Abstract
Aim:
To develop a homogeneous assay for high-throughput screening (HTS) of inhibitors of phosphodiesterase 10 (PDE10).
Methods:
Purified human PDE10 enzyme derived from E coli, [3H]-cAMP and yttrium silicate microbeads were used to develop an HTS assay based on the scintillation proximity assay (SPA) technology. This method was applied to a large-scale screening campaign against a diverse compound library and subsequent confirmation studies. Preliminary structure-activity relationship (SAR) studies were initiated through limited structural modifications of the hits.
Results:
The IC50 value of the control compound (papaverine) assessed with the SPA approach was comparable and consistent with that reported in the literature. Signal to background (S/B) ratio and Z' factor of the assay system were evaluated to be 5.24 and 0.71, respectively. In an HTS campaign of 71 360 synthetic and natural compounds, 67 hits displayed reproducible PDE10 inhibition, of which, 8 were chosen as the scaffold for structural modifications and subsequent SAR analysis.
Conclusion:
The homogeneous PDE10 SPA assay is an efficient and robust tool to screen potential PDE10 inhibitors. Preliminary SAR studies suggest that potent PDE10 inhibitors could be identified and developed through this strategy.
Keywords: phosphodiesterase 10 (PDE10), papaverine, high-throughput screening, psychosis
Introduction
The cyclic nucleotides, cyclic-adenosine monophosphate (cAMP) and cyclic-guanosine monophosphate (cGMP), function as intracellular second messengers regulating a vast array of biological processes. Given the important role of cyclic nucleotides in signal transduction and physiological pathways, therapeutic benefits may be derived from the use of compounds that affect the regulation of cyclic nucleotide signaling1. Phosphodiesterases (PDEs), which regulate cyclic nucleotide signaling by hydrolyzing cAMP and cGMP, may play pivotal roles in cell signaling. To date, 11 known families of PDEs encoded by 21 different genes have been identified2. The PDE families are distinguished functionally based on cyclic nucleotide substrate specificity, mechanisms of regulation and sensitivity to inhibitors. Due to differential expression and functionality throughout the organism, different PDE isozymes exert distinct physiological functions3.
PDE10 is recognized as a unique family based on its primary amino acid sequence and distinct enzymatic activity. It displays dual activities on hydrolysis of both cAMP and cGMP, with an affinity for cAMP (Km=0.05 μmol/L) higher than that for cGMP (Km=3 μmol/L)4,5. Its mRNA is highly expressed in the testis and brain6. Loss of PDE10 activity in the striatum of transgenic mice led to dysregulation of cAMP and cGMP levels, thereby implying an important role in Huntington's disease7. Furthermore, Siuciak and colleagues revealed that PDE10A is involved in the regulation of striatal output, possibly by reducing the sensitivity of medium spiny neurons to glutamatergic excitation8. These results support the hypothesis that PDE10A inhibition may present a novel treatment for psychosis.
Small chemical modulators for PDE10 are of great value in both investigating enzyme properties and developing therapeutics, as exemplified by the PDE10 inhibitor, papaverine albeit it is not PDE10 selective. It was found that conditioned avoidance response in rats and mice as well as PCP- and amphetamine-stimulated locomotor activity in rats could be suppressed by papaverine9. Moreover, PDE10A inhibition caused by papaverine effectively improved executive function deficits associated with experimental schizophrenia in rats10. Selective PDE10A inhibitors TP-10 and MP-10 have been reported to decrease psychomotor activity, reverse deficits in prepulse inhibition and inhibit conditioned avoidance response (CAR) in rodents, implying potential antipsychotic activities11,12. These compounds also improved cognitive performance in domains impaired in schizophrenia and negative symptoms in rodents11,12,13. As mentioned above, it is believed that novel PDE10 inhibitors would serve not only as potential drug candidates to treat psychosis, but also as important tools to elucidate the mechanisms of action related to PDE10.
To discover and characterize novel chemical modulators for PDEs from large compound libraries, approaches amenable for high-throughput screening (HTS) are necessary. Obviously, there is a strong need to seek new assay systems that could offer advantages such as high throughput, robustness and low cost.
In the present study, we describe the development and validation of a robust scintillation proximity assay (SPA) for identification and characterization of human PDE10 inhibitors. A HTS campaign of 71 360 synthetic and natural compounds using this assay system was carried out. As a result, a number of novel inhibitors structurally distinct from the compounds known to modulate human PDE10 function were discovered. Eight initial hits with similar structures were chosen as the scaffold for structural modifications and subsequent structure-activity relationship (SAR) analyses.
Materials and methods
Reagents
Papaverine was provided by H Lundbeck A/S. HEPES was obtained from Invitrogen (Carlsbad, CA, USA). MgCl2 and Tween 20 were purchased from Shanghai Chemical Reagents Co, Ltd (Shanghai, China). Isoplate™ mircotiter plate was the product of PerkinElmer (Boston, MA, USA). SPA yttrium silicate beads and [3H]-cAMP (59 Ci/mmol) were procured from GE Healthcare (Little Chalfont, Buckinghamshire, UK).
PDE10 assay
The SPA assay for PDE10 was modified according to the method described previously14. Briefly, compounds (2 μL per well) dissolved in 100% dimethylsulphoxide (DMSO) were pre-plated onto Isoplate™ mircotiter plates. The wells were incubated in 48 μL PDE10 solution containing 50 mmol/L HEPES, 10 mmol/L MgCl2, 0.03 % Tween 20, pH=7.6. The volume of PDE10 used was determined by enzymatic activity that provides a high signal window but does not deplete the substrate.
After 10 min incubation at room temperature, 50 μL [3H]-cAMP (20 nmol/L) per well was added to the assay plates. The plates were then placed at a shaking table (450 r/min×10 min) and incubated for 40 min at room temperature. Fifty microliter STOP-mixture (SPA beads dissolved in water to a concentration of 10 mg/mL) was added to each well thereafter and incubated for 10 min with shaking at 450 r/min before counting on the Microbeta scintillation counter (PerkinElmer).
HTS campaign
The compound library used for the screening of human PDE10 inhibitors consisted of 71,360 pure synthetic and natural compounds. Samples dissolved in 100% DMSO were applied to the primary screening, with an average final concentration of 3.3 μmol/L for each compound. In each 96-well assay plate, 6 wells were used as IC100 controls (10 μmol/L papaverine), 4 wells as IC50 controls (300 nmol/L papaverine), and 6 wells as IC0 controls (2% DMSO). Compounds showing greater than 70% inhibition relative to IC0 were considered as “hits”.
Data analysis
Data were analyzed using GraphPad Prism software (GraphPad, San Diego, CA, USA). Non-linear regression analyses were performed to generate dose-response curves and to calculate IC50 values. Percentage responses of hits were compared with that of 10 μmol/L papaverine, which was defined as 100%. Z' factor, which estimates the suitability of the SPA assay to HTS, was assessed as previously described15. The equations of S/B=Msignal/Mbackground and CV=100×(SD/M) were applied to calculate the signal/background ratio (S/B) and the coefficient of variation (CV) values of the assay, respectively.
Results
Assay validation
We first used various amounts of the PDE10 enzyme extract to assess the optimal protein concentration for the SPA assay. Both signal (IC0; using DMSO) and background (IC100; using 10 μmol/L papaverine) were investigated, and the optimal PDE10 amount was determined to be 5 nL/well that resulted in a signal to background (S/B) ratio of 6.2 (Figure 1). The effects of reaction time on the assay signal and background are shown in Figure 2. Not only did the signal but also the background increase along with prolonged [3H]-cAMP incubation time. As a result, a substrate incubation time of 40 min led to a better S/B ratio (5.36) and was thus used in the HTS campaign. DMSO is the most commonly employed solvent to dissolve organic compounds in HTS. At concentrations of up to 2% DMSO did not affect the assay performance (data not shown). Under these optimized conditions, the IC50 value of the PDE10 inhibitor papaverine was 364 nmol/L (Figure 3).
Figure 1.
Reactivity characteristics of papaverine with different amounts of PDE10. Serial titration of PDE10 was made to determine the optimal protein concentration.
Figure 2.
Effects of different substrate incubation time on signal and background readout in the scintillation proximity assay system with 2% DMSO or 10 μmol/L papaverine present. Data (mean±SEM) are representative of three independent experiments.
Figure 3.
Dose-response curve of papaverine on PDE10 activity measured by the scintillation proximity assay with optimal conditions, from which IC50 value was calculated (n=3, mean±SEM).
Assay performance
In order to apply the SPA assay system to HTS, both signal evoked by [3H]-AMP, the product of PDE10 enzyme reaction, and background (10 μmol/L papaverine) were studied. As shown in Figure 4, the coefficient of variation (CV) values were 5.5% for total signal and 12.5% for background, respectively. The Z' factor for the assay was 0.71 with an S/B ratio of 5.24. These characteristics indicate that the system is of high quality and well-suited to HTS15.
Figure 4.
Z' factor of the scintillation proximity assay on PDE10 was determined at optimized conditions. Forty-eight replicates of signal (2% DMSO, black circle) and background (10 μmol/L papaverine, white circle) were investigated.
Identification of PDE10 inhibitors
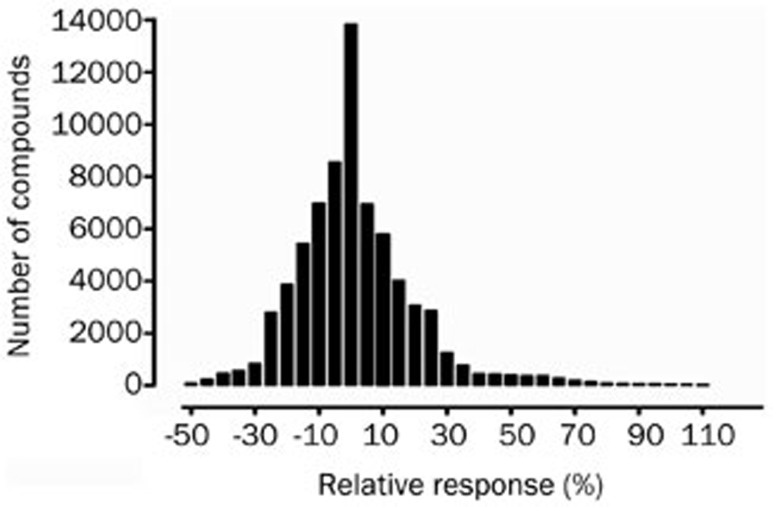
The result of the HTS campaign for PDE10 inhibitors is shown in Figure 5. Of the 71 360 samples initially screened for potential inhibitory effect on PDE10, 692 hits (0.97%) displaying greater than 70% positive modulation activity relative to papaverine were discovered. In the HTS campaign, the Z' factors varying between 0.69 and 0.74 conferred the assay quality requirement of above 0.5. In the secondary confirmation study (initial hits in duplicates), 67 compounds demonstrated consistently inhibition compared to papaverine (9.68% of the initial hits or 0.09% final hit rate). Among them, 8 synthetic chemicals with similar structures displayed IC50 values below 0.4 μmol/L (Table 1). The scaffold of these novel PDE inhibitors was chosen as the starting point for medicinal chemistry efforts.
Figure 5.
HTS campaign of 71 360 compounds using the scintillation proximity assay. Results are expressed as relative response compared to 10 μmol/L papaverine by the number of hit compounds at each 5% interval.
Table 1. Novel small molecule inhibitors of the human PDE10 identified by a scintillation proximity assay-based high-throughput screening campaign.
| Compound | Structure | MW | IC50 (nmol/L) |
|---|---|---|---|
| A-021-011 (d) |  |
480.52 | 17.1 |
| A-095-011 (d) |  |
523.54 | 129.9 |
| 096-oa5-011 |  |
435.53 | 65.4 |
| 096-oa5-020 |  |
437.50 | 106.8 |
| A8 (558) 046-SH2-011 |  |
511.65 | 307.1 |
| D7 (544) 064-SH2-053 |  |
518.55 | 125.7 |
| IMMLG-5061 |  |
524.65 | 91.0 |
| IMMLG-5178 |  |
496.59 | 84.3 |
MW, molecular weight.
Structural modifications
Limited medicinal chemistry efforts were made to modify the structures of the initial hits. Of the 25 analogues made16, 7 were tested for inhibitory properties on PDE10 and none of which showed any improvements in terms of bioactivity (Table 2).
Table 2. Structural modifications and inhibition on human PDE10.
| Compound | Structure | MW | IC50 (nmol/L) |
|---|---|---|---|
| 5g |  |
448.49 | 40 |
| lw-IV-28-1 |  |
468.56 | 45 |
| lw-IV-30-2 |  |
504.43 | 403 |
| 5f |  |
425.50 | 353 |
| 10c |  |
544.99 | 410 |
| 5c |  |
470.54 | 180 |
| lw-V-40 |  |
448.49 | 162 |
MW, molecular weight.
Discussion
Due to its large variety of biological activities, studies on PDE10 have become an emerging area that attracts scientists from different disciplines. Apart from potential value of using PDE10 as a drug target, exploration of small molecule inhibitors of the enzyme could provide powerful tools in further understanding of the physiological roles of this phosphodiesterase subtype. Here we report a rational approach to discover novel PDE10 inhibitors by use of a robust SPA assay.
According to our prior experience in the development of SPA-based receptor binding HTS methods17,18, efforts were made to expand its application to PDE10, one of the phosphodiesterases. The assay is based on the observation that linear nucleotides bind preferentially to SPA yttrium silicate beads compared to cyclic nucleotides in the presence of zinc sulphate. The conversion of [3H]-cAMP to [3H]-AMP catalyzed by PDE10 results in an increase in radioactivity bound to the beads thereby enhancing the signal.
In order to apply the SPA assay to a HTS setting, many factors should be considered to maximize the signal and the S/B ratio. As shown in Figure 1, varying the amount of PDE10 per well influenced the total signal and background of this assay. The substrate incubation time of 40 min was chosen for the HTS campaign as it resulted in a robust total signal and the highest S/B ratio (Figure 2). DMSO tolerance should also be examined as it can limit the maximum concentration of a compound to be examined. In this case, up to 2% DMSO exhibited no obvious effect on the assay performance. The Z' factor is a useful indicator for assessing the quality of HTS assays. In general, a Z' value above 0.5 suggests that an assay is robust enough for use in HTS settings. The SPA system described in this paper consistently displayed a Z' value equal or above 0.69. This, and in conjunction with other parameters such as S/B ratio and CV values, suggest that the assay system employed is well suited to HTS. Subsequent HTS campaign using this system led to the discovery of 67 confirmed hits. Among them, 8 with similar structures displayed consistent inhibitory effects on PDE10 with IC50 values below 0.4 μmol/L. Limited structural modifications did not yield more potent analogues but this medicinal chemistry efforts in still ongoing.
In summary, we have developed and validated a SPA-based assay to identify novel PDE10 inhibitors. The approach may also be applicable to identify and evaluate modulators of other PDEs. Moreover, the newly discovered PDE10 inhibitors have distinct structural features that differ from those reported elsewhere. Indeed, knowledge obtained from the present studies will facilitate the pursuit to develop new PDE10 inhibitors.
Author contribution
Qun-yi LI, Ming-kai XU, Gang LIU, and Claus Tornby CHRISTOFFERSEN performed experiments; Qun-yi LI and Ming-wei WANG analyzed the data; Qun-yi LI and Ming-wei WANG wrote the paper.
Acknowledgments
This study was supported in part by grants from H Lundbeck A/S and the Ministry of Science and Technology of China (2009ZX09302-001, 2012ZX09304011 and 2013ZX09507002), Shanghai Science and Technology Development Fund (11DZ2292200). We are indebted to to Dale E MAIS for critical review of this manuscript.
References
- Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100:1569–78. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]
- Vezzosi D, Bertherat J. Phosphodiesterases in endocrine physiology and disease. Eur J Endocrinol. 2011;165:177–88. doi: 10.1530/EJE-10-1123. [DOI] [PubMed] [Google Scholar]
- Chan S, Yan C. PDE1 isozymes, key regulators of pathological vascular remodeling. Curr Opin Pharmacol. 2011;11:720–4. doi: 10.1016/j.coph.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K, Snyder PB, Uher L, Rosman GJ, Ferguson K, Florio VA. Isolation and characterization of PDE10A, a novel human 3′,5′-cyclic nucleotide phosphodiesterase. Gene. 1999;234:109–17. doi: 10.1016/s0378-1119(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ, Beavo JA. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci U S A. 1999;96:7071–6. doi: 10.1073/pnas.96.12.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishige K, Kotera J, Omori K. Striatum- and testis-specific phosphodiesterase PDE10A: isolation and characterization of a rat PDE10A. Eur J Biochem. 1999;266:1118–27. doi: 10.1046/j.1432-1327.1999.00963.x. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Robertson HA, Denovan-Wright EM. Striatal phosphodiesterase mRNA and protein levels are reduced in Huntington's disease transgenic mice prior to the onset of motor symptoms. Neuroscience. 2004;123:967–81. doi: 10.1016/j.neuroscience.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Fujiwara RA, James LC, Williams RD, et al. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: evidence for altered striatal function. Neuropharmacology. 2006;51:374–85. doi: 10.1016/j.neuropharm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, Harms JF, Lebel LA, McCarthy SA, Chambers L, et al. Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology. 2006;51:386–96. doi: 10.1016/j.neuropharm.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Murphy ER, Baxter MG. PDE10A inhibition reverses subchronic PCP-induced deficits in attentional set-shifting in rats. Eur J Neurosci. 2005;21:1070–6. doi: 10.1111/j.1460-9568.2005.03937.x. [DOI] [PubMed] [Google Scholar]
- Grauer SM, Pulito VL, Navarra RL, Kelly MP, Kelley C, Graf R, et al. Phosphodiesterase 10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J Pharmacol Exp Ther. 2009;331:574–90. doi: 10.1124/jpet.109.155994. [DOI] [PubMed] [Google Scholar]
- Menniti FS, Chappie TA, Humphrey JM, Schmidt CJ. Phosphodiesterase 10A inhibitors: a novel approach to the treatment of the symptoms of schizophrenia. Curr Opin Investig Drugs. 2007;8:54–9. [PubMed] [Google Scholar]
- Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, et al. Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther. 2008;325:681–90. doi: 10.1124/jpet.107.132910. [DOI] [PubMed] [Google Scholar]
- Coste H, Grondin P. Characterization of a novel potent and specific inhibitor of type V phosphodiesterase. Biochem Pharmacol. 1995;50:1577–85. doi: 10.1016/0006-2952(95)02031-4. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Liang W, Liu G. Synthesis of mono- and di-fluorinated benzimidazoles. Chin J Chem. 2011;29:983–90. [Google Scholar]
- Meng T, Su HR, Binkert C, Fischli W, Zhou L, Shen JK, et al. Identification of non-peptidic neuromedin U receptor modulators by a robust homogeneous screening assay. Acta Pharmacol Sin. 2008;29:517–27. doi: 10.1111/j.1745-7254.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang YY, Lu HL, Li QY, Zhou CH, Wang MW. Rhodanine derivatives as novel peroxisome proliferator-activated receptor gamma agonists. Acta Pharmacol Sin. 2007;28:2033–9. [PubMed] [Google Scholar]