Abstract
Loss of cell-cell adhesion and cell polarity is commonly observed in tumors of epithelial origin and correlates with their invasion into adjacent tissues and formation of metastases. Growing evidence indicates that loss of cell polarity and cell-cell adhesion may also be important in early stage of cancer. In first part of this review, we delineate the current understanding of the mechanisms that establish and maintain the polarity of epithelial tissues and discuss the involvement of cell polarity and apical junctional complex components in tumor pathogenesis. In the second part we address the clinical significance of cell polarity and junctional complex components in cancer diagnosis and prognosis. Finally, we explore their potential use as therapeutic targets in the treatment of cancer.
Keywords: cell polarity, epithelial tumors, differential diagnosis
Introduction
In humans, more than 80% of all tumors are carcinomas and originate from epithelial tissues1. A characteristic hallmark for almost carcinomas is the loss of epithelial morphology and the acquisition of a mesenchymal-like phenotype, through a process called epithelial-to-mesenchymal transition (EMT)2, 3. Activated during embryogenesis and adult tissue remodelling4, 5, EMT is a complex molecular program by which epithelial cells shed their differentiated characteristics and acquire mesenchymal features, including motility, invasiveness and a heightened resistance to apoptosis6, 7.
A strong correlation between malignancy and loss of epithelial organization has been histologically documented for almost types of tumor deriving from epithelial cells8, 9, 10, and disruption of cell-cell adhesion per se has been found to promote the development of some cancers11, 12. Thus, understanding the molecular mechanisms that regulate tissue organization and how such mechanisms are disrupted during neoplastic transformation, could provide important and useful insights to be exploited for diagnostic and therapeutic purposes.
Epithelial cells and the apical junctional complex
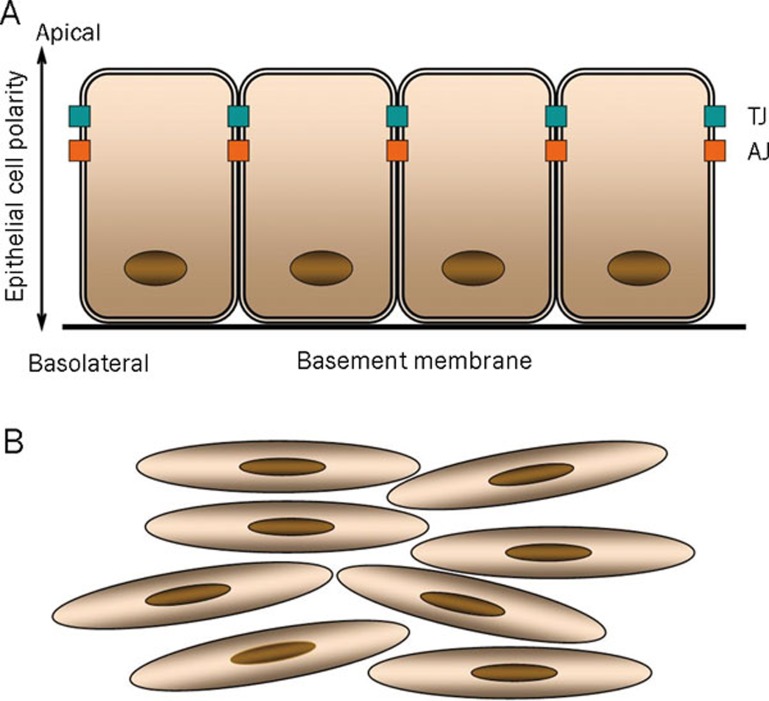
Generally located at the interface between the organism and the outside world or at the free surface of tubes or cavities as in the case of digestive, respiratory, urinary and reproductive tract, epithelial cells are organized in mono- or multi-layered sheets in which any single cell is strictly in contact with neighboring cells by specific cell-cell adhesion structures. To allow a correct sheet alignment, each epithelial cell presents a well-defined orientation with an apical pole directly in contact with the luminal space and a basal pole in contact with basement membrane (Figure 1A). In contrast, mesenchymal cells display a fusiform or spindle-like morphology, do not form organized cell layers, are not polarized, contact the neighboring cells only focally, are not associated with the basement membrane and tend to be highly mobile (Figure 1B).
Figure 1.
Epithelial cells (A) are organized in mono- or multi-layered sheets in which any single cell is strictly in contact with neighboring cells by specific cell-cell adhesion structures: tight junction (TJ) and adherens junction (AJ). Conversely, mesenchymal cells (B) display a fusiform or spindle-like morphology, do not form organized cell layers, are not polarized, contact the neighboring cells only focally, are not associated with the basement membrane and tend to be highly mobile.
The characteristic polarization of epithelial cells is obtained by an asymmetric distribution of cellular components along the internal apicobasal axis. This property, known as apico-basolateral polarity, is part of a crucial differentiation process, termed epithelial polarity program that governs the spatial asymmetry required for a correct epithelial cell morphology and tissue homeostasis13. Schematically, the epithelial polarity program utilizes three cellular machineries dynamically interplaying: the polarized trafficking machinery, the domain-identity machinery and the 3D-organization machinery14. The polarized trafficking machinery is an adaptation of secretory and endocytic systems to sort and deliver proteins and lipids to apical and basolateral plasma membrane domains; the domain-identity machinery governs a highly conserved set of proteins and lipids to the task of generating and maintaining the 'identity' of the apical and basolateral domains; the 3D-organization machinery controls cytoskeleton organization and coordinates extracellular signals with the polarized trafficking and domain-identity machineries.
Among the cellular processes under the domain-identity machinery control there is the establishment of the apical junctional complex, which is formed by two specific cell-cell membrane structures: adherens (AJs) and tight junctions (TJs). Also known as zonula adherens (ZA) and zonula occludens (ZO), these structures are located in the upper portion of a polarized epithelial cell and are composed of transmembrane proteins that interact outside with homotypically molecules in the adjacent cells, and several intracellular scaffolding proteins and signaling molecules connected with the cytoskeletal network15, 16. Whereas the main function of AJs is to provide a strong cell-cell adhesion, TJs form a continuous, circumferential, belt-like, selective barrier to solutes leakage across the cellular sheet, and serve, at the same time, as a boundary between apical and basolateral membrane domains to prevent the diffusion of integral proteins and lipids from one to the other domain17. As well, TJs are also critical for the polarized location of ion channels, receptors, and enzymes to the membrane domains necessary for structurally and functionally developed epithelia, a function referred to as the “fence” function. In addition, to these barrier and fence functions, TJs are an important site for regulation of epithelial cell differentiation and proliferation due to the interactions of some scaffolding proteins with a large number of signaling molecules18, 19.
Initially described as membrane “kissing points” (the external leaflets of the lateral plasma membrane of opposing cells appear fused), TJs are located at the apical end of the basolateral domain just above AJs that form a continuous adhesion belt with a sealing function apparently less stronger than that of TJs because the opposing cell membranes are 15−20 nm distant (Figure 2). However, electron microscopy has revealed that the intercellular space of the AJs is not empty but stuffed by the extracellular portion of several transmembrane proteins, and that at the cytoplasmic side, they are characterized by a “plaque” into which actin microfilaments are particularly condensed and connected with the actin filaments forming the cytoskeleton20.
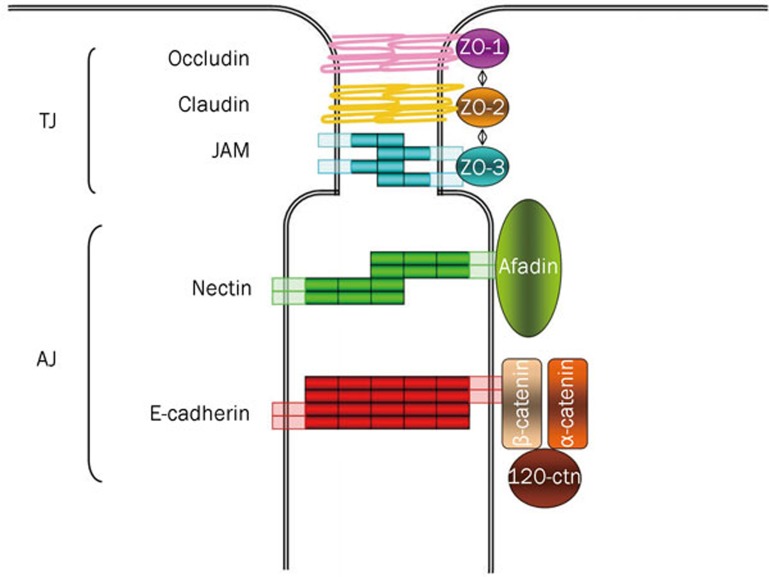
Figure 2.
Tight and adherens junctions components. Tight junctions (TJs) are the most apical intercellular junctions in epithelial cells. Their major transmembrane components are the claudins, occludin and adhesion molecules (JAMs) whereas their cytoplasmic components are zonula occludens (ZOs) proteins. Adherens junction (AJs) are localized just below TJs; their transmembrane components are cadherins and nectins that complex respectively the cytoplasmic components β-catenin and afadin.
Both TJs and AJs are composed of a variety of membrane-localized cell adhesion molecules (CAMs) and cytoplasm-localized adaptor proteins that are responsible, respectively, for the mutual recognition and adhesion of adjacent cells in an epithelial layer and for the anchorage of TJ and AJ structure to the cytoskeleton (Figure 2).
The molecular composition of TJs
To date four types of CAMs or transmembrane proteins have been found in TJs: claudin protein family (mammals express about 24 claudins)21, occludin22, tricellulin23 and junctional adhesion molecules (JAMs)24 (Table 1).
Table 1. Apical junctional complex components.
| Cell adhesion molecules (transmembrane proteins) | Adaptor proteins (cytoplasmic proteins) | |
|---|---|---|
| Tight junction | Claudins (about 24 members) | Proteins without PDZ domains • Cingulin |
| Occludin Tricellulin | • Symplekin • Junction-enriched and junction-associated protein (JEAP) | |
| Proteins with PDZ domains | ||
| Junctional adhesion molecules | MAGUK protein family | |
| • JAM-1 | • ZO-1 | |
| • JAM-2 | • ZO-2 | |
| • JAM-3 | • ZO-3 | |
| • Pals1 | ||
| MAGUK inverted proteins | ||
| • MAGI-1 | ||
| • MAGI-2 | ||
| • MAGI-3 | ||
| multi-PDZ domain proteins | ||
| • MUPP-1 | ||
| • PATJ | ||
| Adherens junction | Cadherins (over 80 members) | Catenins |
| Nectins | • α-catenin | |
| • Nectin-1 | • β-catenin | |
| • Nectin-2 | • p120 catenin | |
| • Nectin-3 | Afadin | |
| • Nectin-4 |
Claudins, occludin and tricellulin are tetraspan proteins that exhibit two extracellular loops, through which they establish contact with homotypic molecules located in the corresponding TJ region of adjacent cells (Figure 2). Experimental evidence from knock-out animals, fibroblasts and epithelial cell lines transfected with different claudin constructs, as well as clinical evidence from human pathological conditions25, 26 indicate that claudin proteins constitute the molecular backbone of TJs and are responsible for the tissue-specific barrier properties of such structures according to the different claudins combination27. On the contrary, the role of occludin on TJ structure and function remains rather controversial since occludin knock-out mice are viable and exhibit TJs with an apparent normal morphology22. Finally, tricellulin, the latest TJ integral protein to be identified, is concentrated at contact points of three epithelial cells and it appears to play a crucial role in the paracellular barrier mechanism23.
JAMs belong to a family of type I proteins characterized by two extracellular immunoglobulin-like domains, a single transmembrane region and a cytoplasmic domain that ends with a canonical PDZ domain. The latter is a module of about 80−90 amino acid residues, that derived its name from the first three proteins in which it was found: postsynaptic density protein PSD-95, the Drosophila lethal disc large tumor suppressor protein Dlg and the TJ scaffold protein zonula occludens 1, ZO-1. The length of the cytoplasmic tail differs among JAMs, and this difference affects their association to different sets of molecules containing one or more PDZ domains. JAMs appear essential for TJs assembly28 and growing evidence indicates that they contribute to the regulation of paracellular permeability.
At the cytoplasmic side of TJs, a wide spectrum of scaffold proteins is found. Named peripheral proteins or cytoplasmic adaptor proteins, they can be classified according to the presence or absence of PDZ domains in their sequences, a feature that gives them the possibility of establishing specific protein-protein interactions (Table 1).
Among the cytoplasmic adaptor proteins without PDZ domains there are cingulin29, symplekin30 and the junction-enriched and junction-associated protein (JEAP), a component of TJs specifically expressed in exocrine cells but not in the epithelial cells of the small intestine31.
Among the cytoplasmic adaptor proteins with one or more PDZ domains, at least three subgroups can be distinguished. The first one corresponds to the membrane-associated guanylate kinase (MAGUK) protein family and it includes ZO-1, ZO-2, ZO-3, and Pals132, 33. The establishment of cultured epithelial cells in which the expression of ZO-1 and ZO-2 is suppressed by RNA interference, has given crucial clues for understanding the role of these proteins in TJ strand formation: ZO-1 and ZO-2 are essential for the polymerization of claudins and to determine their correct localization at the uppermost portion of the lateral membrane34.
The second subgroup corresponds to the MAGUK inverted proteins MAGI-1, MAGI-2, and MAGI-3 in which, differently from ZO proteins, the guanylate kinase domain is located at the amino portion of the molecule and PDZ domains are at the carboxyl region35. The experimental evidence that MAGI proteins can bind and counteract viral oncoproteins and may interact with PTEN (phosphatase and tensin homologue) tumor suppressor suggests possible role of MAGIs in the recruitment of growth suppressors36, 37, 38.
The third subgroup includes the multi-PDZ domain protein 1 (MUPP-1) and Pals1-associated TJ protein (PATJ) that, respectively, contain 13 and 10 PDZ domains39, 40. MUPP-1 is exclusively localized to the TJ of polarized epithelial cells, where it binds to claudin-1 via PDZ10 domain and to claudin-8 via PDZ9 domain. Like ZO-1, MUPP-1 also links to the membrane-associated JAM-1 protein suggesting a pivotal role of MUPP-1 as multivalent scaffolding protein to recruit several other proteins and participate in the regulation of epithelial cell growth and differentiation.
The molecular composition of AJs
AJs consist of two basic adhesive units: the cadherin/catenin and nectin/afadin complexes (Figure 2)16. Classic cadherins are type I, single-pass transmembrane glycoproteins that mediate Ca2+-dependent intercellular adhesion41. They consist of over 80 members, among which, E-cadherin is primarily expressed in epithelia. Specific adhesive binding is conferred by the extracellular portion that serves as a template and engages an identical molecule on the surface of neighboring cells. Conversely, the cytoplasmic domain, highly conserved in length, sequence and interaction partners, mediates key structural and signaling activities by the interaction with some cytoplasmic adaptor proteins belonging to the class of catenins (specifically, α-catenin, β-catenin or the highly homologous γ-catenin/plakoglobin and p120 catenin)42, 43, 44. In particular, an E-cadherin-β-catenin complex is required for the transport of newly synthesized E-cadherin molecules to the plasma membrane where they contribute to the formation of structured AJs45. Once at the plasma membrane, the E-cadherin-β-catenin complex rapidly recruits α-catenin, which is essential to reinforce the association of E-cadherin to filamentous actin (F-actin) by linking other actin-binding proteins, including α-actinin, zyxin and vinculin42, 46. Cadherins also associate with p120 catenin that specifically prevents cadherin degradation enhancing its surface clustering and helping the formation of strong and more “compacted” structures47.
In addition to the association with F-actin, α-catenin binds to afadin, the main cytoplasmic binding partner of nectins, recruiting in this way, the second basic adhesive unit (nectin/afadin) that cooperatively organizes AJs48.
The four nectins till now identified, are members of the immunoglobulin (Ig) superfamily of calcium-independent cell adhesion molecules and are characterized by an extracellular domain with three IgG-like loops, a single transmembrane region, and a cytoplasmic tail that binds to afadin through a C-terminal PDZ binding domain49. Nectin-1, nectin-2, and nectin-3 are ubiquitously expressed in a variety of cells including epithelial cells, neurons, and fibroblasts whereas nectin-4 is mainly expressed in the placenta50. Experimental evidence indicates that similar to cadherins, nectins are thought to form cis-homodimers and trans-homodimers with similar molecules of adjacent cells51 (Figure 2).
Cell polarity complexes and formation of the junctional complex
A key event in the establishment of cell-cell junctional complex is the polarization of epithelial cells. In fact, upon establishing a cell-cell contact and before forming AJs and TJs, epithelial cells must reorganize their cytoskeleton and polarize trafficking routes52. However, to correctly assemble and localize the junctional complex components, a set of evolutionarily conserved polarity proteins that cross-regulate one each other and interplay with cytoskeleton structures is required (Figure 3).
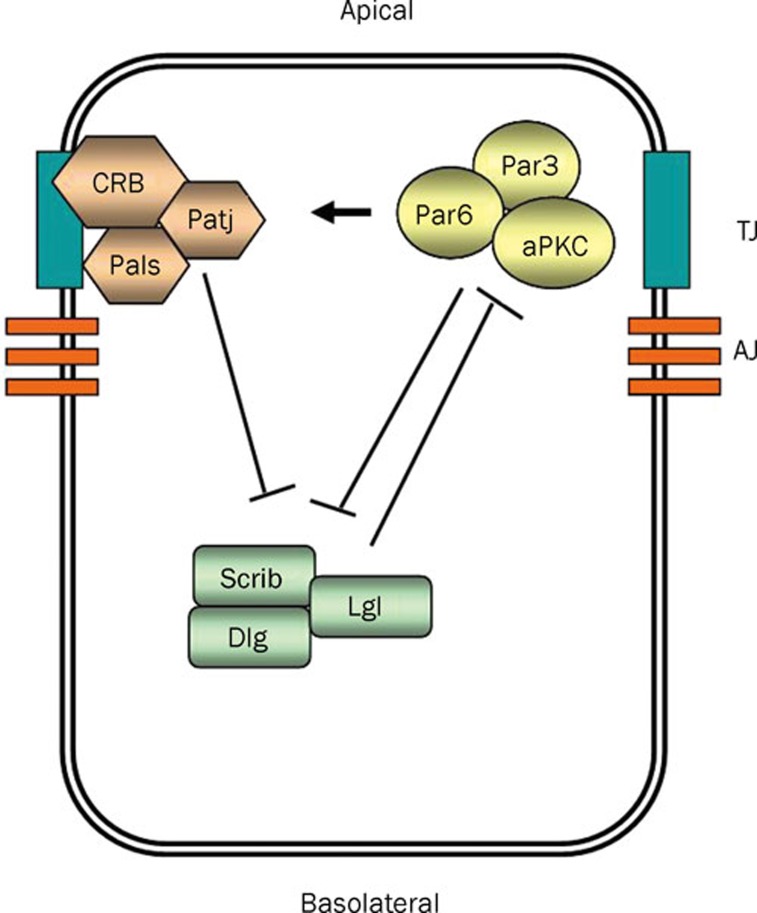
Figure 3.
Epithelial cells are polarized along their apicobasal axis, due to the action of three polarity complexes. PAR (Par3/Par6/aPKC) and CRB (Crumbs/Pals1/Patj) complexes are localized to the apical region and promote apical membrane identity. SCRIB complex is localized to the basolateral region, antagonizes the function of the PAR complex and promotes basal membrane identity. SCRIB complex is reciprocally inactivated by aPKC-dependent phosphorylation of Lgl protein.
Originally identified by genetic experiments in Drosophila melanogaster and Caenorhabditis elegans, in mammals, core polarity regulators are organized in three distinct polarity complexes: (1) the PARtition (PAR) (Par3/Par6/atypical protein kinase (aPKC)) complex; (2) the CRB (Crb/Pals1/Patj) complex; and (3) the SCRIB (Scrib/Dlg/Lgl) complex53, 54, 55.
The PAR complex was initially identified in Caenorhabditis elegans mutants (partitioning-defective) as involved in the regulation of the anterior-posterior cell polarity of the one-cell embryo56. In mammals, it is composed of two scaffold proteins (Par3 and Par6) and aPKC that form a ternary complex able to bind JAMs and nectins through the PDZ-domain of Par3. PAR complex is localized to the apical junction domain and significant evidence indicates that it has a critical role in TJ formation and epithelial polarization55, 57.
The CRB and SCRIB complexes were initially identified in the fruit fly Drosophila melanogaster, as responsible for epithelial defects: fly embryos the lack Crumbs and stardust proteins fail to form a zonula adherens (the Drosophila homolog of TJ) and display a disruption of the apico-basal polarity54. In mammals, Crumbs3, one of the three mammalian homologs of Crumbs protein, is localized to the apical membrane where it forms a complex with Pals1 (the mammalian homolog of Drosophila stardust protein), and Patj (Pals-associated tight junction protein) the mammalian homolog of Drosophila Dlt (Disc lost)58. By the binding of the amino terminus of Pals1 and the PDZ domain of Par6, the CRB complex interacts with PAR complex and together regulate TJ assembly59.
The mammalian SCRIB complex is composed of three proteins, Scribble (Scrib), Disc large (Dlg) and Lethal giant larvae (Lgl)60. While Scrib colocalizes with Dlg and overlaps with the AJs, the localization of Lgl depends on its phosphorylation status, which is mediated by aPKC. In fact, phosphorylated Lgl is unable to localize to the membrane and is inactive61.
In addition to these three core complexes, further components and protein kinases, including Rac1/Cdc42 GTPase, are increasingly being implicated in the organization of cell polarity54.
Based on the work mainly performed in Drosophila and Caenorhabditis elegans, the formation of apical polarity seems to be a hierarchical and interactive process in which PAR and CRB complexes cooperate in establishing the apical domain and in the assembly of TJs, whereas SCRIB complex should define the basolateral plasma membrane domain.
Experimental evidence indicates that, in mammals, the earliest stage of junction biogenesis consists of the trans-interaction of nectins that recruit E-cadherin and JAMs to the apical side of AJs51. JAMs and nectins then recruit Par3 protein, which in turn recruits Par6, the second element of the PAR complex characterized by a binding site for aPKC62. Experimental evidence indicates that the recruitment of aPKC by Par6 and the following phosphorylation of Par3, that allows the activation of the ternary complex, is a critical step for the maturation of the junctional complex into distinct TJ and AJs. In fact, the localization of active PAR complex to the apical domain is stabilized by the CRB complex whose distribution is reciprocally dependent on the PAR complex. As a consequence, CRB complex forces TJs to remain lateroapical by maintaining the PAR complex in this plasma membrane region63, 64. At the same time, the basolaterally located SCRIB complex antagonizes the apical localization of the active PAR complex. In fact, Lgl protein, one of the SCRIB complex components, competes with Par3 for binding to the PAR complex, thereby sequestering the PAR complex away from the apical junctional domain. Such an antagonist function of SCRIB complex is controlled by aPKC as the aPKC-dependent phosphorylation of Lgl protein inactivates the SCRIB complex54 (Figure 3).
Apical junctional complex and gene expression
In addition to their role in establishing and maintaining a correct epithelial phenotype, several components of the apical junctional complex have also been found able to actively influence gene expression. In fact, accumulating evidence indicated that some nontransmembrane proteins involved in the formation of the apical junctional complex can shuttle between plasma membrane and nucleus where they participate in the formation of chromatin-associated complexes and hence in the control of gene transcription.
Initially reported for β-catenin, concerning to its role in Wingless (Wnt) signaling pathway65, this shuttling activity has then been broadened to other TJs proteins (principally ZO-1 and ZO-2)66, 67 and some cell polarity complex components including Par3, Par6 and Dlg168, 69, 70 suggesting for these apical polarity proteins a powerful role as epigenetic factors71, 72.
As recently proposed by Lelièvre73, the impact of the nuclear localization of apical polarity proteins on gene transcription should depend on the integrity of cell-cell junctions. In fact, if the apical junctional complex is intact, some apical proteins move into the nucleus and participate in the transcription repression of the genes that have to be silenced in order to maintain a differentiated phenotype. Conversely, when apical junctional complex is disrupted certain transcription regulators, normally compartmentalized at cell-cell junctions by apical polarity proteins, are free to move into the nucleus where they induce apical proteins to switch from nuclear repressors to nuclear promoters. Such an hypothesis has recently been supported by the finding that nuclear over-expression of symplekin, a transcriptional regulation normally localized at TJs, promotes tumorigenesis in the human colon and that the regulation of claudin-2 expression is instrumental in this effect74.
Disassembly of junctional complex and cancer
Disruption of intercellular junctions and alterations in cell polarity are specific hallmarks of epithelial cancer cells. In fact, most human tumors arising in epithelial tissues, gradually lose their polarized morphology and acquire a more peculiar mesenchymal phenotype according to a process termed epithelial-mesenchymal transition (EMT)75.
Physiologically associated with embryogenesis and wound healing in the adult, EMT is a process characterized by the loss of cell-cell adhesion and apicobasal cell polarity and the concomitant acquisition of a fibroblastoid motile phenotype. When pathologically activated during tumor development, EMT provides cells with ability to escape from the primary tumor mass and to migrate to distant region where they metastasize76.
Disruption of cell-cell adhesion is principally due to the disassembly of the junctional complex that may be induced by a direct modification of the any junctional components (transmembrane and cytoplasmic proteins) or by indirect effects through the actin cytoskeleton that controls the integrity of TJs and AJs. In particular, alterations in the structure and function of TJs have been reported in adenocarcinomas of various organs, including colon and breast77, 78, whereas absent or defective TJs have also been associated with the development of the neoplastic phenotype in endometrial cells79. TJs dysfunction has also been described in inflammatory processes that end up in cancerous transformation. For example, Crohn's disease and ulcerative colitis are inflammatory illnesses involving the gastrointestinal tract in which an abnormal paracellular permeability defect, probably induced by cytokines and growth factors associated to inflammation including interleukin-1, interleukin-4, interleukin-13, transforming growth factor-α, insulin-like growth factor-I and II, interferon-γ, tumor necrosis factor-α and hepatocyte growth factor, appears to precede the development of both syndromes80. Interestingly, it has also been shown that the transforming potential of some oncoproteins, encoded by human adenovirus, papillomavirus (HPV), T-cell leukaemia virus type, depends in part on their ability to interact with TJ-associated proteins of epithelial cells81.
As previously illustrated, alterations in some apical proteins and transcription regulators compartmentalization, due to TJs and AJs disassembly, may have a dramatic impact on gene transcription with the switch in the apical proteins activity from nuclear repressors to nuclear promoters as supported by the observation that ZO-1 protein is found at the plasma membrane in noninvasive cells whereas it is located in the nucleus of invasive cells82. Similarly, the dissociation of E-cadherin-catenin complex results in an increase of the cytosolic pool of β-catenin, the key regulator of Wnt signaling pathway, that drives cell proliferation once migrated to the nucleus83. It is important to note that, in addition to transforming growth factor-β (TGF-β), Wnt is one of the extracellular growth factors that have been shown to trigger epithelial dedifferentiation and EMT by the downregulation of epithelial genes and the upregulation of genes coding for fibroblastoid-associated features as N-cadherin, the typical mesenchymal marker84, 85, 86. That, according to a process known as “cadherin switch” during which cells shift to express different isoforms of this transmembrane component87.
Also transmembrane and cytoplasmic proteins with at least a PDZ domain have been found target of some oncoproteins including HPV E6 protein, one of the major oncogenic determinants of the sexually transmitted HPV, the primary etiologic agent of cervical cancers. HPV E6 protein has a functional PDZ binding motif that interacts with the corresponding PDZ domain of the TJ proteins MUPP-1, MAGI-1, MAGI-2, MAGI-3, and PATJ36, 88. Through such a binding E6 targets these proteins as well as the Dlg and Scrib molecules to ubiquitin-mediated degradation in the proteosome89, 90, 91. In this way, expression of HPV E6 protein in epithelial cells destabilizes TJs, generates ZO-1 mislocalization, disrupts AJs and promotes carcinogenesis.
Cell polarity complexes dysregulation and cancer
The first hint that polarity proteins might contribute to cancer was provided by experiments in Drosophila which showed that knockout of any member of the Scribble complex results in the loss of apicobasal cell polarity, mislocalization of apical junctions and failure to form the zonula adherens, and neoplastic overgrowth of imaginal discs, the ectodermal structure from which originate epithelia, that lead to death at the larval stage suggesting for these proteins an oncosuppressive key function92, 93.
Also in mammals, several reports describe a strong correlation between decreased expression of mammalian homologues of Scrib, Lgl and Dlg and tumor progression. Mice lacking Lgl1, one of the two Lgl homologues, show severe brain dysplasia, accompanied by loss of cell polarity in neuroepithelial cells94, whereas loss or aberrant localization of Lgl2 is associated with human gastric epithelial dysplasia and adenocarcinomas95. In addition, as already described, ubiquitin-mediated degradation of Dlg and Scrib proteins may contribute to the development of the HPV-induced cervical carcinoma91, 96. It should be noted that cancer promoting viruses, such as HPV also destabilize the CRB/PAR complexes interaction required for TJs formation and induce epithelial depolarization97 suggesting for CBR components a role as tumor suppressors98.
Recent findings also implicate the PAR complex in human carcinogenesis. Gene amplification and elevated activity of aPKC have been detected in ovarian, lung and colon cancer and correlated with a poor prognosis99, 100, 101. In addition, as SCRIB complex components are known to cooperate with those of PAR complex in the assembly of TJs, dysregulation of Scrib, Lgl and Dlg proteins affects also PAR complex activity. As the members of PAR complex play a pivotal role in signaling pathways that control polarity and proliferation, PAR complex implication in human carcinogenesis is not surprising102. An example of the link between growth factors signaling, cell polarity and cancer is provided by the ErbB2 receptor tyrosine kinase signaling pathway in breast cancer, in which an overexpression or amplification of the gene is found in 25%-30% of cases. It has recently been demonstrated that after activation, the ErbB2 receptor binds directly to Par6 protein, leading to the competitive recruitment of aPKC and the disruption of the apicobasal polarity103. Further recent insights into the regulation of cell polarity during EMT have converged on PAR complex. In fact, in rat intestinal epithelial cells, upon exposure to TGF-β, Par3 protein level has been found decreased, concomitantly to E-cadherin suppression and the induction of mesenchymal markers. The decrease in Par3 mediated by TGF-β resulted in a redistribution of Par3-aPKC complex from the membrane to the cytoplasm leading to the disorganization of PAR complex and loss of polarity104.
Also mutations in tumor suppressor proteins, such as von Hippel-Lindau protein (VHL), may affect PAR complex efficiency and junctional complex assembly. In fact, as demonstrated by Okuda et al105, VHL, a tumor suppressor involved in the von Hippel-Lindau syndrome which leads to the development of hemagioblastoma, clear-cell renal carcinoma and pheochromocytomas, mediates ubiquitination of a variety of proteins, including activated aPKC, and it regulates the assembly of intercellular junctions. Because VHL tumor suppressor simultaneously affects cell polarity and growth, inactivating mutations result in an abnormal AJs and TJs organization and cell proliferation as evident in VHL-negative cancer cells.
Cell polarity and cell-cell adhesion proteins in cancer diagnosis and prognosis
Due to their multiple links to cancer, many structural and regulatory components of the junctional complex may represent valuable tumor biomarkers. Even though the acquisition of new insights is increasing, at present the largest body of clinical evidence derived from studies on E-cadherin expression. Changes in the normal expression pattern of the E-cadherin/catenin complex have been found in various human cancers from epithelial origin where a partial or total loss of E-cadherin expression correlates with loss of differentiation characteristics, increased tumor grade and metastatic behavior, and poor prognosis. Such a downregulation of E-cadherin may occur as a consequence of gene mutation or epigenetic silencing. Thus, for example, mutations in CDH1, the gene encoding human E-cadherin, are found in more than 50% of stomach cancers106 whereas, in lobular breast cancer, E-cadherin expression is irreversibly lost due to loss of heterozygosis (LOH) in more than 85% of cases; an LOH which is frequently associated with epigenetic silencing of the remaining CDH1 allele via promoter hypermethylation or transcriptional repression12. The prevalence of E-cadherin loss in non-invasive precursor lesions as lobular carcinomas in situ of the breast or pancreas adenomas107 highlights the importance of E-cadherin as a tumor and invasion suppressor and it suggests E-cadherin loss as a critical step in the transition from adenoma to carcinoma.
Also the expression of occludin, one of the major transmembrane components of TJs, has been found decreased with disease progression. For example, moderately and poorly differentiated gastric carcinomas show a reduced occludin mRNA and corresponding protein level when compared to well differentiated ones108. Similarly, loss of occludin has been found in human breast cancer109, in unpolarized prostate cancer cells of Gleason grades 4 and 5110, and in grades 2 and 3 endometrioid carcinoma with respect to grade 179.
More debated is the diagnostic/prognostic role of claudins. In fact, because of their tissue-specific pattern of expression, a causal relationship between claudins expression/localization and cancer has not been established. In some cancers, including prostate, breast, melanocytic neoplasia, esophageal squamous cell carcinoma and lung111, 112, 113, 114, 115, a decreased expression of some claudins, especially claudin-1, has been associated with cancer progression, recurrence and a shorter disease-free survival, suggesting a cancer invasion/metastasis suppressive role. Conversely, in other cancers such as papillary thyroid, oral squamous cell carcinoma, ovarian, colon, melanoma and gastric116, 117, 118, 119, 120, 121, overexpression of claudins has been associated with a more aggressive phenotype.
With regards to the clinical relevance of cytoplasmic proteins, such as ZOs and JAMs proteins, several studies have reported that their reduced expression correlates with dedifferentiation in tumors arising from various compartments of the biliary tract and progression of clear cell renal carcinoma or endometrial cancer122, 123, 124. However, ZO-1, which is generally considered as a tumor suppressor, has unexpectedly been found overexpressed and associated with N-cadherin in melanoma125, overexpressed and aberrantly localized in primary and metastatic pancreatic cancer126, whereas JAM-A has been found overexpressed and positively correlated with poor prognosis in breast cancer patients127.
About cell polarity complex components recent data provide evidence that aPKC is overexpressed in gastric cancer and associated with unfavorable prognosis128. In addition, in a very recent study, aPKC overexpression has been found associated with the loss of Lgl2, a component of the SCRIB polarity complex, in foveolar-type gastric dysplasia suggesting their combined evaluation useful to refine dysplasia diagnosis129. The immunohistochemical determination of Lgl2 expression has also provided to be useful in separating pancreatic intraepithelial neoplasia-3 and pancreatic ductal adenocarcinoma from lower-grade pancreatic intraepithelial neoplasias; in fact, while loss or abnormal Lgl2 expression is seen in pancreatic intraepithelial neoplasia-3 and adenocarcinoma, pancreatic intraepithelial neoplasia-1 and pancreatic intraepithelial neoplasia-2 express the protein in a normal-like basolateral fashion130.
Similarly, the loss of Lgl1 has been associated with several solid tumors including melanoma, prostate, breast, colon, liver and lung cancer131, 132, 133, 134. In these cancers Lgl1 loss is generally associated with higher tumor grade, and is more pronounced in lymph node or distant metastases.
Also Scrib and Dlg, the other two members of the SCRIB complex, have been found of clinical relevance. In fact, an emerging finding is the association of Dlg1 with HPV-induced gynecological malignancies: in later-stage and poorly differentiated tumors, cytoplasmic Dlg1 protein level increases while it decreases at the membrane sites of cell-cell adhesion135, 136. A similar trend of early-stage overexpression and mislocalization has been described in colon carcinogenesis: in adenomatous polyps and well- and moderately differentiated adenocarcinomas, Dlg1 protein level increased but become more diffuse with an increase cytoplasmic staining137 suggesting its potential use as selective biomarker. Similarly even though preliminary, the evidence that alterations in Scrib protein expression and localization are associated with some cancer of epithelial origin including endometrial, cervical and colorectal neoplasias138, 139. In particular in the former, Scrib protein alterations have been found associated with clinical stage, histopathological differentiation, and lymph node metastasis140 whereas in cervical carcinoma the histochemical analysis showed a dramatic decrease in the expression of Scrib with the progression of disease from normal uterine cervical tissues to invasive cervical cancers through the precursor lesions139. Finally, in colorectal carcinoma the overexpression and distribution of Scrib were observed to extensively overlap with the cytoplasmic accumulation of β-catenin and an intense immunoreactivity of Scrib was often observed in small adenomas suggesting that Scrib could be involved in an early step of colon carcinogenesis and supporting the experimental finding of a link between cell polarity disruption and EMT140.
While no clear evidence of a direct link between CRB complex components and tumor features and clinical outcome, it has now emerged, Par6 protein, a PAR complex component, has recently been found overexpressed in breast cancer-derived cell lines and in both precancerous breast lesions and advanced primary human breast cancers, especially in estrogen receptor-positive ones141.
Although, as reported above, several polarity and junctional complex components have been found singly associated with initiation or progression in many cancers of epithelial origin, an integrated picture of the mechanisms controlling their expression and activity during cancer development is lacking. To this aim, novel approaches such as gene expression profile analysis are needed to investigate the associations between the expression of genes coding for cell polarity and cell-cell adhesion determinants and the biologic features of preneoplastic and neoplastic lesions. In such a perspective, for example, we have investigated whether, with respect to normal tissue, epithelial or mixed malignant pleural mesothelioma (MPM) were associated with specific patterns of expression of a selected set of genes related to EMT and cell polarity and adhesion142. Using active and passive Principal Components Analysis-based biplots, to visualize specific patterns of expression for epithelial and mixed MPMs, we found that epithelial MPMs, mixed MPMs and normal tissues were characterized by different patterns of expression of genes contributing to a better characterization of MPM histologies, thus suggesting the possibility of using gene expression profile analysis in refining the morphological diagnosis also in other controversial pathological situations as for example preneoplastic lesions.
Cell polarity and cell-cell adhesion proteins as therapeutic targets
Blocking cell proliferation in neoplasias and preventing EMT in preneoplastic lesions eventually restoring normal epithelial morphology represent the main goal of differentiation therapy. Several attempts, targeting cell polarity and adhesion components, have been made at experimental level with interesting and promising results. For example, it has been observed that, in suspension 3D breast tumor spheroids, the retrovirally-induced reexpression of claudin-1, downregulated or completely lost in breast cancer cells in vitro, resulted in plasma membrane homing of the protein and reconstitution of paracellular flux143. Similarly, in several cancer cell lines, the treatment with demethylators and histone deacetylase inhibitors or retinoids was able to reexpress occludin (silenced by CpG island hypermethylation on its promoter region) with a decreased cellular invasiveness and motility, thereby abrogating the metastatic potency of cancer cells144.
Conversely, as regard to claudins which appear overexpressed in tumors, the use of siRNA to silence gene expression has provided a significant reduction in cell proliferation, reduction in tumor growth and a significant increase in the number of apoptotic cells145. A negative control of claudins overexpression has also been achieved using monoclonal antibody against specific members of the family and promising results have been obtained in pancreatic and ovarian cancers146, 147. The use of monoclonal antibodies has also been applied to inhibit the activity of JAM proteins and in particular to block the proangiogenic activity of JAM-3, another component of TJs that in tumor cells mediates the homophilic interaction with endothelial cells. In a murine model of lung carcinoma, monoclonal antibody against JAM-3 was able to inhibit the growth of lung pulmonary tumor grafts by reducing tumor vasculature148.
In addition to the immunotherapeutic strategy, the potential of claudins as targets for cancer therapy has been highlighted by the observation that treatment with Clostridium perfringens enterotoxin (CPE) elicits a rapid and specific cytolysis of breast, ovarian, pancreatic and prostate carcinoma cells. This toxin, responsible for the gastrointestinal symptoms associated with Clostridium perfringens food poisoning, is released into the intestinal lumen where it binds specifically to claudin-3 and -4 present in the intestinal epithelial. This triggers the formation of a large multiprotein membrane pore that ultimately results in cell lysis. Since claudin-3 and -4 are overexpressed in numerous carcinomas, this condition represents a unique opportunity for innovative therapy using CPE149, 150.
As regard polarity complex components, preliminary data on the ternary Par6/Par3/aPKC complex as a novel target for therapeutic intervention are emerging. In particular, Par6 protein appears a promising target both in early and late stage of breast cancers141 whereas aPKC, as well as being a new prognostic indicator, has been proposed as a novel therapeutic target for the treatment of gastric cancer128.
Conclusion and future perspectives
Increasing evidence clearly shows that the loss of apico-basal polarity and changes in cellular junctions can be initiating events in tumor formation and that they are hallmark of malignant transformation. Although in recent years there has been a significant advance regarding our understanding of the physiologic mechanisms governing cell polarity, the exact sequence of events leading to cell polarity dysregulation are still being uncovered whereas it is clear that the expression of genes coding for core polarity and junctional adhesion components can be directly or indirectly transcriptionally regulated by several factors including growth factors, oncogene products, tumor suppressors and EMT inducers.
Of particular interest, the emerging relationship among EMT, cell polarity and stem cell phenotype based on the observation that the proteins regulating cell polarity also have an important function in asymmetric cell division, a critical event that guarantees the two essential properties of stem cells: self-renewal and differentiation151. In fact, studies in Drosophila have shown that the asymmetric localization of cell-fate determinants is controlled by PAR and SCRIB complexes and that mutations in their components provoke defects in asymmetric stem cell division which finally results in tumor development93, 94.
Also in adult somatic stem cells, which play a central role in epithelial homeostasis, it is thought that polarity is particularly important with respect to fate decisions on stem cell division and that the failure in establishing or regulating stem cell polarity might result in neoplastic transformation152. The recent demonstration that Par3 influences the progenitor cell compartment during mammary gland morphogenesis153 supports this hypothesis and strengthens the urgency to thoroughly investigate the relationship between apico-basal polarity and progenitor/stem cells. That taking into consideration also the recently proposed link between EMT activation and the acquisition by tumor cells of a stem-like phenotype154.
Interfering with the signaling pathways that promote the loss of epithelial integrity may be one of the most promising avenues for the development of new therapeutic strategies to impair tumor progression and to revert preneoplastic lesion toward a more differentiated phenotype.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–4. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thompson EW, Newgreen DF. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition. Cancer Res. 2005;65:5991–5. doi: 10.1158/0008-5472.CAN-05-0616. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Micalizzi DS, Ford HL. Epithelial-mesenchymal transition in development and cancer. Future Oncol. 2009;5:1129–43. doi: 10.2217/fon.09.94. [DOI] [PubMed] [Google Scholar]
- Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–6. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Haley J, Kalluri R, Muthuswamy SK, Thompson E. Epithelial-mesenchymal transition. Cancer Res. 2008;68:9574–7. doi: 10.1158/0008-5472.CAN-08-2316. [DOI] [PubMed] [Google Scholar]
- Lee M, Vasioukhin V. Cell polarity and cancer-cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121:1141–50. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clemens JA, Williams ED, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- Moreno-Buono G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–69. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr Opin Cell Biol. 2005;17:499–5. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–57. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–32. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Balda MS. Epithelial tight junctions, gene expression and nucleojunctional interplay. J Cell Sci. 2007;120:1505–11. doi: 10.1242/jcs.005975. [DOI] [PubMed] [Google Scholar]
- Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–8. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Harris TJC, Tepass U. Adherens junctions: from molecules to morphogenesis. Nature Rev Mol Cell Biol. 2010;11:502–4. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A. 1999;96:511–6. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi M, Furuse K, Furuse H, Sasaki S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–45. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113:2363–74. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1–deficient mice. J Cell Biol. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dymanic behaviour of paired claudin strands within opposing plasma membranes. Proc Natl Acad Sci U S A. 2003;100:3971–76. doi: 10.1073/pnas.0630649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehder D, Iden S, Nasdala I, Wegener J, Brickwedde MK, Vestweber D, et al. Junctional adhesion molecule-A participates in the formation of apico-basal polarity through different domains. Exp Cell Res. 2006;312:3389–403. doi: 10.1016/j.yexcr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Citi S, Amorosi A, Franconi F, Giotti A, Zampi G. Cingulin, a specific protein component of tight junctions, is expressed in normal and neoplastic human epithelial tissues. Am J Pathol. 1991;138:781–9. [PMC free article] [PubMed] [Google Scholar]
- Keon BH, Schifer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–18. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Kakizaki M, Ono Y, Morimoto K, Takeuchi M, Inoue Y, et al. JEAP, a novel component of tight junctions in exocrine cells. J Biol Chem. 2002;277:5583–7. doi: 10.1074/jbc.M110154200. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann NY Acad Sci. 2009;1165:113–20. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight SW, Shin K, Fogg VC, Fan S, Liu C-J, Roh M, et al. Loss of PALS1 expression leads to tight junction and polarity defects. Mol Biol Cell. 2004;15:1981–90. doi: 10.1091/mbc.E03-08-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–63. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, et al. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene. 1999;18:7810–5. doi: 10.1038/sj.onc.1203153. [DOI] [PubMed] [Google Scholar]
- Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, et al. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21:5088–96. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dowbenko D, Spencer S, Laura R, Lee J, Gu Q, et al. Interaction of the tumor suppressor PTEN/MMAC with a PDZ Domain of MAGI3, a novel membrane-associated guanylate kinase. J Biol Chem. 2000;275:21477–85. doi: 10.1074/jbc.M909741199. [DOI] [PubMed] [Google Scholar]
- Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye MB, Yuan XJ, et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci U S A. 2000;97:4233–8. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Straight S, Margolis B. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol. 2005;168:705–11. doi: 10.1083/jcb.200408064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi M, Hamazaki Y, Kobayashi Y, Itoh M, Tsukita S, Furuse M, et al. Similar and distinct properties of MUPP1 and Patj, two homologous PDZ domain-containing tight-junction proteins. Mol Cell Biol. 2009;29:2372–89. doi: 10.1128/MCB.01505-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin M, Yap AS. Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J Mol Histol. 2004;35:839–44. doi: 10.1007/s10735-004-1833-2. [DOI] [PubMed] [Google Scholar]
- Kobielak A, Fuchs E. α-Catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–25. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Kimelman D. Mechanistic insights from structural studies of β-catenin and its binding partners. J Cell Sci. 2007;120:3337–44. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- Reynolds AB. p120-catenin: Past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Stewart DB, Nelson WJ. Coupling Assembly of the E-cadherin/β-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol. 1999;144:687–99. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai S, Correia J, Feng Y, Figueiredo J, Sun SX, Longmore GD, et al. α-Catenin mediates initial E-cadherin-dependent cell–cell recognition and subsequent bond strengthening. Proc Natl Acad Sci U S A. 2008;105:18331–6. doi: 10.1073/pnas.0806783105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–76. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, et al. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–76. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol. 2008;24:309–42. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94:655–67. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie K, Shimizu K, Sakisaka T, Ikeda W, Takai Y. Roles and modes of action of nectins in cell–cell adhesion. Semin Cell Dev Biol. 2004;15:643–56. doi: 10.1016/j.semcdb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta. 2008;1778:770–93. doi: 10.1016/j.bbamem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Humbert PO, Down LE, Russell SM. The scribble and Par complexes in polarity and migration: friends or foes. Trends Cell Biol. 2006;16:622–30. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–30. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–87. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Mortn DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C elegans embryos. Cell. 1988;52:311–20. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–22. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C, Medina E, Delgrossi MH, Michel D, Arsanto JP, Le Bivic A. hINADL/PATJ, a homolog of discs lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. J Biol Chem. 2002;277:25408–15. doi: 10.1074/jbc.M202196200. [DOI] [PubMed] [Google Scholar]
- Michel D, Arsanto JP, Massey-Harroche D, Béclin C, Wijnholds J, Le Bivic A. PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J Cell Sci. 2005;118:4049–57. doi: 10.1242/jcs.02528. [DOI] [PubMed] [Google Scholar]
- Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, et al. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene. 2003;22:9225–30. doi: 10.1038/sj.onc.1207154. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–7. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock F, Perrimon N. Molecular mechanisms of epithelial morphogenesis. Annu Rev Cell Dev Biol. 2002;18:463–93. doi: 10.1146/annurev.cellbio.18.022602.131838. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Apicobasal polarization: epithelial form and function. Curr Opin Cell Biol. 2003;15:747–52. doi: 10.1016/j.ceb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2007;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci U S A. 1996;93:10779–84. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H. The tight junction protein zo-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem. 2003;278:2692–700. doi: 10.1074/jbc.M206821200. [DOI] [PubMed] [Google Scholar]
- Fang L, Wang Y, Du D, Yang G, Tak Kwok T, Kai Kong S, et al. Cell polarity protein Par3 complexes with DNA-PK via Ku70 and regulates DNA double-strand break repair. Cell Res. 2007;17:100–16. doi: 10.1038/sj.cr.7310145. [DOI] [PubMed] [Google Scholar]
- Cline EG, Nelson WJ. Characterization of mammalian Par 6 as a dual-location protein. Mol Cell Biol. 2007;27:4431–43. doi: 10.1128/MCB.02235-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Dubash AD, Sharek L, Carr HS, Frost JA, Burridge K. The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization and influences their tumor suppressor activity. Mol Cell Biol. 2007;27:8683–97. doi: 10.1128/MCB.00157-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouly G, Abad PC, Knowles DW, Lelièvre SA. The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J Cell Sci. 2007;120:1596–1606. doi: 10.1242/jcs.03439. [DOI] [PubMed] [Google Scholar]
- Traweger A, Lehner C, Farkas A, Krizbai IA, Tempfer H, Klement E, et al. Nuclear Zonula occludens-2 alters gene expression and junctional stability in epithelial and endothelial cells. Differentiation. 2008;76:99–106. doi: 10.1111/j.1432-0436.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- Lelièvre SA. Tissue polarity-dependent control of mammary epithelial homeostasis and cancer development: an epigenetic perspective. J Mammary Gland Biol Neoplasia. 2010;15:49–63. doi: 10.1007/s10911-010-9168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert M, Papin M, Bonnans C, Darido C, Raye WS, Garambois V, et al. Symplekin promotes tumorigenicity by up-regulating claudin-2 expression. Proc Natl Acad Sci U S A. 2010;107:2628–33. doi: 10.1073/pnas.0903747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–6. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Gupta A, Massaguè J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Hoover KB, Liao S-Y, Bryant PJ. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol. 1998;153:1767–73. doi: 10.1016/S0002-9440(10)65691-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfed DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–31. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- Tobioka H, Isomura H, Kokai Y, Tokunaga Y, Yamaguchi J, Sawada N. Occludin expression decreases with the progression of human endometrial carcinoma. Hum Pathol. 2004;35:159–64. doi: 10.1016/j.humpath.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–20. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- Javier RT. Cell polarity proteins: common targets for tumorigenic human viruses. Oncogene. 2008;27:7031–46. doi: 10.1038/onc.2008.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polette M, Gilles C, Nawrocki-Raby B, Lohi J, Hunziker W, Foidart JM, et al. Membrane-type 1 matrix metalloproteinase expression is regulated by zonula occludens-1 in human breast cancer cells. Cancer Res. 2005;65:7691–8. doi: 10.1158/0008-5472.CAN-04-4230. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr Opin Genet Dev. 2006;16:51–9. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Huber M, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Thiery J, Sleeman J. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype. Nature Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–35. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- Storrs CH, Silverstein SJ. PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6. J Virol. 2007;81:4080–90. doi: 10.1128/JVI.02545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Glaunsinger B, Pim D, Javier R, Banks L. HPV E6 and MAGUK protein interactions: determination of the molecular basis for specific protein recognition and degradation. Oncogene. 2001;20:5431–9. doi: 10.1038/sj.onc.1204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus e6 proteins and the e6ap ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–53. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol D, Kuhne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene. 1999;18:5487–96. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–59. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–25. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–71. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisovsky M, Dresser K, Baker S, Fisher A, Woda B, Banner B, et al. Cell polarity protein Lgl2 is lost or aberrantly localized in gastric dysplasia and adenocarcinoma: an immunohistochemical study. Mod Pathol. 2009;22:977–84. doi: 10.1038/modpathol.2009.68. [DOI] [PubMed] [Google Scholar]
- Massimi P, Narayan N, Cuenda A, Banks L. Phosphorylation of the disc large tumor suppressor protein controls its membrane localization and enhances its susceptibility to HPV E6-induced degradation. Oncogene. 2006;25:4276–85. doi: 10.1038/sj.onc.1209457. [DOI] [PubMed] [Google Scholar]
- Latorre IJ, Roh MH, Frese KK, Weiss RS, Margolis B, Javier RT. Viral oncoprotein-induced mislocalization of select PDZ proteins disrupts tight junctions and causes polarity defects in epithelial cells. J Cell Sci. 2005;118:4283–93. doi: 10.1242/jcs.02560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CM, Tan TT, Mathew R, Nelson D, Mukherjee C, Degenhardt K, et al. Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression. Cancer Res. 2008;68:4105–15. doi: 10.1158/0008-5472.CAN-07-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP, et al. Atypical PKCi contributes to poor prognosis through loss of apical–basal polarity and Cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:12519–24. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NR, Jamieson L, Yu W, Zhang J, Gokmen-Polar Y, Sier D. Protein kinase Ciota is required for Ras transformation and colon carcinogenesis in vivo. J Cell Biol. 2004;164:797–802. doi: 10.1083/jcb.200311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, Fields AP. Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem. 2005;280:31109–15. doi: 10.1074/jbc.M505402200. [DOI] [PubMed] [Google Scholar]
- Aranda V, Nolan ME, Muthuswamy SK. Par complex in cancer: a regulator of normal polarity joins the dark side. Oncogene. 2008;27:6878–87. doi: 10.1038/onc.2008.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, et al. Par6–aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nature Cell Biol. 2006;8:1235–48. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- Wang X, Nie J, Zhou Q, Liu W, Zhu F, Chen W, et al. Downregulation of Par-3 expression and disruption of Par complex integrity by TGF-beta during the process of epithelial to mesenchymal transition in rat proximal epithelial cells. Biochim Biophys Acta. 2008;1782:51–9. doi: 10.1016/j.bbadis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Okuda H, Saitoh K, Hirai S, Iwai K, Takaki Y, Baba M, et al. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J Biol Chem. 2001;276:43611–7. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- Graziano F, Humar B, Guilford P. The role of E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Ann Oncol. 2003;14:1705–13. doi: 10.1093/annonc/mdg486. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Ohtani S, Terashima M, Satoh J, Soeta N, Saze Z, Kashimura S, et al. Expression of tight-junction-associated proteins in human gastric cancer: down-regulation of claudin-4 correlates with tumor aggressiveness and survival. Gastric Cancer. 2009;12:43–51. doi: 10.1007/s10120-008-0497-0. [DOI] [PubMed] [Google Scholar]
- Martin TA, Mansel RE, Jiang WG. Loss of occludin leads to the progression of human breast cancer. Int J Mol Med. 2010;26:723–34. doi: 10.3892/ijmm_00000519. [DOI] [PubMed] [Google Scholar]
- Busch C, Hanssen TA, Wagener C, O'Brink B. Down-regulation of CEACAM1 in human prostate cancer: correlation with loss of cell polarity, increased proliferation rate, and Gleason grade 3 to 4 transition. Hum Pathol. 2002;33:290–8. doi: 10.1053/hupa.2002.32218. [DOI] [PubMed] [Google Scholar]
- Sheehan GM, Kallakury BVS, Sheehan CE, Fisher HAG, Kaufman RP, Ross JS. Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas. Hum Pathol. 2007;38:564–9. doi: 10.1016/j.humpath.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Tokés AM, Kulka J, Paku S, Szik A, Páska C, Novák PK, et al. Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res. 2005;7:R296–305. doi: 10.1186/bcr983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn ML, Goncharuk VN, Diwan AH, Zhang PS, Shen SS, Prieto VG. Loss of claudin-1 expression in tumor-associated vessels correlates with acquisition of metastatic phenotype in melanocytic neoplasms. J Cutaneous Pathol. 2005;32:533–6. doi: 10.1111/j.0303-6987.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Kusumi T, Sato F, Kawasaki H, Shibata S, Ohashi M, et al. Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed Res. 2008;29:71–6. doi: 10.2220/biomedres.29.71. [DOI] [PubMed] [Google Scholar]
- Chao YC, Pan SH, Yang SC, Yu SL, Che TF, Lin CW, et al. Claudin-1 is a metastatic suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Resp Crit Care Med. 2009;179:123–33. doi: 10.1164/rccm.200803-456OC. [DOI] [PubMed] [Google Scholar]
- Nemeth J, Nemeth Z, Tatrai P, Peter I, Somoracz A, Szasz AM, et al. High expression of claudin-1 protein in papillary thyroid tumor and its regional lymph node metastasis. Pathol Oncol Res. 2010;16:19–27. doi: 10.1007/s12253-009-9182-9. [DOI] [PubMed] [Google Scholar]
- Oku N, Sasabe E, Ueta E, Yamamoto T, Osaki T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006;66:5251–7. doi: 10.1158/0008-5472.CAN-05-4478. [DOI] [PubMed] [Google Scholar]
- Kleinberg L, Holth A, Trope CG, Reich R, Davidson B. Claudin upregulation in ovarian carcinoma effusions is associated with poor survival. Hum Pathol. 2008;39:747–57. doi: 10.1016/j.humpath.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kinugasa T, Huo Q, Higashi D, Shibaguchi H, Kuroki M, Tanaka T, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27:3729–34. [PubMed] [Google Scholar]
- Leotlela PD, Wade MS, Duray PH, Rhode MJ, Brown HF, Rosenthal DT, et al. Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene. 2007;26:3846–56. doi: 10.1038/sj.onc.1210155. [DOI] [PubMed] [Google Scholar]
- Wu YL, Zhang S, Wang GR, Chen YP. Expression transformation of claudin-1 in the process of gastric adenocarcinoma invasion. World J Gastroenterol. 2008;14:4943–8. doi: 10.3748/wjg.14.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth Z, Szasz AM, Somoracz A, Tatrai P, Nemeth J, Gyorffy H, et al. Zonula occludens-1, occludin, and E-cadherin protein expression in biliary tract cancers. Pathol Oncol Res. 2009;15:533–9. doi: 10.1007/s12253-009-9150-4. [DOI] [PubMed] [Google Scholar]
- Gutwein P, Schramme A, Voss B, Abdel-Bakky MS, Doberstein K, Ludwig A, et al. Downregulation of junctional adhesion molecule-A is involved in the progression of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2009;380:387–91. doi: 10.1016/j.bbrc.2009.01.100. [DOI] [PubMed] [Google Scholar]
- Koshiba H, Hosokawa K, Kubo A, Tokumitsu N, Watanabe A, Honjo H. Junctional adhesion molecule A expression in human endometrial carcinoma. Int J Gynecol Cancer. 2009;19:208–13. doi: 10.1111/IGC.0b013e31819bc6e9. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol. 2005;166:1541–54. doi: 10.1016/S0002-9440(10)62370-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeff J, Shi X, Bode HP, Hoover K, Shrikhande S, Bryant PJ, et al. Altered expression and localization of the tight junction protein ZO-1 in primary and metastatic pancreatic cancer. Pancreas. 2001;23:259–65. doi: 10.1097/00006676-200110000-00006. [DOI] [PubMed] [Google Scholar]
- McSherry EA, McGee SF, Jirstrom K, Doyle EM, Brennan DJ, Landberg G, et al. JAM-A expression positively correlates with poor prognosis in breast cancer patients. Int J Cancer. 2009;125:1343–51. doi: 10.1002/ijc.24498. [DOI] [PubMed] [Google Scholar]
- Takagawa R, Akimoto K, Ichikawa Y, Akiyama H, Kojima Y, Ishiguro H, et al. High expression of atypical protein kinase C lambda/iota in gastric cancer as a prognostic factor for recurrence. Ann Surg Oncol. 2010;17:81–8. doi: 10.1245/s10434-009-0708-x. [DOI] [PubMed] [Google Scholar]
- Lisovsky M, Ogawa F, Dresser K, Woda B, Lauwers GY. Loss of cell polarity protein Lgl2 in foveolar-type gastric dysplasia: correlation with expression of the apical marker aPKC-zeta. Virchows Arch. 2010;457:635–42. doi: 10.1007/s00428-010-0990-9. [DOI] [PubMed] [Google Scholar]
- Lisovsky M, Dresser K, Woda B, Mino-Kenudson M. Immunohistochemistry for cell polarity protein lethal giant larvae 2 differentiates pancreatic intraepithelial neoplasia-3 and ductal adenocarcinoma of the pancreas from lower-grade pancreatic intraepithelial neoplasias. Hum Pathol. 2010;41:902–9. doi: 10.1016/j.humpath.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Grifoni D, Garoia F, Schimanski CC, Schmitz G, Laurenti E, Galle PR, et al. The human protein Hugl-1 substitutes for Drosophila Lethal giant larvae tumour suppressor function in vivo. Oncogene. 2004;23:8688–94. doi: 10.1038/sj.onc.1208023. [DOI] [PubMed] [Google Scholar]
- Kuphal S, Wallner S, Schimanski CC, Bataille F, Hofer P, Strand S, et al. Expression of Hugl-1 is strongly reduced in malignant melanoma. Oncogene. 2006;25:103–10. doi: 10.1038/sj.onc.1209008. [DOI] [PubMed] [Google Scholar]
- Schimanski CC, Schmitz G, Kashyap A, Bosserhoff AK, Bataille F, Schafer SC, et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene. 2005;24:3100–9. doi: 10.1038/sj.onc.1208520. [DOI] [PubMed] [Google Scholar]
- Lu X, Feng X, Man X, Yang G, Tang L, Du D, et al. Splicing of Hugl-1 is associated with hepatocellular carcinoma progression. Clin Cancer Res. 2009;15:3287–96. doi: 10.1158/1078-0432.CCR-08-2078. [DOI] [PubMed] [Google Scholar]
- Lin HT, Steller MA, Aish L, Hanada T, Chishti AH. Differential expression of human Dlg in cervical intraepithelial neoplasias. Gynecol Oncol. 2004;93:422–8. doi: 10.1016/j.ygyno.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Cavatorta AL, Fumero G, Choihy D, Aguirre R, Nocito AL, Giri AA, et al. Differential expression of the human homologue of Drosophila discs large oncosuppressor in histologic samples from human papillomavirus-associated lesions as a marker for progression to malignancy. Int J Cancer. 2004;111:373–80. doi: 10.1002/ijc.20275. [DOI] [PubMed] [Google Scholar]
- Gardiol D, Zacchi A, Petrera F, Stanta G, Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int J Cancer. 2006;119:1285–90. doi: 10.1002/ijc.21982. [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Zhan W, Dan L. hScrib, a human homolog of Drosophila neoplastic tumor suppressor, is involved in the progress of endometrial cancer. Oncol Res. 2010;18:593–9. doi: 10.3727/096504010x12767359114045. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Yano T, Nakagawa K, Takizawa S, Suzuki Y, Yasugi T, et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer. 2004;90:194–9. doi: 10.1038/sj.bjc.6601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Kito K, Takeuchi T, Imai Y, Murase R, Ueda N, et al. Human scribble accumulates in colorectal neoplasia in association with an altered distribution of beta-catenin. Hum Pathol. 2007;38:1273–81. doi: 10.1016/j.humpath.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, et al. The polarity protein par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 2008;68:8201–9. doi: 10.1158/0008-5472.CAN-07-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarsa C, Bassani N, Ambrogi F, Zabucchi G, Boracchi P, Biganzoli E, et al. Epithelial-mesenchymal transition, cell polarity and stemness-associated features in malignant pleural mesothelioma. Cancer Lett. 2011;302:136–43. doi: 10.1016/j.canlet.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Hoevel T, Macek R, Swisshelm K, Kubbies M. Re-expression of the TJ protein CLDN1 induces apoptosis in breast tumor spheroids. Int J Cancer. 2004;108:374–83. doi: 10.1002/ijc.11571. [DOI] [PubMed] [Google Scholar]
- Osanai M, Murata M, Nishikiori N, Chiba H, Kojima T, Sawad N. Epigenetic silencing of occludin promotes tumorigenic and metastatic properties of cancer cells via modulations of unique sets of apoptosis-associated genes. Cancer Res. 2006;66:9125–33. doi: 10.1158/0008-5472.CAN-06-1864. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bao Y, Peng W, Goldberg M, Love K, Bumcrot DA, et al. Claudin-3 gene silencing with siRNA suppresses ovarian tumor growth and metastasis. Proc Natl Acad Sci U S A. 2009;106:3426–30. doi: 10.1073/pnas.0813348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Nakano M, Suzuki M, Kawamoto S, Furuya A, Ohta S, Nakamura K, et al. Characterization and evaluation of the antitumour activity of a dual-targeting monoclonal antibody against claudin-3 and claudin-4. Anticancer Res. 2010;30:4555–62. [PubMed] [Google Scholar]
- Suzuki M, Kato-Nakano M, Kawamoto S, Furuya A, Abe Y, Misaka H, et al. Therapeutic antitumor efficacy of monoclonal antibody against Claudin-4 for pancreatic and ovarian cancers. Cancer Sci. 2009;100:1623–30. doi: 10.1111/j.1349-7006.2009.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamagna C, Hodivala-Dilke KM, Imhof BA, Aurrand-Lions M. Antibody against junctional adhesion molecule-c inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:5703–10. doi: 10.1158/0008-5472.CAN-04-4012. [DOI] [PubMed] [Google Scholar]
- Saeki R, Kondoh M, Kakutani H, Matsuhisa K, Takahashi A, Suzuki H, et al. A claudin-targeting molecule as an inhibitor of tumor metastasis. J Pharmacol Exp Ther. 2010;334:576–82. doi: 10.1124/jpet.110.168070. [DOI] [PubMed] [Google Scholar]
- Cocco E, Casagrande F, Bellone S, Richter CE, Bellone M, Todeschini P, et al. Clostridium perfringens enterotoxin carboxy-terminal fragment is a novel tumor-homing peptide for human ovarian cancer. BMC Cancer. 2010;10 doi: 10.1186/1471-2407-10-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Näthke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–24. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- Florian MC, Geiger H. Concise review: polarity in stem cells, disease, and aging. Stem Cells. 2010;28:1623–9. doi: 10.1002/stem.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. The Par3/aPKC interaction is essential for end bud remodelling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 2009;23:1450–60. doi: 10.1101/gad.1795909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The Epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]