-linked O-antigen intermediates, have relaxed specificity for their substrates (Feldman et al. 1999; Marolda et al. 2004; Alaimo et al. 2006). In our study, we demonstrate that the murJ homolog in B. cenocepacia K56-2 is indispensable for viability. A viable mutant in this gene could only be obtained using a conditional expression system. Under non-permissive conditions the mutant rapidly ceased growing and individual bacterial cells lost their bacillary shape and showed indications of osmotic fragility, suggesting a cell wall defect. Biochemically, the mutant accumulates radiolabeled PG intermediates, which is consistent with the flipping function attributed to this family of proteins. We also demonstrated that MurJ proteins from both B. cenocepacia and E. coli are functional homologs, since each of these proteins could restore viability in conditional mutant strains of both species. Furthermore, despite being only 50% related in their primary amino acid sequencing, the E. coli and B. cenocepacia MurJ proteins afforded similar predicted three-dimensional structures that were virtually superimposable including the predicted spatial location of residues that are critical to function. Therefore, our results with B. cenocepacia MurJ bring additional support to the notion that MurJ functions as lipid II flippase, as initially proposed for E. coli (Inoue et al. 2008; Ruiz 2008).
Materials and methods
Bacterial strains and growth conditions
Strains and plasmids used in this study are listed in Table I. Bacteria were grown at 37°C in Luria-Bertani (LB) medium, SOB medium (2% tryptone, 0.5% yeast extract, 0.05% sodium chloride, 0.24% magnesium sulfate, 0.0186% potassium chloride) or M9 minimal medium, as required. Antibiotics were added to reach final concentrations as follows: 50 μg trimethoprim mL−1 for E. coli and 100 μg mL−1 for B. cenocepacia and 40 μg kanamycin mL−1 for E. coli. Ampicillin at 200 μg mL−1 and polymyxin at 20 µg mL−1 were used for triparental mating to select against donor and helper E. coli strains. Rhamnose and glucose were added to final concentrations of 0.2% (w/v) as needed. Antibiotics and chemicals were purchased from Sigma Chemical (St. Louis, MO, USA).
Recombinant DNA methods
The primers used in this study are listed in Table III. The construction of pSC200 was previously reported (Cardona and Valvano 2005). DNA ligations, restriction endonuclease digestions and agarose gel electrophoresis were performed according to standard techniques (Sambrook et al. 1990). Restriction enzymes, T4 DNA polymerase and T4 DNA ligase were purchased from Roche Diagnostics, Dorval, Quebec, Canada. PCR amplifications were carried out using the HotStar HiFidelity polymerase (Qiagen). Colony-PCR was performed with Taq polymerase (Qiagen). Amplifications were done according to the manufacturer's instructions and optimized for each primer pair. DNA sequencing was performed at the sequencing facility in York University (Toronto, Canada).
Construction of a murJBc conditional mutant
A 300-bp fragment of the upstream region of the murJBc gene (BCAL2764) was amplified using primers 6122 and 6123 and the amplicon cloned into pSC200 behind the plasmid-borne rhamnose promoter. Transformation of the ligation mixture was carried out in E. coli GT115 competent cells by the calcium chloride method (Cohen et al. 1972). Transformants were selected on LB agar plates containing 50 μg trimethoprim mL−1. Resistant colonies were screened by colony-PCR. Mobilization of plasmids into B. cenocepacia was conducted by triparental mating (Craig et al. 1989) using the pRK2013 helper plasmid (Figurski and Helinski 1979) on SOB plates containing 0.2% (w/v) rhamnose. Exconjugants were then isolated by plating on LB agar plates supplemented with 100 μg trimethoprim mL−1, 200 μg ampicillin mL−1, 20 μg polymyxin mL−1 and 0.2% rhamnose (Ortega et al. 2007). This experiment resulted in the isolation of the conditional mutant strain YFM1 (Prha::murJBc), in which the murJ gene is only expressed in the presence of rhamnose (see Results and discussion).
Complementation experiments
A plasmid constitutively expressing MurJBc was constructed by amplifying the gene with primers 6491 and 6388. Amplicons were digested with NdeI–XbaI and cloned into a similarly digested pDA12, resulting in pDA12-murJBc. We also constructed another plasmid, pEXT21-murJBc, in which murJ gene expression was placed under the control of the lac promoter using the primers Q369 and 6388. The murJ homolog from E. coli K-12 was amplified with primers 6578 and 6579 and also cloned into pDA12. For complementation in B. cenocepacia, pDA12-murJBc or pDA12-murJEc was introduced into the conditional mutant strain YFM1 by triparental mating as described above. For complementation in E. coli, pDA12-murJBc was introduced by electroporation into the E. coli mutant NR1152 (Para::murJEc) (Ruiz 2008) and transformants selected on 25 μg ampicillin mL−1, 30 μg tetracycline mL−1 and arabinose 0.2%. Also, pEXT21-murJBc was introduced by electroporation into NR1152 and selection was made on plates containing 80 μg spectinomycin mL−1. For induction of MurJBc expression, an overnight culture of NR1152 (pEXT21-murJBc) was diluted to an OD600 of 0.2 in the presence of the appropriate antibiotics, incubated at 37°C for 2 h up to an OD600 of 0.6 at which time IPTG (isopropyl-β-d-1-thiogalactopyranoside) was added to a final concentration of 0.1 mM, and the culture was incubated for further 3 h.
Conditional viability by rhamnose depletion
The conditional viability of mutant YFM1 (Prha::murJBc) was assessed after an overnight growth at 37°C in M9 minimal medium supplemented with 0.5% yeast extract, 1 μM CaCl2, 1 μM MgSO4, 0.2% rhamnose and 100 μg trimethoprim mL−1. An aliquot of the overnight culture was centrifuged and the bacterial pellet was washed three times with sterile phosphate-buffered saline (PBS), resuspended in PBS, and adjusted to an OD600 of 1. Ten 10 μL drops of the undiluted suspension and 10−1, 10−2, 10−3 and 10−4 dilutions were deposited onto M9 agar plates supplemented with 0.2% glucose or 0.2% rhamnose and incubated at 37°C for 24 h. The same procedure was done to test the complementation of NR1152 by murJBc except that the M9 agar plates were supplemented with either 0.2% arabinose or 0.05% fucose (Ruiz 2008). The essentiality of murJBc was further demonstrated by monitoring growth in liquid medium in a rhamnose depletion assay (Ortega et al. 2007). Washed, rhamnose-depleted cells were diluted to an OD600 of 0.15 in M9 medium supplemented with 0.5% yeast extract and 0.2% glucose, and incubated at 37°C for 4 h with shaking. Growing cells were aliquoted to 100-well plates containing fresh medium with 0.2% glucose to give an OD600 of 0.05 and monitored for growth for an additional 12 h in a Bioscreen C automated growth curve reader (MTX Lab Systems, Inc., Vienna, VA) at 37°C with constant, low shaking with OD600 readings taken every 30 min.
Microscopy
Samples of depleted cultures grown in the absence of rhamnose were taken after 6 h, placed on 0.8% agarose slides, covered by a coverslip and examined by phase-contrast microscopy. Other samples from the same cells were fluorescently stained with Syto 9 and propidium iodide (LIVE/DEAD BacLight bacterial viability kit; Molecular Probes, Invitrogen Detection Technologies, Eugene, OR). Samples were also examined by electron microscopy after fixing with 2.5% glutaraldehyde and staining with 2% uranyl acetate and lead citrate, as previously described (Schmerk et al. 2011). Grids were visualized with a Philips 410 transmission electron microscope at 60 kV.
Killing curves using β-lactam antibiotics and E-tests
Washed rhamnose-depleted cells were diluted to an OD600 of 0.15 in M9 minimal medium supplemented with either 0.2 or 0.05% rhamnose and allowed to grow for 4 h. Then, cultures were diluted to ∼2 × 105 cfu mL−1 in M9 minimal media and dispensed in 100-well plates in 300 μL volumes. Either ceftazidime, imipenem or chloramphenicol was previously added to the 100-well plate to give final concentrations ranging from 2 to 128 µg mL−1 in two-fold increments. Plates were incubated in a Bioscreen C automated growth curve reader for 24 h with OD600 readings taken every 30 min. For E-tests, depleted cells were washed and the OD600 adjusted to 0.5 McFarland opacity standard. A sterile cotton swab was dipped into the bacterial suspension and streaked all over the surface of the Müller-Hinton agar plates in three directions to obtain an even distribution of the inoculum. The E-test strips (BioMérieux Clinical Diagnostics) for ceftazidime and imipenem were deposited onto the inoculated plates by means of a sterile forceps and the plates were incubated at 37°C for 48 h.
Detection of cytoplasmic content leakage using anti-RNA polymerase antibody
One-milliliter volumes of rhamnose-depleted cultures at an OD600 of 1 were centrifuged for 1 min at × 13,000g; cells were resuspended in 20 µL distilled water and 10 µL of 3× loading dye, and boiled for 5 min to prepare whole-cell lysates. To examine the culture supernatants, 35 mL of depleted culture was centrifuged for 15 min at 7000 × g at 4°C. Supernatant was collected, filter-sterilized and proteins were precipitated overnight at 4°C in 10% trichloroacetic acid and protease inhibitor. Then, the mixture was centrifuged at 10,000 × g for 30 min at 4°C. The pellet was washed with 5 mL acetone and centrifuged for another 30 min. Acetone was removed and pellet was suspended in 150 µL sodium phosphate buffer 0.1 M (pH 7). The resulting suspension was boiled for 5 min. Five-microliter aliquots of boiled samples for both the whole-cell lysate and the supernatant were separated by gel electrophoresis using 14% (w/v) SDS–polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was rinsed with Tris-buffered saline (TBS), pH 8, and then blocked overnight with 10% (v/v) western blocking reagent (Roche) in TBS at 4°C. The membrane was then incubated with monoclonal antibody to E. coli RNA-polymerase α-subunit (Neoclone) diluted 1:10,000 for 1.5 h at room temperature. The membrane was washed with TBS and then incubated at room temperature for 20 min with anti-mouse IgG antibody conjugated to Alexa Fluor 680 (Molecular Probes) diluted 1:20,000. Proteins were visualized using a Licor Infrared Imaging System with the Odyssey software. A separate gel was also prepared for Coomassie blue staining.
Detection of accumulation of PG precursors using [3H]-DAP
To increase the incorporation of [3H]-diaminopimelic acid (DAP) into growing cells, we constructed a strain with a lysA (BCAM2076) deletion using the method of Flannagan et al. (2008) for the construction of unmarked, non-polar deletions in B. cenocepacia. lysA is responsible for decarboxylation of DAP into lysine, and its deletion would result in the direct incorporation of exogenously added DAP to the PG (Wientjes et al. 1985). A murJ conditional mutant was constructed in the ΔlysA background strain. The resulting strain, YFM2 (Prha::murJ ΔlysA), was grown overnight in M9 minimal medium containing rhamnose and 100 µg trimethoprim mL−1. Cells were washed as indicated above, diluted 1:100 into fresh M9 minimal medium containing glucose, methionine, lysine and threonine and grown under aeration at 37°C for 4 h to OD600 of 0.6 (approximately four generations). Cells were diluted to an OD600 of 0.1 in fresh medium containing glucose, methionine, lysine and threonine and labeled for 2 h at 37°C with 5 µCi of [3H]-DAP (30 Ci/mmol; American Radiolabeled Chemicals) (Wientjes et al. 1985). After 2 h, the cells were exposed again to 5 µCi of [3H]-DAP for another 2 h. At the end of the labeling period, cells were placed on ice and pelleted at 4°C for 5 min at 13,000 × g. Pellets were resuspended in 10 µL of ice-cold water and immediately frozen until they were subjected to paper chromatography (Ramey and Ishiguro 1978; Ruiz 2008). Labeled cells were spotted onto Whatman 3MM paper (57×49 cm), and labeled PG precursors were separated by ascending chromatography by development with isobutyric acid:1M NH4OH(5:3; v/v) for 36 h. Paper was dried and cut into 1-cm squares that were counted in vials containing scintillation mixture (Ready Safe; Beckman Coulter).
Lipid II extraction of radiolabeled cells
Radiolabeled cells were resuspended in 100 µL of PBS (pH 7.4), followed by the addition of 125 µL of chloroform and 250 µL of methanol. After periodic Vortex mixing for 10 min at room temperature, the homogenates were centrifuged at 4,000 × g for 10 min and then the supernatants were converted to a two-phase Bligh–Dyer system by adding 125 µL chloroform and 125 µL PBS (Bligh and Dyer 1959;Guan et al. 2005). Since Lipid II partitions into the upper aqueous phase of a two-phase Bligh–Dyer system at the neutral pH of PBS, this aqueous layer was separated and counted in vials containing scintillation mixture (Bligh and Dyer 1959;Guan et al. 2005). To confirm the presence of [3H]-DAP-labeled lipid II in the aqueous phase, this layer was also spotted onto Whatman 3MM paper for separation of lipid II by ascending chromatography. The identity of lipid II was confirmed by mass spectrometry (MS) analysis using matrix-assisted laser desoprtion/ionization-time of flight (MALDI-TOF). Lipid II was extracted as previously described (Guan et al. 2005) and dissolved in 100 μL of chloroform/methanol (2:1, v/v). One-microliter of this mixture was loaded on the target and covered by 1 μL of the 5-chloro-2-mercaptobenzothiazole (20 mg/mL) matrix. The target was inserted in a Bruker Autoflex MALDI-TOF spectrometer. Data acquisition and analysis were performed using the Flex Analysis software.
Site-directed mutagenesis
murJBc was PCR amplified from genomic B. cenocepacia using the primers Q294 (introducing a Flag-tag at the N-terminal) and 6388. The PCR product was cut with NdeI and XbaI and ligated to pDA12 cut with the same restriction enzymes to create pYM29 (pDA12-FLAGmurJBc), which is used as a template. Five point mutations were created individually in pDA12-FLAGmurJBc as previously described (Hamad et al. 2012). Complementary primers were designed to contain the desired mutation (Ala), flanked by unmodified sequence to anneal to the same sequence on opposite strands of the template plasmid. Primers from Q295 to Q304 (Table III) were used in pairs to create Ala point mutations in five selected residues, R18, R24, D39, R52 and R274 which correspond to similar residues in the E. coli murJ (Butler et al. 2013) to create the plasmids pYM31–pYM35, respectively. The plasmids were introduced into the strain YFM1 (Prha::murJBc) by triparental mating and tested for their ability to complement YFM1 under non-permissive conditions. MurJ protein expression was detected on total membranes that were prepared as described before (Patel et al. 2012) with the only modification that the overnight culture was diluted to an OD600 of 0.2 then incubated at 37°C to grow for about 6 h to reach an OD600 of 0.8–1. Protein concentration was measured using a nanodrop (Nanovue Plus, GE Healthcare). Flag-tagged MurJ was revealed by immunoblotting using a 1:10,000 dilution of the mouse anti-Flag monoclonal antibody. Proteins were visualized using a Licor Infrared Imaging System with the Odyssey software.
Supplementary Data
Supplementary data for this article are available online at http://glycob.oxfordjournals.org/.
Funding
This research was supported by a grant from the Canadian Institutes of Health Research. Y.F.M. was supported by the Mission Sector, Ministry of Higher Education, Egypt, and is currently supported by an International PhD Scholarship from Queen's University, Belfast.
Conflict of interest
None declared.
Abbreviations
DAP, [3H]-diaminopimelic acid; GlcNAc, N-acetyl glucosamine; LB, Luria-Bertani; PBS, phosphate-buffered saline; PG, peptidoglycan; MurNAc, N-acetyl muramic acid; MS, mass spectrometry; MALDI-TOF, matrix-assisted laser desoprtion/ionization-time of flight; TBS, Tris-buffered saline.
Supplementary Material
Acknowledgements
We are grateful to N. Ruiz for generously providing us with the strain NR1152, S. F. Koval for assistance with the electron microscopy and K. Patel for help with the structural modelling. We also thank all the lab members for useful and critical discussions.
References
- Alaimo C, Catrein I, Morf L, Marolda CL, Callewaert N, Valvano MA, Feldman MF, Aebi M. Two distinct but interchangeable mechanisms for flipping of lipid-linked oligosaccharides. Embo J. 2006;25:967–976. doi: 10.1038/sj.emboj.7601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert DF, Flannagan RS, Valvano MA. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect Immun. 2008;76:1979–1991. doi: 10.1128/IAI.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreteau H, Kovac A, Boniface A, Sova M, Gobec S, Blanot D. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:168–207. doi: 10.1111/j.1574-6976.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bouhss A, Trunkfield AE, Bugg TD, Mengin-Lecreulx D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev. 2008;32:208–233. doi: 10.1111/j.1574-6976.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- Butler EK, Davis RM, Bari V, Nicholson PA, Ruiz N. Structure-function analysis of MurJ reveals a solvent-exposed cavity containing residues essential for peptidoglycan biogenesis in Escherichia coli. J Bacteriol. 2013;195:4639–4649. doi: 10.1128/JB.00731-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona ST, Valvano MA. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid. 2005;54:219–228. doi: 10.1016/j.plasmid.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Cohen SN, Chang AC, Hsu L. Nonchromosomal antibiotic resistance in bacteria: Genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci USA. 1972;69:2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig FF, Coote JG, Parton R, Freer JH, Gilmour NJ. A plasmid which can be transferred between Escherichia coli and Pasteurella haemolytica by electroporation and conjugation. J Gen Microbiol. 1989;135:2885–2890. doi: 10.1099/00221287-135-11-2885. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn DM, St Pierre R, Linn T. A set of compatible tac promoter expression vectors. Gene. 1996;177:133–136. doi: 10.1016/0378-1119(96)00289-2. [DOI] [PubMed] [Google Scholar]
- Ehlert K, Holtje JV. Role of precursor translocation in coordination of murein and phospholipid synthesis in Escherichia coli. J Bacteriol. 1996;178:6766–6771. doi: 10.1128/jb.178.23.6766-6771.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghachi M, Bouhss A, Barreteau H, Touze T, Auger G, Blanot D, Mengin-Lecreulx D. Colicin M exerts its bacteriolytic effect via enzymatic degradation of undecaprenyl phosphate-linked peptidoglycan precursors. J Biol Chem. 2006;281:22761–22772. doi: 10.1074/jbc.M602834200. [DOI] [PubMed] [Google Scholar]
- Fay A, Dworkin J. Bacillus subtilis homologs of MviN (MurJ), the putative Escherichia coli lipid II flippase, are not essential for growth. J Bacteriol. 2009;191:6020–6028. doi: 10.1128/JB.00605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman MF, Marolda CL, Monteiro MA, Perry MB, Parodi AJ, Valvano MA. The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O antigen assembly is independent of the chemical structure of the O repeat. J Biol Chem. 1999;274:35129–35138. doi: 10.1074/jbc.274.49.35129. [DOI] [PubMed] [Google Scholar]
- Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Linn T, Valvano MA. A system for the construction of targeted unmarked gene deletions in the genus Burkholderia. Environ Microbiol. 2008;10:1652–1660. doi: 10.1111/j.1462-2920.2008.01576.x. [DOI] [PubMed] [Google Scholar]
- Guan Z, Breazeale SD, Raetz CR. Extraction and identification by mass spectrometry of undecaprenyl diphosphate-MurNAc-pentapeptide-GlcNAc from Escherichia coli. Anal Biochem. 2005;345:336–339. doi: 10.1016/j.ab.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hamad MA, Di Lorenzo F, Molinaro A, Valvano MA. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia. Mol Microbiol. 2012;85:962–974. doi: 10.1111/j.1365-2958.2012.08154.x. [DOI] [PubMed] [Google Scholar]
- Hamad MA, Skeldon AM, Valvano MA. Construction of aminoglycoside-sensitive Burkholderia cenocepacia strains for use in studies of intracellular bacteria with the gentamicin protection assay. Appl Environ Microbiol. 2010;76:3170–3176. doi: 10.1128/AEM.03024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MV, Orr DC. Mode of action of ceftazidime: Affinity for the penicillin-binding proteins of Escherichia coli K12, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother. 1983;12:119–126. doi: 10.1093/jac/12.2.119. [DOI] [PubMed] [Google Scholar]
- Henriques AO, Glaser P, Piggot PJ, Moran CP., Jr Control of cell shape and elongation by the rodA gene in Bacillus subtilis. Mol Microbiol. 1998;28:235–247. doi: 10.1046/j.1365-2958.1998.00766.x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sato T, Wachi M, Jung HK, Ishino F, Kobayashi Y, Matsuhashi M. Structural similarity among Escherichia coli FtsW and RodA proteins and Bacillus subtilis SpoVE protein, which function in cell division, cell elongation, and spore formation, respectively. J Bacteriol. 1989;171:6375–6378. doi: 10.1128/jb.171.11.6375-6378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Murata Y, Takahashi H, Tsuji N, Fujisaki S, Kato J. Involvement of an essential gene, mviN, in murein synthesis in Escherichia coli. J Bacteriol. 2008;190:7298–7301. doi: 10.1128/JB.00551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F, Jung HK, Ikeda M, Doi M, Wachi M, Matsuhashi M. New mutations fts-36, lts-33, and ftsW clustered in the mra region of the Escherichia coli chromosome induce thermosensitive cell growth and division. J Bacteriol. 1989;171:5523–5530. doi: 10.1128/jb.171.10.5523-5530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F, Park W, Tomioka S, Tamaki S, Takase I, Kunugita K, Matsuzawa H, Asoh S, Ohta T, Spratt BG, et al. Peptidoglycan synthetic activities in membranes of Escherichia coli caused by overproduction of penicillin-binding protein 2 and RodA protein. J Biol Chem. 1986;261:7024–7031. [PubMed] [Google Scholar]
- Islam ST, Lam JS. Wzx flippase-mediated membrane translocation of sugar polymer precursors in bacteria. Environ Microbiol. 2013;15:1001–1015. doi: 10.1111/j.1462-2920.2012.02890.x. [DOI] [PubMed] [Google Scholar]
- Khattar MM, Begg KJ, Donachie WD. Identification of FtsW and characterization of a new ftsW division mutant of Escherichia coli. J Bacteriol. 1994;176:7140–7147. doi: 10.1128/jb.176.23.7140-7147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol MA, de Kroon AI, Rijkers DT, Killian JA, de Kruijff B. Membrane-spanning peptides induce phospholipid flop: A model for phospholipid translocation across the inner membrane of E. coli. Biochemistry. 2001;40:10500–10506. doi: 10.1021/bi010627+. [DOI] [PubMed] [Google Scholar]
- Kol MA, van Laak AN, Rijkers DT, Killian JA, de Kroon AI, de Kruijff B. Phospholipid flop induced by transmembrane peptides in model membranes is modulated by lipid composition. Biochemistry. 2003;42:231–237. doi: 10.1021/bi0268403. [DOI] [PubMed] [Google Scholar]
- Ling JM, Moore RA, Surette MG, Woods DE. The mviN homolog in Burkholderia pseudomallei is essential for viability and virulence. Can J Microbiol. 2006;52:831–842. doi: 10.1139/w06-042. [DOI] [PubMed] [Google Scholar]
- Marolda CL, Vicarioli J, Valvano MA. Wzx proteins involved in biosynthesis of O antigen function in association with the first sugar of the O-specific lipopolysaccharide subunit. Microbiology. 2004;150:4095–4105. doi: 10.1099/mic.0.27456-0. [DOI] [PubMed] [Google Scholar]
- Mercer KL, Weiss DS. The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J Bacteriol. 2002;184:904–912. doi: 10.1128/jb.184.4.904-912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi T, van Dam V, Sijbrandi R, Vernet T, Zapun A, Bouhss A, Diepeveen-de Bruin M, Nguyen-Disteche M, de Kruijff B, Breukink E. Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. Embo J. 2011;30:1425–1432. doi: 10.1038/emboj.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu HC. Clinical perspectives on imipenem. J Antimicrob Chemother. 1983;12(Suppl. D):149–153. doi: 10.1093/jac/12.suppl_d.149. [DOI] [PubMed] [Google Scholar]
- Ortega X, Hunt TA, Loutet S, Vinion-Dubiel AD, Datta A, Choudhury B, Goldberg JB, Carlson R, Valvano MA. Reconstitution of O-specific lipopolysaccharide expression in Burkholderia cenocepacia strain J2315, which is associated with transmissible infections in patients with cystic fibrosis. J Bacteriol. 2005;187:1324–1333. doi: 10.1128/JB.187.4.1324-1333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega XP, Cardona ST, Brown AR, Loutet SA, Flannagan RS, Campopiano DJ, Govan JR, Valvano MA. A putative gene cluster for aminoarabinose biosynthesis is essential for Burkholderia cenocepacia viability. J Bacteriol. 2007;189:3639–3644. doi: 10.1128/JB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KB, Toh E, Fernandez XB, Hanuszkiewicz A, Hardy GG, Brun YV, Bernards MA, Valvano MA. Functional characterization of UDP-glucose:Undecaprenyl-phosphate glucose-1-phosphate transferases of Escherichia coli and Caulobacter crescentus. J Bacteriol. 2012;194:2646–2657. doi: 10.1128/JB.06052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey WD, Ishiguro EE. Site of inhibition of peptidoglycan biosynthesis during the stringent response in Escherichia coli. J Bacteriol. 1978;135:71–77. doi: 10.1128/jb.135.1.71-77.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmert M, Biegert A, Hauser A, Soding J. HHblits: Lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat Methods. 2011;9:173–175. doi: 10.1038/nmeth.1818. [DOI] [PubMed] [Google Scholar]
- Rodloff AC, Goldstein EJ, Torres A. Two decades of imipenem therapy. J Antimicrob Chemother. 2006;58:916–929. doi: 10.1093/jac/dkl354. [DOI] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick PA, Arcondeguy T, Kennedy CK, Kahn D. glnD and mviN are genes of an essential operon in Sinorhizobium meliloti. J Bacteriol. 2001;183:2682–2685. doi: 10.1128/JB.183.8.2682-2685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N. Bioinformatics identification of MurJ (MviN) as the peptidoglycan lipid II flippase in Escherichia coli. Proc Natl Acad Sci USA. 2008;105:15553–15557. doi: 10.1073/pnas.0808352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1990. [Google Scholar]
- Schmerk CL, Bernards MA, Valvano MA. Hopanoid production is required for low-pH tolerance, antimicrobial resistance, and motility in Burkholderia cenocepacia. J Bacteriol. 2011;193:6712–6723. doi: 10.1128/JB.05979-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Matsuzawa H, Matsuhashi M. Cluster of mrdA and mrdB genes responsible for the rod shape and mecillinam sensitivity of Escherichia coli. J Bacteriol. 1980;141:52–57. doi: 10.1128/jb.141.1.52-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano MA. Undecaprenyl phosphate recycling comes out of age. Mol Microbiol. 2008;67:232–235. doi: 10.1111/j.1365-2958.2007.06052.x. [DOI] [PubMed] [Google Scholar]
- Valvano MA, Hanuszkiewicz A. Bussum, The Netherlands: Bentham Science Publishers Ltd; 2012. Proteins involved in the membrane translocation of O-antigen lipopolysaccharide. In: Andrade M, editor. An overview on the chemistry and biochemistry of carbohydrates. Mini-Reviews in Carbohydrate Chemistry p. 261–269. [Google Scholar]
- van Dam V, Sijbrandi R, Kol M, Swiezewska E, de Kruijff B, Breukink E. Transmembrane transport of peptidoglycan precursors across model and bacterial membranes. Mol Microbiol. 2007;64:1105–1114. doi: 10.1111/j.1365-2958.2007.05722.x. [DOI] [PubMed] [Google Scholar]
- van Heijenoort J. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology. 2001;11:25R–36R. doi: 10.1093/glycob/11.3.25r. [DOI] [PubMed] [Google Scholar]
- Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, Revets H, Lauwers S, Gillis M, Kersters K, et al. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- Vasudevan P, McElligott J, Attkisson C, Betteken M, Popham DL. Homologues of the Bacillus subtilis SpoVB protein are involved in cell wall metabolism. J Bacteriol. 2009;191:6012–6019. doi: 10.1128/JB.00604-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wientjes FB, Pas E, Taschner PE, Woldringh CL. Kinetics of uptake and incorporation of meso-diaminopimelic acid in different Escherichia coli strains. J Bacteriol. 1985;164:331–337. doi: 10.1128/jb.164.1.331-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.