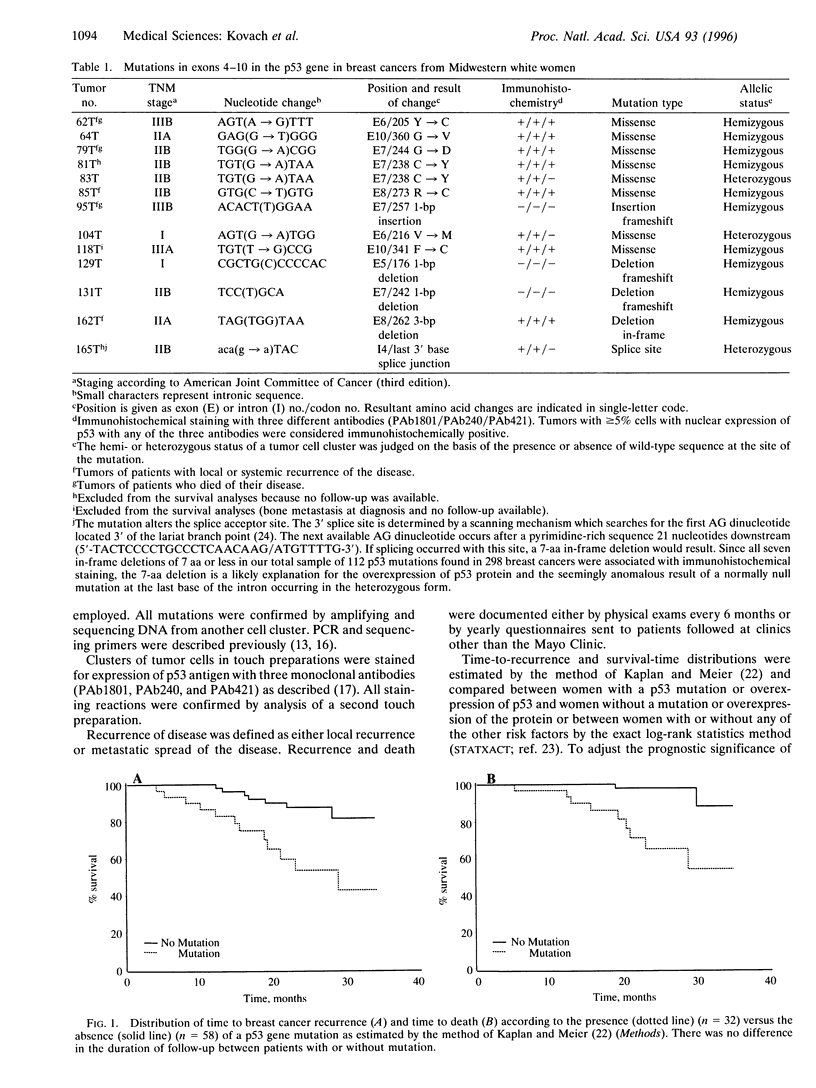
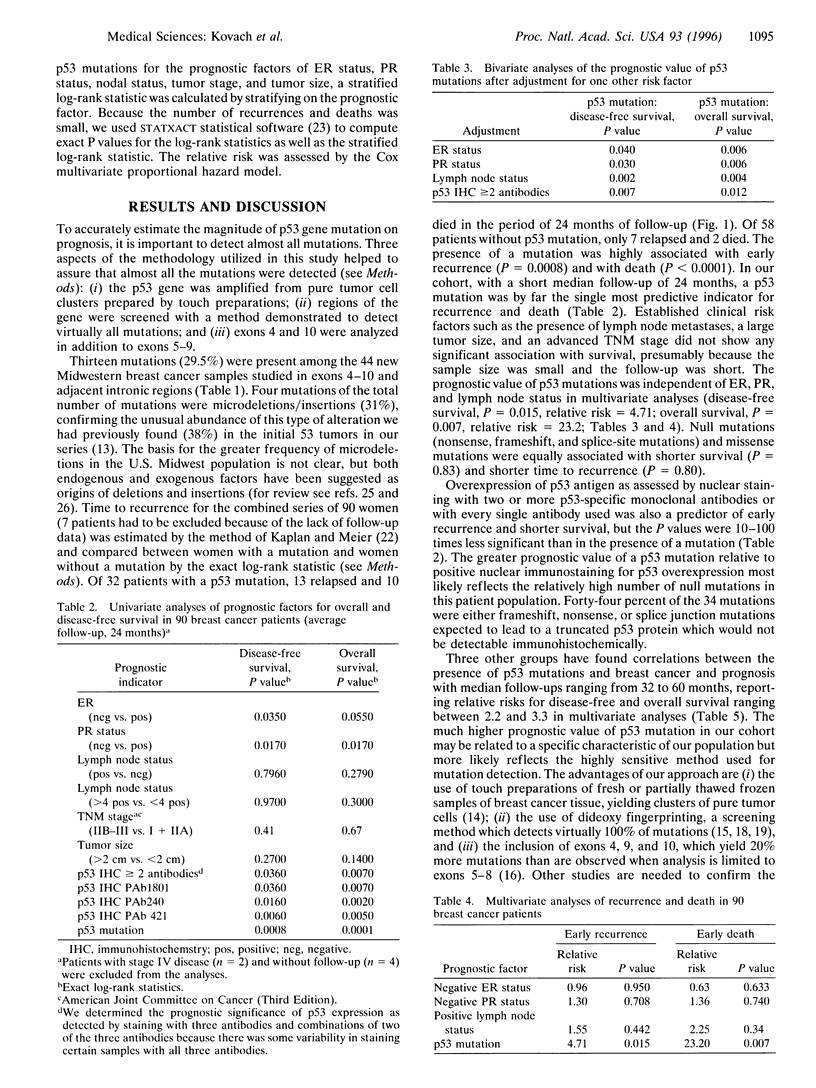
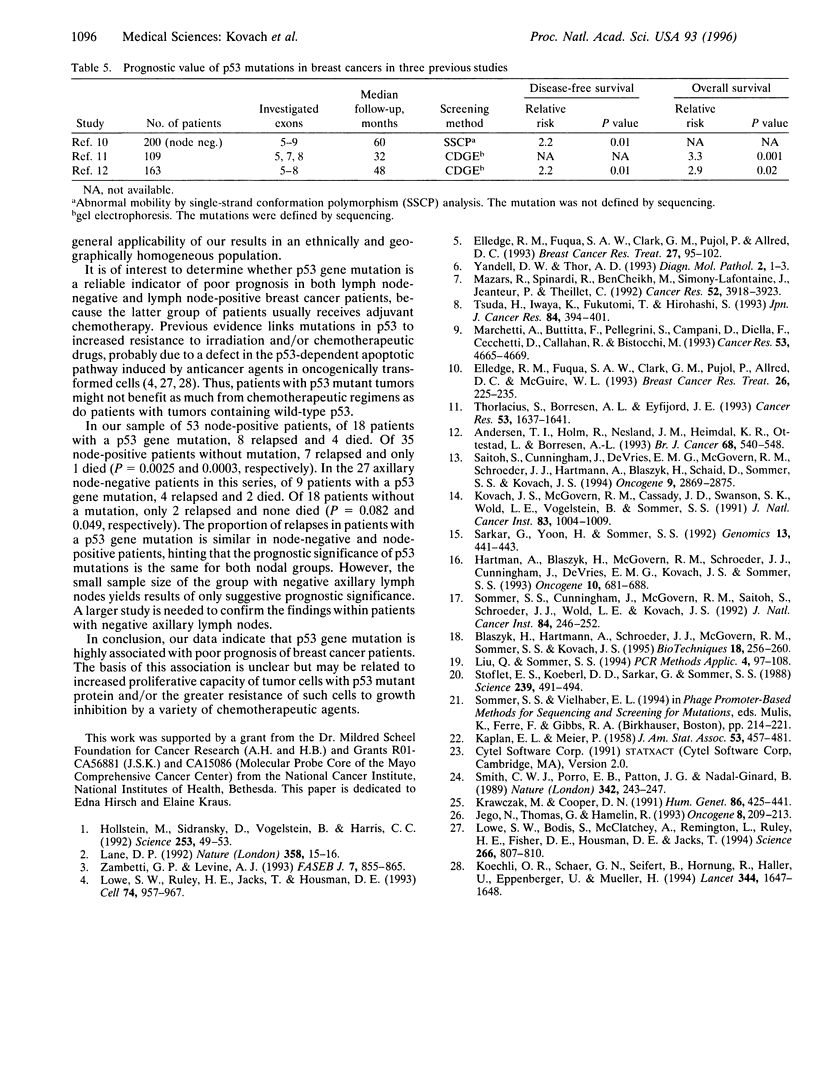
Abstract
Human cancer cells with a mutated p53 tumor-suppressor gene have a selective growth advantage and may exhibit resistance to ionizing radiation and certain chemotherapeutic agents. To examine the prognostic value of mutations in the p53 gene, a cohort of 90 Midwestern Caucasian breast cancer patients were analyzed with methodology that detects virtually 100% of all mutations. The presence of a p53 gene mutation was by far the single most predictive indicator for recurrence and death (relative risks of 4.7 and 23.2, respectively). Direct detection of p53 mutations had substantially greater prognostic value than immunohistochemical detection of p53 overexpression. Analysis of p53 gene mutations may permit identification of a subset of breast cancer patients who, despite lack of conventional indicators of poor prognosis, are at high risk of early recurrence and death.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen T. I., Holm R., Nesland J. M., Heimdal K. R., Ottestad L., Børresen A. L. Prognostic significance of TP53 alterations in breast carcinoma. Br J Cancer. 1993 Sep;68(3):540–548. doi: 10.1038/bjc.1993.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszyk H., Hartmann A., Schroeder J. J., McGovern R. M., Sommer S. S., Kovach J. S. Rapid and efficient screening for p53 gene mutations by dideoxy fingerprinting. Biotechniques. 1995 Feb;18(2):256–260. [PubMed] [Google Scholar]
- Elledge R. M., Fuqua S. A., Clark G. M., Pujol P., Allred D. C., McGuire W. L. Prognostic significance of p53 gene alterations in node-negative breast cancer. Breast Cancer Res Treat. 1993;26(3):225–235. doi: 10.1007/BF00665800. [DOI] [PubMed] [Google Scholar]
- Elledge R. M., Fuqua S. A., Clark G. M., Pujol P., Allred D. C. William L. McGuire Memorial Symposium. The role and prognostic significance of p53 gene alterations in breast cancer. Breast Cancer Res Treat. 1993;27(1-2):95–102. doi: 10.1007/BF00683196. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Blaszyk H., McGovern R. M., Schroeder J. J., Cunningham J., De Vries E. M., Kovach J. S., Sommer S. S. p53 gene mutations inside and outside of exons 5-8: the patterns differ in breast and other cancers. Oncogene. 1995 Feb 16;10(4):681–688. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Jego N., Thomas G., Hamelin R. Short direct repeats flanking deletions, and duplicating insertions in p53 gene in human cancers. Oncogene. 1993 Jan;8(1):209–213. [PubMed] [Google Scholar]
- Koechli O., Schaer G. N., Seifert B., Hornung R., Haller U., Eppenberger U., Mueller H. Mutant p53 protein associated with chemosensitivity in breast cancer specimens. Lancet. 1994 Dec 10;344(8937):1647–1648. doi: 10.1016/s0140-6736(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Kovach J. S., McGovern R. M., Cassady J. D., Swanson S. K., Wold L. E., Vogelstein B., Sommer S. S. Direct sequencing from touch preparations of human carcinomas: analysis of p53 mutations in breast carcinomas. J Natl Cancer Inst. 1991 Jul 17;83(14):1004–1009. doi: 10.1093/jnci/83.14.1004. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Cooper D. N. Gene deletions causing human genetic disease: mechanisms of mutagenesis and the role of the local DNA sequence environment. Hum Genet. 1991 Mar;86(5):425–441. doi: 10.1007/BF00194629. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Liu Q., Sommer S. S. Parameters affecting the sensitivities of dideoxy fingerprinting and SSCP. PCR Methods Appl. 1994 Oct;4(2):97–108. doi: 10.1101/gr.4.2.97. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Marchetti A., Buttitta F., Pellegrini S., Campani D., Diella F., Cecchetti D., Callahan R., Bistocchi M. p53 mutations and histological type of invasive breast carcinoma. Cancer Res. 1993 Oct 1;53(19):4665–4669. [PubMed] [Google Scholar]
- Mazars R., Spinardi L., BenCheikh M., Simony-Lafontaine J., Jeanteur P., Theillet C. p53 mutations occur in aggressive breast cancer. Cancer Res. 1992 Jul 15;52(14):3918–3923. [PubMed] [Google Scholar]
- Saitoh S., Cunningham J., De Vries E. M., McGovern R. M., Schroeder J. J., Hartmann A., Blaszyk H., Wold L. E., Schaid D., Sommer S. S. p53 gene mutations in breast cancers in midwestern US women: null as well as missense-type mutations are associated with poor prognosis. Oncogene. 1994 Oct;9(10):2869–2875. [PubMed] [Google Scholar]
- Sarkar G., Yoon H. S., Sommer S. S. Dideoxy fingerprinting (ddE): a rapid and efficient screen for the presence of mutations. Genomics. 1992 Jun;13(2):441–443. doi: 10.1016/0888-7543(92)90266-u. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Porro E. B., Patton J. G., Nadal-Ginard B. Scanning from an independently specified branch point defines the 3' splice site of mammalian introns. Nature. 1989 Nov 16;342(6247):243–247. doi: 10.1038/342243a0. [DOI] [PubMed] [Google Scholar]
- Sommer S. S., Cunningham J., McGovern R. M., Saitoh S., Schroeder J. J., Wold L. E., Kovach J. S. Pattern of p53 gene mutations in breast cancers of women of the midwestern United States. J Natl Cancer Inst. 1992 Feb 19;84(4):246–252. doi: 10.1093/jnci/84.4.246. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Thorlacius S., Börresen A. L., Eyfjörd J. E. Somatic p53 mutations in human breast carcinomas in an Icelandic population: a prognostic factor. Cancer Res. 1993 Apr 1;53(7):1637–1641. [PubMed] [Google Scholar]
- Tsuda H., Iwaya K., Fukutomi T., Hirohashi S. p53 mutations and c-erbB-2 amplification in intraductal and invasive breast carcinomas of high histologic grade. Jpn J Cancer Res. 1993 Apr;84(4):394–401. doi: 10.1111/j.1349-7006.1993.tb00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandell D. W., Thor A. D. p53 analysis in diagnostic pathology. Biologic implications and possible clinical applications. Diagn Mol Pathol. 1993 Mar;2(1):1–3. doi: 10.1097/00019606-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Zambetti G. P., Levine A. J. A comparison of the biological activities of wild-type and mutant p53. FASEB J. 1993 Jul;7(10):855–865. doi: 10.1096/fasebj.7.10.8344485. [DOI] [PubMed] [Google Scholar]


