Abstract
AtPeps are a family of small peptides in Arabidopsis that are believed to act as endogenous amplifiers of the plant’s innate immune response. In our recent study,10 we showed that in Arabidopsis leaf disks, bacterial MAMPs (microbe-associated molecular patterns) such as the flagellin derived elicitor flg22, greatly enhanced the release of reactive oxygen species (ROS) triggered by a subsequent AtPep-perception. This enhanced ROS production could be a hallmark either of improved local defense or of the initiation of ROS-based systemic signaling. Here, we established a superior ROS detection system based on a new derivative of luminol (L-012). With this sensitive system we were able to show that chitin, too, acts as an enhancer of AtPep-triggered ROS, linking this specific defense response amplification also to the recognition of fungal pathogens. In addition we used the more sensitive ROS assay to transfer the experimental setup from cut leaf disks to unwounded seedlings. Thereby we revealed that wounding is not a prerequisite to enable the MAMP-induced enhancement of the AtPep-triggered ROS response.
Keywords: innate immunity, reactive oxygen species, elicitor, chitin, AtPep
The first line of plant defense against pathogens is based on the recognition of conserved microbe-associated molecular patterns (MAMPs) by specific membrane-bound receptors leading to the activation of pattern-triggered immunity (PTI).1,2 Recently, a family of seven endogenous peptides in Arabidopsis, named AtPeps, was shown to activate PTI-like responses when binding their respective receptors PEPR1 and PEPR2.3-6 Therefore, and since the expression of the PEPRs as well as the AtPep-precursor proteins PROPEPs is induced under conditions of biotic stress, AtPeps are believed to act as an additional endogenous defense system, amplifying the defense response locally and/or spreading this response to distal, non-infected tissue.1,7 Moreover, recent studies suggest an interaction of AtPep-signaling with the defense hormone ethylene to maintain PTI responses after the initial MAMP recognition.8,9 Further underlining the role of AtPeps as amplifiers of defense, we recently showed that one particular AtPep-triggered defense response, the production of reactive oxygen species, is greatly enhanced upon previous MAMP recognition.10 This increased ROS production seems to be Pep-specific, since no similar effect was observed for MAMPs, where previous elicitor recognition did not lead to an increased production of ROS. Remarkably, this MAMP-triggered enhancement of subsequent responses to AtPeps was exclusive for ROS and did not affect other responses connected to PTI, such as MAPK activation, ethylene production or defense gene expression.
To analyze this MAMP-mediated enhancement of AtPep-triggered ROS production in greater detail we established a more sensitive ROS detection system based on a luminol derivative (L-012, Wako Chemicals). As shown in Figure 1, also with the new derivative of luminol we detected the strong enhancement of AtPep-triggered ROS by previous treatment with flg22, the active epitope of bacterial flagellin.
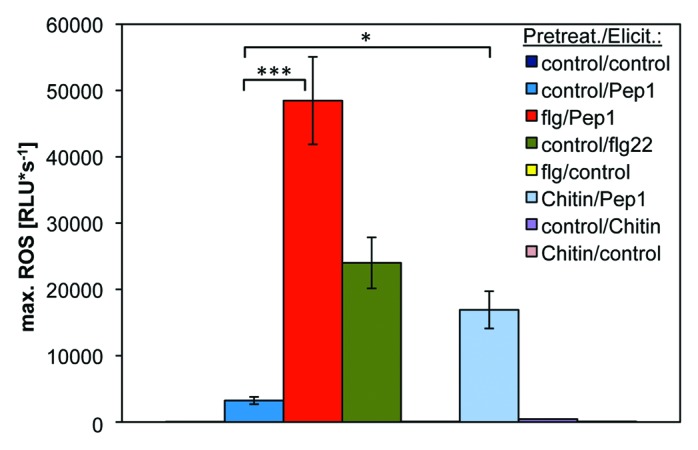
Figure 1. Chitin pretreatment strongly enhances the AtPep-triggered ROS response in Arabidopsis leaf disks. Arabidopsis leaf disks were pretreated, as described previously,10 with either 1 µM of flg22, chitin or a control solution for 16 h and then treated with 1 µM of the indicated elicitor or without any peptide (control). Columns represent averages of the peak values of ROS production of eight biological replicates detected with the new luminol derivative L-012. Error bars depict 1 SE of the mean. Asterisks represent t-test results (*, p < 0.05; **, p < 0.01). RLU = relative light units.
To investigate if the observed ROS enhancement might be restricted to bacterial MAMPs we then took this more sensitive ROS detection system and used a fungal elicitor molecule, chitin, which strongly differs from flg22 in its chemical composition and the plants perception system used to detect it. Whereas flg22 is a small peptide composed of 22 amino acids, chitin is an oligomer of N-acetyl-D-glucosamine subunits. Moreover, in contrast to FLS2, the receptor for flg22, which utilizes leucine-rich repeats (LRRs) to detect the peptide, the Arabidopsis chitin receptor CERK1 contains three lysine motif (LysM) domains for chitin perception.11 In addition, CERK1 does not require interaction with the co-receptor BAK1 for full activity, whereas FLS2 does.12 Finally, also the PTI signature elicited by chitin is slightly different from the one triggered by flg22.13 For example, chitin elicitation generally induces a much weaker and less transient ROS production in comparison to the ROS production triggered by flg22 (Fig. 1).10,14
Leaf disks only treated with chitin (Fig. 1, control/chitin) showed a very weak ROS response at roughly a third of the intensity compared with AtPep1-treated leaf disks but still significantly higher than the control treatment (Fig. 1, control/control). However, despite of a much weaker ROS-inducing capacity than flg22, chitin pretreatment strongly enhanced a subsequent AtPep-triggered ROS production (Fig. 1, control/Pep1 vs. chitin/Pep1). Thus, we can conclude that the AtPep-ROS enhancing effect is neither specific for bacterial MAMPs, since it is also triggered by fungal MAMPs, nor specific for BAK1-associated MAMP perception systems, indicating that it might be a more general phenomenon.
Since we always measured the ROS response in cut leaf disks, we could never fully exclude the potential necessity of wounding to enable this effect. Previously, alternative setups using unwounded seedlings did not produce ROS levels detectable with the standard luminol-based detection system. Here, with the new assay based on the more sensitive luminol derivative L-012 we were able to detect the comparably low levels of ROS produced by seedlings upon AtPep1 or flg22 treatment (Fig. 2A).
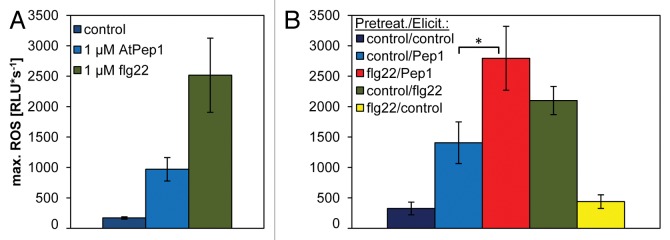
Figure 2. Elicitor-triggered and MAMP-enhanced AtPep-triggered ROS produced by Arabidopsis seedlings. (A) ROS response of 5-d-old Arabidopsis seedlings exposed to 1 µM of the respective elicitor. Elicitor treatment and ROS quantification was performed directly after harvesting the seedlings from 0.5-strength MS plates. (B) Seedlings display an enhanced AtPep-triggered ROS after flg22 pretreatment. Seedlings were pretreated with either 1 µM of flg22 or a control solution for 16 h and then treated with 1 µM of the indicated elicitor or without any peptide (control). (A and B) Columns represent averages of the peak values of ROS production of eight biological replicates detected with the new luminol derivative L-012. Error bars depict 1 SE of the mean. Asterisks represent t-test results (*, p < 0.05). RLU = relative light units.
Using the same setup (except for the luminol) as described in Flury et al. (2013),10 we observed that 5-d-old seedlings, pretreated with flg22 for 16 h, clearly showed an increased AtPep-triggered ROS response compared with seedlings pretreated with a control solution (Fig. 2B). Therefore, we can now exclude the possibility that wounding is a factor in the MAMP-induced enhancement of the AtPep-ROS response. Moreover, our data shows that already young seedlings are capable to react to MAMPs with a ROS burst, although in our assay the maximal ROS production measured in relative light units (RLUs) of a leaf disk treated with flg22 reached 25,000 RLUs (Fig. 1) whereas a 5-d-old seedling produced upon the same treatment a maximum of only 2,500 RLUs (Fig. 2A). The latter response was not detectable with the previous luminol-based ROS detection system.
Since plants are believed to have numerous endogenous elicitor molecules apart from AtPeps,1 it will be intriguing to find out whether this enhanced ROS production upon MAMP perception is restricted to AtPeps or whether the ROS production triggered by other endogenous elicitors can be similarly enhanced.
Glossary
Abbreviations:
- MAMPs
microbe-associated molecular patterns
- ROS
reactive oxygen species
- PTI
pattern-triggered immunity
- PRR
pattern-recognition receptor
- PEPR
AtPep-receptor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25346
References
- 1.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 2.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi Y, Pearce G, Ryan CA. The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc Natl Acad Sci USA. 2006;103:10104–9. doi: 10.1073/pnas.0603729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem. 2010;285:13471–9. doi: 10.1074/jbc.M109.097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22:508–22. doi: 10.1105/tpc.109.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA. 2006;103:10098–103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CA, Huffaker A, Yamaguchi Y. New insights into innate immunity in Arabidopsis. Cell Microbiol. 2007;9:1902–8. doi: 10.1111/j.1462-5822.2007.00991.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Wu Y, Yang F, Zhang Y, Chen S, Xie Q, et al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc Natl Acad Sci USA. 2013;110:6205–10. doi: 10.1073/pnas.1215543110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tintor N, Ross A, Kanehara K, Yamada K, Fan L, Kemmerling B, et al. Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc Natl Acad Sci USA. 2013;110:6211–6. doi: 10.1073/pnas.1216780110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flury P, Klauser D, Schulze B, Boller T, Bartels S. The anticipation of danger: MAMP perception enhances AtPep-triggered oxidative burst. Plant Physiol. 2013 doi: 10.1104/pp.113.216077. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Liu Z, Song C, Hu Y, Han Z, She J, et al. Chitin-induced dimerization activates a plant immune receptor. Science. 2012;336:1160–4. doi: 10.1126/science.1218867. [DOI] [PubMed] [Google Scholar]
- 12.Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem. 2010;285:9444–51. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felix G, Baureithel K, Boller T. Desensitization of the perception system for chitin fragments in tomato cells. Plant Physiol. 1998;117:643–50. doi: 10.1104/pp.117.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chinchilla D, et al. Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci USA. 2009;106:15973–8. doi: 10.1073/pnas.0905532106. [DOI] [PMC free article] [PubMed] [Google Scholar]


