Abstract
Previously, we established a model in which physiologically adequate function of the autologous β cells was recovered in non-obese diabetic (NOD) mice after the onset of hyperglycemia by rendering them hemopoietic chimera. These mice were termed antea-diabetic. In the current study, we addressed the role of T regulatory (Treg) cells in the mechanisms mediating the restoration of euglycemia in the antea-diabetic NOD model. The data generated in this study demonstrated that the numbers of Treg cells were decreased in unmanipulated NOD mice, with the most profound deficiency detected in the pancreatic lymph nodes (PLNs). The impaired retention of the Treg cells in the PLNs correlated with the locally compromised profile of the chemokines involved in their trafficking, with the most prominent decrease observed in SDF-1. The amelioration of autoimmunity and restoration of euglycemia observed in the antea-diabetic mice was associated with restoration of the Treg cell population in the PLNs. These data indicate that the function of the SDF-1/CXCR4 axis and the retention of Treg cells in the PLNs have a potential role in diabetogenesis and in the amelioration of autoimmunity and β cell regeneration in the antea-diabetic model. We have demonstrated in the antea-diabetic mouse model that lifelong recovery of the β cells has a strong correlation with normalization of the Treg cell population in the PLNs. This finding offers new opportunities for testing the immunomodulatory regimens that promote accumulation of Treg cells in the PLNs as a therapeutic approach for type 1 diabetes (T1D).
Keywords: β cell regeneration, T regulatory cell trafficking, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is a disease with multi-organ, microangiopathic and neurological pathology. Mainly affecting young people, it is associated with high morbidity and premature mortality. The insulin deficiency underlying all clinical manifestations of T1D is a result of the destruction of β cells by the autoreactive mononuclear cells that invade the islets of Langerhans. The major milestones in solving the problem of insulin deficiency are: (i) the discovery of insulin;1,2 (ii) pancreata and islets of Langerhans transplantation;3,4 and (iii) development of insulin-supplying devices.5,6 All of these contribute to symptomatic therapies that restore the insulin deficiency.
Until recently, approaches aiming at restraining the autoimmunity that causes β cell destruction were not considered a practical venue for solving the problem of insulin deficiency in T1D because the majority of β cells in the islets of Langerhans are destroyed by the time of disease diagnosis.7 The finding that insulin-secreting cells could fully recover under conditions that restrain their devastation inspired exploration of the immunomodulatory approaches for therapy of T1D.8,9,10,11,12,13,14
To further explore the concept that suppression of autoimmunity could become an adequate condition for recovery of the autologous insulin-secreting tissue, we established a model in which recovery of the physiological function of the autologous β cells was achieved in non-obese diabetic (NOD) mice by induction of allogeneic hemopoietic chimerism after the onset of hyperglycemia. The therapeutic regimen utilized in this model consisted of transplantation of allogeneic bone marrow (BM) in combination with the islets of Langerhans, which were MHC-matched to the donor's BM. The choice for induction of hemopoietic chimerism as the means for constraining autoimmunity in NOD mice was based on our previous investigations, which demonstrated, along with other studies, that allogeneic chimerism induced prior to the onset of hyperglycemia in the NOD mouse model of T1D abrogates autoimmunity, arrests progression of insulitis, and hence, precludes the clinical onset of the disease.15,16,17 Transplantation of the donor's BM MHC-matched islets of Langerhans under the recipient's kidney capsule was utilized to sustain euglycemia until recovery of a physiologically sufficient mass of the autologous β cells. The full recovery of the autologous β cell function was achieved in this model. Euglycemia was sustained in these mice after removal of the kidney with transplanted islets and lasted for their lifetime with no recurrence of autoimmunity (as shown by the absence of insulitis in the autologous islets of Langerhans). We termed these ‘cured from diabetes' animals antea-diabetic (Latin for ‘formerly') mice.18
The current study was conducted to elucidate the immunological mechanisms mediating recovery of euglycemia observed in the NOD mice subjected to induction of hemopoietic chimerism after the onset of hyperglycemia (antea-diabetic model). We hypothesized that regeneration of the autologous β cells sustaining euglycemia in the antea-diabetic mice was possible as the result of the attenuation of autoimmune reactions mediated by the T regulatory (Treg) cell population in the pancreatic lymph nodes (PLNs) of these animals. Treg cells represent a distinct Foxp3+/CD25+/CD4+ T-cell subpopulation and are involved in physiological and pathological immune responses. Evidence is accumulating in support of Treg cell relevance to the pathogenesis of T1D.19,20,21,22,23,24,25 Deviations in the expression of different chemokines and their receptors, as well as trafficking patterns of the Treg cells, were demonstrated in T1D both in humans and in the NOD mouse model.26,27,28,29,30
Data generated in this study demonstrate that the numbers of Treg cells are decreased in the PLNs of the NOD mice and that the expression of the relevant chemokines, especially SDF-1, is downregulated. The Treg cell population was recovered following induction of allogeneic chimerism in the antea-diabetic model. This observation supports our conclusions that: (i) the lack of the Treg cells and the impaired function of the CXCR4/SDF-1 axis in the PLNs of the NOD mice may play a role in diabetogenesis; and (ii) that an improved Treg cell accumulation in the PLNs reflects recovery of the peripheral tolerance in the PLNs of the antea-diabetic NOD mice and is part of the mechanisms leading to the resumption of euglycemia in these animals. In light of the recent finding that full regeneration of the β cells to their physiologic capacity could be achieved in the murine model of T1D after the onset of hyperglycemia,8,9,10,11,12,13,18 immunomodulatory protocols aiming at promoting Treg cell retention in the PLNs can be considered possible means for induction of a condition favoring β cell regeneration in T1D.
Materials and methods
Mice
C57BL/10J (B10), NOD/LtJ (NOD) and NOD.129S7 (B6)-Rag1tm1Mom/J (NOD.Rag1−/−) female mice were obtained from the Jackson Laboratory. IcrTac:ICR (ICR) female mice were obtained from the Taconic Laboratory. B10 and ICR mice were used as autoimmunity-free control animals. The mice were housed under specific pathogen-free conditions in the Animal Facility at Children's Hospital of Pittsburgh in accordance with the National Institute of Health regulations. This study was approved by the Animal Care and Use Committee of Children's Hospital of Pittsburgh of the UPMC Health System.
Antea-diabetic mouse model
This model was described previously.18 Briefly, spontaneously diabetic (glucose >300 mg/dl in three consecutive daily blood test readings) NOD mice were subjected to an allogeneic BM transplantation from autoimmunity-free (B10) donors. The next day, 300 islets of Langerhans, MHC-matched to the donor's bone marrow, were transplanted under the kidney capsule of the chimeric animals. Euglycemia was recovered in the diabetic chimerae the day following islet transplantation due to the insulin secreted by the transplanted islets. After 16 weeks, the graft-bearing kidneys were removed and the animals remained euglycemic. NOD mice that resumed euglycemia 16 weeks after the onset of hyperglycemia followed by induction of hemopoietic chimerism were termed antea-diabetic mice. There was no recurrence of either hyperglycemia or insulitis in these animals for 7 months (the length of observation).
Study groups
The majority of NOD mice (approximately 80% in our colony) became hyperglycemic between the ages of 12–25 weeks. One of objectives of this study was to examine the distribution of the Treg cells in different lymphoid organs in the untreated NOD mice (with respect to age and hence stage of the diabetogenesis) in comparison with the antea-diabetic mice, which resumed euglycemia following an allogeneic BM transplantation, performed after the clinical onset of diabetes. The study groups were young (2 and 5 weeks old), hyperglycemic (12–25 weeks old), antea-diabetic (up to 52 weeks of age) and age-matched, autoimmunity-free controls. In the experiments addressing patterns of chemokine expression in Treg cells, two groups, 6- and 12-week-old animals, of unmanipulated NOD mice were assessed at the beginning and the advanced stages of insulitis, respectively. Autoimmunity-free ICR mice served as the control group in this set of experiments.
Preparation of allogeneic chimera
The basic protocol was adapted from previously described methods.31 NOD recipient mice were gamma-irradiated with a non-lethal (700 cGy) dose of total body irradiation and were reconstituted within 5 h via an intravenous injection with 40×106 T cell-depleted BM cells harvested from B10 mice. For T-cell depletion, a rabbit anti-mouse brain-associated Thy-1 (anti-CD90) antiserum, which is toxic to T lymphocytes, and guinea pig complement were used (Inter-Cell Technologies, Inc., Somerville, NJ, USA).
Islets of Langerhans transplantation
Islets were harvested from B10 donors and transplanted into chimeric (B10>NOD) animals. Pancreatic islet isolation was performed using a modified collagenase digestion procedure.3 Briefly, 3 ml of cold Hanks solution containing 2.0 mg/ml of collagenase (Boehringer-Mannheim, Indianapolis, IN, USA) was injected into the pancreatic duct. After pancreatectomy, the islets were purified from the digested tissue by discontinuous density gradient cell separation using Ficoll gradients (1.108, 1.096, 1.069 and 1.037) (Sigma, St Louis, MO, USA) and hand-counted under a stereomicroscope. Each NOD mouse received approximately 300 islets transplanted beneath the renal capsule. Graft acceptance and function were monitored by testing blood and urine glucose levels and later by histological examination of the renal subcapsular grafts for insulitis, and insulin and Glut-2 content were measured after the kidneys with islets grafts were removed by a live surgery procedure. All surgical procedures were performed aseptically under isoflurane anesthesia.
Blood glucose measurement
A glucometer (Precision QID, Medisense) was used to measure blood glucose from tail vein samples, which was expressed in mg/dl glucose. Mice were considered diabetic with a level of 300 mg/dl or above on three consecutive daily readings.
Characterization of chimera by flow cytometry
The level of chimerism was assessed by flow cytometry as a percentage of peripheral blood leukocytes bearing donor and host MHC class I molecules (H-2Kb for B10 and H-2Kd for NOD) after staining with directly labeled fluorescent H-2KbFZ (Clone AF6-88.5) and H-2KdPE (Clone SF1-1.1) monoclonal antibodies (mAbs). Both mAbs were purchased from BD Biosciences (San Jose, CA, USA). To isolate leukocytes from the peripheral blood, a red cell lysis buffer was applied (RCL Buffer; Sigma). Flow analysis was performed on a Becton Dickinson FACS Vantage SE. To characterize donor's vs. recipient's hemopoiesis derivation of the Treg cells in the chimeric animals, a three-color staining was carried out with anti-donor and anti-recipient MHC-Class I mAbs (as above) and anti-Foxp3APC (Clone FJK-16s) mAb (eBioscience, San Diego, CA, USA).
Morphological evaluation of pancreata
Pancreata were fixed in 10% neutral buffered formalin for 2–3 days, routinely processed and embedded in paraffin. Five-micron-thick paraffin sections (from two levels 100 µm apart, two sections of each level) were stained with hematoxylin–eosin (H&E). Morphological evaluation was performed by a pathologist (VS), using previously published criteria.32
FACS analysis
Lymph nodes, spleens and thymi were harvested from diabetic NOD and control B10 mice and were rendered to the single-cell suspensions. Three-color staining was performed with directly labeled CD45RBPE, CD4APC and CD25FZ mAbs purchased from BD Biosciences. Two-color staining was done with CD4PECy5 mAb, purchased from BD Biosciences, and Foxp3APC mAb purchased from eBioscience. Flow cytometric analysis was performed on a Becton Dickinson FACS Vantage SE.
Spleen cell transfer experiments
Immunocompromised NOD.Rag1−/− mice were used as recipients for the transfer of splenocytes from the antea-diabetic NOD mice. These recipient mice are typically used for induction of diabetogenesis, as they do not have endogenous T or B lymphocytes.33 Typically, disease can be transferred to NOD.Rag1−/− mice by intraperitoneal injection of splenocytes from spontaneously diabetic NOD mice. Recipients become hyperglycemic in 3–6 weeks. In our study, a single-cell suspension was prepared from spleens harvested from the antea-diabetic NOD mice. Each recipient NOD.Rag1−/− mouse received 30×106 splenocytes via an intraperitoneal injection.
Immunofluorescence
Pancreata were fixed in 2% paraformaldehyde for 3 h, incubated overnight in 30% sucrose at 4 °C and snap-frozen in optimal cutting compound (Sakura, Finetek, Torrance, CA, USA) on liquid nitrogen. Immunofluorescent staining was performed using a standard protocol with primary and secondary fluorescently labeled antibodies. Briefly, sections were blocked in non-immune goat serum in bovine serum albumin (BSA) for 45 min at room temperature. After five washes with BSA, an additional blocking was done with goat anti-mouse Fab-fragments (Jackson ImmunoResearch Lab, West Grove, PA, USA) at 1∶70 for 1 h, followed by five washes in BSA. Primary antibodies in BSA were added to sections for 1 h at room temperature: mouse anti-insulin (Sigma) 1∶1000 and rabbit anti-Glut2 (Calbiochem, La Jolla, CA, USA) 1∶200. Sections were washed five times in BSA, and then secondary antibodies in BSA were added for 1 h at room temperature: goat anti-mouse Cy3 (Jackson ImmunoResearch Lab, West Grove, PA, USA) 1∶3000 and goat anti-rabbit Alexa 488 (Molecular Probes, Invitrogen, Carlsbad, CA, USA) 1∶500. The sections were washed three times in BSA and three times in phosphate-buffered saline. Nuclear staining was achieved with nuclear dye Draq5, diluted 1∶2000 (Axxora LLC, San Diego, CA, USA), and color was designated as green or blue. Coverslips were applied using Gelvatol, and the slides were viewed on an Olympus FluoView 500 confocal microscope (Melville, NY, USA).
Real-Time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Quantitation of transcripts was performed by real-time quantitative RT-PCR (qRT-PCR) using a Bio-Rad iCycler Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). At specified time points, total RNA was extracted from the cells of the thymus, spleen, pancreatic and other pancreatic lymph nodes (OLNs) by the Trizol Method of RNA isolation. Briefly, according to manufacturer's instructions, tissue samples were lysed with 0.5 ml of Trizol reagent (Invitrogen) per ∼5×105 cells. Samples were centrifuged at 13 000g at 4 °C for 15 min following the addition of chloroform (1/5 volume of Trizol). Extracted RNA was quantified by spectrophotometric analysis, and the quality was determined according to the A260/280 ratio of 2.0 per the manufacturer's recommendation (Invitrogen). One microgram of total DNase-treated (DNA-free Ambion 1906) RNA was reverse-transcribed into cDNA using either SuperScript III First-Strand Synthesis SuperMix (Invitrogen) or GoScript Reverse Transcription System (Promega, Madison, WI, USA), random hexamer primers, and oligo(dT)20 primers according to the manufacturer's protocol. Gene expression was analyzed in triplicate using a specific primer probe set (accession numbers are as follows: Foxp3 NM_054039.1; TGF-β NM_011577.1; IL-10 NM_010548.1; CTLA-4 NM_009843.2; CD62L NM_001164059.1; SDF1 NM_021704.3; CCL22 NM_009137.2; CCL5 NM_031116; CCL19 NM_011888.2; VCAM NM_011693.3, http://www.ncbi.nlm.nih.gov/nuccore (Applied Biosystems, Foster City, CA, USA)) following the manufacturer's protocol. An internal control gene, Hprt (accession number NM_009843.2 (Applied Biosystems)), was used to normalize mRNA loading. The ΔΔCT method was used to determine the fold change between samples. Briefly, the ΔCT for each condition was determined by subtracting the gene threshold (CT) value for HPRT from the CT for target genes. The ΔΔCT was calculated by subtracting the ΔCT for each experimental sample from the ΔCT of the autoimmunity-free control sample; thus, the data are expressed as 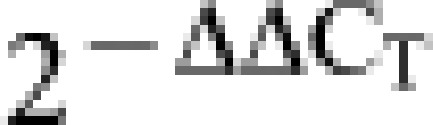 relative to the control sample.
relative to the control sample.
Cells processing and transportation
The experiments assessing the mRNA expression of the Treg cell-associated chemokine molecules were performed in the Laboratory of the Department of Bioscience Technologies, Jefferson School of Health Professions at Thomas Jefferson University, utilizing the cells shipped from the Division of Immunogenetics, Children's Hospital of Pittsburgh, University of Pittsburgh School of Medicine. Lymph nodes and spleens were collected from euthanized animals. Tissues were rendered into single cell suspensions and fixed in RNAlater Storage Solution (Sigma-Aldrich, St Louis, MO, USA) for shipping.
Statistical analysis
Data collected from real-time qRT-PCR of Foxp3 and chemokine transcripts were analyzed relative to autoimmunity-free control mice (fold-change) to determine the levels in NOD and antea-diabetic mice. An unpaired t-test was used to perform comparisons between autoimmunity-free control mice and NOD or antea-diabetic mice. Statistical significance was set at P<0.05.
Results
Dynamics of pathology in the pancreata of antea-diabetic NOD mice
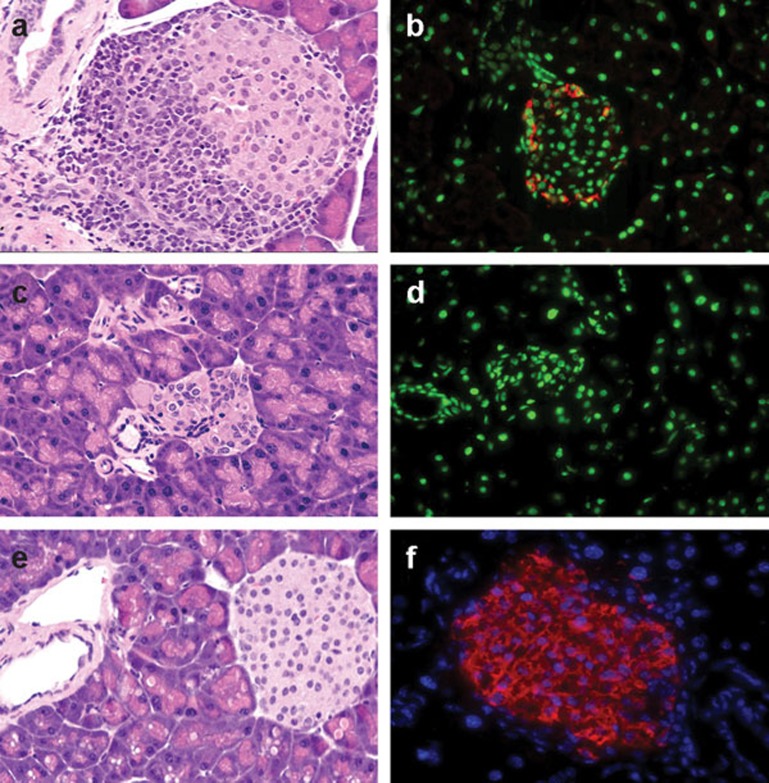
The dynamics of the autoimmune and reparative processes in the islets of Langerhans in the antea-diabetic NOD mice were assessed by morphologic evaluation. The sections of pancreata were stained with H&E and for insulin and were evaluated for the size and appearance of the islets, presence and extent of insulitis, and the production of insulin. Pancreata were collected from antea-diabetic NOD mice at two time points: 3 and 16 weeks after the onset of hyperglycemia and BM transplantation. In samples obtained 3 weeks after induction of chimerism, the pronounced insulitis, which was affecting the majority of islets prior to intervention, was fully eradicated. However, the overall morphological appearance of the pancreatic tissue was not normal. The total islets volume was significantly reduced, and existing islets, although insulitis-free, were significantly reduced in size (Figure 1c).18 They were also negative for insulin staining (Figure 1d). Islets in the pancreatic samples harvested from antea-diabetic mice 16 weeks after BM transplantation showed positive staining for insulin. Their size and overall morphology were undistinguishable from that of normal controls (Figure 1e and f). At this time point, 16 weeks after the BM transplantation, some mice were subjected to surgical removal of the islet graft-bearing kidneys and were monitored afterwards for their blood glucose levels. These mice remained euglycemic in 100% of cases for 7 months (the length of observation).
Figure 1.
Dynamics of pathology in the Islets of Langerhans in the antea-diabetic model. (a, b) Pancreas harvested from a hyperglycemic mouse prior to therapeutic intervention. (a) Islet with advanced insulitis. H&E, light microscopy, ×400. (b) The majority of islets were negative for insulin staining. The shown islet is one of very few with positive staining for insulin in the remaining functional β cells (red). Nuclei are stained with Draq5 and are designated as green, confocal image, ×400. (c, d) Pancreas harvested from mice 3 weeks after induction of hemopoietic chimerism following onset of hyperglycemia. (c) Islets are insulitis-free but morphologically altered and reduced in size. H&E, light microscopy, ×400. (d) Staining for insulin is negative; nuclear staining designated as green. Confocal image, ×400. (e, f) Pancreas harvested 16 weeks after induction of hemopoietic chimerism. (e) Insulitis-free islet, normal size and morphology. H&E, light microscopy, ×400. (f) Positive staining for insulin (red); nuclear staining designated as blue, confocal image, ×640. H&E, hematoxylin–eosin.
Recovery of peripheral tolerance was achieved in the antea-diabetic model
Next, we investigated whether mechanisms of central versus peripheral tolerance were involved in the amelioration of autoimmunity and the resulting regeneration of the β cells observed in the antea-diabetic model. To address this question, antea-diabetic mice were assessed for the presence of the diabetogenic cells in the secondary lymphoid organs. Splenocytes from antea-diabetic NOD mice (experimental group) and from hyperglycemic NOD mice (positive control group) were injected intraperitoneally into immunocompromised NOD.Rag1−/− mice. In two independent experiments, seven of eight NOD.Rag1−/− mice developed hyperglycemia in 3–6 weeks after transplantation of splenocytes from the antea-diabetic mice. All of the NOD.Rag1−/− mice that received cells from the positive controls (hyperglycemic NOD mice) became diabetic in the same time frame. These results demonstrated that diabetogenic cells persisted but were silenced (prevented from invading the islets) in antea-diabetic mice, while in NOD.Rag1−/− recipients, they were able to initiate destruction of β cells, eventually leading to hyperglycemia. These data indicate that the peripheral rather than central tolerance was involved in restraining the autoimmune reactions in the antea-diabetic model. Based on these results, we suggested that restoration of a ‘tolerance-guarding' function of the Treg cells played a role in the amelioration of autoimmunity observed in the PLNs of the antea-diabetic mice.
Numbers of Treg cells are decreased in the lymphoid tissues of NOD mice
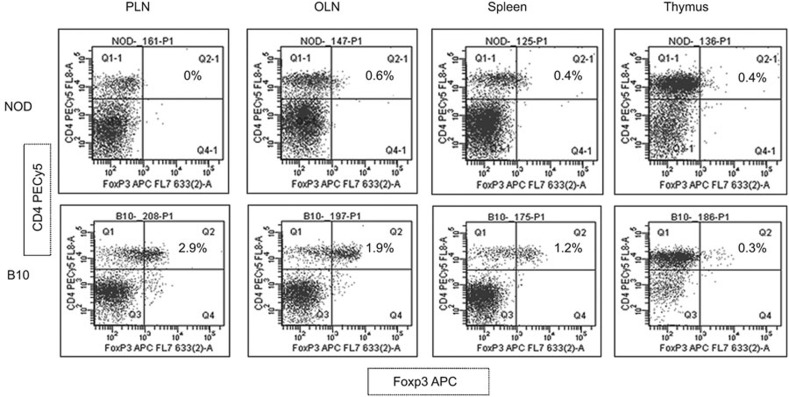
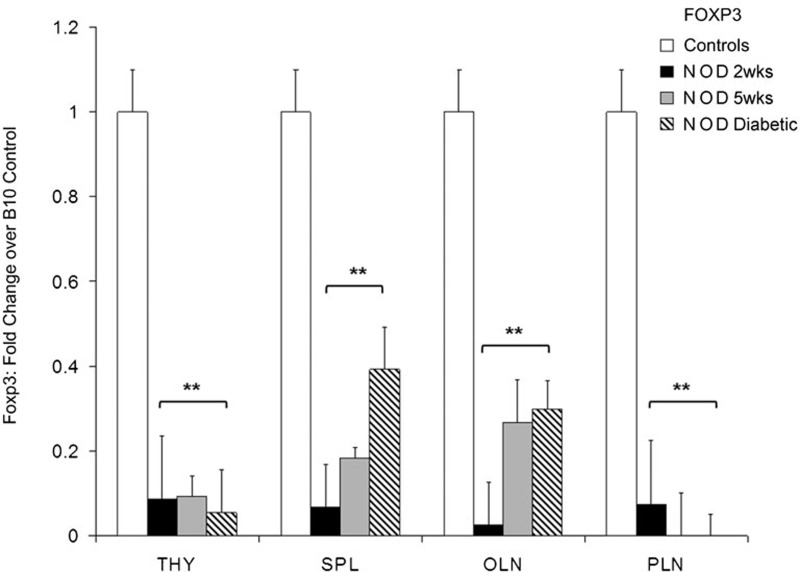
To elucidate the validity of our hypothesis that enhancement of the mechanisms of peripheral tolerance in the antea-diabetic mice is associated with the quantity and function of Treg cells in the PLNs, we first determined whether Treg cell presence is compromised in the untreated NOD mice compared to the control autoimmunity-free B10 mice. We began this assessment by utilizing FACS analysis first based on the CD45RBLow/CD4+/CD25+ profile (not shown) and later the CD4+/Foxp3+ phenotype (Figure 2). The numbers of Treg cells were notably reduced in all secondary lymphoid tissues studied (PLNs, OLNs and spleen) with the most pronounced decrease observed in the PLNs (0%) when assessed by either panel in both sets of experiments. No difference in the Treg cell numbers based on flow cytometry was found in the thymi of diabetic NOD and control animals. Thus, Treg cells were undetectable in the PLNs of the diabetic NOD mice by flow cytometric analysis. This observation was also confirmed by immunohistochemistry (not shown). In further experiments, we used a more sensitive real-time qRT-PCR approach for Treg cell evaluation. Foxp3 gene expression is specific for Treg cells34 and was used as a marker to examine the distribution of Treg cells in the subsequent experiments by real-time qRT-PCR. The levels of Foxp3 transcripts were assessed in the thymus, spleen, PLNs and OLNs of the NOD mice at different ages and were presented as fold changes over the autoimmunity-free control mice (Figure 3). Expression of Foxp3 was significantly decreased, although to a different extent, in all studied tissues (thymus, spleen and all lymph nodes) of NOD mice at 2 and 5 weeks of age, as well as after the onset of hyperglycemia. In the PLNs of the NOD mice, no Foxp3 transcripts were detected in mice of 5 weeks of age and after the onset of hyperglycemia (n=9, P<0.05; Figure 3). Thus, both FACS analysis and real-time qRT-PCR confirmed that the Treg cell population was undetectable in the PLNs of the NOD mice.
Figure 2.
Undetectable levels of Treg cells in PLNs of the NOD mice (FACS analysis). Lymphocytes from PLNs, OLNs, spleen and thymus from both NOD and B10 control mice were subjected to FACS analysis based on the CD4+/Foxp3+ phenotype. Foxp3APC is shown on the X-axis versus CD4PECy5 on the Y-axis. A decreased presentation of CD4+/Foxp3+ Treg cells is demonstrated in all secondary lymphoid tissues with the most profound drop (0%) found in the PLNs. No difference in the presence of these cells was shown in the thymus of the NOD mice compared to that in the control animals. NOD, non-obese diabetic; OLN, other pancreatic lymph node; PLN, pancreatic lymph node; Treg, T regulatory.
Figure 3.
Distribution of Foxp3 transcripts among secondary lymphatic tissues of NOD mice at different ages and after onset of hyperglycemia. Transcript quantities were determined by specific primer-probe pairs in real-time qRT-PCR analysis in the THY, SPL, PLNs and OLNs. Fold change over autoimmunity-free control mice (n=4) was determined for different age groups of mice: (i) 2 weeks, n=6; (ii) 5 weeks, n=5; and (iii) diabetic, n=9. For relative gene expression of autoimmunity-free control groups: (i) 2 weeks, n=3; (ii) 5 weeks, n=5; and (iii) 12–25 weeks, n=4. Statistical significance was determined by the unpaired parametric t-test. *P<0.05; **P<0.01, relative to baseline autoimmunity-free control. NOD, non-obese diabetic; OLN, other pancreatic lymph node; PLN, pancreatic lymph node; qRT-PCR, Real-Time quantitative reverse transcriptase polymerase chain reaction; SPL, spleen; THY, thymus; Treg, T regulatory.
Treg cell population was recovered in the PLNs of the antea-diabetic mice
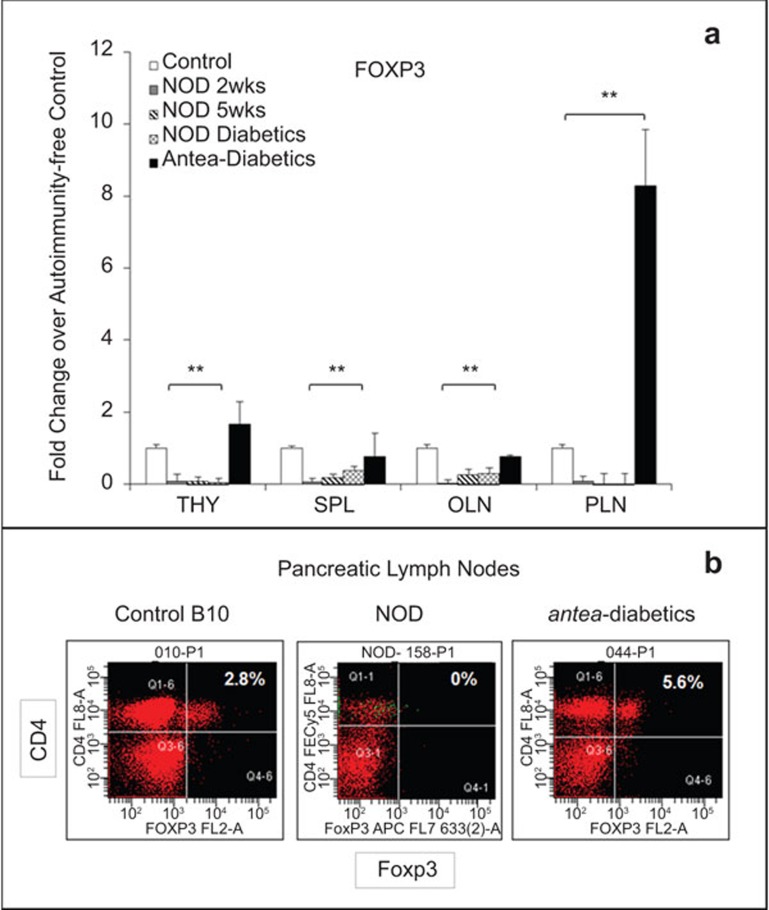
In the unmanipulated NOD mice, a decrease of the CD4+/Foxp3+ cells was shown by FACS analysis and was further confirmed by a marked reduction of Foxp3 transcripts, as determined by qRT-PCR, in all secondary lymphoid tissues of NOD mice at 2 and 5 weeks of age and after the onset of hyperglycemia, compared to autoimmunity-free controls. In the PLNs of these animals, 0% of cells were identified as Treg cells by FACS analysis, and no Foxp3 transcripts were detected by qRT-PCR. In the antea-diabetic NOD mice, which resumed euglycemia 16 weeks after the onset of hyperglycemia and the BM transplantation, the levels of Foxp3 transcripts were significantly increased, although to different degrees in all examined lymphoid tissues, compared to untreated NOD mice (n=3, P<0.05; Figure 4a). The most prominent increase was observed in the PLNs, which was up to eightfold higher than the values of the control animals. In correlation with these findings, the flow cytometry data also showed that the population of Treg cells was recovered in the PLNs of the antea-diabetic mice, reaching levels exceeding that in the control mice (5.6% Figure 4b). In the thymus, the levels of Foxp3 exceeded the average levels of the control mice by approximately 40%. The levels of Foxp3 in the spleen and OLNs were 30% and 20% below the average levels of the controls, respectively, even though they showed some increase compared to diabetic animals. These data demonstrate a correlation between the increase of Treg cell retention in the PLNs and restoration of euglycemia achieved in the antea-diabetic mice. This observation suggests an essential role that Treg cells may play in both amelioration of autoimmune reactions in the vicinity of the islets of Langerhans and in the following β cell regeneration.
Figure 4.
Recovery of the Treg cell population in the PLNs of the antea-diabetic NOD mice. (a) Numbers of the Foxp3 transcripts were assessed by real-time qRT-PCR in the lymphocytes collected from THY, SPL, PLN and OLN. The transcripts were determined as fold changes over the autoimmunity-free control mice (n=4). NOD groups: (i) 2 weeks old: n=6; (ii) 5 weeks old: n=6; Hyperglycemic NOD mice: n=9; and (iii) antea-diabetic NOD mice: n=3. For the autoimmunity-free controls, four mice per group were used. Statistical significance was determined by the unpaired parametric t-test. *P<0.05; **P<0.01, relative to baseline autoimmunity-free control. (b) FACS analysis of the CD4+/Foxp3+ Treg cells in the PLNs of the antea-diabetic mice in comparison with control B10 and unmanipulated NOD mice. NOD, non-obese diabetic; OLN, other pancreatic lymph node; PLN, pancreatic lymph node; qRT-PCR, Real-Time quantitative reverse transcriptase polymerase chain reaction; SPL, spleen; THY, thymus; Treg, T regulatory.
Decreased expression of chemokines regulating Treg cell trafficking was observed in NOD mice
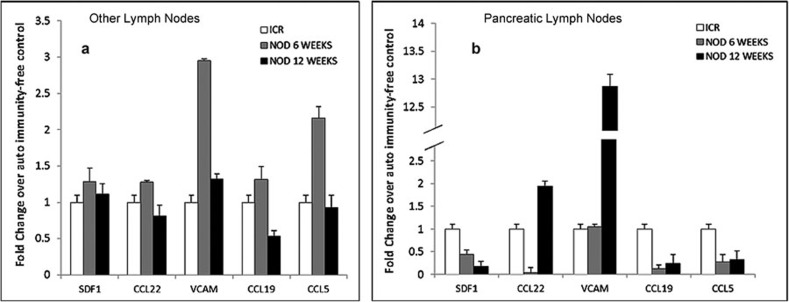
As is true for other types of cells, trafficking patterns of Treg cells are defined by the chemokines in the local tissues of their ‘destination' and the expression of corresponding receptors on the surface of the Treg cells. To elucidate the mechanisms involved in the altered distribution of Treg cells in NOD mice, five chemokines were selected based on their role in Treg cell trafficking patterns under physiological conditions. The expression of chemokines was assessed with respect to the stage of diabetogenesis. The PLNs and OLNs were collected from two groups of unmanipulated NOD mice at 6 and 12 weeks of age: 6-week-old NOD mice represent the early stage of diabetogenesis when insulitis is at the beginning phase, while at 12 weeks, these animals show very advanced insulitis with some animals reaching the point of destruction of β cells resulting in hyperglycemia. Because, according to our data, Treg cells in the PLNs of the NOD mice are not detectable, and hence, the assessment of their chemokine receptor phenotype is not feasible, we evaluated the expression of a panel of their corresponding chemokines in the unfractioned cell populations of their lymph nodes. Quantitation of the transcripts of genes encoding SDF1, CCL22, CCL5, CCL19 and VCAM-I was performed by real-time qRT-PCR using RNA extracted from PLNs and OLNs of the NOD mice. The levels of expression of CCL5, CCL19 and SDF-1 were significantly decreased in the PLNs of NOD mice with the most profound deviation observed for SDF-1 (n=6, P<0.05; Figure 5).
Figure 5.
Expression of chemokines involved in the regulation of Treg cell trafficking was decreased in the PLNs of the NOD mice. Lymphocytes were collected from the PLNs and OLNs of the unmanipulated NOD mice and were assessed by Real-Time qRT-PCR for expression of SDF-1 (CXCL12), CCL22, VCAM-I, CCL19 and CCL5. The transcripts were determined as fold changes over the autoimmunity-free control mice (n=4). NOD groups: (i) 6 weeks old, n=6; and (ii) 12 weeks old, n=6. Statistical significance was determined by the unpaired parametric t-test. *P<0.05; **P<0.01, relative to baseline autoimmunity-free control. NOD, non-obese diabetic; OLN, other pancreatic lymph node; PLN, pancreatic lymph node; qRT-PCR, Real-Time quantitative polymerase chain reaction; Treg, T regulatory.
Origin (host vs. donor BM) of the Treg cells recovered from the PLNs of the antea-diabetic NOD mice
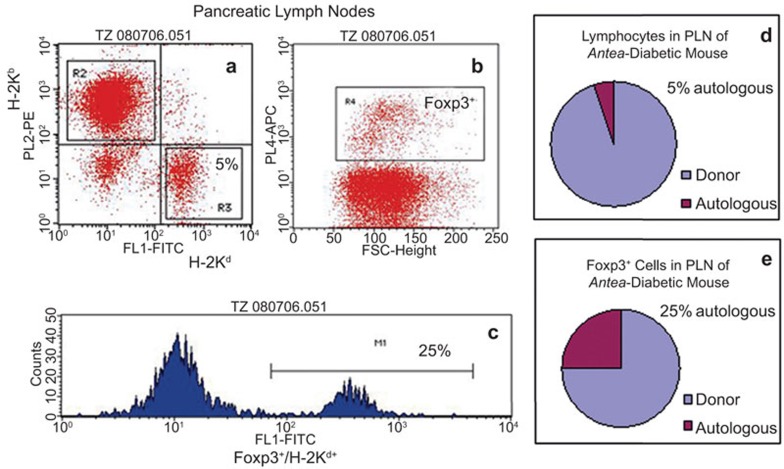
Previously, we reported that allogeneic chimerism, required to ameliorate autoimmunity adequate for sustaining β cell regeneration and recovery of euglycemia in the antea-diabetic model, is equally efficient at low and high levels.17 To elucidate whether the population of Treg cells recovered in the antea-diabetic mice was of donor or recipient origin, we examined animals with a high (∼95%) level of donor chimerism in the current study. To determine this origin, expression of MHC class I molecules was examined on Treg cells. A level of 95% donor chimerism implies that lymphocytes of the autologous origin in the PLNs (as in any other tissue, organ and peripheral circulation) would comprise 5% of the total number of lymphocytes (Figure 6a and d). However, three-color flow cytometric analysis of the lymphocytes harvested from the PLNs of the antea-diabetic mice, stained with anti-H-2Kd, anti-H-2Kb (anti-MHC class I of NOD and B10 mice, respectively) and anti-Foxp3 mAbs, revealed that 25% of cells among Foxp3+ cells were autologous, H-2Kd-positive cells (Figure 6b, c and e).
Figure 6.
Donor versus recipient MHC class I expression on the Treg cells in PLN of the antea-diabetic mice. Three-color flow cytometric analysis of the PLN cells stained with anti-H-2Kd, anti-H-2Kb (anti-MHC Class I of NOD and B10 mice, respectively) and anti-Foxp3 mAbs. (a) Distribution of the autologous (H-2Kd-positive) and donor-derived (H-2Kb-positive) lymphocytes in the PLNs of the antea-diabetic mouse. (b) Gate showing Foxp3+ population. (c) H-2Kd histogram derived from Foxp3+ gate shown in b. (d, e) Graphical illustration of the original data presented in a–c. NOD, non-obese diabetic; PLN, pancreatic lymph node; Treg, T regulatory.
Discussion
In the previously established antea-diabetic model, restoration of euglycemia as the result of amelioration of autoimmunity and regeneration of the autologous β cells was achieved in the hyperglycemic NOD mice subjected to allogeneic hemopoietic chimerism. To elucidate the mechanisms mediating these processes, we assessed whether the recovery of central or peripheral tolerance was attained in this model. Detection of the diabetogenic cells in the antea-diabetic mice, which remained euglycemic and did not have recurrence of insulitis for seven months (the length of observation), indicated that a suppressive peripheral mechanism might have prevented the diabetogenic cells from invading pancreatic islets in these animals. We hypothesized that the Treg cell population in the PLNs of the antea-diabetic mice may play a role in the amelioration of autoimmunity and regeneration of the autologous β cells in this model. The role of Treg cell dysfunction in the pathogenesis of autoimmune disorders including T1D is strongly supported by accumulating evidence in experimental models.19,20,22,23,24,25 Whether there is a distinct pattern of Treg cell deviation in T1D patients is still not clear.35,36,37 In the studies referenced above, the numbers of Treg cells were assessed in the peripheral blood of T1D patients. The discrepancy regarding alterations in the Treg cell numbers in the peripheral circulation of the T1D patients reported in these publications might reflect fluctuations among individuals. Furthermore, to the best of our knowledge, characterization of Treg cell populations in the PLNs, which would be of importance in T1D, has not been addressed yet in a clinical setting.
To explore our hypothesis that the Treg cell population in the PLNs plays a role in recovery of euglycemia in the antea-diabetic mice, we characterized the Treg cell distribution in a variety of lymphoid tissues of the antea-diabetic mice in comparison to both unmanipulated NOD mice and the autoimmunity-free, age-matched controls, with an emphasis on the PLNs in which the most pronounced changes were expected in T1D.38 The FACS analysis and qRT-PCR results showed that the retention of Treg cells is compromised in all secondary lymphoid organs, reaching undetectable levels in the PLNs of the untreated NOD mice. These data were in agreement with other publications addressing the distribution of Treg cells in NOD mice.39 In the thymus, the FACS data showed no difference in the Foxp3-positive cell numbers in NOD versus B10 control mice, while the qRT-PCR data demonstrated a significant decrease of Foxp3 transcripts in NOD mice. These results suggest that the expression of the Foxp3 gene was downregulated in Treg cells in the thymus of NOD mice, while the numbers of these cells remained comparable to that in the control animals.
In the antea-diabetic NOD mice that resumed euglycemia after induction of hemopoietic chimerism, the levels of Foxp3 transcripts assessed by qRT-PCR were increased in all studied lymphoid tissues compared to the untreated NOD mice. In the PLNs, the observed rise was the most prominent, reaching levels up to eightfold higher compared to that in normal controls. FACS analysis also demonstrated that the population of CD4+/Foxp3+ cells in the antea-diabetic mice, which was undetectable in the unmanipulated NOD mice, reached levels exceeding that in the control group. In the thymus, the numbers of Foxp3 transcripts exceeded the normal levels, while in OLNs and spleen, the Foxp3 transcripts, although slightly increased when compared to untreated NOD mice, were still below the normal levels. The spleen was of special interest because the inoculum of splenocytes was used in the diabetes transfer experiments. In this cell population, the Foxp3-positive cells remained at a level of approximately 30% lower compared to autoimmunity-free controls. This could explain why they did not hinder the effect of the diabetogenic splenocytes and did not preclude the successful transfer of diabetes into NOD.Rag1−/− mice by splenocytes obtained from the antea-diabetic mice. Thus, an increase in the numbers of the Foxp3-positive Treg cells was found in the antea-diabetic mice within all studied lymphoid tissues, to different extents. The major focus of this study was to evaluate the dynamics of the Treg cell population in the PLNs of these animals. Based on both FACS and real-time qRT-PCR data, a significant shift was observed in the of Treg cells in the PLNs from undetectable levels in NOD mice to values above normal in the antea-diabetic mice. These results indicate a strong link between recovery of the Treg cell population in the PLNs, amelioration of autoimmunity and regeneration of β cells in the antea-diabetic mice. These data are supportive of our hypothesis that the Treg cell population is involved in enhancing the mechanisms of peripheral tolerance in the vicinity of the islets of Langerhans and in providing an adequate condition for the unhindered regeneration of the β cells in the antea-diabetic model.
The next objective of this study was to explore the aberrations in the repertoire of chemokines in the reticulocytes and endothelial cells in the PLN of the NOD mice that could contribute to the impaired retention of Treg cells in the PLN. We tested the expression of chemokines, which are involved in the regulation of the trafficking patterns of Treg cells, in the PLNs and OLNs of the NOD mice in comparison with autoimmunity-free control mice. The numbers of transcripts of three of these chemokines (CCL5, CCL19 and SDF-1) were significantly decreased in the PLNs of the NOD mice, with the most profound inhibition observed for SDF-1. SDF-1 is of a special relevance to the current study because it has been shown that a polymorphism in SDF-1 in humans is associated with early onset T1D.40,41 Furthermore, Thivolet and co-authors26 have shown that CXCR4 expression was significantly reduced in the PLNs of 12-week-old NOD mice and that the SDF-1/CXCR4 axis has a protective effect in diabetes-transfer experiments. This study also demonstrated that AMD3100, a specific CXCR4 antagonist, can block the ability of the CXCR4-positive T cells to abolish the transfer of diabetes by diabetogenic effector cells. These data are in agreement with our in vivo observations. Our interpretation of the data generated in this study is as follows. The role of the CXCR4/SDF-1 axis in the retention of hematopoietic stem cells is well established.42 Our data demonstrate the lack of Treg cells in correlation with the downregulation of the expression of the chemokine SDF-1 in the PLNs of the diabetic NOD mice. The resumption of euglycemia in the antea-diabetic mice is associated with the recovery of the Treg cell population in the PLNs of these animals. These data, taken together with findings by Thivolet et al., indicate that impaired function of the CXCR4/SDF-1 axis may play an essential role in Treg cell retention in the PLNs and hence in diabetogenesis.
The population of Treg cells recovered from the PLNs of the antea-diabetic mice was determined to be a mixture of cells of the donor's and the recipient's BM origin. The autologous subset of these cells was comparable to that in normal controls. However, whether autologous, donor-derived or both, whether Treg cell populations represent a major contributing factor to amelioration of autoimmunity, as observed in the islets of Langerhans of the antea-diabetic NOD mice, remains an open question.
In summary, we are reporting herein (i) that the PLNs of NOD mice lack Treg cells, which correlates with the locally decreased expression of chemokines involved in their trafficking, with the most prominent decrease shown for SDF-1; and (ii) that recovery of euglycemia in the antea-diabetic NOD mice is associated with the restoration of the Treg cell population in the PLNs. Euglycemia, achieved in the antea-diabetic mice, continued throughout their lifetime, which suggests the feasibility of long-lasting recovery of the Treg cell population in the PLNs of the NOD mice. These data provide a basis for the understanding of the role of Treg cells and the CXCR4/SDF-1 axis in diabetogenesis, amelioration of autoimmunity and regeneration of the autologous β cells in the antea-diabetic model. In light of the accumulating evidence that Treg cells can suppress the transfer and onset of T1D,16,17,43,44,45,46 our findings reported here imply that an immunomodulatory regimen that improves the function of the CXCR4/SDF-1 axis and subsequent retention of Treg cells in the PLNs might become an alternative therapeutic approach for T1D.
Acknowledgments
Grants from Juvenile Diabetes Research Foundation, JDRF #1-2004-580 (T.Z.) and JDRF # 7-2005-1154 (M.T. and T.Z.) and Grant from Intramural Grant Program, Jefferson School of Health Professions, Thomas Jefferson University, 2010-2011 (T.Z.) supported this study.
References
- Kleiner IS, Meltzer SJ. Retention in the circulation of dextrose in normal and depancreatized animals, and the effect of an intravenous injection of an emulsion of pancreas upon this retention. Proc Natl Acad Sci USA. 1915;1:338–341. doi: 10.1073/pnas.1.6.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus: preliminary report. 1922. CMAJ. 1991;145:1281–1286. [PMC free article] [PubMed] [Google Scholar]
- Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- Selam JL. External and implantable insulin pumps: current place in the treatment of diabetes. Exp Clin Endocrinol Diabetes. 2001;109 Suppl 2:S333–S340. doi: 10.1055/s-2001-18592. [DOI] [PubMed] [Google Scholar]
- Tanna S, Joan Taylor M, Sahota TS, Sawicka K. Glucose-responsive UV polymerised dextran-concanavalin A acrylic derivatised mixtures for closed-loop insulin delivery. Biomaterials. 2006;27:1586–1597. doi: 10.1016/j.biomaterials.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gepts W. 1965. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes. 2004;53:1700–1705. doi: 10.2337/diabetes.53.7.1700. [DOI] [PubMed] [Google Scholar]
- Suri A, Calderon B, Esparza TJ, Frederick K, Bittner B, Unanue ER. Immunological reversal of autoimmune diabetes without hematopoietic replacement of beta cells. Science. 2006;311:1778–1780. doi: 10.1126/science.1123500. [DOI] [PubMed] [Google Scholar]
- Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology. 2007;148:5136–5144. doi: 10.1210/en.2007-0358. [DOI] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. Dreams for type 1 diabetes: shutting off autoimmunity and stimulating beta-cell regeneration. Endocrinology. 2010;151:2971–2973. doi: 10.1210/en.2010-0538. [DOI] [PubMed] [Google Scholar]
- Alipio Z, Liao W, Roemer EJ, Waner M, Fink LM, Ward DC, et al. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci USA. 2010;107:13426–13431. doi: 10.1073/pnas.1007884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo L, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreze DV, Gaedeke JW, Leiter EH. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: defective regulation of cytokine receptors and protein kinase C. Proc Natl Acad Sci USA. 1993;90:9625–9629. doi: 10.1073/pnas.90.20.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorina TD, Kaufman CL, Ildstad ST.Mixed allogeneic chimerism for the prevention of autoimmune diabetes, and the reversal of insulitis in the non-obese diabetic (NOD) mouse. J Immunol 19931508 part II): A80 [Google Scholar]
- Li H, Kaufman CL, Boggs SS, Johnson PC, Patrene KD, Ildstad ST. Mixed allogeneic chimerism induced by a sublethal approach prevents autoimmune diabetes and reverses insulitis in nonobese diabetic (NOD) mice. J Immunol. 1996;156:380–388. [PubMed] [Google Scholar]
- Zorina TD, Subbotin VM, Bertera S, Alexander AM, Haluszczak C, Gambrell B, et al. Recovery of the endogenous beta cell function in the NOD model of autoimmune diabetes. Stem Cells. 2003;21:377–388. doi: 10.1634/stemcells.21-4-377. [DOI] [PubMed] [Google Scholar]
- Tian B, Hao J, Zhang Y, Tian L, Yi H, O'Brien TD, et al. Upregulating CD4+CD25+FOXP3+ regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy. Transplantation. 2009;87:198–206. doi: 10.1097/TP.0b013e3181933261. [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol. 2008;28:677–684. doi: 10.1007/s10875-008-9242-z. [DOI] [PubMed] [Google Scholar]
- Homann D, von Herrath M. Regulatory T cells and type 1 diabetes. Clin Immunol. 2004;112:202–209. doi: 10.1016/j.clim.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- D'Alise AM, Auyeung V, Feuerer M, Nishio J, Fontenot J, Benoist C, et al. The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors. Proc Natl Acad Sci USA. 2008;105:19857–19862. doi: 10.1073/pnas.0810713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- Aboumrad E, Madec AM, Thivolet C. The CXCR4/CXCL12 (SDF-1) signalling pathway protects non-obese diabetic mouse from autoimmune diabetes. Clin Exp Immunol. 2007;148:432–439. doi: 10.1111/j.1365-2249.2007.03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspord C, Czerkinsky C, Durand A, Stefanutti A, Thivolet C. Alpha4 integrins and L-selectin differently orchestrate T-cell activity during diabetes prevention following oral administration of CTB-insulin. J. Autoimmun. 2002;19:223–232. doi: 10.1006/jaut.2002.0610. [DOI] [PubMed] [Google Scholar]
- Leng Q, Nie Y, Zou Y, Chen J. Elevated CXCL12 expression in the bone marrow of NOD mice is associated with altered T cell and stem cell trafficking and diabetes development. BMC Immunol. 2008;9:51. doi: 10.1186/1471-2172-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai C, Csaszar A, Czinner A, Szabo T, Panczel P, Madacsy L, et al. Chemokine receptor CCR2 and CCR5 polymorphisms in children with insulin-dependent diabetes mellitus. Pediatr Res. 199;46:82–84. doi: 10.1203/00006450-199907000-00014. [DOI] [PubMed] [Google Scholar]
- Tonkin DR, Haskins K. Regulatory T cells enter the pancreas during suppression of type 1 diabetes and inhibit effector T cells and macrophages in a TGF-beta-dependent manner. Eur J Immunol. 2008;39:1313–1322. doi: 10.1002/eji.200838916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildstad ST, Wren SM, Bluestone JA, Barbieri SA, Sachs DH. Characterization of mixed allogeneic chimeras. Immunocompetence, in vitro reactivity, and genetic specificity of tolerance. J Exp Med. 1985;162:231–244. doi: 10.1084/jem.162.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorina TD, Subbotin VM, Bertera S, Alexander AM, Haluszczak C, Styche AJ, et al. Distinct characteristics and features of allogeneic chimerism in the NOD mouse model of autoimmune diabetes. Cell Transplant. 2002;11:113–123. [PubMed] [Google Scholar]
- Soderstrom I, Bergman ML, Colucci F, Lejon K, Bergqvist I, Holmberg D. Establishment and characterization of RAG-2 deficient non-obese diabetic mice. Scand J Immunol. 1996;43:525–530. doi: 10.1046/j.1365-3083.1996.d01-70.x. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam AL, Vendrame F, Dotta F, Gottlieb PA. CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24:55–62. doi: 10.1016/j.jaut.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- Dubois-Laforgue D, Hendel H, Caillat-Zucman S, Zagury JF, Winkler C, Boitard C, et al. A common stromal cell-derived factor-1 chemokine gene variant is associated with the early onset of type 1 diabetes. Diabetes. 2001;50:1211–1213. doi: 10.2337/diabetes.50.5.1211. [DOI] [PubMed] [Google Scholar]
- Ide A, Kawasaki E, Abiru N, Sun F, Fukushima T, Takahashi R, et al. Stromal-cell derived factor-1 chemokine gene variant is associated with type 1 diabetes age at onset in Japanese population. Hum Immunol. 2003;64:973–978. doi: 10.1016/s0198-8859(03)00176-9. [DOI] [PubMed] [Google Scholar]
- Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- Seung E, Iwakoshi N, Woda BA, Markees TG, Mordes JP, Rossini AA, et al. Allogeneic hematopoietic chimerism in mice treated with sublethal myeloablation and anti-CD154 antibody: absence of graft-versus-host disease, induction of skin allograft tolerance, and prevention of recurrent autoimmunity in islet-allografted NOD/Lt mice. Blood. 2000;95:2175–2182. [PubMed] [Google Scholar]
- Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]