Abstract
A plethora of factors is involved in the maturation of newly synthesized proteins, including chaperones, membrane targeting factors, and enzymes. Many factors act cotranslationally through association with ribosome-nascent chain complexes (RNCs), but their target specificities and modes of action remain poorly understood. We developed selective ribosome profiling (SeRP) to identify substrate pools and points of RNC engagement of these factors. SeRP is based on sequencing mRNA fragments covered by translating ribosomes (general ribosome profiling, RP), combined with a procedure to selectively isolate RNCs whose nascent polypeptides are associated with the factor of interest. Factor–RNC interactions are stabilized by crosslinking, the resulting factor–RNC adducts are then nuclease-treated to generate monosomes, and affinity-purified. The ribosome-extracted mRNA footprints are converted to DNA libraries for deep sequencing. The protocol is specified for general RP and SeRP in bacteria. It was first applied to the chaperone trigger factor and is readily adaptable to other cotranslationally acting factors, including eukaryotic factors. Factor–RNC purification and sequencing library preparation takes 7–8 days, sequencing and data analysis can be completed in 5–6 days.
Keywords: Ribosome profiling, Translation, Chaperones, Cotranslational folding, Nascent polypeptide processing
INTRODUCTION
Nascent polypeptide chains undergo a variety of cotranslational processing and maturation steps1,2. N-terminal enzymatic modifications, such as the removal of N-terminal methionines by methionine aminopeptidases3 and N-terminal acetylation by N-acetyltransferases4,5, occur in both prokaryotes and eukaryotes. Structurally divergent ribosome-associated molecular chaperones, which include trigger factor (TF) in bacteria and the Hsp70- and Hsp40-based ribosome-associated complex (RAC) and the nascent polypeptide-associated complex (NAC) in eukaryotes, stabilize or assist in the folding of nascent polypeptides2,6–8. Cotranslational membrane targeting of polypeptides is carried out, both in prokaryotes and eukaryotes, by the signal recognition particle (SRP)9. Ultimately, these cotranslational processes determine the fate and functionality of newly synthesized proteins.
Little is known of how these factors engage nascent chains in a selective manner, and how their activities are coordinated with the translation machinery, as well as with one another. The identity of the substrate pool of each factor, the timing of factor engagement with nascent chains, and the dependence of factor engagement upon environmental and cellular stresses are open issues of central importance for understanding protein biogenesis.
On the basis of a previously described ribosome profiling (RP) protocol for eukaryotic cells10, we provide a modified protocol to analyze translation in bacteria. Table 1 gives an overview of the differences between RP in prokaryotes and eukaryotes. Based on our RP protocol, we also describe a detailed protocol for selective ribosome profiling (SeRP), which enables the monitoring of cotranslational interaction events between ribosome-associated or nascent chain-associated factors and their native substrates for both prokaryotes and eukaryotes. Not only does this method identify nascent substrates bound by each factor, it also resolves the issue of when factor engagement occurs during peptide synthesis. Moreover, correlation of the binding events with features of the nascent chain can help identify parameters that control nascent chain interaction. Finally, by comparing the interaction profiles of various factors, SeRP can reveal the sequence of interactions necessary for the maturation of individual polypeptides.
Table 1. Major differences in the protocol of general RP applied to prokaryotes or to eukaryotes.
All steps listed are based on the procedures presented in this protocol for prokaryotes and in the protocol by Ingolia et al.10 for eukaryotes.
| Prokaryotes | Eukaryotes | |
|---|---|---|
| Cell harvest | - Rapid harvest, including filtration - Conventional harvest, including chloramphenicol pretreatment and centrifugation |
- Harvesting without freezing - Harvesting with flash-freezing |
| Lysis | Mixer milling | Trituration through 26-G needle |
| Nucleic acid digestion | MNase, 1 h, RT | RNase I, 45 min, RT |
| Ribosome recovery | - Sucrose cushion centrifugation - Sucrose gradient centrifugation |
Sucrose cushion centrifugation |
| rRNA depletion | Bacteria-specific oligonucleotides | Mouse-specific and human-specific oligonucleotides |
| Footprint fragment extraction | Acid-phenol extraction | miRNeasy kit |
| Data analysis | - Casava - Cutadapt - Bowtie for rRNA removal - Bowtie for genome alignment - Python scripts for complete analysis presented in all figures |
- Casava - Bowtie for rRNA depletion - TopHat for genome alignment |
RP and SeRP — an overview
RP reports on cellular gene expression levels more accurately than mRNA abundance measurements, such as microarray analyses or mRNA-seq alone, because it also captures translational regulation11,12. In RP, polysomes are digested with a nuclease, which results in monosomes that protect ~30 nucleotides (nt)-long mRNA fragments from degradation. Depending on the analysis, these ribosome-protected footprints can provide different types of information. The sequence of each ribosome footprint represents one ribosome carrying one nascent chain of a defined length. Therefore, ribosome footprints, as a whole, resemble the total translatome. Moreover, the detection of footprints outside known open reading frames can lead to the identification of previously unknown and very short genes11–14. The abundance of ribosomes at all positions along mRNAs, referred to as read density, provides information about the relative translation speed and the occurrence of pausing sites within genes11,15, whereas the sum of read densities for each gene reports on relative gene expression levels. Finally, the distribution of ribosomes along an average message can be inferred by averaging read densities across all genes, a procedure referred to as a meta-gene analysis11,12.
SeRP is a combination of RP11,12 with a selective purification of a subset of ribosome-nascent chain complexes (RNCs) that are engaged by the factor of choice. Ribosomal footprints derived from SeRP directly reveal the interactome of the factor, i.e. the nascent chains that are bound by the factor during synthesis. The ratio of read densities of interactome (derived from SeRP) and total translatome (derived from RP) calculated for every position along mRNAs provides the relative enrichment efficiency of factor-bound RNCs over the total of translating ribosomes. To achieve the best comparison between RP and SeRP data, we recommend that the monosomes obtained for interactome studies be derived from the pool used for translatome studies. Fig. 1 gives an overview of the entire procedure. Recently, a variant of SeRP using conformation-specific antibodies was applied to study the folding propensity of a specific nascent polypeptide of varying length16.
Figure 1.
Outline of the procedure for general ribosome profiling (RP) in bacteria (in black) and selective ribosome profiling (SeRP) of factor–RNCs (in red). * marks the two mutually exclusive options of in vivo and ex vivo crosslinking.
Analysis of TF function by SeRP
We developed SeRP to analyze the nascent interactome of the chaperone TF11. We demonstrated that TF binds the vast majority of nascent chains during translation, except for nascent inner membrane proteins, which are targeted to the translocon by SRP early in translation11. We identified outer membrane β-barrel proteins as prominent cotranslational substrates of TF. In addition, we found that, in contrast to existing models17, TF is not pre-bound to ribosomes (via transient association with ribosomal protein L2318) before the nascent chains emerge from the tunnel exit. Instead it is recruited only after nascent chains have reached an average length of more than 100 amino acids11. This finding gave support to the idea that nascent chains engage maturation factors in a yet to be defined order of binding events.
Experimental setup
Cell growth and harvest
Translation is tightly controlled in response to environmental growth conditions, resulting in subtle changes in ribosome occupancy caused by the modification of growth conditions, for instance temperature, aeration, and type of medium. The high sensitivity of RP enables the detection and investigation of such changes. Accordingly, variation in ribosome occupancy can be observed when cells are grown on different days under similar but not identical conditions. Thus, for maximal translational fidelity and reproducibility, we recommend growing cells on the same day under identical conditions in rich, defined media supplemented with amino acids, and in the absence of antibiotics that interfere with translation.
Both in RP and SeRP polysomes must be kept intact during cell harvest and polysome preparation. Also, the association of factors with RNCs must be preserved during SeRP. We developed two independent harvesting protocols in which the translation status is preserved. In the first, referred to as conventional harvest, cells are pretreated with the translation inhibitor chloramphenicol to arrest mRNA translation by ribosomes and poured over ice to quickly cool them before cell harvest by centrifugation. The cell pellet is then resuspended and flash-frozen in liquid nitrogen. To verify whether chloramphenicol addition immediately inhibits translation, we performed pulse-labeling experiments in minimal media and isolated RNCs from total lysate by sucrose cushion ultracentrifugation. Incorporation of radioactive methionine into proteins only occurred when 35S-methionine was pulsed before adding chloramphenicol (Supplementary Fig. 1, lane 1), but not when it was done simultaneously or after, as reflected by the constant, low radioactive background signals (lanes 2–4). Thus, translational stalling by chloramphenicol is faster than detectable methionine incorporation.
The second cell harvesting protocol, referred to as rapid harvest, was developed to avoid the use of translation inhibitors during harvest. Here, cells are filtered in a pre-warmed glass filtration system using a nitrocellulose membrane and flash-frozen in liquid nitrogen. During filtration (which takes about one minute), cells collected at the filter surface are constantly flushed with growth media, thereby preventing them from sensing, and responding to, growth-limiting amino acid levels or increasing cell densities. The cell layer is then quickly scraped off the membrane with a scoopula and the entire scoopula, which holds the scraped cells, is transferred immediately into liquid nitrogen. This is the most critical step of the procedure, and it should be completed within a few seconds. In our experience, if this takes too long, polysomes can be lost due to the rapid adaptation of translation in response to the changing environment19.
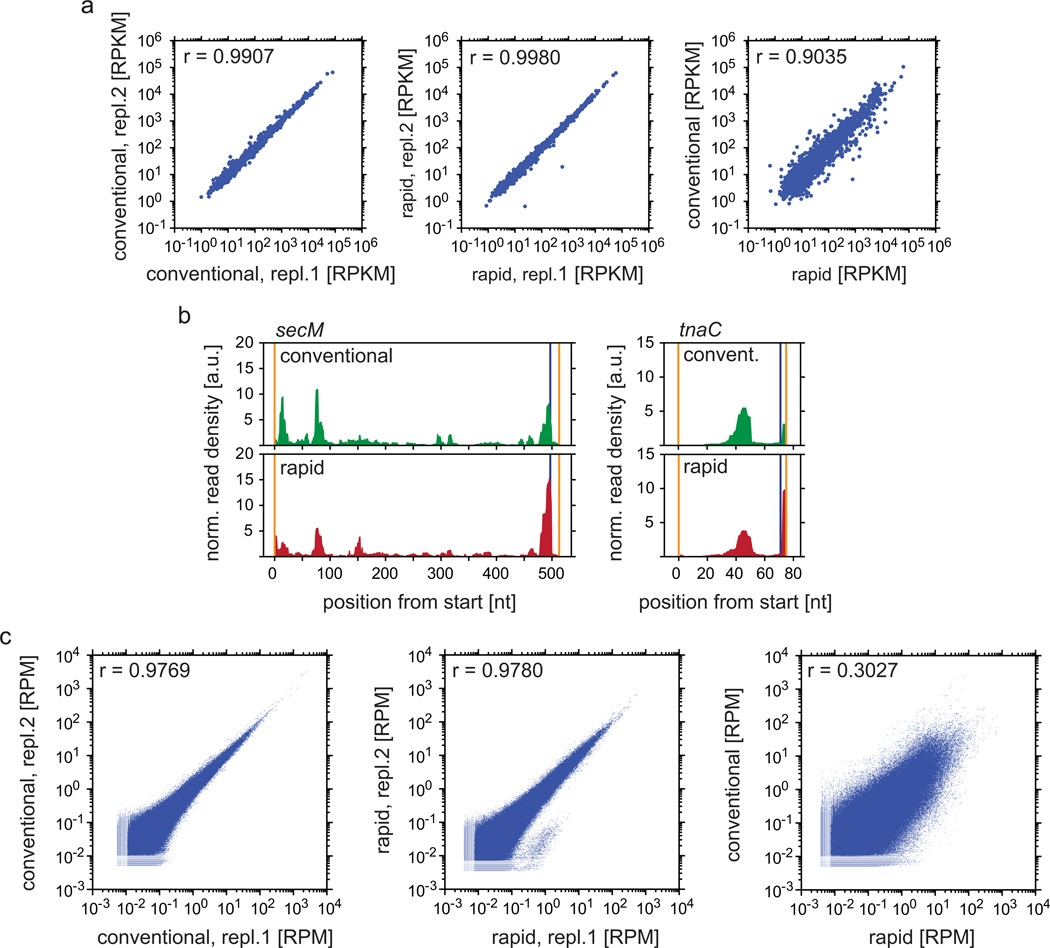
We prepared samples according to both harvesting protocols on the same day and compared the translatome after sequencing. Expression levels from cells harvested the same way in two separate experiments were highly correlated (r = 0.99), whereas the correlation of gene expression levels between conventionally and rapidly harvested cells was lower (r = 0.90; Fig. 2a). Upon rapid harvest of cells grown in LB medium, ribosomes mostly accumulate before or at serine codons due to serine depletion15. Upon conventional harvest, the accumulation of paused ribosomes at serine codons and at native stalling sites, e.g. secM20 and tnaC21 (Fig. 2b), was lower, indicating a shift or loss of ribosomes during the conventional harvest. The observed effect might be due to differences in stalling efficiencies of chloramphenicol at specific codons22. This discrepancy is reflected by a lower correlation of global read densities from cells harvested using the two different protocols, whereas data collected in the same harvesting conditions were highly correlated (Fig. 2c).
Figure 2.
Translatome analyses of cells harvested according to the conventional or the rapid harvesting method. E. coli MC4100 ∆tig::Kan + pTrc-tig-TEV-Avi cells were grown in LB medium and harvested as described in the protocol for conventional (step 1, option A) or rapid harvest (step 1, option C). After lysis, the lysate was crosslinked ex vivo with DSP or EDC (step 7, option B), polysomes were digested, and ribosomes were isolated in a sucrose gradient ultracentrifugation (step 18, option B). Then footprint fragments were isolated and used to prepare a sequencing library (see Supplementary Methods including rRNA depletion). Sequencing was performed on Illumina GAII. Data were analyzed as described in the basic analysis using phred+64 quality score (steps 35–40), followed by the specific data analysis (steps 41–59).
(a) Analysis of gene expression levels performed according to steps 42–49. Left and middle panel: two replicates each harvested according to the same method; right panel: comparison of two different harvesting methods. (b) Analysis of read densities along the individual open reading frames of secM and tnaC known to contain native stalling sites. Samples were prepared using EDC as crosslinker. Steps 42–44 and 50–52 were implemented. Then read densities along secM and tnaC were normalized to the expression level by dividing each position within the open reading frame by the sum of read densities within this open reading frame multiplied with the length of the open reading frame. Orange vertical lines mark start and stop of genes, blue vertical lines represent the position of the native stalling site. (c) Results of the analysis of read densities along all protein coding regions following implementation of steps 42–45 and 50–55.
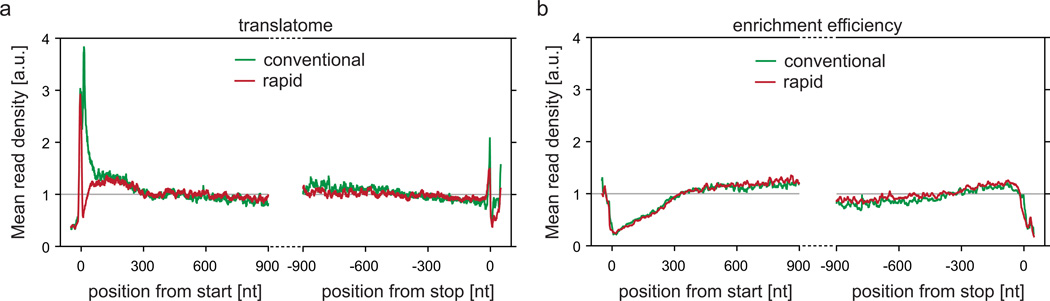
In a meta-gene analysis, differences in the average distribution of ribosomes along transcripts were also observed (Fig. 3a). As seen in eukaryotic RP experiments using cycloheximide as translation inhibitor10, we find a pronounced accumulation of ribosomes about 6 codons downstream of the initiation site, which indicates that initiation is not prevented during chloramphenicol pretreatment but ribosomes are stalled shortly thereafter. Finally, we analyzed how cell harvesting conditions affect the TF interactome. Importantly, the ratio of average read densities between the interactome and translatome was independent of how cells were harvested (Fig. 3b). We conclude that cell growth and harvest procedures have a strong impact on the translatome and recommend the rapid harvest without chloramphenicol pretreatment as the superior procedure. If pretreatment with chloramphenicol or other translation inhibitors is not problematic, this step may be included in the rapid harvesting protocol before filtration. In such cases, the risk of losing polysomes or inducing translational adaptation before freezing the cells is minimal. Still, the conventional harvest approach may be the only available option for microorganisms that cannot be rapidly filtered.
Figure 3.
The distribution of ribosomes along an average message (Meta-gene analyses) from cells harvested according to the conventional or the rapid harvesting method. Data obtained from translatome samples of E. coli MC4100 ∆tig::Kan + pTrc-tig-TEV-Avi cells harvested via conventional or rapid harvest were prepared and sequenced as described for Fig. 2. (a) Meta-gene analyses from start and stop codon performed according to steps 42–43, 45 and 56–58. (b) TF enrichment efficiency (ratio of interactome and translatome) based on meta-gene analyses from start and stop codon. Here, ribosomes for the interactome sample were isolated in a sucrose cushion centrifugation (step 18, option A) and subjected to affinity purification and TEV cleavage. Meta-gene analyses were calculated separately for interactome and translatome samples as described in (a). For TF enrichment efficiency, the ratio of interactome and translatome was calculated for every position along the average message.
Stabilization of nascent chain interactions by chemical crosslinking
After harvest, cells are lysed in a mixer mill while frozen. For translatome analyses, the protocol proceeds directly to the nuclease digestion described in the next paragraph. However, in the case of SeRP, mechanistic details of how the factor of choice interacts with RNCs should be considered. Most factors interact only transiently with translating ribosomes due to short half-life times of the relevant complexes. As a consequence, upon affinity purification of factor-bound RNCs, repeated binding and release cycles of the factor may create an artificial equilibrium that does not resemble the in vivo situation. This issue can be solved by the rapid stabilization of in vivo-formed complexes and by interfering with the formation or stability of new interactions. The method of choice to stabilize such complexes needs to fulfill several requirements: (i) It must be fast enough to prevent the onset of a new equilibrium after translation has ended or was stalled; (ii) it should not introduce a bias by stabilizing preferentially particular complexes; (iii) it must be specific enough to avoid the formation of artificial complexes; (iv) it must stabilize complexes throughout the purification procedure; (v) it should not stabilize complexes of ribosomes with factors that do not interact with the nascent polypeptide. In the case of factors whose substrate binding is ATP/GTP-dependent (e.g. Hsp70 chaperones and SRP), in vivo interactions can be stabilized and new interactions can be prevented by rapid ATP depletion using either apyrase or hexokinase and glucose23,24. However, a universally applicable and effective method, which is also effective on ATP-independent factors (e.g. TF and NAC), is chemical crosslinking. Destabilizing non-native complexes or preventing their formation is most effectively achieved by choosing the appropriate conditions during ultracentrifugation when isolating monosomes (see ‘Isolation of monosomes’).
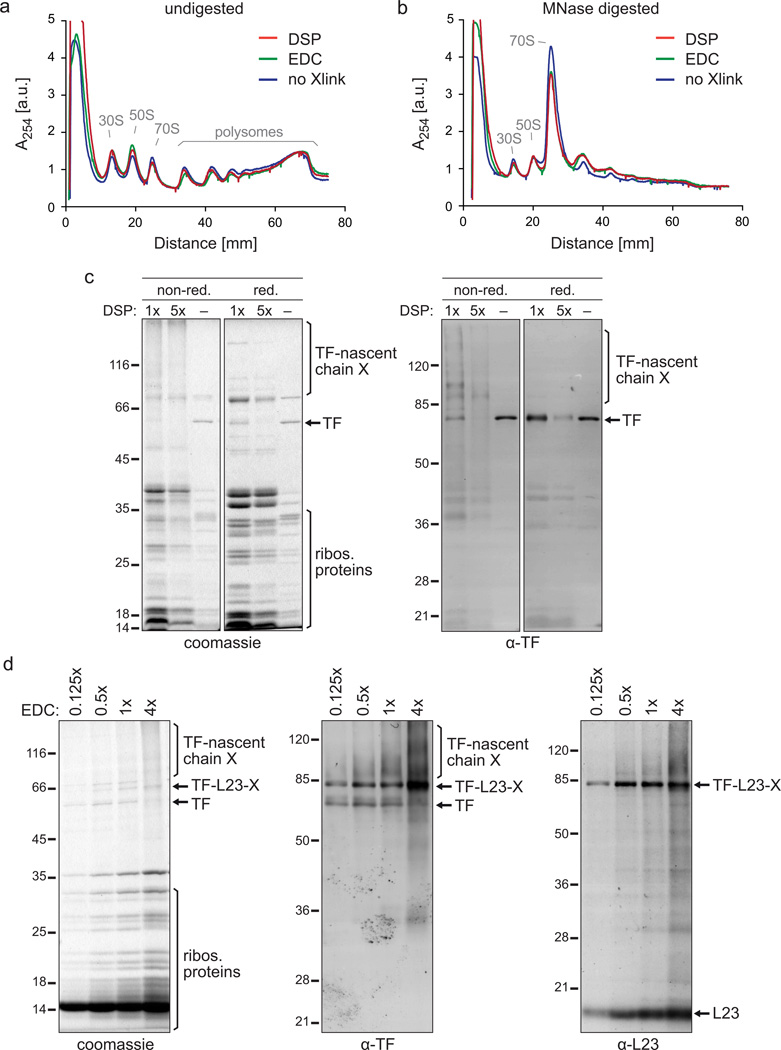
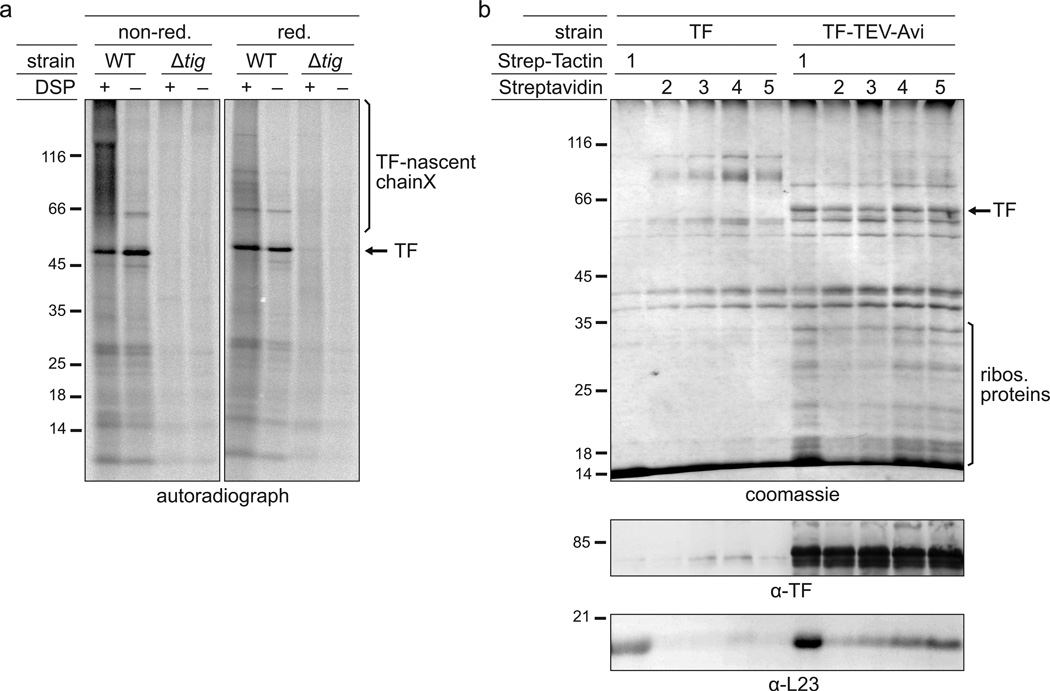
We developed comprehensive protocols for crosslinking a desired factor to RNCs after cell lysis (ex vivo crosslinking) or in vivo. For ex vivo crosslinking, the frozen cell powder is thawed in buffer containing the crosslinker to prompt rapid crosslinking. We tested a variety of different crosslinkers and obtained the best results in terms of efficiency and specificity using N-hydroxysuccinimide (NHS) ester derivatives, such as the non-water-soluble dithiobis [succinimidyl propionate] (DSP). DSP covalently links primary amines together, i.e. lysine residues and N-termini of nascent chains, and has a spacer arm length of 12 Å, which contains a disulfide bond. Consequently, adding a reducing agent to the crosslinked sample can reverse the crosslink, a feature that can be exploited for troubleshooting purposes (see ‘Troubleshooting’) or even as an elution strategy following affinity purification (see ‘Factor–RNC purification’). We carefully titrated the amount of DSP with the goal of (i) retaining a normal polysome profile (Fig. 4a), (ii) not interfering with the downstream RNA digestion (Fig. 4b), and (iii) enabling high crosslinking efficiency and recovery of crosslinked TF–RNCs (Fig. 4c). Use of a five-fold excess of DSP with respect to its optimal concentration reduced the amount of purified TF–RNCs, which was made evident by (i) reduced levels of TF-nascent chain crosslinks (appearing as a high-molecular-weight smear above the TF band under non-reducing conditions), (ii) decreased levels of TF under reducing conditions, and (iii) a reduction in the amount of co-purified ribosomal proteins (Fig. 4c). We observed a similar decrease in efficiency of purified TF–RNCs when we reduced the DSP concentration to one-fifth of the optimal concentration (data not shown).
Figure 4.
The impact of crosslinking on the purification of TF–RNCs. (a,b) Polysome profiles in the absence of crosslinker or after DSP or EDC ex vivo crosslinking and sucrose gradient centrifugation. Depicted are data from experiments in which E. coli MC4100 cells were grown in LB medium and harvested as described in step 1, option C. The lysate was either crosslinked ex vivo with DSP or EDC (step 7, option B) or left untreated (step 7, option A). Undigested (a) and digested (b) lysates were run on a sucrose gradient (step 18, option B). The digestion was performed with a reduced MNase concentration of 15 U/A260 to partially retain di- and trisomes for comparison. ‘30S’ and ‘50S’ depict the peaks of the small and large ribosomal subunits, respectively. The monosome peak is labeled with ‘70S’. To compare polysome profiles quantitatively, the curves were normalized to the same area underneath all ribosomal peaks. (c) Gel analysis of the DSP crosslinker titration. 200 ml of E. coli MC4100 ∆tig::Kan + pTrc-tig-TEV-Avi cells were grown and harvested according to step 1, option A. Cells were resuspended in 2 ml of buffer A (50 mM HEPES pH 7.5, 1 M potassium acetate, 10 mM MgAc2, 1 mM PMSF, 1 mM chloramphenicol, 0.4% Triton X100, 0.1% NP-40, 1 mg/ml lysozyme, 2.5 µg/ml RNase-free DNase I). Lysis and purification of TF–RNCs was performed as described including DSP ex vivo crosslinking (step 7, option B) and sucrose cushion centrifugation (step 18, option A) with the following exceptions: For crosslinking either 3 mg of DSP (‘1×’), 15 mg of DSP (‘5×’) or DSMO only (‘−‘) were used. Ultracentrifugation was done with 1 M potassium acetate instead of 1 M NaCl in the sucrose cushion buffer causing the high amount of non-crosslinked TF that copelleted without DSP addition (‘−‘). For AP only half of Strep-Tactin slurry and TEV protease were used. Either non-reducing (‘non-red.’) or reducing (‘red.’) sample buffer was used for SDS-PAGE. Gels were stained with coomassie or used for western blotting employing a polyclonal α-TF antibody. Crosslinks are abbreviated with ‘X’. (d) Gel analysis of the EDC crosslinker titration. E. coli MC4100 ∆tig::Kan + pTrc-tig-TEV-Avi cells were grown in 1 l LB, harvested as described in step 1, option A, and resuspended in 6 ml of lysis buffer. Ex vivo crosslinking (step 7, option B) was performed with 2.5 mM (‘0.125×’), 10 mM (‘0.5×’), 20 mM (‘1×’) and 80 mM (‘4×’) EDC. TF–RNCs were purified as described, including sucrose cushion centrifugation (step 18, option A), eluted from the affinity matrix by boiling in reducing sample buffer, and analyzed by SDS-PAGE or western blotting using polyclonal antibodies against TF and L23. Crosslinks are abbreviated with ‘X’.
Another useful crosslinker is the water-soluble carbodiimide 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC). EDC is an irreversible zero-length crosslinker that covalently links primary amines with carboxyl groups. Apart from TF-nascent chain crosslinks observed for DSP, EDC also generated considerable crosslinks between TF and its binding partner, ribosomal protein L23 (Fig. 4d), which was not observed for DSP. We tested the pull-down efficiency of TF–RNCs after crosslinking with different EDC concentrations, 0.125, 0.5, 1 and 4 times the standard concentration reported in the Procedure below. Increasing EDC concentration led to higher crosslinking efficiency (Fig. 4d). However, overcrosslinking resulted in reduced quality or even a complete loss of detectable polysomes (data not shown). Therefore, we recommend performing a careful titration of the crosslinker concentration as exemplified in Fig. 4c,d. In addition, to avoid introducing a potential bias due to the use of one specific crosslinker, we recommend using at least two different crosslinkers with different specificity and chemical properties. In the case of TF-SeRP, we did not observe substantial differences between EDC and DSP crosslinking11.
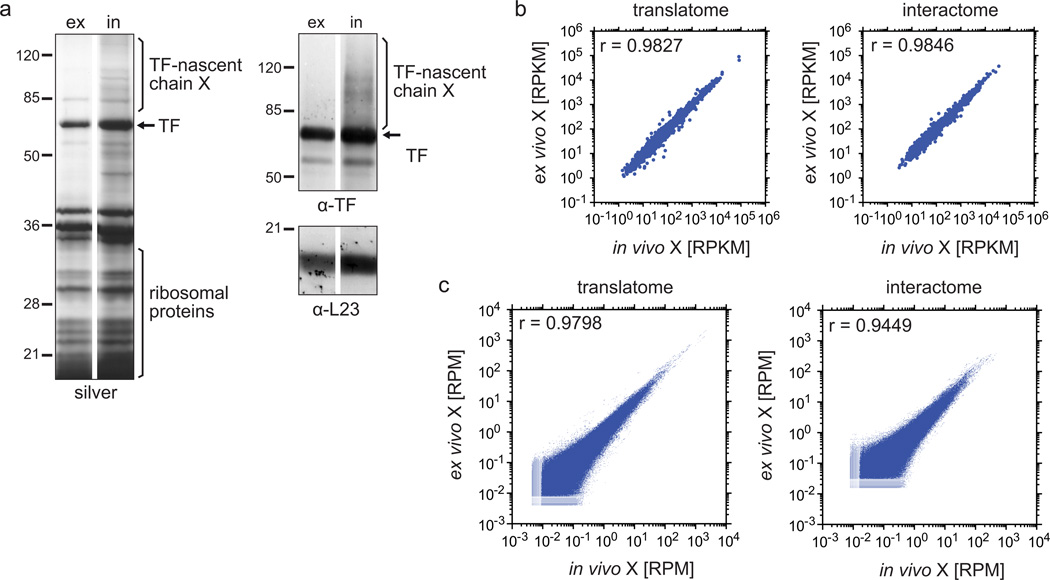
For the in vivo crosslinking protocol, only crosslinkers that penetrate the cell membrane can be used, like DSP and disuccinimidyl suberate (DSS). However, they usually react with amino or carboxyl groups of amino acids that are highly abundant in rich growth media. Therefore, in vivo crosslinking requires cells to be grown in minimal media lacking amino acids. We compared the crosslinking and pull-down efficiency of TF–RNCs after ex vivo or in vivo DSP crosslinking. In the first case, cells were treated with chloramphenicol and harvested by centrifugation followed by lysis and crosslinking (conventional harvest). In the second case, cells were treated with chloramphenicol (for 5 s) followed by crosslinking (30 s), quenching (30 s), harvest by centrifugation, and lysis. Filtration could be used as an alternative harvesting method. In both cases, TF-bound RNCs were purified after nuclease digestion and ribosome isolation. In vivo crosslinking yielded slightly higher amounts of TF–RNCs than ex vivo crosslinking (Fig. 5a). Still, genome-wide measurements showed good agreement between the two crosslinking approaches (Fig. 5b,c). In addition, read densities along individual genes as well as meta-gene analyses were similar (Fig. 6a–c), indicating that both crosslinking methods are suitable alternatives. However, the in vivo crosslinking procedure has several disadvantages: (i) It requires higher amounts of crosslinker; (ii) the selection of crosslinkers that penetrate the membrane is limited; (iii) it is unclear to what extent cells can sense the crosslinker and respond to this stress by changing the translatome, thus in vivo crosslinking requires pretreatment with chloramphenicol to preserve the translational status; (iv) the most frequently used amine-crosslinkers (such as DSP or DSS) react with free amino acids present in most growth media, limiting analysis to prototrophic cells grown in minimal media.
Figure 5.
Comparison of samples crosslinked in vivo and ex vivo in translatome and interactome analyses. (a) Gel analysis of the TF–RNC purification after ex vivo and in vivo crosslinking. For ex vivo crosslinking, E. coli MC4100 ∆tig::Kan + pTrc-tig-TEV-Avi cells grown in LB medium were harvested as described in step 1, option A, and the lysate was crosslinked ex vivo with DSP (step 7, option B). In vivo crosslinking was performed on cells grown in M9 minimal medium (step 1, option B and step 7, option A). After affinity purification and TEV elution, samples were treated with reducing sample buffer before being loaded onto an SDS-PAGE for silver stain and western blots using antibodies against TF and L23. Pictures from ex vivo and in vivo crosslinking were derived from the same gels and blots, but samples in between were cut out for this illustration. Crosslinks are abbreviated with ‘X’. (b,c) Scatter plots of gene expression levels and read densities comparing ex vivo and in vivo crosslinking. E. coli MC4100 ∆tig::Kan + pTrc-tig-TEV-Avi were grown in M9 minimal medium and treated as described in the protocol, including step 1, option B and step 7, option A for in vivo crosslinking, and step 1, option A and step 7, option B for ex vivo crosslinking. Ribosomes were isolated through a sucrose cushion centrifugation (step 18, option A) or sucrose gradient centrifugation (step 18, option B) for interactome and translatome, respectively. All downstream steps were done as described in the protocol and in the legend of Fig. 2, including the calculation of gene expression levels (b) and read densities in protein coding regions (c). Crosslinks are abbreviated with ‘X’.
Figure 6.
Comparison of translatome and interactome samples after in vivo and ex vivo crosslinking. Samples crosslinked in vivo and ex vivo were prepared as described in the legend of Fig. 5. Crosslinks are abbreviated with ‘X’. (a) Read densities along individual open reading frames of icd and ompC known to represent native TF substrates11 were analyzed for translatome and interactome as described in the legend of Fig. 2b. (b,c) Meta-gene analyses for translatomes (b) and TF enrichment efficiencies as the ratios of meta-gene analyses for interactome and translatome (c) were calculated as described in the legend of Fig. 3a and b, respectively.
Lysate clearing and nuclease digestion
In the next step, the lysate is cleared from cell debris and membranes by centrifugation. We observed that a small amount of ribosomes co-pellet during this clearance step, potentially because of the interaction with the translocon or membranes upon cotranslational protein translocation. This loss of ribosomes was not affected by crosslinking (data not shown). Although this clearance step can, in principle, affect the translatome and interactome, it does not affect the relative factor-enrichment efficiency in SeRP. Still, experimenters should consider omitting this step for RNCs that potentially interact with membranes.
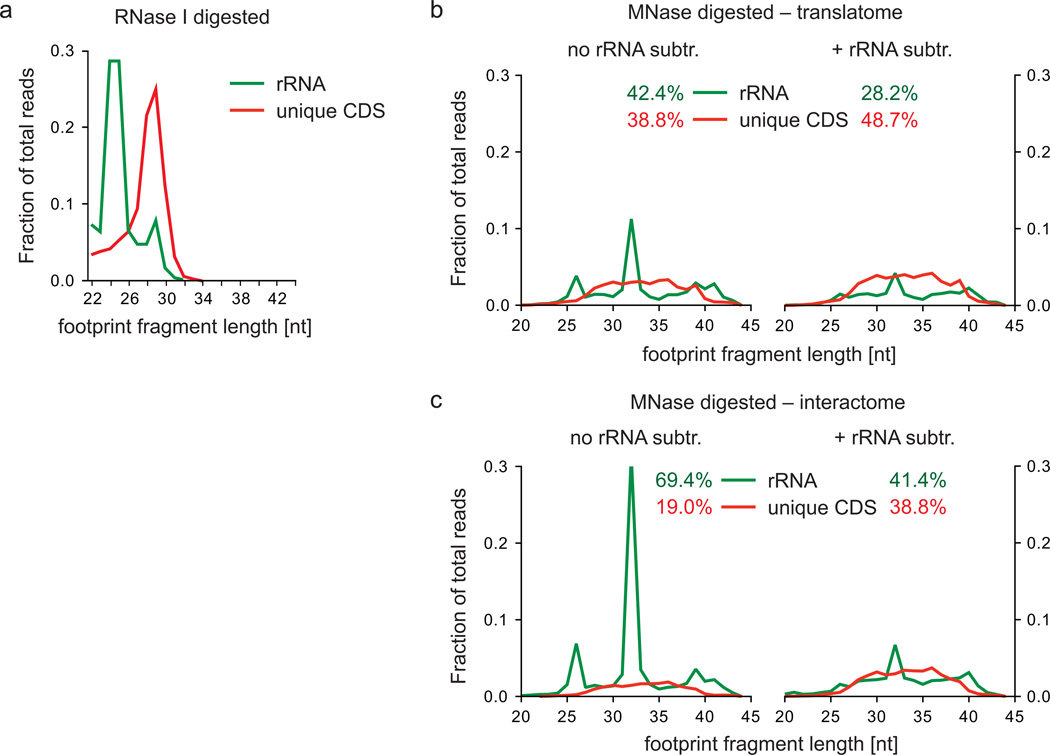
The clarified lysate is then digested with a nuclease to generate monosomes. While RNase I is frequently used in eukaryotic cells10, this enzyme is inactive in bacteria25. Therefore, we use micrococcal nuclease (MNase) from Staphylococcus aureus. MNase can also be used in eukaryotic lysates and, in fact, leads to a reduced amount of ribosomal RNA (rRNA) contamination compared to RNase I11. Furthermore, its activity can be regulated by calcium ions. A disadvantage of MNase is the sequence bias of its catalytic activity, which is 30-fold increased proximal to A or T26. Accordingly, ~80% of the sequenced mRNA fragments start with an A or T at the 5' end, and the generated fragments are more heterogeneous in length when compared to fragments derived from yeast lysates using RNase I12 (Fig. 7a–c). As a consequence, the use of MNase usually does not provide sub-codon resolution. Nonetheless, its use facilitated the identification of serine and Shine-Dalgarno-like sequences as major causes of ribosome pausing15.
Figure 7.
Footprint fragment lengths and rRNA contamination variation according to digestion conditions. (a) Footprint fragments and rRNA contamination of RNase I digested yeast lysate (data from Ingolia et al.12) were plotted according to their lengths as fractions of total reads. (b,c) MNase-digested bacterial footprint fragments derived from translatome (b) or interactome (c) samples were prepared as described in the legend of Fig. 2 without (left panel) and after (right panel) rRNA depletion during the preparation of the deep sequencing library (steps 76-83 in the Supplementary Methods). Read lengths were plotted as fractions of total reads calculated according to step 41.
The required amount of MNase has to be determined carefully as increased nuclease activity (either caused by elevated amounts of enzyme, pH variation, or increased digestion time) primarily results in increased rRNA contamination. Therefore, the activity of each new batch of MNase should be determined using an MNase activity assay (box 1). Slight overdigestion was sometimes observed in interactome analyses, probably due to the incomplete inactivation of MNase upon ethylene glycol tetraacetic acid (EGTA) addition (Fig. 7b,c). rRNA contamination can be reduced by including an additional step in the library preparation protocol to remove the most prominent rRNA fragments using antisense oligonucleotides (see ‘Preparation of a footprint fragment library’). By contrast, using insufficient amounts of MNase causes less stringent cleavage of ribosomal footprint fragments, which results in an increase in the length of footprint reads15. This effect may reduce the overall yield of ribosome footprints in the size-selective gel purification procedure and provide less accurate information on ribosome position on mRNAs (see 'Preparation of a footprint fragment library').
Box 1.
Assaying the activity of MNase TIMING 2 h
-
1
Prepare MNase solutions for the activity assay according to option A, for purchased MNase, or option B, for self-purified MNase (as described in Box 2). For most accurate measurements and consistent results, it is best to compare the activity with a previously used MNase of known concentration. If such a reference is not available, we recommend titrating MNase in the first RP- or SeRP-experiment and using this as a reference for the next activity assay.
A. Preparation of a solution of purchased MNase
-
Dissolve each vial of lyophilized MNase in 40 µl of 10 mM Tris pH 8.0. This solution corresponds roughly to a concentration of 375 U/µl.
CRITICAL STEP For the activity test of purchased MNase, we recommend combining at least five vials of dissolved MNase to have a reasonable amount of protein.
PAUSE POINT This MNase solution can be stored at −80 °C for at least one year.
B. Preparation of a solution of self-purified MNase
-
Adjust the concentration of the self-purified protein (produced as in Box 2) to approximately 14 mg/ml.
PAUSE POINT This MNase solution can be stored at −80 °C for at least one year.
-
2
Prepare the substrate master mix (72×):
Component Volume for 1×
mix (µl)Volume for
72× mix (µl)End
concentration100 mM Tris pH 8.0 5.0 360 10 mM 100 mM CaCl2 5.0 360 10 mM 2 mg/ml DNA 5.0 360 0.2 mg/ml RNase-free water 35.0 2520 -
3
Prepare serial dilutions A–F of new (purchased or self-purified) MNase and an MNase reference in an end volume of 15 µl in 10 mM Tris pH 8.0 according to the pipetting table below:
Dilution Volume of
MNase (µl)MNase
sourceVolume of
Tris pH 8.0 (µl)A 1.0 stock 14.0 B 9.6 Dilution A 5.4 C 7.5 Dilution B 7.5 D 7.5 Dilution C 7.5 E 7.5 Dilution D 7.5 F 7.5 Dilution E 7.5 G 7.5 Dilution F 7.5 H 0.0 - 15.0 -
4
Prepare one enzyme master mix (5×) per MNase dilution C–H for both new MNase and MNase reference. Each dilution is measured in four replicates:
Component Volume for 1×
mix (µl)Volume for
5× mix (µl)End
concentration100 mM Tris pH 8.0 5.0 25.0 10 mM MNase dilution 1.0 5.0 Depends on dilution RNase-free water 44.0 220.0 -
5
Distribute 50 µl of the substrate master mix into rows 1–4 of the microplate with a multi-channel pipette.
-
6
Add 50 µl of enzyme master mix to the specified wells (see table below; black wells: dilutions of new MNase; red wells: dilutions of MNase reference). Rapidly mix with a multi-channel pipette.
CRITICAL STEP Try to mix and start the measurement as fast as possible to record the initial slope at maximal substrate concentration.


-
7
Automix in the plate reader for 10 s before the first measurement.
-
8
Take kinetic measurements of absorbance at 260 nm for 1 h at 25 °C. Collect readings every 30 s or 60 s.
-
9
Use nonlinear regression analysis based on an exponential equation with one phase association [Y=Y0 + (A-Y0)*(1-e(−K*x))] to fit the readings. Calculate initial slopes for x(0) and x(1) from the equation for the four replicates. One enzyme unit is defined as an increase of 0.005 A260 units per minute or via the comparison with the MNase reference.
As SeRP experiments analyzing TF function required large amounts of MNase, we developed an MNase overexpression and purification protocol (box 2; Supplementary Fig. 2a,b). We compared the self-purified MNase with the commercially available MNase in RP experiments and found that the enzymes have comparable activities and produce footprints of similar lengths (Supplementary Fig. 2c–e), but using the new MNase slightly reduced the percentage of rRNA contamination (data not shown).
Box 2.
Overproduction and purification of MNase with N-terminal OmpA signal sequence and C-terminal His-tag
CRITICAL The molecular weight of mature His6-tagged MNase is 20.1 kDa. The isoelectric point (pI) of the mature His6-tagged protein is 9.4 (Supplementary Fig. 2).
Cell growth
-
1
Inoculate 4× 1.5 l of LB medium containing 1.5 ml of 50 mg/ml kanamycin per 1.5 l in 5-l Erlenmeyer flasks with a 15-ml culture of E. coli BL21(DE3) transformed with plasmid pET24a-ompA-nucB(MNase) in stationary phase per 1.5 l (for design of the construct, refer to Supplementary Fig. 2a).
-
2
Grow till an OD600 of 0.6 at 30 °C (3–3.5 h).
-
3
Collect 1 ml of culture that will be used as an uninduced control for SDS-gel analysis.
-
4
Centrifuge the uninduced control at 16,000 g for 1 min at 4 °C in a tabletop centrifuge. Discard the supernatant.
-
5
Resuspend the pellet from step 4 in 70 µl of reducing sample buffer.
-
6
Incubate for 10 min at 95 °C in a thermomixer. Keep on ice until SDS-gel analysis (steps 10–11). The sample can be stored at −20 °C for years.
-
7
Add 1.5 ml of 1 M IPTG per Erlenmeyer flask to the culture from step 2 to induce MNase expression.
-
8
Grow the cultures for 5 h at 30 °C (cultures reach an OD600 of ~2.0).
-
9
Collect 250 µl of the cultures that will be used as induced control for SDS-gel analysis and proceed as in steps 4–6.
-
10
Centrifuge uninduced and induced controls from steps 6 and 9, respectively, at 20,000 g for 2 min at RT in a tabletop centrifuge. Do not remove the supernatant.
-
11
Load 10 µl of each supernatant of uninduced and induced sample (from step 10; corresponding to ~0.1*109 cells) to a 14% SDS-gel. Run and stain the gel with coomassie to check the MNase expression.
-
12
Centrifuge the cultures from step 8 in 1-l centrifuge tubes at 3620 g (4500 rpm) for 10 min at 4 °C in an F9 rotor. Discard the supernatant.
-
13
Transfer the cell pellets from 3 l of culture into a 50-ml falcon tube using a rubber scraper.
-
14
Flash-freeze the cell pellets in the falcon tube in liquid nitrogen and store them at −80 °C.
PAUSE POINT The cells can be kept at −80 °C for up to 6 months.
Protein purification
-
15
Thaw the cells by resuspending each cell pellet (from step 14) (one per 3 l of culture) in lysis buffer MNase to 35 ml total volume.
-
16
Disrupt the cells in a French pressure cell at 8,000 lb/in2.
-
17
Collect 5 µl of the total cell lysate from step 16 for SDS-gel analysis. Add the same volume of reducing sample buffer.
-
18
Incubate for 10 min at 95 °C in a thermomixer. Keep on ice until SDS-gel analysis (steps 31–32). The sample can be stored at −20 °C for years.
-
19
Centrifuge the total lysate from step 16 in SS34 tubes at 30555 g (15900 rpm) for 30 min at 4 °C in an F21 rotor. Recover the supernatant as clarified cell lysate.
-
20
Collect a sample of the clarified cell lysate for SDS-gel analysis as in steps 17–18.
-
21
Use a HisTrap FF column for the His-tag purification. Equilibrate with ~10 column volumes (CV) of ultrapure water using a 50 ml syringe. Equilibrate with 10 CV of lysis buffer MNase.
CRITICAL Never let the column run dry and never let air bubbles get into the column.
-
22
Pool the clarified lysate (~60 ml) from step 19. Slowly pass it through the column. Keep the flow-through.
-
23
Collect a sample of the flow-through for SDS-gel analysis as in steps 17–18.
-
24
Wash the column with 3× 20 ml aliquots of wash buffer MNase. Collect the wash fractions in separate falcon tubes.
-
25
Check that the last wash fraction does not contain any proteins anymore in a quick Bradford test: Pipet 100 µl of 1× Bradford solution in one drop onto Parafilm, add 5 µl of the wash fraction. If the color turns slightly blue, wash again with 20 ml of wash buffer.
-
26
Collect a sample of the last wash fraction for SDS-gel analysis as in steps 17–18.
-
27
Wash the column with 2 CV of lysis buffer MNase to adjust the salt concentration.
-
28
Elute MNase from the column with 4× 20 ml aliquots of elution buffer MNase. Check the protein concentration of the elution fractions with a quick Bradford test. Most protein elutes in fractions 1 and 2.
-
29
Collect samples of all elution fractions for SDS-gel analysis as in steps 17–18.
-
30
Wash the column with 20 CV of wash buffer MNase.
-
31
Centrifuge the samples collected for SDS-gel analysis (from steps 18, 20, 23, 26, and 29) at 20,000 g for 2 min at RT in a tabletop centrifuge. Do not remove the supernatant.
-
32
Load 1 µl of the samples from step 31 to a 14% SDS-gel. Run and stain the gel with coomassie to check the protein content and purity.
-
33
(OPTIONAL) If there is still a high amount of MNase in the flow-through, repeat steps 22–32 as often as desired.
-
34
Pool the desired fractions containing MNase.
-
35
Concentrate the protein with centrifuge filter units (cut-off 10 kDa) to approximately 14 mg/ml.
-
36
Dialyze the protein solution against 5 l of dialysis buffer MNase overnight at 4 °C. Use tubing with 6–8 kDa cutoff.
-
37
Clean the column by uploading 10 CV of 500 mM imidazole followed by 10 CV of ultrapure water.
-
38
Regenerate the column by uploading, in the order specified, 10 CV of regeneration buffer MNase, 10 CV of ultrapure water, and 1 CV of 0.1 M NiSO4. Incubate NiSO4 on the column for 10 min at RT.
-
39
Add to the column 10 CV of ultrapure water and 4 CV of 20% (vol/vol) ethanol. The column can now be stored for at least one year at 4 °C.
-
40
Measure the final protein concentration after dialysis (from step 36) by Bradford. The yield should be 350–400 mg.
-
41
Collect a sample after dialysis (from step 36) for SDS-gel analysis as in steps 17–18 and step 31. Load 1 µl to a 14% SDS-gel. Run and stain the gel with coomassie to check for the protein content and purity.
-
42
From our experience the MNase purity is sufficient after running one HisTrap-column chromatography experiment. Should a higher purity be preferred, use cation exchange chromatography (resource S column) in addition. Use dialysis buffer MNase as low salt buffer and dialysis buffer MNase with 500 mM NaCl instead of 25 mM as high salt buffer for the salt gradient.
-
43
Aliquot the protein solution to 100 or 200-µl aliquots and flash-freeze them in liquid nitrogen.
PAUSE POINT The protein can now be stored at −80 °C for at least one year.
Isolation of monosomes
For determining translatomes by RP, monosomes are most effectively isolated by either sucrose gradient or sucrose cushion centrifugation. Sucrose cushion centrifugation pellets most ribosomal particles, whereas a sucrose gradient centrifugation enables the selective purification of monosomes from subunits and polysomes. On the other hand, sucrose cushion centrifugation enables researchers to process larger amounts of cell lysates and is less demanding in terms of the instrumentation required. Following monosome enrichment, footprint fragments can be directly isolated.
For interactome analyses in SeRP experiments, we also recommend isolating monosomes first and using them as starting material for the pull-down of factor–RNCs. Although the purification of factor–RNCs can be performed using total lysate, starting with purified ribosomes has several advantages. First, the starting material for the factor–RNC purification directly resembles the total translatome. Second, ribosome isolation can eliminate excess free factor that is not associated with RNCs. Although free factors in principle do not interfere with the outcome of the RNC purification, they will compete for binding sites during affinity purification. This competition reduces the yield of purified complexes and must be compensated by scaling up the amount of affinity matrix, which increases the probability of unspecific binding. Finally, the ribosome isolation procedure can help eliminate factor–RNCs that formed during the purification procedure. Prerequisite to eliminate such interactions is a higher stability of in vivo formed complexes, for instance as a consequence of chemical crosslinking. Non-crosslinked complexes can then be stripped away, for example by performing the ribosome purification step in high salt concentrations. For the purification of TF–RNCs in SeRP, we chose to perform a sucrose cushion centrifugation with a concentration of 1 M NaCl to recover enough material as input for the purification and to reduce the amount of uncrosslinked TF.
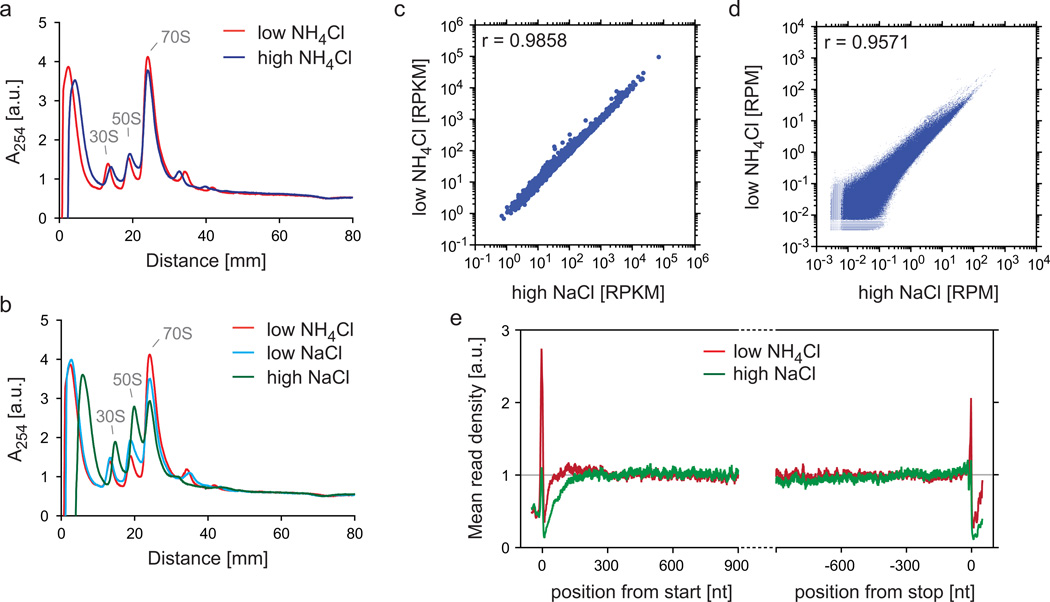
We explored the impact of different salts and salt concentrations on ribosome distribution along mRNAs by monitoring polysome profiles. The amount of NH4Cl salt in the gradient buffers (100 mM or 1 M) had a minimal impact on the polysome profiles (Fig. 8a). Similarly, a low concentration of NaCl (100 mM) resulted in only modest differences compared to a low concentration of NH4Cl (100 mM) (Fig. 8b). By contrast, elevating the NaCl concentration from 100 mM to 1 M changed the polysome profile dramatically (Fig. 8b). In the presence of 1 M NaCl the abundance of 70S ribosomes was greatly reduced and the amount of 50S subunits increased to a similar extent to the 70S ribosome reduction, which suggests that NaCl partially disassembles monosomes. This salt effect was similarly observed in sucrose cushion centrifugations with an even more pronounced effect occurring with high salt NaCl (Supplementary Fig. 3). To explore whether high NaCl concentration solely affects inactive ribosomes or translating ribosomes as well, we compared the translatome from samples prepared via sucrose cushion centrifugation containing high NaCl concentration or low NH4Cl concentration. Gene expression levels and read densities were similar between the samples (Fig. 8c,d). However, comparison of meta-gene analyses revealed a specific loss of reads close to the start and at the stop codon if sample preparation included a purification step with 1 M NaCl (Fig. 8e), which suggests that ribosomes at the beginning and end of a transcript are less salt resistant. Therefore, we recommend a careful titration of the salt concentration and propose using a high concentration of NaCl only if it is necessary for the depletion of non-crosslinked factor, as in the case of TF. In general, we recommend using the same buffer and centrifugation conditions for the purification of ribosomes for both translatome and interactome analysis.
Figure 8.
Impact of salt concentrations on the stability of ribosomes during ribosome purification. (a,b) Comparison of polysome profiles using different salt conditions. E. coli MC4100 cells grown in LB medium were harvested via the rapid harvest protocol (step 1, option C). The lysate was thawed according to step 7, option A and digested with MNase at a concentration of 15 U/A260, as described in the legend of Fig. 4a,b. Digested lysate was loaded onto sucrose gradients (step 18, option B) with different salts or salt concentrations: 100 mM NH4Cl (‘low NH4Cl’), 1 M NH4Cl (‘high NH4Cl’), 100 mM NaCl (‘low NaCl’), and 1 M NaCl (‘high NaCl’). ‘30S’ and ‘50S’ depict the peaks of the small and large ribosomal subunits, respectively. The monosome peak is labeled with ‘70S’. Polysome profiles were normalized to the area under the curves as explained in the legend of Fig. 4a,b. (c–e) Comparison of translatomes prepared under different salt conditions. Lysates were prepared and digested as in (a,b). Digested lysates were loaded onto sucrose cushions containing either 1 M NaCl (‘high NaCl’) or 100 mM NH4Cl (‘low NH4Cl’). Sequencing libraries (without rRNA depletion) were prepared and data were analyzed as described in the protocol. Gene expression levels (c), read densities in protein coding regions (d), and meta-gene analyses (e) were performed as described in in the legends to Fig. 2a, 2c, and 3a, respectively.
Factor–RNC purification
The specific purification of factor–RNCs is one of the key steps in SeRP. The purification can be carried out via immunoprecipitation (IP) or affinity purification (AP), but which approach to be used has to be determined for each factor. An IP is simpler because it does not require any tagging of the factor. Whether IP can be implemented heavily depends on the availability of an antibody that binds the folded factor stably enough to remain bound through the several washing steps necessary for the specific purification of the factor–RNCs. If the antibody recognizes the unfolded factor during its synthesis, it will also facilitate the purification of ribosomes synthesizing the factor.
The first attempts at purifying TF–RNCs we made were based on IP using a polyclonal α-TF antibody together with protein A sepharose beads (CL-4B, GE Healthcare). To specifically track the isolation of nascent or newly synthesized polypeptides, we performed radioactive labeling experiments, followed by in vivo DSP crosslinking, sucrose cushion centrifugation and IP. Using this approach, we were able to specifically pull down TF–RNCs from an E. coli MC4100 wild-type strain upon DSP crosslinking (Fig. 9a, lanes 1 and 5). We also found out that without crosslinking mainly free TF was purified (Fig. 9a, lanes 2 and 6). As expected, no background binding of ribosomes was detectable in a TF deletion strain (Δtig; Fig. 9a, lanes 3, 4, 7, and 8). However, when scaling up the IP reaction, we observed a higher background of unspecifically bound ribosomes, which could not be reduced by additional washing steps without losing TF–RNCs (data not shown). Therefore, we chose AP as preferred approach for TF–RNC purification.
Figure 9.
Evidence that translating ribosomes can be specifically pulled down with TF. (a) Autoradiograph of a Co-IP experiment with radioactively labeled nascent chains. Radioactive labeling experiments were performed as described in the legend of Supplementary Fig. 1 for sample 1, including controls of non-crosslinked samples and E. coli MC4100 ∆tig::Kan strain. After ultracentrifugation resuspended ribosomes were subjected to IP using 50 µl of a 50% protein A sepharose slurry (GE Healthcare, CL-4B) and 10 µl of a polyclonal α-TF antibody (lab collection). After incubation for 1 h at 4 °C the matrix was washed twice for 10 min each with wash buffer and once with phosphate buffered saline containing 1 mM chloramphenicol and 10 mM MgAc2. TF–RNCs were eluted by boiling in non-reducing (‘non-red.’) or reducing (‘red.’) sample buffer and separated on a 10% tricine gel. The gel was coomassie-stained and dried for autoradiography. (b) Different affinity matrices vary in their efficiency to pull down TF–RNCs. 1 l of MC4100 ∆tig::Kan + pTrc-tig or pTrc-tig-TEV-Avi cells were grown in M9 media to an OD600 of 0.45. Translation was arrested with 1 mM chloramphenicol, followed by in vivo crosslinking using 2.5 mM DSP for 30 min at 37 °C and crosslinker quenching with 20 mM Tris pH 7.5 for 5 min. Cells were harvested by centrifugation and resuspended in 6 ml of buffer B Lysis and nuclease digestion were performed as described in Supplementary Fig. 1. Ultracentrifugation was done as described in step 18, option A with 1 M potassium acetate instead of 1 M NaCl in the sucrose cushion buffer. The pellet was washed once with buffer C and resuspended in 5 ml of buffer C overnight on ice. Ribosomes were split into five aliquots and incubated with different affinity matrices for 1 h at 4 °C on an overhead roller: 120 µl of a 50% slurry of Strep-Tactin sepharose (1) or 120 µl of four different Dynabeads (Invitrogen, # 658.01D), M270 Streptavidin (2), M280 Streptavidin (3), MyOne Streptavidin C1 (4), and MyOne Streptavidin T1 (5). Beads were washed three times with buffer C. TF–RNCs were eluted by boiling in reducing sample buffer and analyzed by SDS-PAGE and western blotting using polyclonal antibodies against TF and L23.
AP requires fusing an affinity tag to the factor of interest. Provided that the functional integrity of the factor is preserved, we recommend using C-terminal tags, as they prevent the isolation of RNCs engaged in the synthesis of the factor itself. Suitable tags should be small, confer high affinity towards the matrix, and not interfere with factor function or ribosome interaction. In this respect, poly-histidine tags should be avoided, as they, in our experience, often promote or stabilize ribosome interactions. In particular, low-pH buffers can facilitate electrostatic interactions between the negatively charged ribosomal surface and the positively charged histidine tag. For TF analysis, we chose the AviTag (Avidity LCC), a sequence that is biotinylated in vivo by the endogenous biotin ligase BirA. The biotinylated AviTag binds with extremely high affinity to streptavidin, avidin (dissociation constant <10−14 M), and Strep-Tactin (IBA GmbH). This high affinity enables the use of extensive washing steps. However, depending on the amount of protein synthesized, biotinylation may not be very efficient, resulting in a subpopulation of factor that cannot bind to the affinity matrix (see ‘Troubleshooting’). Biotinylation efficiency may then be increased by supplementing the growth medium with biotin and expressing the biotin-ligase BirA from a plasmid (pBirAcm, Avidity LCC).
Using TF–RNCs, we tested different affinity matrices to evaluate their binding efficiency. We compared Strep-Tactin sepharose (IBA, Göttingen, Germany) with four different streptavidin matrices (Dynabeads, M270, M280, MyOne C1, and MyOne T1, Invitrogen, 658.01D). The amount of purified Avi-tagged TF was indistinguishable between the different matrices (Fig. 9b). However, the amount of co-purified RNCs varied extensively, with binding properties of Strep-Tactin sepharose being most suitable to pull down the high-molecular-weight fraction of the TF–RNCs. Furthermore, background binding of ribosomes derived from a control strain expressing untagged TF was lower with Strep-Tactin sepharose than with any of the streptavidin matrices. For the purification of other factor–RNCs, the most efficient beads need to be identified in a similar way.
The specificity of the AP can be increased by inserting a TEV-protease cleavage site between the factor and the AviTag. This stratagem enabled us to specifically elute TF–RNCs in a short time and under mild conditions using TEV protease (box 3 and Supplementary Fig. 4 for purification of TEV protease). The eluate is then directly used for phenol-chloroform extraction of the mRNA footprint fragments. Such highly specific two-step purification of factor–RNCs cannot be achieved by IP. If factor–RNCs are stabilized by chemical crosslinking using DSP or any other cleavable crosslinker, RNCs can be eluted from the matrix by cleaving the spacer arm of the crosslink (in case of DSP through the addition of reducing agent). Otherwise, ribosomal footprint fragments are eluted directly from the beads via phenol-chloroform extraction. Implementation of this procedure might, however, increase the background noise by co-purifying footprint fragments derived from non-specifically bound ribosomes.
Box 3.
Overproduction and purification of TEV protease with C-terminal His-tag
CRITICAL The molecular weight of TEV protease (including His6-tag) is 33.4 kDa. The pI of the protein (including His6-tag) is 8.8 (Supplementary Fig. 4).
Cell growth
-
1
Inoculate 1.5 l of TB medium containing 1.5 ml of 100 mg/ml ampicillin and 1.02 ml of 50 mg/ml chloramphenicol in a 5 l Erlenmeyer flask with a 15-ml culture of strain E. coli BL21(DE3) Star-Rosetta transformed with plasmid pTH24TEVsh42 in stationary phase (grown at 30 °C).
-
2
Grow the culture at 30 °C until an OD600 of 0.45–0.6 has been reached (~6 h).
-
3
Collect a 1-ml sample of the culture to be used as uninduced control for SDS-gel analysis.
-
4
Centrifuge the uninduced control sample at 16,000 g for 1 min at 4 °C in a tabletop centrifuge. Discard the supernatant.
-
5
Resuspend the cell pellet from step 4 in 70 µl of reducing sample buffer.
-
6
Incubate for 10 min at 95 °C in a thermomixer. Keep on ice until SDS-gel analysis (steps 11–12). The sample can be stored at −20 °C for years.
-
7
Shift the incubation temperature of the culture from step 2 to 20 °C.
-
8
Add 1 ml of 1 M IPTG to the culture to induce the expression of TEV protease.
-
9
Grow the culture for 16 h at 20 °C (culture will reach stationary phase).
-
10
Take a 100-µl sample of the culture from step 9 as induced control and proceed as in steps 4–6.
-
11
Centrifuge the uninduced and induced controls from steps 6 and 10, respectively, at 20,000 g for 2 min at RT in a tabletop centrifuge. Do not remove the supernatant.
-
12
Load 10-µl aliquots of each uninduced and induced sample (from step 11; corresponding to ~0.1*109 cells) to a 14% SDS-gel. Run and stain the gel with coomassie to check TEV protease expression.
-
13
Centrifuge the culture from step 9 in 1-l centrifuge tubes at 3620 g (4500 rpm) for 10 min at 4 °C in an F9 rotor. Discard the supernatant.
-
14
Transfer the cell pellets into one 50-ml falcon tube using a rubber scraper.
-
15
Flash-freeze the cell pellet in the falcon tube in liquid nitrogen and store at −80 °C.
PAUSE POINT The cells can be kept at −80 °C for up to 6 months.
Protein purification: Recovery of clarified cell lysate
-
16
Thaw the cells by resuspending the cell pellet from step 15 in lysis buffer TEV to 70 ml of total volume.
-
17
Disrupt 2× 35 ml of cells in a French pressure cell at 8,000 lb/in2.
-
18
Collect a 5-µl aliquot of the total cell lysate from step 17 for SDS-gel analysis. Add to the sample the same volume of reducing sample buffer.
-
19
Incubate the sample from step 18 for 10 min at 95 °C. Keep on ice until SDS-gel analysis (steps 44–45). The sample can be stored at –20 °C for years.
-
20
Centrifuge the total lysate from step 17 in SS34 tubes at 30,555 g (15,900 rpm) for 30 min at 4 °C in an F21 rotor. Recover the supernatant (clarified cell lysate).
-
21
Collect a sample of the clarified cell lysate for SDS-gel analysis as described in steps 18–19.
Streptomycin sulfate precipitation to precipitate nucleic acids
-
22
Weigh 2 g of streptomycin sulfate and dissolve it in 10 ml of lysis buffer TEV.
-
23
Stir the clarified cell lysate from step 20 in a beaker placed in ice.
-
24
Slowly add 7 ml of the streptomycin solution from step 22 to the lysate.
-
25
Stir the resulting solution for 20 min while the beaker is kept in ice.
-
26
Centrifuge the solution from step 25 in SS34 tubes at 12,000 g (10,000 rpm) for 30 min at 4 °C in an F21 rotor. Recover the supernatant (nucleic-acid free lysate).
-
27
Collect a sample of the nucleic-acid free lysate for SDS-gel analysis as described in steps 18–19.
His-tag purification
-
28
Use a Ni-IDA resin (Protino) for His-purification. Weigh 3 g of Protino in a 50-ml conical tube.
-
29
To equilibrate the Protino resin, add 30 ml of lysis buffer TEV; invert the tube to resuspend Protino; let Protino settle down for a few minutes; remove the supernatant by pipetting. Repeat this equilibration step another two times.
-
30
Add the nucleic-acid free lysate from step 26 to the Protino.
-
31
Invert the Protino plus lysate prepared in step 30 for 20 min on an overhead roller at 4 °C.
-
32
Put the tube on ice. Let the Protino settle down.
-
33
Remove the supernatant (unbound fraction) by pipetting.
-
34
Collect a sample of the unbound fraction for SDS-gel analysis as described in steps 18–19.
-
35
Add 30 ml of wash buffer TEV1 to the Protino; invert a few times to resuspend the Protino; place the tube on ice and let the Protino settle down; remove the supernatant by pipetting. Repeat this procedure once more.
-
36
Add 30 ml of wash buffer TEV2 to the Protino; invert a few times to resuspend the Protino; place the tube on ice and let the Protino settle down; remove the supernatant by pipetting.
-
37
Check that the last wash fraction does not contain any proteins anymore in a quick Bradford test: Pipet 100 µl of 1× Bradford solution in one drop onto Parafilm and add 5 µl of the wash fraction. If the color turns slightly blue, wash again with 30 ml of wash buffer TEV2 as in step 36.
-
38
Collect a sample of the last wash fraction for SDS-gel analysis as described in steps 18–19.
-
39
Add 20 ml of wash buffer TEV2 to the Protino. Invert a few times to resuspend the Protino. Pour or pipet the Protino slurry into a chromatography column. Let the buffer run through the column.
CRITICAL STEP Always stop the flow before the column runs dry.
-
40
Add 30 ml of wash buffer TEV2 to the column and let the buffer run through the column.
-
41
Add 30 ml of elution buffer TEV. Elute the protein slowly in different fractions. Check the concentration of the protein in the eluate with a quick Bradford test. Usually the first 5 ml are empty for protein and can be discarded.
-
42
Collect samples from different elution fractions for SDS-gel analysis as described in steps 18–19.
-
43
Wash the Protino with 100 ml of wash buffer TEV2.
-
44
Centrifuge the samples from steps 19, 21, 27, 34, 38, and 42 at 20,000 g for 2 min at RT in a tabletop centrifuge. Do not remove the supernatant.
-
45
Load 4 µl of each sample from step 44 to a 14% SDS-gel. Run and stain the gel with coomassie to check the protein content and purity. Pool the desired fractions.
-
46
(OPTIONAL) If there is still a high amount of TEV protease in the unbound fraction (sample collected in step 34), repeat steps 30–45.
-
47
Clean the Protino with 60 ml of 500 mM imidazole and 60 ml of ultrapure water.
-
48
Regenerate the Protino with, in the specified order, 60 ml of ultrapure water, 20 ml of 0.1 M NiSO4, 60 ml of ultrapure water, 40 ml of 20% (vol/vol) ethanol, and 100 ml ultrapure water. The Protino can be stored at 4 °C for at least one year.
-
49
Dialyze the pooled fractions from step 45 or 46 against 5 l of dialysis buffer TEV overnight at 4 °C. Use tubing with 6–8 kDa cutoff.
Gel filtration
CRITICAL Because TEV protease precipitates in buffers with a salt concentration below 200 mM, we do not recommend ion exchange chromatography but gel filtration as third purification step.
-
50
Concentrate the protein solution from step 49 with centrifuge filter units (cut-off 10 kDa) to reduce the volume to 4 ml for gel filtration.
-
51
To prepare the gel filtration column, wash the column with ultrapure water and dialysis buffer TEV.
-
52
Filter the sample. Inject the sample via the sample loop. Collect 1.5-ml fractions.
-
53
Collect 10-µl samples from every second fraction with significant absorption (e.g. fractions 45–61) for SDS-gel analysis. Treat the samples as in steps 18–19 and step 44.
-
54
Load 10-µl of the samples prepared in step 53 to a 14% SDS-gel. Run and stain the gel with coomassie to check the protein content.
-
55
Pool the fractions containing TEV protease based on the results from SDS-gel analysis.
-
56
Measure the protein concentration by Bradford.
-
57
Concentrate the protein again with centrifuge filter units to achieve a concentration of approximately 4.5 mg/ml.
-
58
Add glycerol to a final concentration of 45% (vol/vol). Aliquot to 200-µl aliquots and store at –20 °C.
PAUSE POINT The protein is stable at –20 °C for at least three months.
Preparation of a footprint fragment library
After the isolation of ribosomes, mRNA footprint fragments are extracted and converted into a deep-sequencing library (Fig. 10a). This procedure is based on the previously published RP protocol for eukaryotic cells10, so we focus on the adjustments needed for RP of prokaryotes. A detailed protocol for the preparation of a footprint fragment library is included in the Supplementary Methods.
Figure 10.
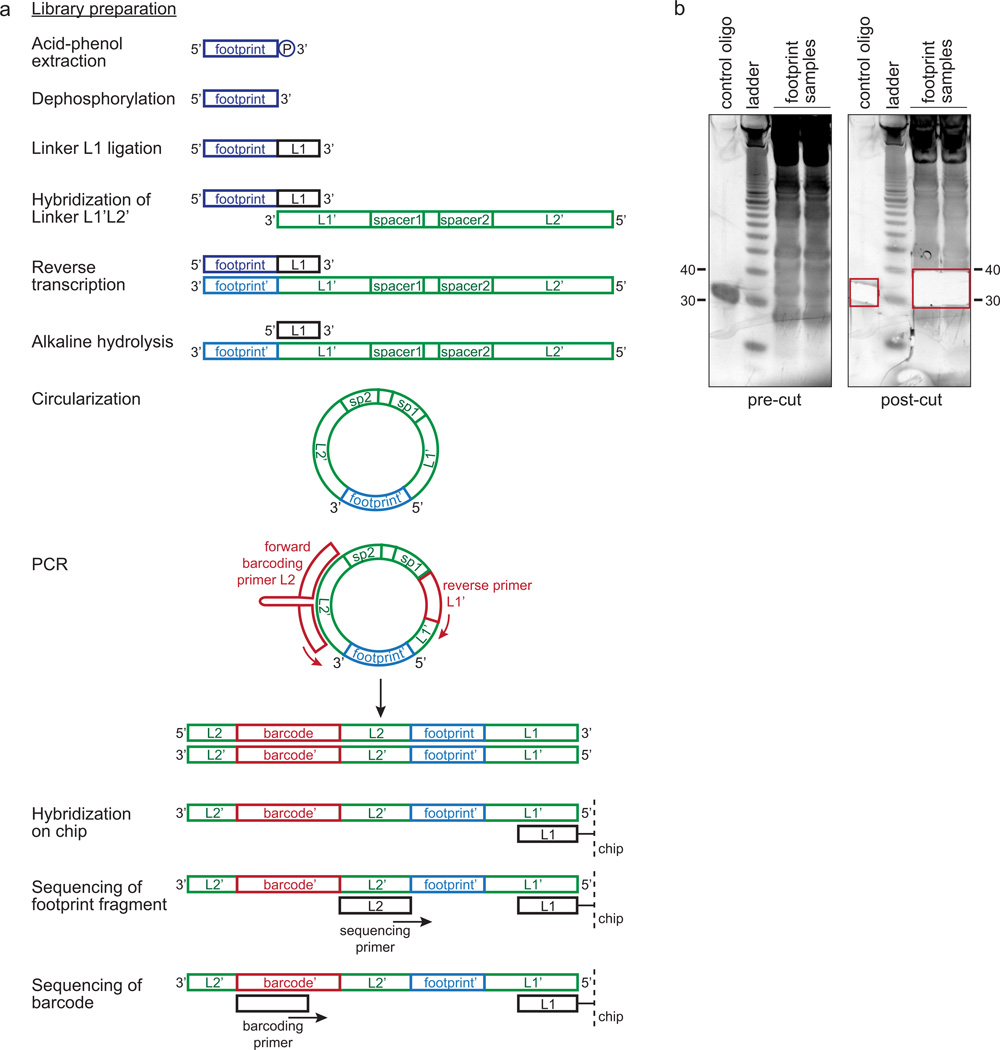
Preparation of a deep sequencing library. (a) Schematic of the library preparation protocol. See text and Supplementary Methods for details. (b) Size selection of footprint fragments. Isolated footprint fragments were loaded on a 15% TBE-urea polyacrylamide gel (steps 18 and following in the Supplementary Methods). The gel was stained with SYBR gold (pre-cut) and the region of interest (marked with the red box) was excised (post-cut). A phosphorylated RNA control oligonucleotide was included as internal control of the method.
mRNA footprint fragments are isolated either using a miRNA isolation kit (Qiagen, miRNeasy kit, 217004) as described by Ingolia et al.10 or by acid phenol extraction. The extracted RNA is then run on a denaturing polyacrylamide gel and footprint fragments are excised. Apart from the conditions of the MNase digestion, the size range that is excised from the gel is the major determinant of footprint length distribution. A nuclease protection assay using radioactively labeled mRNA showed the accumulation of ~30 nt-long footprint fragments upon increasing MNase concentration (see Supplementary Fig. 2b inset in Li et al.15). This evidence agrees well with an average footprint length of ~31 nt in our RP experiments. Therefore, we recommend excising a gel fragment that contains RNAs of 25–40 nt in size (Fig. 10b). In special cases, it can be desirable to excise a gel region containing smaller RNAs, for instance if a different nuclease is used or a more vigorous nuclease digestion is performed. However, the Gaussian distribution we obtained in our RP experiments suggest that most footprint fragments lie within the described range (Fig. 7b,c).
The isolated fragments are subsequently dephosphorylated at their 3’ end using T4 polynucleotide kinase and then run on a Bioanalyzer Small RNA Chip to measure concentration and quality (Supplementary Fig. 5a). 5’-adenylated DNA-Linker L1 is ligated to the 3’ end of the footprint fragments with truncated T4 RNA Ligase 2. This enzyme specifically ligates the adenylated 5’ end of DNA or RNA to the dephosphorylated 3’ end of RNA, that is the footprint fragment. Furthermore, the 3’ end of linker L1 is blocked with a dideoxyribose to avoid circularization of the linker and the consecutive ligation of several linker molecules. Ligation products are separated from non-ligated footprints and linker by denaturing polyacrylamide gel electrophoresis and are excised from the gel (Supplementary Fig. 5b). Linker L1’L2’ is hybridized to Linker L1, after which footprint fragments are reverse transcribed using Superscript III. The RNA pool is hydrolyzed by high pH and temperature. Reverse-transcribed products are separated from the non-ligated linker by denaturing polyacrylamide gel electrophoresis and the single-stranded DNA is excised from the gel (Supplementary Fig. 5c). Next, DNA is circularized by CircLigase. The amount of rRNA-derived DNA can be depleted with the help of biotinylated antisense oligonucleotides that are pulled out via magnetic Dynabeads (Fig. 7b,c). This step can be skipped if the samples contain only small amounts of contaminating rRNA, which depends on the amount and activity of the nuclease added. In Supplementary Table 1 is reported a list of E. coli-specific antisense oligonucleotides. The usage of the rRNA depletion method does not influence the distribution of all non-rRNA reads in ORFs (Supplementary Fig. 6). A PCR is performed to amplify the circularized, single-stranded DNA and to introduce a barcode using Phusion polymerase (see Supplementary Table 2 for PCR primers; Illumina compatible oligonucleotides are listed in Supplementary Table 3). PCR products are gel-purified (Supplementary Fig. 5d) and quantified with a Bioanalyzer High Sensitivity DNA chip (Supplementary Fig. 5e). Finally, they are sequenced with sequencing and barcoding primers.
Sequencing analysis
Sequencing reads are separated according to their barcodes. The Cutadapt algorithm27 is then used to trim linker L1 from the 3’ end of the reads. The reads are aligned to a reference sequence containing only rRNA genes, and unaligned reads, which now lack the rRNA sequences, are aligned to the genome using Bowtie28, or, if eukaryotic SeRP data are analyzed, Bowtie 229. We recommend using Bowtie on data from experiments on prokaryotic samples, as it works best for small genomes and sequencing reads with an average length of 35 nt. When analyzing data from eukaryotic samples, we recommend using Bowtie 2, because it takes into account insertions and deletions that occur during splicing. Furthermore, Bowtie 2 is more efficient for larger genomes. We generally perform all further processing steps with our self-written python scripts that are provided as Supplementary Notes (Supplementary Notes 1–14).
A critical step in the analysis (step 42 in the protocol; Supplementary Note 2) involves the use of a specific scoring system, a center-weighted strategy for counting each read. Center-weighting can better locate the position of the ribosomal A-site and P-site than a simple counting at the 5’ end of the read, because only the midpoint (center) of a footprint fragment is scored. Center-weighting also takes into account the heterogeneous read lengths after MNase digestion and size selection. To this end, every footprint receives the same score regardless of length. If the read is longer than the defined minimum length (23 nt), the position of the ribosome cannot be clearly assigned. To this end, 11 nt from either end are removed and the score of the footprint is distributed equally among the remaining nt. This scoring system was successfully used to verify well-known pause sites, like stop codons and nascent chain-mediated stalling sites15. In addition, it facilitated the detection of pausing at serine codons upon starvation and of Shine-Dalgarno-like sequences as general ribosome pausing sites15. Center-weighted scores can be used for downstream analyses, which include the calculation of gene expression levels (steps 46–49, Supplementary Notes 4–5), normalized read densities along the genome or in protein coding regions (steps 50–55, Supplementary Notes 6–11), and the average read density in a meta-gene analysis (steps 56–58, Supplementary Notes 12–13). Comparisons of interactome and translatome samples can be performed by calculating factor-enrichment efficiencies along the genome (step 59, Supplementary Note 14).
Comparison of SeRP with other approaches
SeRP can better capture cellular factor–RNC binding events in terms of both precision and scale than conventional methods to determine nascent chain interactions. In classical in vitro crosslinking experiments, the appearance of crosslinking products is used to explore interactions of factors with selected nascent chains. The use of stalled ribosomes exposing nascent chains of defined lengths30 enables researchers to determine the minimal length of the nascent chain required for factor binding. However, such in vitro experiments may not accurately reflect the dynamics of interactions occurring in the cell. For example, results from studies using stalled nascent chains have suggested that TF and SRP can coexist on ribosomes and compete for nascent substrates31,32. SeRP experiments instead revealed that TF probably binds only after SRP has been released from translating ribosomes11,33.
Other attempts to identify in vivo substrates of factors interacting with nascent chains (in particular chaperones) have relied on co-purifications and/or analyses of protein aggregates formed in deletion mutants24,34–37. Identification of such co-purification products and aggregates through state-of-the-art mass spectrometry instrumentation has been highly informative, yielding the identities of hundreds of substrates. These approaches, however, cannot differentiate between contacts made during translation and those made after it (i.e., cotranslational versus posttranslational substrates) and they do not provide any information on the coordination of interactions with the translation process (i.e., at what length nascent chains are contacted).
Two studies were recently performed in yeast to identify the cotranslational substrates of the ribosome-associated factors NAC, SSB, and SRP38,39. In these studies, complexes of factors associated with translating ribosomes were purified. Using DNA microarrays, co-purified, full-length mRNAs were used to identify the nascent substrates of these factors. Though these studies provided insight into the cotranslational interactomes of three nascent chain-associated proteins, they could not determine the nascent chain length requirements for the interactions to occur.
New applications of SeRP
Although crosslinking and purification efficiencies may vary for every factor that is analyzed, SeRP should be readily adaptable to the investigation of other cotranslationally-acting factors, both nascent chain–associated and ribosome-associated. These factors, in principle, include all nascent chain–interacting chaperones, membrane targeting factors, processing enzymes, and proteases. By comparing individual substrate specificities, and, more importantly, by determining the time frames of individual binding and release events, it should be possible to unravel the order of binding of various cotranslationally acting proteins and to determine how they affect each other’s function. Forcing cells to grow in suboptimal conditions (e.g. via chromosomal deletions or stress) can report on cellular plasticity of protein biogenesis, including how substrate pools change and binding specificities of cotranslationally acting proteins are adjusted to cellular needs.
Limitations
The main challenge of SeRP is to stabilize and purify complexes of the factor of interest with ribosomes and nascent chains without introducing a bias. Biases may be introduced by protein tags that affect functionality, by chemical crosslinking due to amino acid specificity of most crosslinkers, and by long purification protocols that facilitate either loss or establishment of new interactions.
An important question when studying the interactions of factors with ribosomes and nascent chains is whether the detection of an interaction with a ribosome in all cases reflects an interaction also with the nascent chain. This question is of particular relevance for factors that are present in approximately equimolar concentration to ribosomes. In these cases, factors could be bound to ribosomes irrespective of specific nascent chain interaction. Such limitation of SeRP can be overcome by developing selective crosslinking or purification conditions that differentiate between these types of binding events and select complexes involving nascent chain interactions (e.g., high-salt washing). In the case of TF, the two crosslinkers DSP and EDC enabled the stabilization of only nascent chain interactions (DSP) or of both ribosome and nascent chain interactions (EDC)11. A lack of reads at the beginning of protein coding regions (until the ribosome has translated an N-terminal fragment of the nascent chain that can be recognized by the factor) can be inferred as a reasonable indication of specific factor recruitment (Fig. 3b, 6c).
Another limitation of the present protocol might be low yield of purified complexes. This low yield can be due to very weak and transient interactions, low abundance of the factor, or a limited substrate pool. Transient interactions might be stabilized by low temperatures, by chemical crosslinking, or, if the interactions are controlled by nucleotide binding, by quick hydrolysis of the nucleotide pool24 (see ‘Troubleshooting’).
Finally, the purification procedure itself might be a limitation of the protocol. IP has the advantage that the endogenous protein present in the wild type is analyzed. However, this procedure is often associated with limited purity and quantity of the isolated complexes. Small tag sequences facilitating high-affinity purification procedures may, therefore, be the better alternative, as long as the tag does not interfere with function or ribosome interaction.
MATERIALS
REAGENTS
Bacto Tryptone (BD, 211699)
Bacto Yeast Extract (BD, 212720)
NaCl (Roth, 9265)
MOPS EZ Rich Defined Medium Kit (Teknova, M2105)
Na2HPO4*2H2O (Applichem, A3567)
KH2PO4 (Applichem, 1043)
NH4Cl (Roth, 298)
MgSO4 (Roth, P027)
CaCl2 (Roth, T885)
Thiamine (Sigma, T4625)
Glucose (Roth, 6780)
D(+)-Biotin (Roth, 3322)
NaOH (Roth, P031) CAUTION Sodium hydroxide is corrosive. Wear gloves and eye protection.
Chloramphenicol (Sigma, C0378)
Absolute ethanol p.A. (AppliChem A1613) CAUTION Ethanol is flammable. Keep away from sources of ignition.
Dithiobis [succinimidyl propionate] (DSP, Thermo Scientific, 22858)
Dimethylsulfoxide (DMSO, Sigma, 154938)
Tris (Roth, 4855)
Diethylpyrocarbonate (DEPC, Roth, K028) CAUTION DEPC is carcinogenic. Handle with care. Wear gloves.
KOH (Roth, 6751) CAUTION Potassium hydroxide is corrosive. Wear gloves and eye protection.
HEPES (AppliChem, A1069)
MgCl2 (Roth, A537)
Isopropanol (2-propanol) (Sigma, 33539) CAUTION Isopropanol is flammable. Keep away from sources of ignition.
Phenylmethylsulfonylfluoride (PMSF, Roth, 6367)
Triton X100 (Roth, 3051)
Nonidet P 40 Substitute (NP-40, Sigma, 74385)
RNase-free DNase I (Roche, 04716728001)
HCl 37% (wt/wt) (VWR, 20252.335) CAUTION Hydrochloric acid is corrosive. Wear gloves and eye protection.
1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC, Thermo Scientific, 22980)
Glycine (Roth, 3790)
NaHCO3 (Roth, 6885)
Superase•In RNase inhibitor (Ambion, AM2696)
Micrococcal nuclease (Nuclease S7) (Roche, 10107921001 or box 2)
Ethylene glycol tetraacetic acid (EGTA, AppliChem, A0878)
Sucrose (Sigma, 16104)
Strep-Tactin Sepharose 50% suspension (IBA, 2-1201-025)
DNA, sodium salt from salmon testes (Sigma-Aldrich, D1626)
Kanamycin (Applichem, A1493)
Isopropyl β-D-1-thiogalactopyranoside (IPTG, Roth, CN08)
Colloidal coomassie staining solution (Roth, Roti-Blue quick, 4829)
Imidazole (Roth, 3899) CAUTION Imidazole is corrosive. Wear gloves and eye protection.
Glycerol (Applichem, A3561)
Leupeptin (Boehringer, 1017128)
Aprotinin (Gerbu, 1302)
Pepstatin A (Applichem, A2205)
Methanol (Sigma, 32213) CAUTION Methanol is toxic and flammable. Handle with care. Wear gloves. Keep away from sources of ignition.
Ethylenediaminetetraacetic acid (EDTA, di-sodium-di-hydrate, Roth, 8043)
SDS (Roth, CN30) CAUTION SDS is carcinogenic. Handle with care. Wear gloves.
β-mercaptoethanol (Roth, 4227) CAUTION β-mercaptoethanol is toxic. Handle with care. Wear gloves.
Bromophenole blue (Roth, A512)
Bradford (Bio-Rad, Protein assay, 500-0006) CAUTION Bradford is toxic. Handle with care. Wear gloves.
NiSO4*6H2O (Roth, T111) CAUTION NiSO4 is toxic. Handle with care. Wear gloves.
Ampicillin (Roth, K029)
Terrific broth (TB) powder (Roth, X972)
Streptomycin sulfate (Sigma, S6501)
EQUIPMENT
UV/Vis Spectro-photometer (GE Life Sciences, Novaspec Plus, 80-2117-50)
Ice cube trays (Fisher&Paykel, 874107)
Centrifuge (Sorvall/Thermo Scientific, RC-6 Plus Superspeed, 46915); rotor F9 (Thermo Scientific, Fiberlite F9-4x1000y, 76981); centrifuge tubes 1 l (Nalgene, polypropylene, 3141-1006)
Conical tubes, 15 ml (Sarstedt, 62.554.512)
Conical tubes, 50 ml (Sarstedt, 62.547.254)
Centrifuge for conical tubes (Heraeus / Thermo Scientific, Multifuge 3SR Plus, 75004371)
Serological pipette, 10 ml (Greiner Bio-One, Cellstar, 607107)
Syringe, 50 ml (BD, 300865)
Filtering equipment: glass filter holder with glass funnel (1 l), vacuum base and cap, stainless steel screen, gasket and spring clamp (Millipore, 90 mm, XX1009020); ground joint flask 1 l (Millipore, XX1504705)
Scoopula (Fisher Scientific, 14-357Q)
Nitrocellulose membrane, 0.2 µm (Roth, Roti-NC, HP40.1)
Needle 0.9 × 40 mm (Braun, Sterican, 4657519)
Mixer mill (Retsch, MM400, 20.745.0001)
Stainless steel jars (Retsch, 10 ml, 01.462.0236, and 50 ml, 01.462.0216)
Stainless steel grinding balls (Retsch, 12 mm, 05.368.0037, and 25 mm, 05.368.0105)
Magnetic stirrer (Heidolph MR Hei-Mix L, P/N 505-00000-00)
Magnetic stir bar (Roth, PK74.1)
Reaction tubes, 1.5 ml (Sarstedt, 72.690.001)
Tabletop centrifuge, refrigerated (Eppendorf, 5417R, 5407 000.317)
Nanodrop spectrophotometer (Thermo Scientific, NanoDrop2000, ND2000)
Water bath, polycarbonate (Lauda, 006 T, LCZ 0628), with thermostat (Lauda, A 100, LCE 0225)
Ultracentrifuge (Sorvall WX90, 46901); rotor Type 45 Ti (Beckman, 339160); polycarbonate bottles 70 ml, 38 × 102 mm (Beckman, 355655); rotor Type SW 40 Ti (Beckman, 331302); Open-top polyclear tubes (Seton, 7031)
Syringe filters, 0.22 µm (Sarstedt, 83.1826.001)
Gradient station (BIO-COMP, 153)
Overhead roller (Neolab, Intelli-Mixer, 7-0045)
Thermomixer (Eppendorf, Thermomixer comfort, 5355 000.011)
Tabletop centrifuge (Eppendorf, Centrifuge 5424, 5424 000.614)
Vortex Genie 2 (Scientific Industries, SI-0256)
Cutadapt program (http://code.google.com/p/cutadapt/)
Bowtie program (http://bowtie-bio.sourceforge.net/index.shtml)
Python (http://www.python.org/download/)
UV-Star Microplate µClear (Greiner Bio-One, 655801)
Multi-channel pipette, 20–200 µl (Gilson, Pipetman, F14404)
SpectraMax M2 (Molecular Devices)
Incubator (Kuhner, Climo-Shaker ISF1-X)
French Pressure Cell Press (SIM-AMINCO)
Rotor F21 (Piramoon Technologies/Thermo Scientific, F21-8x50, 46923)
Centrifuge tubes (Nalgene, oak ridge polypropylene, 3119-0050)
Rubber Scraper (Durawear, 5922)
HisTrap FF crude columns, 5 ml column volume (GE, 17-5286-01)
Laboratory film (Parafilm, PM-996)
Centrifuge filter units (Sartorius, Vivaspin6, cutoff 10 kDa, VS2001)
Dialysis tubing (Fisherbrand, 6–8 kDa cutoff, 21-152-3)
Protino resin (Macherey-Nagel, Ni-IDA, 745210)
Chromatography column (Bio-Rad, Econo-Pac polypropylene columns, 732-1010EDU)
ÄKTApurifier (GE Healthcare); gel filtration column (GE Healthcare, HiLoad 16/60, Superdex 200, separates proteins between 10 and 600 kDa, dead volume ~24 ml, 17106901)
REAGENT SETUP
CRITICAL Dissolve all chemicals in ultrapure water in the indicated concentrations, unless noted otherwise. Adjust the pH if necessary.
NaCl 5 M. Autoclave or sterile filter. Store at room temperature (RT), that is 22 °C. The solution is stable for years.
MgSO4 1 M. Autoclave or sterile filter. Store at RT. The solution is stable for years.
CaCl2 1 M. Autoclave or sterile filter. Store at RT. The solution is stable for years.
Glucose 20% (wt/vol). Autoclave or sterile filter. Store at RT. The solution is stable for at least six months.
NaOH 10 N. Store at RT. The solution is stable for years.
KOH 10 N. Store at RT. The solution is stable for years.
HEPES 1 M, adjust pH to 7.0 with KOH. Sterile filter. Store at RT. The solution is stable for at least six months.
MgCl2 2 M. Autoclave or sterile filter. Store at RT. The solution is stable for years.
Absolute ethanol 70% (vol/vol) and 20% (vol/vol). Store at RT. The solution is stable for at least one year.
Triton X100 20% (vol/vol). Sterile filter. Store at RT. The solution is stable for at least three months.
NP-40 10% (vol/vol). Sterile filter. Store at RT. The solution is stable for at least three months.
Tris 2 M, adjust pH to 7.0, 7.5 and 8.0 with HCl. Autoclave or sterile filter. Store at RT. The solution is stable for at least one year.
NaHCO3 1 M. Sterile filter. Store at RT. The solution is stable for years.
Glycine 2 M, adjust pH to 8.0 with NaOH. Sterile filter. Store at −20 °C. The solution is stable for at least one year.
EGTA 0.5 M, adjust pH to 8.0 with NaOH. Autoclave or sterile filter. Store at RT. The solution is stable for years.
NH4Cl 2 M. Autoclave or sterile filter. Store at RT. The solution is stable for years.
RNase-free water. Add 1 ml of DEPC to 1 l of ultrapure water, shake vigorously for 1 min, incubate overnight under the hood with open lid, autoclave the next day to inactivate DEPC. Store at RT. The solution is stable for years.
LB medium. For 1 l weigh 10 g of NaCl, 5 g of Bacto Yeast Extract, and 10 g of Bacto Tryptone. Dissolve in 1 l of deionized water. Autoclave. Store at RT. Medium is stable for at least three months.
Rich defined medium. Prepare according to manufacturer’s instructions.
M9 minimal medium. For 1 l weigh 7.6 g of Na2HPO4*2H2O, 3 g of KH2PO4, 0.5 g of NaCl, 1 g of NH4Cl, and 10 mg of thiamine. Dissolve in 900 ml of deionized water. Add 1 mM MgSO4, 0.1 mM CaCl2 and adjust the volume to 980 ml with deionized water. Autoclave. Store at RT. Medium is stable for at least three months. Add 20 ml of 20% (wt/vol) glucose before usage.
Biotin 40 mg/ml. Weigh 400 mg of biotin and dissolve in 400 µl of 10 N NaOH. Slowly add 4 ml of 1 M HEPES 7.0 and adjust volume with ultrapure water to 10 ml. Sterile filter. Store at 4 °C. The solution is stable for at least one year.
Chloramphenicol 50 mg/ml. Weigh 1 g of chloramphenicol, dissolve in 20 ml of 70% (vol/vol) ethanol. Store at −20 °C. The solution is stable for at least three months.
Chloramphenicol-ice. For 1 l add 2 ml of 50 mg/ml chloramphenicol to 1 l of ultrapure water. Fill into the ice cube trays. Store at −20 °C. The ice is stable for at least three months.
DSP 250 mM. Dissolve 101 mg of DSP in 1 ml of DSMO. Prepare fresh for every experiment.
PMSF 100 mM. Dissolve 174 mg of PMSF in 10 ml of isopropanol. Store at −20 °C. The solution is stable for at least six months.
Lysis buffer. 50 mM HEPES pH 7.0, 100 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 1 mM chloramphenicol, 1 mM PMSF, 0.4% (vol/vol) Triton X100, 0.1% NP-40 (vol/vol) in RNase-free water. Always prepare fresh and keep on ice until use.
HEPES 0.5 M pH 4.5. Dilute 1 M HEPES pH 7.0 to 0.5 M and adjust pH to 4.5 with HCl. Sterile filter. Store at RT. The solution is stable for at least six months.
Lysis buffer EDC. Lysis buffer with 50 mM HEPES pH 4.5 instead of pH 7.0. Always prepare fresh and keep on ice until use.
EDC 1 M. Equilibrate the EDC container to RT before opening it. Dissolve 192 mg of EDC in 1 ml of Lysis buffer EDC. Prepare fresh for every experiment.
Micrococcal nuclease. Dissolve 1 vial of lyophilized powder in 40 µl of 10 mM Tris pH 8.0. Alternatively, purify the protein according to box 2. Perform an MNase activity assay to determine the exact activity (box 1). Store at −80 °C. The enzyme is stable for up to one year.
2× lysis buffer without calcium. 100 mM HEPES pH 7.0, 200 mM NaCl, 20 mM MgCl2, 2 mM chloramphenicol, 2 mM PMSF, 0.8% (vol/vol) Triton X100, 0.2% (vol/vol) NP-40 in RNase-free water. Always prepare fresh and keep on ice until use.
Sucrose cushion buffer. 50 mM Tris pH 7.0, 1 M NaCl, 10 mM MgCl2, 1 mM chloramphenicol, 1 mM PMSF, 25% (wt/vol) sucrose in RNase-free water. Always prepare fresh and keep on ice until use.
Wash buffer. 50 mM Tris pH 7.0, 200 mM NaCl, 11 mM MgCl2, 1 mM chloramphenicol, 1 mM PMSF, 1 mM EGTA, 0.4% (vol/vol) Triton X100, 0.1% (vol/vol) NP-40 in RNase-free water. Always prepare fresh and keep on ice until use.
10% sucrose gradient buffer. 20 mM Tris pH 8.0, 10 mM MgCl2, 100 mM NH4Cl, 1 mM chloramphenicol, 10% (wt/vol) sucrose in RNase-free water. Filter before use. Always prepare fresh and keep on ice until use.
50% sucrose gradient buffer. 20 mM Tris pH 8.0, 10 mM MgCl2, 100 mM NH4Cl, 1 mM chloramphenicol, 50% (wt/vol) sucrose in RNase-free water. Filter before use. Always prepare fresh and keep on ice until use.
Cleavage buffer. 50 mM Tris pH 7.0, 200 mM NaCl, 10 mM MgCl2, 1 mM chloramphenicol in RNase-free water. Always prepare fresh and keep on ice until use.
TEV protease. Purify the protein according to box 3.
DNA (from salmon testes) 2 mg/ml. Store at −20 °C. The solution is stable for years.
Kanamycin 40 mg/ml. Sterile filter. Store at −20 °C. The solution is stable for at least one year.
IPTG 1 M. Sterile filter. Store at −20 °C. The solution is stable for at least one year.
Leupeptin 5 mg/ml. Store at −80 °C. The solution is stable for at least three months.
Aprotinin 10 mg/ml. Store at −80 °C. The solution is stable for at least three months.
Pepstatin A 1 mg/ml. Weigh 10 mg of pepstatin A, dissolve in 10 ml of methanol. Store at −20 °C. The solution is stable for at least three months.
Imidazole 1 M, adjust pH to 7.5 with NaOH. Sterile filter. Store at RT in the dark. The solution is stable for at least three months.
EDTA 0.5 M, adjust pH to 8.0 with NaOH. Autoclave or sterile filter. Store at RT. The solution is stable for years.
NiSO4 0.1 M. Sterile filter. Store at RT. The solution is stable for at least one year.
SDS 20%. Weigh 20 g of SDS, dissolve in 100 ml of hot (approximately 70 °C) ultrapure water. Autoclave. Store at RT. The solution is stable for years.
2× reducing sample buffer. 250 mM Tris pH 7.0, 6% (wt/vol) SDS, 6% (vol/vol) β-mercaptoethanol, 20% (vol/vol) glycerol, 0.02% (wt/vol) bromophenole blue. Store at 4 °C. The solution is stable for at least one year.
2× non-reducing sample buffer. 250 mM Tris pH 7.0, 6% (wt/vol) SDS, 20% (vol/vol) glycerol, 0.02% (wt/vol) bromophenole blue. Store at 4 °C. The solution is stable for years.
Lysis buffer MNase. 50 mM Tris pH 7.5, 25 mM NaCl, 1 mM CaCl2, 5 mM imidazole pH 7.5, 1 mM PMSF, 5% (vol/vol) glycerol, 5 µg/ml leupeptin, 10 µg/ml aprotinin, 1 µg/ml pepstatin A in ultrapure water. Always prepare fresh and keep on ice until use.
Wash buffer MNase. 50 mM Tris pH 7.5, 250 mM NaCl, 10 mM imidazole pH 7.5, 5% (vol/vol) glycerol in ultrapure water. Always prepare fresh and keep on ice until use.
Elution buffer MNase. 50 mM Tris pH 7.5, 25 mM NaCl, 250 mM imidazole pH 7.5, 5% (vol/vol) glycerol in ultrapure water. Always prepare fresh and keep on ice until use.
Regeneration buffer MNase. 20 mM HEPES pH 7.5, 500 mM NaCl, 50 mM EDTA pH 8.0 in ultrapure water. Place on ice before usage. The buffer is stable for at least six months.
Dialysis buffer MNase. 50 mM Tris pH 7.5, 25 mM NaCl, 1 mM EDTA pH 8.0, 5% (vol/vol) glycerol in ultrapure water. Always prepare fresh and keep on ice until use.
TB medium. Weigh 50.8 g of TB, dissolve in 900 ml of deionized water; add 0.8% (vol/vol) glycerol, add 2 mM MgCl2. Adjust volume to 1 l with deionized water; autoclave; store at RT. Medium is stable for at least three months.
Ampicillin 100 mg/ml. Sterile filter. Store at −20 °C. The solution is stable for at least one year.
Lysis buffer TEV. 50 mM Tris pH 7.0, 400 mM NaCl, 2 mM β-mercaptoethanol, 2.5 µg/ml RNase-free DNase I. Always prepare fresh and keep on ice until use.
Wash buffer TEV1. 50 mM Tris pH 7.0, 400 mM NaCl, 2 mM β-mercaptoethanol. Always prepare fresh and keep on ice until use.
Wash buffer TEV2. 50 mM Tris pH 7.0, 600 mM NaCl, 2 mM β-mercaptoethanol. Always prepare fresh and keep on ice until use.
Elution buffer TEV. 50 mM Tris pH 7.0, 400 mM NaCl, 500 mM imidazole pH 7.5. Always prepare fresh and keep on ice until use.
PROCEDURE
CRITICAL This protocol describes SeRP for a TEV-AviTagged factor (exemplified by TF-TEV-Avi) as outlined in Fig. 1. Different options in the protocol can be selected depending on the specific requirements of the experiment. The alternative approach of an immunoprecipitation is described in the legend of Fig. 9a. Alternative matrices for affinity purification are mentioned in the Introduction and in the legend to Fig. 9b.
CRITICAL For general ribosome profiling in bacteria, crosslinking and factor–RNC complex purification are not required. In this case, furthermore, a culture volume of 200 ml per sample is sufficient.
Cell growth and harvest
-
1
Harvest cells according to option A (conventional harvest), if cells cannot be filtered, option B (conventional harvest including in vivo crosslinking) for in vivo crosslinking of cells, or option C (rapid harvest) to avoid the pretreatment with translation inhibitors (see Experimental design for further details).
A. Conventional harvest TIMING 3.5 h
-
Inoculate 500 ml of medium (LB, rich defined, or M9 minimal medium; see Experimental design for details) per sample with a fresh overnight culture to an optical density at 600 nm (OD600) of 0.01. Add 500 µl of 40 mg/ml biotin to the medium for an AviTagged factor in SeRP.
CRITICAL We recommend preparing a non-crosslinked sample in parallel to the crosslinking sample as a control.
-
Grow the culture at 37 °C to an OD600 of 0.45 with 120 rpm shaking.
CRITICAL STEP For E. coli cells the growth temperature can be varied from about 16 to 45 °C according to the specific scientific question addressed in this experiment.
Add 1 ml of 50 mg/ml chloramphenicol. Shake for 1 min.
Fill 500 ml of chloramphenicol-ice into a 1-l centrifuge tube. Add the cell culture to the tube.
Centrifuge the tube at 3620 g (4500 rpm) for 10 min at 4 °C. Discard the supernatant.
Resuspend the cell pellet on ice in 5 ml of lysis buffer. Transfer the suspension to a 15-ml conical tube.
Centrifuge at 3620 g (4500 rpm) for 5 min at 4 °C. Discard the supernatant.
Resuspend the cell pellet on ice in 3 ml of lysis buffer. Add 30 µl of 10 U/µl RNase-free DNase I.
Lodge a 10-ml serological pipette in an empty 30-ml syringe for easier dripping. Drip resuspension into liquid nitrogen. CAUTION Always wear gloves and eye protection when working with liquid nitrogen.
-
Collect the frozen drops of cells in a 50-ml conical tube and store them at −80 °C.
PAUSE POINT The cells can be kept at −80 °C for up to 6 months.
B. In vivo crosslinking and conventional harvest TIMING 3.5 h
-
Inoculate 500 ml M9 minimal medium lacking amino acids per sample with a fresh overnight culture grown in the same medium to an OD600 of 0.01. Add 500 µl of 40 mg/ml biotin to the medium for an AviTagged factor in SeRP.
CRITICAL STEP We recommend preparing a non-crosslinked sample in parallel to the crosslinking sample as a control.
-
Grow the culture at 37 °C to an OD600 of 0.45 with 120 rpm shaking.
CRITICAL For E. coli cells the growth temperature can be varied from about 16 to 45 °C according to the specific scientific question addressed in this experiment.
Add 1 ml of 50 mg/ml chloramphenicol. Shake for 5 s.
Add 5 ml of 250 mM DSP. Shake for 30 s.
Add 12.5 ml of 2 M Tris pH 7.0 to quench the crosslinking reaction. Shake for 30 s.
Fill 500 ml of chloramphenicol-ice into a 1-l centrifuge tube. Add the cell culture to the tube.
Centrifuge the tube at 3620 g (4500 rpm) for 10 min at 4 °C. Discard the supernatant.
Resuspend the cell pellet on ice in 5 ml of lysis buffer. Transfer the suspension to a 15-ml conical tube.
Centrifuge at 3620 g (4500 rpm) for 5 min at 4 °C. Discard the supernatant.
Resuspend the cell pellet on ice in 3 ml of lysis buffer. Add 30 µl of 10 U/µl RNase-free DNase I.
Lodge a 10-ml serological pipette in an empty 30-ml syringe for easier dripping. Drip resuspension into liquid nitrogen. CAUTION Always wear gloves and eye protection when working with liquid nitrogen.
-
Collect the frozen drops of cells in a 50-ml conical tube and store them at −80 °C.
PAUSE POINT The cells can be kept at –80 °C for up to 6 months.
C. Rapid harvest TIMING 4 h
-
Inoculate 5× 200 ml medium (LB, rich defined, or M9 minimal medium; see Experimental design for details) per sample with a fresh overnight culture to an OD600 of 0.01. Add 500 µl of 40 mg/ml biotin to the medium for an AviTagged factor in SeRP.
CRITICAL We recommend preparing a non-crosslinked sample in parallel to the crosslinking sample as a control.
-
Grow the culture at 37 °C to an OD600 of 0.45 with 120 rpm shaking.
CRITICAL STEP For E. coli cells the growth temperature can be varied from about 16 to 45 °C according to the specific scientific question addressed in this experiment.
Pre-warm the filtering equipment, scoopula and nitrocellulose membrane to the growth temperature.
-
Filter the cells from one flask at a time. Rapidly scrape the cells off the membrane with the scoopula. Place the entire scoopula in liquid nitrogen.
TROUBLESHOOTING
-
Dislodge the frozen cells from the scoopula and collect them separately for every culture in a 50-ml conical tube filled with liquid nitrogen. Pierce the lid with a needle and invert to spray out the remaining liquid nitrogen.
CAUTION Always wear gloves and eye protection when working with liquid nitrogen.
-
Drip 650 μl of lysis buffer containing 6.5 µl of 10 U/µl RNase-free DNase I into liquid nitrogen. Collect the frozen drops of buffer and add them to the frozen cell pellets. Keep at −80 °C.
PAUSE POINT The cells can be kept at −80 °C for up to 6 months.
Cell lysis TIMING 1–5 h
-
2
Once the cells have been harvested and frozen, perform lysis in a mixer mill.
-
3
Chill jars (50-ml jars if lysing cells harvested via conventional harvest — with or without in vivo crosslinking — or 10-ml jars for cells harvested by rapid harvest) and grinding balls (25-mm grinding balls if lysing cells harvested via conventional harvest — with or without in vivo crosslinking — or 12-mm grinding balls jars for cells harvested by rapid harvest) in liquid nitrogen.
-
4
Add frozen cells to the jar. Please note that in the case of cells harvested by rapid harvest, cells from the five cultures should be lysed separately.
-
5
Mixer mill five times at 15 Hz for 3 min each time. Chill jars in liquid nitrogen in between.
-
6
Scrape out pulverized cells into a 50-ml conical tube. Please note that in the case of cells harvested via rapid harvest, the cell powder from the five cultures should be combined in one 50-ml conical tube. Store at −80 °C.
CRITICAL STEP Make sure the lysate stays frozen all the time.
PAUSE POINT The lysate can be stored at –80 °C for up to four weeks.
Thawing of the lysate
-
7
Thaw the frozen cell powder according to option A, in the absence of crosslinker, e.g. for general RP or if cells were already crosslinked in vivo (step 1, option B) or option B, if thawing the cell powder in the presence of crosslinker for ex vivo crosslinking (see Experimental design for further details).
A. Thawing in the absence of crosslinker TIMING 15 min
Add 3.5 ml of lysis buffer to the tube containing the frozen cell powder from step 6 at room temperature (RT). Incubate at RT for 10 min. Invert from time to time.
Add 400 µl of 2 M Tris pH 8.0. Incubate at RT for 5 min. Invert from time to time.
B. Thawing for ex vivo crosslinking (mutually exclusive with step 1, option B) TIMING 25 min
Use either DSP or EDC to crosslink the factor to RNCs (see Experimental design for a discussion on these two crosslinking agents). To this end, pipet 3.5 ml of lysis buffer (for DSP crosslinking) or 3.4 ml of lysis buffer EDC (for EDC crosslinking) into a glass beaker on a magnetic stirrer at RT.
Add 37.5 µl of 250 mM DSP or 70 µl of 1 M EDC.
Slowly add about half of the frozen cell powder (i.e. half of the lysate of 500 ml cell culture of conventionally harvested cells or half of 5× 200 ml cell culture of rapidly harvested cells) from step 6 in batches to the beaker. Make sure each batch completely thaws before more is added.
Add again 37.5 µl of 250 mM DSP or 70 µl of 1 M EDC and the second half of the frozen cell powder. This addition will in total take 7 to 10 min. The final volume will be around 7.5 ml resulting in an end concentration of 2.5 mM DSP or 20 mM EDC.
Stir for 5 min at RT.
Quench the crosslinking reaction with 400 µl of 2 M Tris pH 8.0 (for DSP crosslinking) or 1.2 ml of 2 M glycine pH 8.0, 450 µl of 2 M Tris pH 8.0 and 35 µl of 1 M NaHCO3 (for EDC crosslinking). To account for the larger volume in EDC crosslinking, add also 17 µl of 1 M MgCl2 and 9 µl 1 M CaCl2.
Stir for 5 min at RT. Transfer the crosslinked lysate to a 15-ml conical tube.
Nuclease acid digestion TIMING 2 h
-
8
Incubate the lysate on ice for 10 min. In the meanwhile transfer it to 1.5-ml tubes.
-
9
Centrifuge the tubes at 20,000 g for 10 min at 4 °C in a tabletop centrifuge. Combine the supernatants again in a 15-ml conical tube placed on ice.
TROUBLESHOOTING
-
10
Dilute 1 µl of lysate with 99 µl of H2O. Measure A260 by Nanodrop to determine the nucleic acid concentration. Compare absorbance to a blank containing a 1:100 dilution of lysis buffer, because chloramphenicol shows significant absorption at 260 nm.
TROUBLESHOOTING
-
11
Calculate the concentration of clarified lysate (the supernatant from step 9) considering that 1 A260 (blank-corrected, multiplied by dilution factor) unit corresponds to a nucleic acid concentration of 40 µg/ml.
-
12
(OPTIONAL) We recommend to set aside a 1-mg sample of the clarified lysate as undigested control and run in a sucrose gradient centrifugation to check the quality of the polysomes (see step 18, option B).
TROUBLESHOOTING
-
13
Transfer a volume of the lysate equivalent to 18 mg of nucleic acids to a new 15-ml conical tube kept on ice. Dilute with lysis buffer to 7.5 ml.
-
14
Add 40 µl of Superase•In RNase inhibitor.
-
15
Add 1000 U of MNase per mg of nucleic acids (equivalent to 40 U for 1 A260).
-
16
Incubate for 1 h at 25 °C in a water bath. Invert the tube from time to time.
-
17
Quench the reaction with 90 µl of 0.5 M EGTA pH 8.0 to inactivate MNase and transfer the tube to ice immediately.
Monosome isolation
-
18
Isolate ribosomes via option A, if large quantities of ribosomes are required for analysis, or via option B, if only monosomes must be isolated (see Experimental design for further details).
A. Sucrose cushion ultracentrifugation TIMING 5.5 h plus overnight resuspension of ribosomes
Adjust the volume of the digested lysate to 15 ml and the NaCl-concentration to 1 M (‘high salt’): Add 3.75 ml 2× lysis buffer without calcium and 1.05 ml RNase-free water, then slowly add 2.7 ml of 5 M NaCl under constant mixing.
-
Fill a 60-ml polycarbonate ultracentrifuge tube with 45 ml of sucrose cushion buffer. Carefully add on the surface of the buffer solution the cell lysate.
CRITICAL STEP Make sure not to mix the lysate with the sucrose cushion buffer to ensure proper separation of molecules according to their sedimentation behavior.
Pellet ribosomal particles by centrifugation at 235,000 g (45,000 rpm) for 4.5 h at 4 °C in a 45 Ti rotor. Discard the supernatant. Quickly rinse the tube walls and the glassy pellet with a few ml of wash buffer and remove the wash buffer again by pipetting.
Add 1.25 ml of wash buffer to the pellet to resuspend it. Ribosomes can be left on ice overnight for resuspension. Alternatively the pellet can be resuspended manually, which can easily take more than one hour.
Transfer the resuspended ribosomes to a new 1.5-ml tube pre-chilled on wet ice.
Dilute 1 µl of the solution with 99 µl of H2O. Measure A260 by Nanodrop to determine the RNA concentration. Use as blank a 1:100 dilution of wash buffer.
-
Take 100 µg of isolated ribosomes (corresponding to 58 pmol) for total translatome analysis in RP. Use these isolated ribosomes for the preparation of a deep sequencing library (step 1 and following in the Supplementary Methods).
PAUSE POINT Ribosomes for total translatome analysis can be flash-frozen in liquid nitrogen and kept at −80 °C for up to 6 months. Use the rest of the isolated ribosomes directly for selective factor–RNC purification.
B. Sucrose gradient ultracentrifugation TIMING 4 h
Mark one open-top polyclear tube per sample in the middle with a marker block. Fill with 10% sucrose buffer to the mark. Transfer 50% sucrose buffer underneath the 10% buffer up to the mark using a syringe.
Cap the tube. Avoid air bubbles.
- Prepare the sucrose gradient in the gradient station:
- long Sucr 10–50% wv 2St SW40
Remove the cap.
-
Carefully layer the digested lysate (from step 17) (OPTIONAL: the undigested lysate from step 12 and/or the non-crosslinked control from step 1 in addition) onto the gradient. Load a maximum of 1 mg of nucleic acids and a maximal volume of 500 µl of the digested lysate.
CRITICAL STEP Make sure not to disturb the gradient while adding the lysate.
-
Centrifuge tubes at 217,000 g (35,000 rpm) for 2.5 h at 4 °C in an SW40 rotor. Accelerate and decelerate with parameters 7 and 1, respectively.
CRITICAL STEP All six positions of the rotor must be filled with tubes containing sucrose buffer. If there are less than six samples to load on gradients, fill the remaining tubes with sucrose buffer and centrifuge as balance.
-
Fractionate the gradient using the following parameters (gradients from undigested lysates do not need to be fractionated; these gradients are only prepared to visualize the amount and quality of polysomes):
-
-measure absorption at 254 nm
-
-10 measurements per second
-
-piston speed: 0.3 mm/s
-
-distance: 2 mm/fraction
-
-number of fractions: 38–40
TROUBLESHOOTING
-
-
Collect the fractions containing monosomes. The location of the monosome peak can be inferred from the polysome profiles presented in Fig. 4a,b and Fig. 8a,b, respectively.
-
Take 100 µg of isolated monosomes (corresponding to 58 pmol) for total translatome analysis in RP. Use these isolated ribosomes for the preparation of a deep sequencing library (step 1 and following in the Supplementary Methods).
PAUSE POINT Monosomes for total translatome analysis can be flash-frozen in liquid nitrogen and kept at −80 °C for up to 6 months. Use the rest of the isolated ribosomes directly for selective factor–RNC purification.
Selective factor–RNC purification TIMING ~7 h
-
19
Isolate tagged factor–RNCs by affinity purification according to the following steps or implement an immunoprecipitation procedure (see legend of Fig. 9a and Experimental design for additional details).
-
20
Equilibrate the Strep-Tactin sepharose matrix before usage: Transfer 220 µl of the 50% Strep-Tactin sepharose suspension into a 1.5-ml tube using a 1000-µl pipet tip with the tip cut off.
-
21
Centrifuge the sepharose matrix at 500 g for 1 min at 4 °C in a tabletop centrifuge and remove the supernatant.
-
22
Add to the pelleted matrix 1 ml of wash buffer. Incubate for 5 min on an overhead roller at 4 °C. Centrifuge as in step 21, before removing the supernatant once again.
-
23
Repeat washing step 22 two more times.
-
24
Add the ribosome solution (from step 18A vii or step 18B ix) to the Strep-Tactin slurry. Incubate for 1 h on an overhead roller at 4 °C.
-
25
Centrifuge the sepharose matrix as in step 21. Remove the supernatant.
(OPTIONAL) We recommend collecting a sample of this supernatant (unbound fraction) and conducting analyses on it with SDS-PAGE and western blotting.
-
26
Add 1.2 ml of wash buffer to the matrix for washing, incubate for 45 min at 4 °C on an overhead roller, centrifuge as in step 21 and remove the supernatant. Repeat this washing step two more times.
-
27
Add 1.2 ml of cleavage buffer to the matrix, incubate for 45 min at 4 °C on an overhead roller, centrifuge as in step 21 and remove the supernatant.
-
28
Add 120 µl of cleavage buffer plus 10 µl of 2.5 mg/ml TEV protease to elute factor–RNCs from the matrix. Incubate for 30 min on an overhead roller at RT.
-
29
Centrifuge the matrix at 500 g for 1 min at 4 °C. Transfer the supernatant to a new 1.5-ml tube pre-chilled on ice.
-
30
Add an additional 120 µl of cleavage buffer plus 10 µl of 2.5 mg/ml TEV protease to the Strep-Tactin matrix. Incubate for 30 min on an overhead roller at RT and centrifuge as in step 29. Combine this second supernatant with the first supernatant from step 29.
-
31
Add 130 µl of cleavage buffer without TEV protease to the matrix. Incubate for 30 min on an overhead roller at RT and centrifuge as in step 29. Combine the supernatant with supernatants from steps 29 and 30.
-
32
Dilute 1 µl of lysate with 9 µl of H2O. Measure A260 by Nanodrop to determine the RNA concentration. Use as blank a 1:10 dilution of cleavage buffer.
-
33
(OPTIONAL) We recommend collecting a sample of the combined eluate (step 31) and conducting analyses on it with SDS-PAGE and western blotting.
PAUSE POINT The combined eluate containing isolated TF–RNCs (from step 31) can be flash-frozen in liquid nitrogen and kept at −80 °C for up to 6 months.
TROUBLESHOOTING
-
34
Proceed with the preparation of a double stranded DNA library for deep sequencing, which includes size-selection of the footprint fragments, fragment dephosphorylation, linker ligation, reverse transcription and RNA hydrolysis, DNA circularization, rRNA removal, and PCR amplification and addition of barcodes. As these procedures are similar to those described in the protocol by Ingolia et al.10 their step-by-step descriptions are not provided in the main text of this protocol but in the Supplementary Methods starting at step 1. Alternatively, proceed with step 13 of Ingolia et al.10. Finally, samples are sequenced on Illumina HiSeq or GAII instrumentation according to the manufacturer’s protocol (step 96 in the Supplementary Methods). After sequencing data are first analyzed using basic data analysis tools (steps 35–40) followed by specific data analysis using the provided python-based scripts (steps 41–59).
Basic data analysis after sequencing TIMING ~2 d
CRITICAL: The following steps (35–59) describe the computational analysis for prokaryotic ribosome profiling data. For the analysis of eukaryotic data, refer to Ingolia et al.10.
-
35
Preprocess the sequencing data with CASAVA 1.8 (Illumina software package): Discard low quality reads. Sort the sequencing data according to their barcodes.
-
36
Use Cutadapt27 to trim the linker sequence derived from sequencing parts of linker L1 (see Fig. 10a) from the 3’ end of the footprint. Sequences are identified as linker-derived and removed if they match to six or more bases from the 5’ end of the linker. Allow 0.15 mismatches per nucleotide. Sort out reads shorter than 7 nt after trimming. Write reads that could not be trimmed into a new file.
-f fastq -a CTGTAGGCACCATCAATTCGTATGCCGTCTTCTGCTTG -O 6
-m 6 -o <filename_output>.fastq -n 3
--too-short-output=<filename>_too_short.fastq
--untrimmed-output=<filename>_untrimmed.fastq -e 0.15
-
37
Use Bowtie28 for the following alignment steps 38 and 40. Before the first alignment, generate one index of all rRNA genes in Bowtie: Copy the sequences for all rRNA genes behind each other in one file. Save the file as fasta format with ending .fna:
bowtie-build <filename>.fna <name of rRNA index>
-
38
Exclude reads derived from rRNA contaminations from further analysis by aligning all trimmed sequencing reads (from step 36) to the rRNA index. Collect and continue with all unaligned reads. Selection for best alignments is only necessary if the information of rRNA reads is used for further analyses. Selection for unique alignments should not be done here. Allow up to 2 mismatches in the default seed region (28 nt). If sequencing reads were generated with an version older than CASAVA 1.8, a different Phred quality score needs to be specified for the alignment, e.g. --phred64-quals for CASAVA 1.3 and 1.5 (e.g. for sequencing on Illumina GAII without barcodes):
bowtie --best --un <filename_unaligned>.fastq <name of rRNA index> <filename_input>.fastq <filename_output>.map
-
39
Generate an index of the whole genome as reference sequence in Bowtie. Download the .fna file of the complete genome from NCBI (e.g. NC_012759.fna for E. coli MC4100 MuLac (BW2952) or NC_000913.fna for E. coli MG1655):
bowtie-build <filename>.fna <name of genome index>
-
40
Align rRNA-depleted reads from step 38 to the reference genome using Bowtie. Select for unique alignments if desired. Allow up to 2 mismatches in the default seed region (28 nt). Write unaligned reads and reads that align more than once into separate files. Use the default Bowtie output:
bowtie -m 1 --best --strata --un <filename_unaligned>.fastq --max <filename_morealigned>.fastq <name of genome index> <filename_input>.fastq <filename_output>.map
CRITICAL STEP Note that the reported alignment position from Bowtie always represents the left-most character of the alignment. Take this into account for reads aligning to the minus strand.
CRITICAL STEP Bowtie uses zero-based offset. Keep this in mind when comparing the footprint location with published start and stop coordinates of genes, e.g. from NCBI, that usually use one-based numbering.
Specific data analysis TIMING ~2 d
CRITICAL From this step on use python scripts we have written ourselves (see Supplementary Notes 1–14) for further analysis. We describe the most important parts here. Depending on the desired type of data analysis, not all steps need to be performed. For an overview of footprint fragment lengths use step 41; for analyses of gene expression levels employ steps 42–49; for read density analyses use steps 42–45 and 50–55; to calculate meta-gene analyses apply steps 42–43, 45 and 56–58; for the calculation of factor-enrichment efficiencies use steps 42–45, 50–52 and 59.
-
41
Calculate the distribution of read lengths of the footprint fragments according to Supplementary Note 1; input: Bowtie alignment file from step 40.
-
42
Perform center weighting according to Supplementary Note 2; input: Bowtie alignment file from step 40. Calculate the number of reads at each position along the genome (read density) by using ‘center-weighting’ to locate and score only the center of footprint reads in the following way: Divide the pool of footprint reads into reads mapped to the plus strand and reads mapped to the minus strand. The length distribution of footprint reads of MNase-digested prokaryotic samples is considerably broader than that of RNase I digestions in eukaryotic ribosome profiling12,40 (Fig. 7). Therefore, select reads between 23 and 41 nt length. From our experience, selecting only shorter reads does not increase the resolution, probably due to the sequence bias of MNase. To account for varying lengths, subtract 11 nt (minimum length minus 1 divided by 2) from both 5’ and 3’ end of the read. Divide 1 (the score for each footprint fragment) by the remaining length of the footprint center and assign this score to all the positions of the footprint center. Calculate the sum of these center-weighted scores (read density) for every position along the genome.
-
43
Approximately one third of sequencing reads have a mismatch at the first nucleotide, probably due to an untemplated nucleotide addition by reverse transcriptase10. If such a mismatch exists, exclude it from further analysis according to Supplementary Note 2. These read density files for plus and minus strand are used as input files for all downstream processing steps.
-
44
Count the number of total reads for all positions along the genome (sum of center-weighted read densities) on plus and minus strand according to Supplementary Note 3; input: output files from step 43.
-
45
Generate gene lists for plus and minus strand. To this end, download for instance the .ptt file of the desired genome from NCBI. Extract the information about gene name, start and stop coordinates and create one list for plus and one for minus strand.
-
46
Determine the read density per gene as gene expression level according to Supplementary Note 4; input: output files from step 43. Isolate and sum up all center-weighted read densities per protein coding region with the help of the gene lists for the plus and minus strand created in step 45.
-
47
Compare gene expression levels according to Supplementary Note 5; input: output files from steps 46 and 44. Combine read densities per gene from two different samples in one file. Exclude from the analysis genes that do not pass the variability threshold above which the inter-replicate variation is the major source of error and error from counting statistics is negligible. To determine the variability threshold, perform variability analyses from two replicate experiments as described in Ingolia et al.12, Fig. S7. In our analyses the threshold was determined to be approximately 100.
-
48
Divide the sum of read densities for each gene by the total number of mapped reads (in million; from step 44) and by the length of the gene (in kilobases) to calculate RPKM-normalized gene expression levels (RPKM = reads per kilobase of gene length per million mapped reads) as described for Supplementary Note 5.
-
49
Calculate the Pearson correlation coefficient r for gene expression levels from two different samples (from step 48) with the help of a statistics or spreadsheet program or step 54.
-
50
Determine RPM-normalized read densities according to Supplementary Note 6; input: output files from steps 43 and 44. Divide center-weighted read densities at each position by the number of total reads (in million) to calculate RPM-normalized read densities for plus and minus strand (RPM = reads per million mapped reads). These files can then be uploaded to genome browsers like MochiView41 (http://johnsonlab.ucsf.edu/mochi.html).
-
51
Compile complete lists of RPM-normalized read densities including all positions along the genome for plus and minus strand according to Supplementary Note 7; input: output files from step 50. Create such lists by adding all genomic positions without any assigned read density to the RPM-lists and ascribing zero to them.
-
52
If only RPM-normalized read densities in protein coding regions are of interest, select those based on the gene lists created in step 45 and according to Supplementary Note 8; input: output files from step 51.
-
53
Compare RPM-normalized read densities according to Supplementary Note 9; input: output files from step 51 or 52. Combine read densities along the whole genome or in protein coding regions only from two different samples in one file.
-
54
Calculate the Pearson correlation coefficient for read densities from two different samples according Supplementary Note 10; input: output files from step 53.
-
55
Plot the comparison of read densities from two different samples according to Supplementary Note 11; input: output files from step 53.
-
56
Perform meta-gene analyses according to Supplementary Notes 12/13; input: output files from step 43. Calculate the mean read density along an average transcript aligned from start (Supplementary Note 12) or stop (Supplementary Note 13) codon by averaging read densities across all genes based on the gene lists created in step 45: For alignments to the start codon, select center-weighted read densities from, for instance, 50 nt upstream to 1500 nt downstream of each start codon or until the stop codon is reached (for shorter genes). For alignments to the stop codon, begin, for instance, 1500 nt upstream or directly at the start codon (for shorter genes) until 50 nt downstream of the stop codon. If a certain gene length is required, exclude genes shorter than this length (400 nt in TF-SeRP).
-
57
Determine the average read density per base (depending on the expression level) for each gene by summing up all read densities within this gene divided by the length of the gene according to Supplementary Notes 12/13. This approach can be adjusted to special cases. For TF-SeRP, we counted the sum of read densities between nt 280 and 400 and divided it by 120, because of the lack of reads in the beginning of translation for the TF-interactome. Similar adjustments can be performed for other factors, depending on their characteristics of binding to RNCs. For alignments from the start codon, divide the positions from −50 nt to 1500 nt (or to the stop codon if the gene is shorter) by the average read density per base for this gene to normalize all genes to their expression levels. For alignments to the stop codon, use positions −1500 nt (or begin at the start codon if the gene is shorter) to 50 nt.
-
58
Calculate the sum of normalized reads for every single position from −50 to 1500 (stop codon alignments: −1500 to 50) from all genes according to Supplementary Notes 12/13. Divide this sum for every position from −50 to 1500 (stop codon alignments: –1500 to 50) by the number how often this position was counted.
-
59
Calculate the enrichment efficiency as the ratio of read densities from interactome and translatome for every position of RPM-normalized read densities along the whole genome or in protein coding regions according to Supplementary Note 14; input: output files from step 51 or 52. Because many genes are marked with regions lacking continuous read density, read densities at each position can be blurred by including the scores from the neighboring positions. In the case of TF-SeRP, we added the read densities of the ±20 neighboring positions to each individual position along the genome.
TIMING
Step 1: Cell growth and harvest: ~3.5 h (options A/B), ~4 h (option C)
Steps 2–6: Cell lysis: 1–5 h
Step 7: Thawing of the lysate: ~15 min (option A), ~25 min (option B)
Steps 8–17: Nuclease acid digestion: ~2 h
Step 18: Monosome isolation: ~5.5 h plus overnight resuspension of ribosomes (option A), ~4 h (option B)
Steps 19–33: Selective factor–RNC purification: ~7 h
Step 34: See break down of sets of steps in Supplementary Methods: 6–7 d
Steps 35–40: Basic data analysis: ~2 d
Steps 41–59: Specific data analysis: 1–2 d
ANTICIPATED RESULTS
Harvesting by centrifugation typically generates around 7.5 ml of lysate, yielding a nucleic acid concentration of 3.0–3.5 mg/ml. To obtain similar concentrations for rapid filtration, twice the volume of cell culture needs to be filtered (1 l instead of 500 ml). Cells should be scraped off the membrane immediately following filtration, taking at most ten seconds before the cells are plunged into liquid nitrogen. In this context, the priority is to be extremely fast and, therefore, we usually lose about 40–50% of cells during filtration with respect to centrifugation.
After ultracentrifugation the concentration of nucleic acids should be 4–5 mg/ml, which corresponds to 3–4 nmol of isolated ribosomes (1 A260 unit equals roughly 23 nM of purified ribosomes). The expected nucleic acid concentration after TEV cleavage is 0.2–0.3 mg/ml, which corresponds to an amount of 40–60 pmol (70–100 µg) of isolated TF–RNCs. Following acid phenol extraction, 60–85 µg of footprint fragments can be recovered, of which 50 µg are used for size-selection on a polyacrylamide gel. The undiluted concentration of RNA footprint fragments determined by the Small RNA Bioanalyzer run should be 10–15 ng/µl, that is ~150 ng of RNA, which corresponds to 14 pmol of footprints fragments (calculated with an average molecular weight of 340 g/mol for an RNA nucleotide and 31 nt average footprint length). The amount of gel-extracted control oligonucleotide is ~20 pmol. 5–10 pmol of control and footprint fragments are used for the following steps. Ultimately, the undiluted concentration of PCR products determined by the DNA Bioanalyzer run should be 3–4 ng/µl (40–50 nM) before samples are loaded on the sequencing chip.
TROUBLESHOOTING
Step 1C iv:
If the membrane is still wet, the cells cannot be scraped off, and any attempts to this end will cause most cells to be lost. Therefore, the membrane needs to be relatively dry. Still, the cells cannot be left completely dried, because this may induce stress response pathways and subsequently result in a loss of polysomes. Thus, right timing and quick handling is very important here.
Step 9:
If the lysate is too viscous to be removed, it might be due to several reasons: First, genomic DNA might have been insufficiently digested by DNase I. This problem can be overcome by resuspending the pellet, adding 1–2 μl of DNase I to each 1.5-ml tube, and incubating and centrifuging again as described in steps 8 and 9. Second, too much crosslinker might have been used. Check whether this is the case by analyzing the amount of protein in the pellet fraction of crosslinked and non-crosslinked cells using SDS-PAGE. If the amount of protein is higher for crosslinked cells, we recommend reducing the crosslinker concentration.
Step 10:
A low concentration of nucleic acids and proteins in the lysate after centrifugation can be observed, which might be caused by a loss of cells from inefficient scraping of cells off the filter (step 1C iv). Additionally, high concentrations of crosslinker could reduce yields if the lysate was too viscous (see troubleshooting to step 9). Here we recommend a careful titration of crosslinker.
Step 12:
A loss in the amount of polysomes observed at this step is most likely a result of scraping the cells off the membrane during the rapid harvest too slowly (step 1C iv). We also detected a loss of polysomes upon insufficient lysis of cells in the mixer mill, e.g. when other jars were used than the ones recommended in the protocol.
Furthermore, RNase contamination can cause the premature collapse of polysomes, which results in elevated monosome levels. In this case, it is beneficial to add RNase inhibitor to lysate and sucrose gradient buffers before ultracentrifugation. A severe overcrosslinking of the sample can also cause a loss of polysomes. In this case, the crosslinker concentration should be reduced.
Step 18B vii:
In the polysome profile after digest, a small shoulder for the disome peak should be present. If this shoulder is absent, it might indicate that polysomes were overdigested with MNase, which leads to an increased amount of rRNA contamination in the sample. Although such an overdigestion can often be determined from the Bioanalyzer results using a Small RNA chip (step 38 in Supplementary Methods), it can only be fully clarified after sequencing the sample. In this case, MNase should be titrated to lower concentrations. In addition, rRNA depletion (steps 76–83 in the Supplementary Methods) can be included in the library preparation.
On the other hand, small but distinct peaks for disomes and trisomes indicate an insufficiently efficient MNase digestion. This scenario will lead to a loss of footprint fragments with the correct size. Here, we also recommend a careful titration of MNase and a careful evaluation of the experimental conditions upon RNA digestion. MNase activity can be influenced by different parameters, such as pH and digestion time.
Finally, severe overcrosslinking can change the polysome profile dramatically and can even lead to a loss of monosomes. The crosslinker concentration needs to be reduced in this case.
Step 33:
At this stage can be encountered a low yield of purified factor–RNCs, which can have different reasons. First of all, most of the factor might not be associated with RNCs per se. In this case, the amount of cells can be increased or growth conditions may be used that are known to trigger factor interactions with RNCs. Alternatively, existing complexes may not be stable enough and RNCs might be lost during digestion, ultracentrifugation, and purification. This possibility can be explored by quantifying the amount of factor and RNCs that remain after the different purification steps (e.g. in the lysate, after ribosome isolation, and during AP or IP) by SDS-PAGE and western blotting, as shown in Fig. 4c,d, 5a, and 9b. Using a cleavable crosslinker (like DSP) enables one to visualize the samples before and after reversing the crosslink on a gel (Fig. 4c). Stabilization of factor–RNCs may be achieved to some extent by lowering the temperature during the purification or by including or optimizing chemical crosslinking procedures. If implementing these stratagems is not possible, less rigorous washing might reduce the loss of RNCs.
Another possible reason for the observed low yield in factor–RNCs is that the conditions during ultracentrifugation might not have been suitable to deplete free factors, which then compete for binding to the affinity matrix. To circumvent this problem, the salt concentration during ultracentrifugation can be increased. Furthermore, factor–RNCs may exist but cannot be purified. If this is the case it should be tested whether alternative affinity matrices increase the purification yield, as exemplified in Fig. 9b. Alternatively, the stringency during purification may be modified by changing the buffer composition (e.g. detergent and salt concentrations) or by reducing the washing time or the incubation temperature. When using AviTagged factor, we sometimes observed incomplete biotinylation of the AviTag, which decreases the purification yield. Quantification of biotinylation can be performed by analyzing input, unbound and bound fraction of an AP. As such, the ratio between biotinylated and non-biotinylated forms is determined by quantitative western blotting using a streptavidin conjugate (which detects the biotin moiety) and either a factor-specific antibody or a 'mouse anti-C-terminus AviTag antibody' (available from Avidity LCC), which recognizes C-terminal AviTags irrespective of their biotinylation state. Biotinylation efficiency can be increased by addition of biotin to the growth medium and co-expression of biotin ligase.
Although we used up to 100 μg of purified ribosomes for TF-SeRP, a concentration of as little as 5 μg of purified RNCs is in our experience sufficient to generate a sequencing library. Lower amounts of RNCs can still be sufficient for library preparation but the robustness of the procedure is compromised.
Finally, removal of unspecifically bound RNCs upon affinity purification can be improved by a more stringent washing procedure, that is increasing salt and detergent concentration, increasing the washing time and/or steps as well as transferring the matrix to a new reaction tube for every washing step.
Supplementary Material
ACKNOWLEDGEMENTS
We thank B. Zachmann-Brand and N. Reifenberger for cloning and initial experiments; the DKFZ sequencing facility for experimental support; A. Jonasson und B. Haldemann for help in the data analysis; members of the Bukau and Weissman labs for discussions on troubleshooting and comments on the manuscript. This work was supported by NIH (P01 AG10770) and Howard Hughes Medical Institute (J.S.W.); the Peter und Traudl Engelhorn-Stiftung and EMBO (A.B.); SFB638 and FOR1805 of the Deutsche Forschungsgemeinschaft (B.B. and G.K.).
Footnotes
Primary article:
11. Oh E, Becker AH, Sandikci A, Huber D, Chaba R, Gloge F, Nichols RJ, Typas A, Gross CA, Kramer G, Weissman JS, and Bukau B. Cell 2011 Dec 9;147(6):1295–308. doi: 10.1016/j.cell.2011.10.044.
AUTHOR CONTRIBUTIONS
G.K., J.S.W. and B.B. designed the study. A.B. and E.O. performed experiments. E.O. and J.S.W. set up the protocol for general ribosome profiling in bacteria. A.B., G.K., and B.B. established the protocol for selective ribosome profiling. A.B. and E.O. analyzed the data. A.B., G.K., and B.B. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Pechmann S, Willmund F, Frydman J. The ribosome as a hub for protein quality control. Mol Cell. 2013;49:411–421. doi: 10.1016/j.molcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer G, Boehringer D, Ban N, Bukau B. The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat Struct Mol Biol. 2009;16:589–597. doi: 10.1038/nsmb.1614. [DOI] [PubMed] [Google Scholar]
- 3.Giglione C, Boularot A, Meinnel T. Protein N-terminal methionine excision. Cell Mol Life Sci. 2004;61:1455–1474. doi: 10.1007/s00018-004-3466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starheim KK, Gevaert K, Arnesen T. Protein N-terminal acetyltransferases: when the start matters. Trends Biochem Sci. 2012;37:152–161. doi: 10.1016/j.tibs.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Jones JD, O'Connor CD. Protein acetylation in prokaryotes. Proteomics. 2011;11:3012–3022. doi: 10.1002/pmic.201000812. [DOI] [PubMed] [Google Scholar]
- 6.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 7.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 8.Preissler S, Deuerling E. Ribosome-associated chaperones as key players in proteostasis. Trends Biochem Sci. 2012 doi: 10.1016/j.tibs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Egea PF, Stroud RM, Walter P. Targeting proteins to membranes: structure of the signal recognition particle. Curr Opin Struct Biol. 2005;15:213–220. doi: 10.1016/j.sbi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Ingolia NT, Brar GA, Rouskin S, McGeachy AM, Weissman JS. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat Protoc. 2012;7:1534–1550. doi: 10.1038/nprot.2012.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh E, et al. Selective ribosome profiling reveals the cotranslational chaperone action of trigger factor in vivo. Cell. 2011;147:1295–1308. doi: 10.1016/j.cell.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brar GA, et al. High-resolution view of the yeast meiotic program revealed by ribosome profiling. Science. 2012;335:552–557. doi: 10.1126/science.1215110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li GW, Oh E, Weissman JS. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature. 2012;484:538–541. doi: 10.1038/nature10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, et al. Monitoring cotranslational protein folding in mammalian cells at codon resolution. Proc Natl Acad Sci U S A. 2012;109:12467–12472. doi: 10.1073/pnas.1208138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patzelt H, et al. Three-state equilibrium of Escherichia coli trigger factor. Biol Chem. 2002;383:1611–1619. doi: 10.1515/BC.2002.182. [DOI] [PubMed] [Google Scholar]
- 18.Kramer G, et al. L23 protein functions as a chaperone docking site on the ribosome. Nature. 2002;419:171–174. doi: 10.1038/nature01047. [DOI] [PubMed] [Google Scholar]
- 19.Mitkevich VA, et al. Thermodynamic characterization of ppGpp binding to EF-G or IF2 and of initiator tRNA binding to free IF2 in the presence of GDP, GTP, or ppGpp. J Mol Biol. 2010;402:838–846. doi: 10.1016/j.jmb.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Nakatogawa H, Ito K. The ribosomal exit tunnel functions as a discriminating gate. Cell. 2002;108:629–536. doi: 10.1016/s0092-8674(02)00649-9. [DOI] [PubMed] [Google Scholar]
- 21.Gong F, Yanofsky C. Instruction of translating ribosome by nascent peptide. Science. 2002;297:1864–1867. doi: 10.1126/science.1073997. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DN. On the specificity of antibiotics targeting the large ribosomal subunit. Ann N Y Acad Sci. 2011;1241:1–16. doi: 10.1111/j.1749-6632.2011.06192.x. [DOI] [PubMed] [Google Scholar]
- 23.Kerner MJ, et al. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell. 2005;122:209–220. doi: 10.1016/j.cell.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Calloni G, et al. DnaK Functions as a Central Hub in the E. coli Chaperone Network. Cell Rep. 2012;1:251–264. doi: 10.1016/j.celrep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Datta AK, Burma DP. Association of ribonuclease I with ribosomes and their subunits. J Biol Chem. 1972;247:6795–6801. [PubMed] [Google Scholar]
- 26.Dingwall C, Lomonossoff GP, Laskey RA. High sequence specificity of micrococcal nuclease. Nucleic Acids Res. 1981;9:2659–2673. doi: 10.1093/nar/9.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17:10–12. [Google Scholar]
- 28.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merz F, et al. Molecular mechanism and structure of Trigger Factor bound to the translating ribosome. Embo J. 2008;27:1622–1632. doi: 10.1038/emboj.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buskiewicz I, et al. Trigger factor binds to ribosome-signal-recognition particle (SRP) complexes and is excluded by binding of the SRP receptor. Proc Natl Acad Sci U S A. 2004;101:7902–7906. doi: 10.1073/pnas.0402231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raine A, Lovmar M, Wikberg J, Ehrenberg M. Trigger factor binding to ribosomes with nascent peptide chains of varying lengths and sequences. J Biol Chem. 2006;281:28033–28038. doi: 10.1074/jbc.M605753200. [DOI] [PubMed] [Google Scholar]
- 33.Bornemann T, Jockel J, Rodnina MV, Wintermeyer W. Signal sequence-independent membrane targeting of ribosomes containing short nascent peptides within the exit tunnel. Nat Struct Mol Biol. 2008;15:494–499. doi: 10.1038/nsmb.1402. [DOI] [PubMed] [Google Scholar]
- 34.Vorderwulbecke S, et al. Low temperature or GroEL/ES overproduction permits growth of Escherichia coli cells lacking trigger factor and DnaK. FEBS Lett. 2004;559:181–187. doi: 10.1016/S0014-5793(04)00052-3. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Hackert E, Hendrickson WA. Promiscuous substrate recognition in folding and assembly activities of the trigger factor chaperone. Cell. 2009;138:923–934. doi: 10.1016/j.cell.2009.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deuerling E, Schulze-Specking A, Tomoyasu T, Mogk A, Bukau B. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature. 1999;400:693–696. doi: 10.1038/23301. [DOI] [PubMed] [Google Scholar]
- 37.Deuerling E, et al. Trigger Factor and DnaK possess overlapping substrate pools and binding specificities. Mol Microbiol. 2003;47:1317–1328. doi: 10.1046/j.1365-2958.2003.03370.x. [DOI] [PubMed] [Google Scholar]
- 38.del Alamo M, et al. Defining the specificity of cotranslationally acting chaperones by systematic analysis of mRNAs associated with ribosome-nascent chain complexes. PLoS Biol. 2011;9:e1001100. doi: 10.1371/journal.pbio.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willmund F, et al. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell. 2013;152:196–209. doi: 10.1016/j.cell.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Homann OR, Johnson AD. MochiView: versatile software for genome browsing and DNA motif analysis. BMC Biol. 2010;8:49. doi: 10.1186/1741-7007-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Berg S, Lofdahl PA, Hard T, Berglund H. Improved solubility of TEV protease by directed evolution. J Biotechnol. 2006;121:291–298. doi: 10.1016/j.jbiotec.2005.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.