Abstract
The initial inflammatory phase of bone fracture healing represents a critical step for the outcome of the healing process. However, both the mechanisms initiating this inflammatory phase and the function of immune cells present at the fracture site are poorly understood. In order to study the early events within a fracture hematoma, we established an in vitro fracture hematoma model: we cultured hematomas forming during an osteotomy (artificial bone fracture) of the femur during total hip arthroplasty (THA) in vitro under bioenergetically controlled conditions. This model allowed us to monitor immune cell populations, cell survival and cytokine expression during the early phase following a fracture. Moreover, this model enabled us to change the bioenergetical conditions in order to mimic the in vivo situation, which is assumed to be characterized by hypoxia and restricted amounts of nutrients. Using this model, we found that immune cells adapt to hypoxia via the expression of angiogenic factors, chemoattractants and pro-inflammatory molecules. In addition, combined restriction of oxygen and nutrient supply enhanced the selective survival of lymphocytes in comparison with that of myeloid derived cells (i.e., neutrophils). Of note, non-restricted bioenergetical conditions did not show any similar effects regarding cytokine expression and/or different survival rates of immune cell subsets. In conclusion, we found that the bioenergetical conditions are among the crucial factors inducing the initial inflammatory phase of fracture healing and are thus a critical step for influencing survival and function of immune cells in the early fracture hematoma.
Keywords: apoptosis, fracture hematoma model, hypoxia, immune cells, inflammation
Introduction
The musculoskeletal system provides mechanical stability to the human body and a scaffold which protects vital organs from exogenous harm. In addition, bones are important for systemic mineral homeostasis and hematopoiesis.1,2 Bone fractures occur when mechanical forces applied to the bone exceed its stability, thus leading to the disruption of bone tissue.
Bone fracture healing is a complex biological process which is unique in its ability to fully reconstruct bone tissue.3 This process is highly regulated and can be subdivided into three main phases: inflammation, repair and remodeling.4 These stages occur consecutively but in an overlapping manner and can be further refined based on histological, biochemical and biomechanical characteristics.
The involvement of the immune system in mechanisms of bone fracture healing is still poorly comprehended. However, the participation of immune cells and soluble factors derived from them are hypothesized to be decisive for tissue repair like wound healing or bone regeneration.5,6,7,8 Macrophages are critical for the clearance of cell debris and for matrix degradation during tissue regeneration9 and neutrophil recruitment is important in regard to fighting infections10 which are more likely to occur in damaged tissues. Depletion of macrophages and the depletion of all T lymphocytes lead to less effective wound healing, while the depletion of CD8+ T cells exhibits the opposite effect.9,11,12 In contrast to wound healing, fracture healing has been shown to be accelerated in RAG−/− mice lacking B and T cells.13 However, the investigation of RAG−/− mice does not allow the differentiation of subpopulations of the adaptive immune system, which might play very different roles in fracture healing, as is the case for wound healing.
Current concepts assume that the inflammatory phase of bone fracture healing is initiated by the migration of immune cells into the fracture hematoma.
Nevertheless, these hypotheses do not take into account that the trauma itself generates a rapid influx of immune cells into the fracture gap. These cells are released from the bone marrow, as well as from disrupted blood and lymph vessels situated at the fracture site. Hence, immune cells become a critical component of the early fracture hematoma right from the beginning.8 This might be of clinical importance, since it has been shown already in a rat femur fracture model that the early fracture hematoma is highly relevant for the outcome of fracture healing.14 Fracture healing was significantly impaired when the fracture hematoma was removed 30 min subsequent to bone fracture.14 It may be noted that migration of neutrophils into the fracture hematoma was observed 24 h after fracture followed by an invasion of macrophages to the fracture site.15,16 These findings stress the possibility that immune cells and inflammatory molecules present in the very early fracture hematoma play an important role in attracting further cells needed for successful fracture repair. Furthermore, it is likely that the composition of immune cells in the fracture hematoma is specific as compared to other hematomas. For example, the immune cell populations found in fracture hematomas of osteotomized sheep differ profoundly from those seen in corresponding muscle hematomas.17
The disruption of blood vessels and the development of edemas at the fracture site result in a local lack of nutrients and oxygen. As a consequence, the cells in the initial fracture hematoma have to cope with bioenergetically restricted conditions: oxygen level and substrate provision is low, and therefore the ability of cells to produce energy via oxidative phosphorylation and/or glycolysis is severely compromised. We and others have demonstrated that immune cells can adapt to such conditions and thus continue to fulfill their effector functions.18,19,20 Recently, we were also able to show that human cells present in the fracture hematoma do express genes associated with infalammation.21 Moreover, immunologically restricted patients—who are known to often suffer from impaired fracture healing—exhibit an inadequate response to hypoxia in fracture hematomas with an overshooting inflammatory response.22 Thus, the adaptation of immune cells to the compromised bioenergetical conditions as found within the fracture hematoma seems to be essential for the fracture healing outcome.
In order to study the immune cells present in the early inflammatory phase of bone fracture healing and their ability to adapt to bioenergetically restricted conditions, we established a human in vitro fracture hematoma model in our laboratory. This model allowed us to characterize the initial fracture hematoma with regard to its immune cell composition and cytokine expression. Furthermore, we used this model to monitor the change of immune cell populations and their survival and cytokine secretion during early time points (6 and 24 h) following bone fracture dependent on the bioenergetical restrictions found in early fracture hematomas.
Materials and methods
Sample collection
Total hip arthroplasty (THA)-hematomas which formed after the transection of the femur in patients receiving a total hip arthroplasty were collected. Patients with autoimmune diseases, immunosuppressive drugs, chronic infections (e.g., HIV, HBV, HCV, Tbc), cancer, osteoporosis or bone metabolism relevant drugs were excluded from the study. Twenty patients suffering from hip osteoarthritis were included into the study, and the patients' characteristics are described in Table 1. The study has been approved by the local ethical board and all participants gave their written informed consent. THA-hematomas were immediately filtered through a 70-µm cell strainer (BD Biosciences, Heidelberg, Germany) to minimize contamination with bone fragments or fat. Subsequently, the samples were analyzed (THA-hematoma 0 h) or further incubated.
Table 1. Patients' characteristics.
| Gender | Number | Average age (years) | Age range (years) |
|---|---|---|---|
| Male | 10 | 65.8 | 54–74 |
| Female | 10 | 65.9 | 46–78 |
Incubation conditions (normoxia vs. hypoxia and no nutrient supply vs. incubation in RPMI medium with sufficient nutrient supply)
THA-hematomas were incubated under hypoxia at 5% CO2 and <1% O2, balanced with N2 without any additional nutrient supply. Control THA-hematoma cells were first washed and then incubated in RPMI (Invitrogen, Carlsbad, CA, USA). Normoxic controls—both with and without additional nutrient supply—were incubated under 5% CO2 in a humidified atmosphere with 18% O2 for 6 and 24 h.
RNA isolation
Erythrocyte lysis was performed (erythrocyte lysis buffer: 0.01 M KHCO3, 0.155 M NH4Cl, 0.1 mM EDTA, pH 7.5 for 6 min at 4°C) and cells were washed with PBS/BSA. Obtained cells were filtered through a MACS preseparation filter (30 µm; Miltenyi Biotech, Bergisch Gladbach, Germany), centrifuged and resuspended in RLT buffer (Qiagen, Hilden, Germany). Total RNA was extracted (RNeasy Mini Kit; Qiagen), and the quality was assessed using the Bioanalyzer (Agilent Technologies Deutschland GmbH, Böblingen, Germany).
Quantitative PCR (qPCR)
The cDNAs were synthesized by reverse transcription using TaqMan Reverse Transcription Reagents (Applied Biosystems Deutschland GmbH, Darmstadt, Germany). qPCRs were performed using the LightCycler Fast Start DNA Master SYBR Green I Kit (ROCHE Diagnostics—Applied Science, Mannheim, Germany). Data were normalized to the expression of β-actin (ACTB). All primers used were obtained from TIB Molbiol (Berlin, Germany; gene symbol: forward primer, reverse primer): ACTB: gACAg gATgC AgAAg gAgAT CACT, TgATC CACAT CTgCT ggAAg gT; HIF1A: CCATT AgAAA gCAgT TCCgC, TgggT AggAg ATggA gATgC; PGK1: ATggA TgAgg TggTg AAAgC, CAgTg CTCAC ATggC TgACT; VEGF: AgCCT TgCCT TgCTg CTCTA, gTgCT ggCCT TggTg Agg; IFN-γ: TTCAgCTCTgCATCgTTTTg, TCAgCCATCACTTggATgAg; IL6: AAgCA gCAAA gAggC ACTgg, TgggT CAggg gTggT TATTg; IL8: ggACC CCAAg gAAAA CTgg, CAACC CTACA ACAgA CCCAC AC.
Quantification of cytokine concentration
Supernatants from ex vivo samples (0 h) and in vitro cultured samples were stored at −80°C. The concentrations of interleukin (IL)-6, IL-8, interferon-gamma (IFN-γ), vascular endothelial growth factor (VEGF) and monocyte chemotactic protein-1 (MCP-1, CCL2) were quantified by multiplex suspension array (Bio-Rad Laboratories, Munich, Germany) according to the manufacturer's instructions. Data acquisition was conducted using the Bio-Plex suspension system.
Flow cytometric analysis
After erythrocyte lysis, the THA-cells were stained with 2 µg/ml ethidium monoazide (EMA; Molecular Probes) for 20 min on ice followed by 10 min of photofixation (40 W). Active caspases (indicating apoptotic cells) were stained with CaspACE FITC-VAD-FMK (Promega, Madison, WI, USA). Two million cells/ml were incubated with 10 µM FITC-VAD-FMK for 30 min at 37°C. Lymphocytes and granulocytes were defined by forward and side scatters. Cells were acquired and recorded using an LSR II cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, USA).
Statistical analysis
Statistical tests were performed using Graph Pad Prism Software. Differences were compared using the Mann–Whitney U test. Probability values of P<0.05 were considered to be statistically significant (*P<0.05; **P<0.01; ***P<0.001).
Results
To establish a fracture hematoma model, we incubated hematomas generated by the transection of the femur during total hip arthroplasty (THA) under bioenergetically different conditions. The transected femurs served as an artificial fracture and the forming of ex vivo hematomas was defined as time point 0 h (THA-hematoma). As blood supply is interrupted during a fracture, the cells within the fracture hematoma in the fracture gap are considered to cope with bioenergetically restricted conditions, i.e., the supply of the cells with oxygen and substrates to produce energy in form of ATP is assumed to be severely impaired. In order to investigate the influence of hypoxia in more detail, we simulated these conditions present in the fracture gap in vitro by incubating the THA-hematomas under normoxic versus hypoxic conditions for 6 and 24 h.
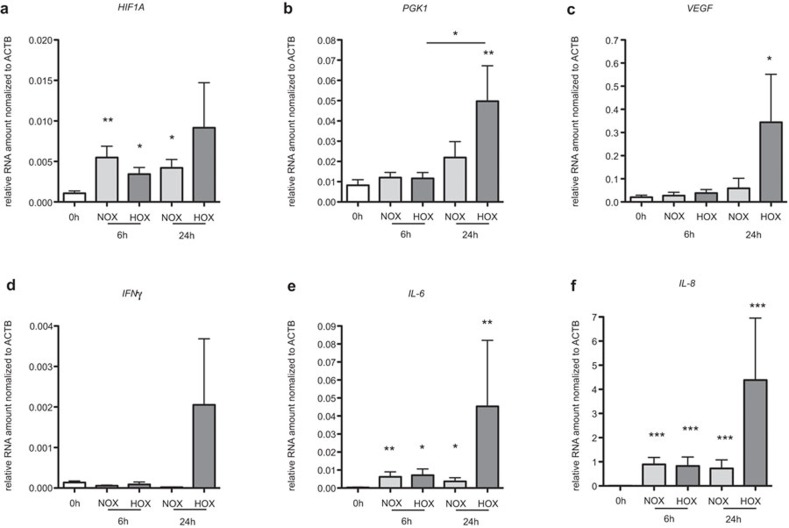
First, we asked ourselves whether or not cells from in vitro cultured THA-hematomas can adapt to hypoxia. HIF1α is the master regulator for the cell to adapt to hypoxia. However, since it is mainly regulated post-translationally, gene expression of HIF1A cannot be used as a marker for cells in a hypoxic environment. Thus, we also analyzed the expression of the HIF1α-target genes PGK1 and VEGF in cells from THA-hematomas which have been incubated under normoxic or hypoxic conditions.
We found the expressions of PGK1 and VEGF to be significantly increased at the RNA level following 24 h of incubation under hypoxia when compared to 0 h (P<0.01 and P<0.05, Figure 1b and c) confirming that THA-hematoma cells adapt to the hypoxic environment on the transcriptional level.
Figure 1.
THA-hematoma cells incubated under hypoxia adapt to low oxygen partial pressure and express mRNA of angiogenic and inflammatory factors. mRNA expression of HIF1α (a), PGK1 (b), VEGF (c), IFN-γ (d), IL-6 (e) and IL-8 (f) were analyzed in THA-hematomas ex vivo (0 h) and after incubation under normoxia or hypoxia after 6 and 24 h. The mRNA expression was normalized to ACTB. Mann–Whitney U test, n=11, *P<0.05; **P<0.01; ***P<0.001. IFN-γ, interferon-gamma; IL, interleukin; THA, total hip arthroplasty; VEGF, vascular endothelial growth factor.
HIF-1α is also known to be involved in the regulation of inflammatory cytokines, and the initial phase of fracture healing is defined to be an inflammatory one. Therefore, we also measured the expressions of the pro-inflammatory cytokines IFN-γ, IL-6 and IL-8 in cells in cultured THA-hematomas. The expression of IFN-γ was almost undetected (close to the detection limit) in all conditions tested except for the 24 h time point where we observed an increased expression in cells cultured under hypoxia (Figure 1d). In contrast, IL-6 and IL-8 expressions increased under both normoxia and hypoxia after 6 and 24 h, when compared to 0 h (Figure 1e and f). Interestingly, the highest expressions of IL-6 and IL-8 were found after 24 h of incubation under hypoxic conditions (P<0.01, P<0.001). In summary, the expressions of PGK1, VEGF, IFN-γ, IL-6 and IL-8 exhibited the highest levels after 24 h of incubation under hypoxia which clearly points to the adaptation of the cells to hypoxia and the development of a pro-inflammatory environment within the hematoma after 24 h.
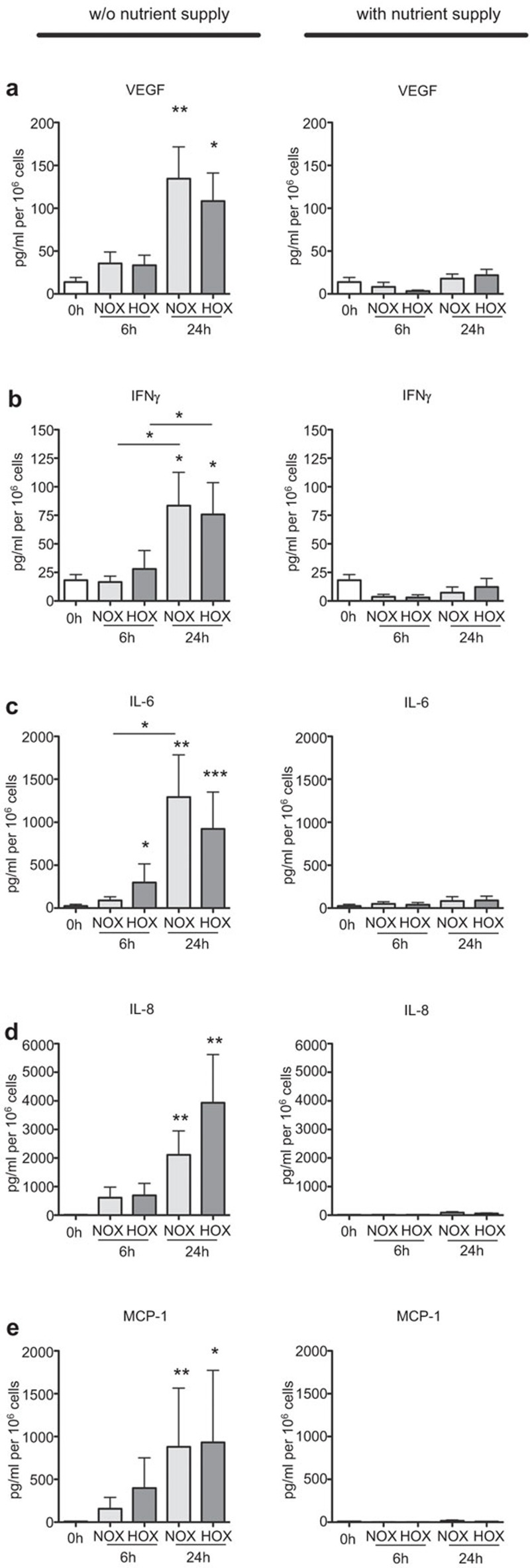
Since we found elevated transcription for the angiogenic and pro-inflammatory genes by THA-cells after 24 h in hypoxia, we assumed that we would also find increased secretion of the corresponding cytokines in the culture supernatants. In addition, we hypothesized an increase in the production of MCP-1 as an important chemoattractant of macrophages. Thus, we determined the protein concentrations of these factors in the supernatants of cultured THA-hematoma cells. As the THA-hematomas were incubated as collected ex vivo (i.e., without the addition of medium or other nutrient supply) first the basal concentrations of these factors at 0 h were measured (Figure 2). After 24 h of incubation, VEGF (Figure 2a, left), IFN-γ (Figure 2b, left), IL-6 (Figure 2c, left), IL-8 (Figure 2d, left) and MCP-1 (Figure 2e, left) showed significantly increased concentrations which were independent of the oxygen availability when compared to the situation at 0 h. The concentration of IL-6 was also significantly increased after 6 h under hypoxia (Figure 2c, left). In contrast to the mRNA data, increased concentrations of all factors investigated were not only found after 24 h of incubation under hypoxia, but also after 24 h of incubation under normoxia. This could point toward a selective survival of cells producing these cytokines under hypoxia, thus, showing the enhanced expression of these factors on the mRNA level. Alternatively, the cells incubated under hypoxia could not only produce higher protein amounts, but also consume these factors to much higher extents. In contrast to the oxygen-independent secretion of angiogenic and pro-inflammatory cytokines, this protein secretion strongly depended on the restriction of nutrients, since RPMI-cultured THA-hematoma cells did not show an increase in the secretion of VEGF, IFN-γ, IL-6, IL-8 and MCP-1 (Figure 2, right). This suggests that the lack of nutrients is among the critical factors which induce the inflammatory phase in the fracture hematoma.
Figure 2.
THA-hematoma cells secrete VEGF, IFN-γ, IL-6, IL-8 and MCP-1. THA-hematomas were incubated as indicated; subsequently, the culture supernatants were analyzed for the concentrations of VEGF (a), IFN-γ (b), IL-6 (c), IL-8 (d) and MCP-1 (e). Mann–Whitney U test, n=19, *P<0.05; **P<0.01. IFN-γ, interferon-gamma; IL, interleukin; MCP-1, monocyte chemotactic protein-1; THA, total hip arthroplasty; VEGF, vascular endothelial growth factor.
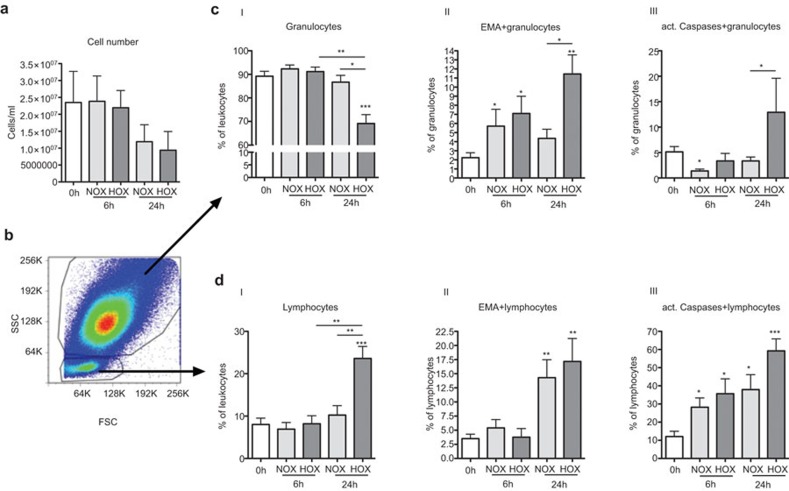
We also analyzed the cellular composition of THA-hematomas in our model including the cell count and the survival of different immune cell subpopulations. We found that cell numbers after 6 h of incubation under normoxic or hypoxic conditions did not differ from those measured ex vivo. However, the cell numbers were almost reduced by half after 24 h of incubation independently of oxygen availability (Figure 3a). Analyses of THA-hematomas by flow cytometry revealed that granulocytes and lymphocytes were the main immune cell population within the hematomas (Figure 3b). The frequency of granulocytes in the hematomas decreased significantly from about 90% at 0 h/ex vivo to less than 70% following 24 h of incubation under hypoxia (P<0.001) (Figure 3c-I). In contrast, the frequency of lymphocytes did increase more than twofold from less than 10% at 0 h to more than 20% (P<0.001) after 24 h of incubation under hypoxia (Figure 3d-I). Thus, although the total cell numbers decreased after 24 h of incubation independently of oxygen availability, the incubation under hypoxic conditions obviously favored the survival of lymphocytes.
Figure 3.
The survival of granulocytes and lymphocytes within THA-hematomas depends on oxygen availability. THA-hematomas were analyzed ex vivo (0 h) and after incubation under normoxia or hypoxia for 6 and 24 h. (a) Total cell numbers of THA-hematoma cells, n=5. (b) A representative scatter dot plot of a THA-hematoma at 0 h. Percentages of granulocytes (c-I) and lymphocytes (d-I) (proportions are shown as percent values) among leukocytes. Frequencies of EMA+ (dead) granulocytes (c-II) and leukocytes (d-II) among granulocytes and lymphocytes, respectively. Proportions of active caspases+ (apoptotic) granulocytes (c-III) and leukocytes (d-III) are shown as percent values of total granulocyte/lymphocyte populations. n=8, Mann–Whitney U test, *P<0.05; **P<0.01; ***P<0.001. EMA, ethidium monoazide; THA, total hip arthroplasty.
To differentiate between necrotic and apoptotic events within these populations, the labeling with EMA (indicating dead cells) and the expression of activated caspases (indicating apoptotic cells) was analyzed in granulocytes and lymphocytes. The initial frequency of necrotic EMA+ granulocytes and lymphocytes was very low ex vivo with ca. 2%–3% (Figure 3c-II and d-II). However, the number of EMA+ granulocytes increased after 6 h independently of oxygen availability. Interestingly, this number reached a plateau for granulocytes cultured under normoxia, but increased significantly under hypoxic conditions after 24 h of incubation (Figure 3c-II). Lymphocytes showed a delayed increase of cell death when compared to granulocytes reaching significance only after 24 h (Figure 3d-II). In addition, cell death of lymphocytes apparently did not depend on oxygen levels in the culture. The delayed kinetic of lymphocyte cell death and the particular high cell death of granulocytes following 24 h of culture under hypoxia could explain the observed effect of a selective increase of the lymphocyte population and the associated decrease in the granulocyte-to-lymphocyte ratio specifically in this bioenergetically restricted condition.
In contrast to the modest frequencies of necrotic cells ex vivo, the frequencies of apoptotic THA-hematoma cells were comparatively high for both granulocytes (∼5%, Figure 3c-III) and lymphocytes (∼10%, Figure 3d-III) before in vitro culture, suggesting that the osteotomy induced apoptosis in these cells. Consistent with the observed results for EMA+ granulocytes, we found the highest frequency of apoptotic granulocytes under the 24 h hypoxia condition showing that the elimination of granulocytes is at least in part an active process (Figure 3c-III). The kinetics show that the bioenergetical conditions—namely the nutrient content and the reduced oxygen amounts—led to a negative selection of granulocytes which at least to some extent is mediated by the initiation of apoptotic programs in these cells. In contrast to granulocytes, apoptotic lymphocytes were already found after 6 h of incubation when compared to the case at 0 h (P<0.05 under normoxia and hypoxia, Figure 3d-III). Moreover, after 24 h, significantly increased numbers were found after the incubation under normoxia (P<0.05) and at higher levels under hypoxia (P<0.001).
Discussion
A highly regulated sequence of events in the early inflammatory phase of fracture healing is a prerequisite for a timely and successful regeneration process.5,6,7,8 The initial fracture hematoma develops immediately after injury and contains various immune cell populations originating from bone marrow, disrupted blood and lymph vessels.8,17 So far, studies on the early inflammatory phase of fracture healing primarily focused on the infiltration of immune cells into already existing fracture hematomas.15,23,24 Here, we aimed at studying the immune cell populations present in the developing fracture hematoma with regard to their cytokine expression, and survival. To this end, we established an in vitro fracture hematoma model by culturing cells from hematomas forming after osteotomies of the femur during THA. These hematomas were harvested from hip osteoarthritis patients ex vivo immediately after osteotomy to obtain an accurate 0-h time point fracture hematoma. Subsequently, these hematomas were incubated for 6 and 24 h without additional nutrient supply under hypoxic or normoxic conditions, in order to mimic the assumed conditions at the fracture site in vivo.21
The master regulator of the adaptation to hypoxia in most cell types including immune cells is the hypoxia inducible factor 1 alpha (HIF-1α).20,25,26,27 However, the transcriptional expression of HIF1A is usually not altered by hypoxia—and this is also what we observed when measuring HIF1A expression in cultured THA-hematoma cells (Figure 1a)—but instead the HIF-1α protein expression is regulated by post-translationally.25 Thus, we used the expression of the HIF-1 target genes PGK1 and VEGF as a parameter for the adaptation of immune cells cultured in a hypoxic environment. The expression of both PGK1 and VEGF were induced in THA-hematoma cells when cultured for 24 h under hypoxia (Figure 1b and c). In addition, the pro-inflammatory cytokine IL-8 has been shown to also mediate angiogenesis acting accordantly with the VEGF expression.26,28,29 The IL8 mRNA was expressed at high levels in cells cultured for 24 h with reduced oxygen availability. Thus, the cells in our in vitro model adapt to hypoxia and express VEGF and IL-8 to counteract the hypoxic condition via induction of angiogenesis. We have previously described the induction of VEGF and IL-8 in ex vivo human fracture hematomas,21,22 thus the enhanced VEGF and IL-8 expression in our models resembles the in vivo situation. Our findings suggest that the expression of VEGF and IL-8 by the cells initially present in the fracture hematoma induce neo-angiogenesis at the fracture site, which is essential for successful bone regeneration.
Furthermore, we had asked whether the cells in our model already initiate an inflammatory response as has been shown for in the initial fracture hematoma.21 We could show that the mRNA expression of the pro-inflammatory factors IFN-γ, IL-6 and IL-8 is highly induced after 24 h under hypoxia (Figure 1d–f). The induction of IFN-γ expression following 24 h of hypoxic incubation is indeed high but did not reach any statistical significance, and this can be explained by the high interindividual variations. Additionally, IL-8 acts as chemoattractant thereby promoting the immigration of further immune cells like neutrophils.26,28,29 These results reflect mRNA expressions found in ex vivo fracture hematomas indicating the usability of our in vitro model.21
Thus, the trauma itself and the consequent hypoxic conditions without any additional nutrient supply—as they were chosen in our in vitro model—seem to be responsible for initial inflammation, angiogenesis and chemoattraction.
In order to corroborate our mRNA results, we also analyzed the concentrations of VEGF, IFN-γ, IL-6, IL-8 and the macrophage chemoattractant MCP-1 in the supernatants at 0 h (THA-hematoma 0 h) and after 6 and 24 h of incubation under hypoxic and normoxic conditions without any additional nutrient supply, respectively. All factors investigated were significantly elevated after 24 h of incubation independently of the oxygen availability (Figure 2, left). In contrast, when the THA-hematoma cells were incubated in medium (sufficient nutrient supply) either under normoxia or hypoxia, there was no comparable cytokine induction (Figure 2, right). But it is of interest that only IL-6 was found to be already significantly increased after 6 h under hypoxia without nutrient supply. This means that the initial injury, in this case the osteotomy of the femur as a model for fracture, and the incubation without additional nutrient supply under hypoxia are among the factors leading to an induction of angiogenesis via VEGF and IL-8 secretion and inflammation as indicated by the secretion of IFN-γ, IL-6, IL-8 and MCP-1. Furthermore the chemoattraction of phagocyting cells is induced via the secretion of MCP-1 and IL-8.30,31 High levels of IL-6, IL-8 and VEGF were also shown in ex vivo fracture hematomas, again supporting the suitability of our in vitro model.24,32 These protein results did not reflect the mRNA data, where the incubation under hypoxia led to high expression of VEGF, IFN-γ, IL-6 and IL-8. We interpret this observation as follows: there is likely a selective survival of cells producing these factors under hypoxia as evidenced by the observed enhanced expression on the mRNA level. Another, not mutually exclusive explanation is that the cells incubated under hypoxic conditions do not only produce higher protein amounts, but also consume these factors to a much higher extent resulting in lower measurable concentrations.
These results enforced the investigation of cell numbers and survival of immune cells in our model. While the cell numbers remained constant within the first 6 h of incubation, they were about halved after 24 h of incubation independently of oxygen availability (Figure 3a). Using flow cytometry, we could show that at 0 h the THA-hematomas predominantly contained granulocytes (about 90%, Figure 3b and c-I). About 10% of the cells were lymphocytes (Figure 3b and d-I). This composition changed after 24 h of incubation under hypoxia, where the frequency of granulocytes had diminished to about 70% and the proportion of lymphocytes was more than doubled to >20% (Figures 3c-I and d-I). This relative increase in lymphocytes is considered to be responsible for the measured increase of mRNA expression of VEGF, IFN-γ, IL-6 and IL-8. A decrease in the proportion of granulocytes and increase of lymphocytes was also shown in an sheep osteotomy model from 1 to 4 h hematomas.17 Furthermore, although the immune cells incubated under hypoxia in our model had to face not only the nutrient lack but also had to adapt to deprivation of oxygen, they were still able to initiate angiogenesis, inflammation and chemoattraction. This matches the ability shown previously where e.g. CD4+ T cells maintain specific effector function under lack of oxygen or nutrients.19
Next, we analyzed necrotic and apoptotic events under the various conditions in our model: while the proportion of dead EMA+ lymphocytes remained stable within the first 6 h, the proportion of dead EMA+ granulocytes increased significantly. This effect was seen independently of oxygen availability. In contrast, after 24 h the proportion of EMA+ granulocytes increased under hypoxia while the frequency of EMA+ lymphocytes increased independently of oxygen availability (Figure 3c-II and d-II). This explains the relatively increased lymphocyte and decreased granulocyte proportions after 24 h of incubation under hypoxia. Interestingly, we could detect a relevant expression of active caspases indicating apoptosis in granulocytes only after 24 h of incubation under hypoxia. In contrast, lymphocytes did express active caspases under all incubation conditions but with the highest expression after 24 h under hypoxia (Figure 3c-III and d-III). The expression of effector molecules combined with the induction of apoptosis points towards an activation of the present immune cells leading to activation induced cell death.33
In conclusion, we could show that our in vitro fracture hematoma model may be suitable for the investigation of initial immunological events when THA-hematomas are incubated under hypoxia. After trauma and under restricted conditions as is assumed to be the case in vivo, i.e., lack of nutrients and low oxygen levels,8 the immune cells within the fracture hematoma 21 and within our in vitro fracture hematoma model are involved in the initiation of angiogenesis, inflammation and chemoattraction. This activation under bioenergetically restricted conditions leads to an initiation of apoptosis after successful contribution to the onset of regeneration. Thus, the cells initially present in the fracture hematoma may exhibit the endogenous ability to initiate the early inflammatory phase of bone regeneration.
Acknowledgments
The authors thank Manuela Jakstadt for her excellent technical assistance. This work was supported in part by the BCRT-Grant I.
References
- Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A. Bone development: overview of bone cells and signaling. Curr Osteoporos Rep. 2011;9:264–273. doi: 10.1007/s11914-011-0078-8. [DOI] [PubMed] [Google Scholar]
- McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60-B:150–162. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- Remedios A. Bone and bone healing. Vet Clin North Am Small Anim Pract. 1999;29:1029–1044. doi: 10.1016/s0195-5616(99)50101-0. [DOI] [PubMed] [Google Scholar]
- Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, et al. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res. 2009;24:251–264. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–675. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010;16:427–434. doi: 10.1089/ten.TEB.2009.0687. [DOI] [PubMed] [Google Scholar]
- Schaffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg. 1998;85:444–460. doi: 10.1046/j.1365-2168.1998.00734.x. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51:2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Efron JE, Frankel HL, Lazarou SA, Wasserkrug HL, Barbul A. Wound healing and T-lymphocytes. J Surg Res. 1990;48:460–463. doi: 10.1016/0022-4804(90)90013-r. [DOI] [PubMed] [Google Scholar]
- Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann JE, Frisch JT, et al. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res. 2011;26:113–124. doi: 10.1002/jbmr.185. [DOI] [PubMed] [Google Scholar]
- Grundnes O, Reikeras O. The importance of the hematoma for fracture healing in rats. Acta Orthop Scand. 1993;64:340–342. doi: 10.3109/17453679308993640. [DOI] [PubMed] [Google Scholar]
- Andrew JG, Andrew SM, Freemont AJ, Marsh DR. Inflammatory cells in normal human fracture healing. Acta Orthop Scand. 1994;65:462–466. doi: 10.3109/17453679408995493. [DOI] [PubMed] [Google Scholar]
- Chung R, Cool JC, Scherer MA, Foster BK, Xian CJ. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 2006;80:1272–1280. doi: 10.1189/jlb.0606365. [DOI] [PubMed] [Google Scholar]
- Schmidt-Bleek K, Schell H, Kolar P, Pfaff M, Perka C, Buttgereit F, et al. Cellular composition of the initial fracture hematoma compared to a muscle hematoma: a study in sheep. J Orthop Res. 2009;27:1147–1151. doi: 10.1002/jor.20901. [DOI] [PubMed] [Google Scholar]
- Dziurla R, Gaber T, Fangradt M, Hahne M, Tripmacher R, Kolar P, et al. Effects of hypoxia and/or lack of glucose on cellular energy metabolism and cytokine production in stimulated human CD4+ T lymphocytes. Immunol Lett. 2010;131:97–105. doi: 10.1016/j.imlet.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Tripmacher R, Gaber T, Dziurla R, Haupl T, Erekul K, Grutzkau A, et al. Human CD4+ T cells maintain specific functions even under conditions of extremely restricted ATP production. Eur J Immunol. 2008;38:1631–1642. doi: 10.1002/eji.200738047. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar P, Gaber T, Perka C, Duda GN, Buttgereit F. Human Early Fracture hematoma is characterized by inflammation and hypoxia. Clin Orthop Relat Res. 2011;469:3118–3126. doi: 10.1007/s11999-011-1865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff P, Gaber T, Schmidt-Bleek K, Senturk U, Tran CL, Blankenstein K, et al. Immunologically restricted patients exhibit a pronounced inflammation and inadequate response to hypoxia in fracture hematomas. Immunol Res. 2011;51:116–122. doi: 10.1007/s12026-011-8235-9. [DOI] [PubMed] [Google Scholar]
- Cornell CN, Lane JM. Newest factors in fracture healing. Clin Orthop Relat Res. 1992;277:297–311. [PubMed] [Google Scholar]
- Hauser CJ, Zhou X, Joshi P, Cuchens MA, Kregor P, Devidas M, et al. The immune microenvironment of human fracture/soft-tissue hematomas and its relationship to systemic immunity. J Trauma. 1997;42:895–903. doi: 10.1097/00005373-199705000-00021. [DOI] [PubMed] [Google Scholar]
- Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis. 2005;64:971–980. doi: 10.1136/ard.2004.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr B, Tang C, Germann G, Longaker MT, Quarto N. Locally applied vascular endothelial growth factor a increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells. 2011;29:286–296. doi: 10.1002/stem.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038–6042. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkilde MM, Schwartz TW. The chemokine system—a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- Bastian O, Pillay J, Alblas J, Leenen L, Koenderman L, Blokhuis T. Systemic inflammation and fracture healing. J Leukoc Biol. 2011;89:669–673. doi: 10.1189/jlb.0810446. [DOI] [PubMed] [Google Scholar]
- Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP. Is human fracture hematoma inherently angiogenic. Clin Orthop Relat Res. 2000;378:224–237. doi: 10.1097/00003086-200009000-00033. [DOI] [PubMed] [Google Scholar]
- Arnold R, Brenner D, Becker M, Frey CR, Krammer PH. How T lymphocytes switch between life and death. Eur J Immunol. 2006;36:1654–1658. doi: 10.1002/eji.200636197. [DOI] [PubMed] [Google Scholar]