Abstract
Adult neurogenesis is an important therapeutic target in treating neurological disorders. Adult neurogenesis takes place in two regions of the brain: Subventricular zone and dentate gyrus in the hippocampus. The progressive understanding on hippocampal neurogenesis in aging and mood disorders increases the demand to explore powerful and subtle interventions on hippocampal neurogenesis. Traditional Chinese herbal medicine provides an abundant pharmaceutical platform for modulating hippocampal neurogenesis. Recent progress in exploring the effects of Chinese herbal medicine and the related mechanisms opens a new direction for regeneration therapy. The current review gives a thorough summary of the research progress made in traditional Chinese herbal formulas, and the effective compounds in Chinese herbs which are beneficial on hippocampal neurogenesis and the possible mechanisms involved.
Keywords: Active components, Hippocampal neurogenesis, Neural progenitor cells, Traditional Chinese herb
INTRODUCTION
Adult neurogenesis is a continuous bioactivity in certain brain regions. This activity is highly reserved during revolution, from oscines to rodents and primates. In mammals, there are two regions in brain continuously generating the new neurons during adulthood, the subventricular zone and the dentate gyrus.[1]
It had been a long-term ambiguity whether adult neurogenesis in the dentate gyrus had substantial functions. In 2008, clear evidence proved that the newly generated neurons in the dentate gyrus projected axons and established synapses with hilar interneurons, mossy cells, and CA3 pyramidal cells, and released glutamate as their main neurotransmitter.[2] The process of maturation and synapse formation of the adult-born dentate granule cells was causally linked to memory and learning in the brain.[3] For instance, many factors known to be beneficial for memory (e.g. running, environment enrichment) also increased the number of new neurons;[4,5,6,7] likewise, factors that impaired memory, such as aging, stress, and several diseases, were associated with lower neurogenesis levels.[8,9] In addition to learning and memory, adult neurogenesis in the dentate gyrus was proved to be involved in psychological disorders such as depression[4] and anxiety.[5] An impairment of adult neurogenesis in the dentate gyrus could be one of the critical factors in the etiology of certain psychiatric disorders.[6]
Given the significance of the adult neurogenesis in the hippocampus, pharmacological interventions on adult neurogenesis are believed to be one of the key strategies to treat psychological disorders and to improve the cognitive functions during aging. In China, over more than 3200 herbs and 300 mineral and animal extracts are used in more than 400 different formulas.[7] Although there exists distinct theories in the history of drug development between the oriental world and the western world,[7] these Chinese herbs and extracts provide an abundant database for the drug screening and development using the modern technologies in modern bioscience research. Great efforts had been made in the last decade to explore the effects of Chinese herbal medicine on promoting adult neurogenesis, and recent progress indicates that these medicines hold promising potential for neural regeneration therapy. This review tries to give a summary and comparison on the recent research articles focusing on the Chinese herbs and adult neurogenesis in the hippocampus.
CHINESE HERBS IN FORMULA AND ADULT NEUROGENESIS
In the history of traditional Chinese medicine (TCM), majority of the TCMs are found to be applied as formulas. Several formulas of TCM have been found to be able to promote adult neurogenesis in the dentate gyrus in stressed animals.[8,9,10] LiuWei Dihuang Tang (六味地黃湯 Liū Wèi Dì Huáng Tāng), a Chinese herbal formula used to treat complications of diabetes and glomerulonephritis, was proved to promote hippocampal neurogenesis in adult rats at a dose of 100 mg/kg, which was thought to be associated with improvement in cognitive function in eight radiating arms.[8] More recently, researchers found that oral treatment of Kami-ondam-tang (加味溫膽湯 Jiā Wèi Wēndăn Tāng) at a dose of 50 mg/kg increased the number of the doublecortin-positive cells in the dentate gyrus in naïve rats, and consequently improved the cognitive functions in mice treated for 2 weeks.[10] Another study using kami-shoyo-san (加味逍遙散 Jiā Wèi Xiāo Yáo Săn) reported that in stressed rats, 20 times of standard dose of kami-shoyo-san was able to reverse the impaired neurogenesis in the hippocampus.[9] The above three studies provide evidence that TCM formulas could enhance neurogenesis in the dentate gyrus, both in physical and pathological conditions. However, each formula contained several components based on the theory of TCM. For example, kami-shoyo-san consisted of nine herbal plants [Paeoniae Radix (白芍 Bái Sháo) 4 g, Bupleuri Radix (柴胡 Chái Hú) 4 g, Atractylodis Macrocephalae Rhizoma (白朮 Bái Zhú) 4 g, Liriopis tuber (麥冬 Mài Dōng) 4 g, Angelicae Gigantis Radix (當歸 Dāng Guī) 4 g, Hoelen (茯苓 Fú Ling) 4 g, Menthae Folium (薄荷腦葉 Bò Hé Năo Yè) 2 g, Glycyrrhizae Radix (甘草 Gān Căo) 2 g, and Zingiberis Rhizoma (生薑 Shēng Jiāng) 6 g].[9] The complex composition of TCMs in formula makes it difficult to further explain which component or components in the formula are beneficial for neurogenesis. It is also unknown whether the effective components have synergistic or antagonistic effects.
EFFECTIVE COMPONENTS IN CHINESE HERBS AND HIPPOCAMPAL NEUROGENESIS
According to the principle of “Jun-Chen-Zuo-Shi (君-臣-佐-使)” in the traditional Chinese medical theory, every component in a certain Chinese medicine formula is essential and plays its own respective role. In view of the complex composition in Chinese medicine formulas, popular research direction in the modern science to promote the application of Chinese herbs for neurogenesis has been carried out to study the effective components in Chinese herbs which are able to stimulate neurogenesis in the dentate gyrus. Therefore, the effects of extracts of Chinese formulas on neurogenesis are widely investigated. Table 1 summarizes that active components from different Chinese herbs that have been proved to enhance the hippocampal neurogenesis under naïve and pathological conditions.
Table 1.
Effective component in Chinese herbs beneficial to hippocampal neurogenesis
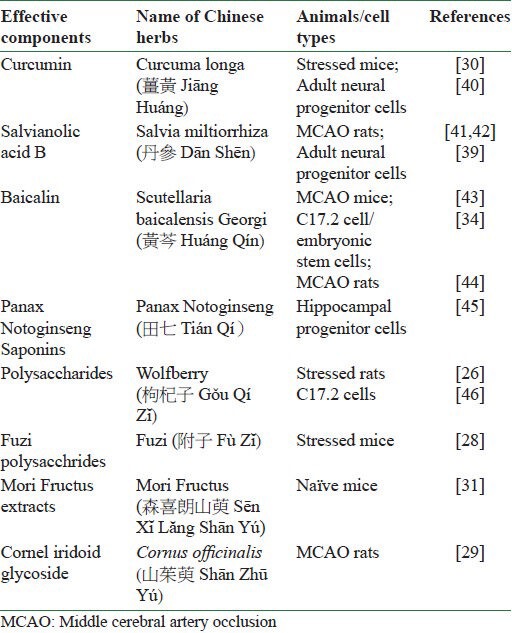
In addition, a few interesting comparison studies were carried out to screen the useful components in a certain Chinese formula. For instance, Buyang Huanwu Decoction (補陽還五湯 Bŭ Yáng Huăn Wŭ Tāng) is a classic formula that has been used for post-stroke disability for 300 years.[11] It contains Radix Astragali Membranaceus (黄芪根 Huáng Qígēn), Radix Angelicae Sinensis (白芷 Bái Zhĭ), Radix Paeonia Rubra (赤芍 Chì Sháo), Rhizoma Chuanxiong (川穹 Chuān Qióng), Semen Persicae (桃仁 Táo Rén), Flos Carthami (紅花 Hōng Huā), and earthworm (蚯蚓 Qiū Yĭn). The components are mixed in order in the ratio of 120:10:10:10:10:10:4.5 (dry weight). Either with or without earthworm, Buyang Huanwu Decoction displayed a similar stimulating effect on neurogenesis in stroke rats. The result pointed out that earthworm was not a necessary component in Buyang Huanwu Decoction to stimulate neurogenesis after ischemic injury.[12] Besides, the findings from the same group showed that Buyang Huangwu Decoction improved the neurological scores and functional recovery in stroke rats as well.[13]
PRETREATMENT OF CHINESE HERBS AND HIPPOCAMPAL NEUROGENESIS
Considering that Chinese herbs can be used for health maintenance and disease prevention according to the TCM theory, pretreatment of Chinese herbs by modern scientific techniques is widely investigated in neurodegenerative disease models. The stroke model and Alzheimer's disease model can be taken as examples. Pretreatment with Salvia miltiorrhiza Bunge (丹參 Dān Shēn) or ginsenoside Rd both significantly decreased the infarct volume and reduced the sequential inflammatory response after transient focal ischemia in rats.[14,15] Pretreatment with ruscogenin also proved to be neuroprotective in mice subjected to middle cerebral artery occlusion (MCAO).[16] In the in vitro studies, pretreatment of dicaffeoylquinic acids from Herba Erigerontis (燈盞花 Dēng Zhăn Huā),[17] baji jiasu from Radix Morinda Officinalis (巴戟天 Bā Jĭ Tiān),[18] jatrorrhizine from Coptidis Rhizome (黃連 Huáng Lián),[19] Houttuyniae Herba (魚腥草 Yú Xīng Căo),[20] isorhynchophylline from Uncaria rhynchophylla (鉤藤 Gōu Téng),[21] and extracts from Lycium barbarum (wolfberry)[22,23] led to the compounds display their protective effects against Aβ cell toxicity in different types of neuronal cells. More interestingly, a study of the extracts from Feverfew (甘菊 Gān Jú) showed that pretreatment of 30 mg/kg Feverfew extracts displayed a better outcome than post-treatment of Feverfew extracts at the same dose in the rat MCAO model. Pretreatment of Feverfew extracts preserved the neuronal morphology better in CA1 subfield and increased the number of CA1 neurons that survived compared to the post-treatment of Feverfew.[24] This study raised a conception that pretreatment of TCM might lead to a better outcome than the regular treatment did in the MCAO animal model. Nevertheless, pretreatment of Chinese herbal medicine to modulate hippocampal neurogenesis has been hardly investigated.
A pilot study carried out in our lab was on pretreatment of polysaccharides from wolfberry (枸杞子 Gǒu Qí Zĭ) in a rat depression model. It had been previously confirmed in our lab that chronic injection of 30 mg/kg, 40 mg/kg, and 50 mg/kg corticosterone caused a significant loss of hippocampal neurogenesis and subsequently induced a depression-like behavior in those corticosterone-treated rats. Furthermore, 14 days of physical exercise successfully reversed the impairment of hippocampal neurogenesis and the depression-like behavior in both 30 mg/kg and 40 mg/kg corticosterone treated rats, but failed to do so in 50 mg/kg corticosterone-treated rats.[25] However, 7 days of pretreatment plus 14 days of treatment of wolfberry were able to enhance the neuronal differentiation of the hippocampal neurogenesis and reverse the depression-like behavior caused by 50 mg/kg corticosterone injection.[26] Our data together indirectly suggested that a 7-day pretreatment plus a 14-day treatment of wolfberry might have a stronger impact on hippocampal neurogenesis than the running exercise had. The underlying mechanism of the beneficial effects of the 7-day pretreatment of wolfberry on hippocampal neurogenesis was totally unclear. Therefore, future studies need to be carried out to investigate how the pretreatment of Chinese herbs can induce factors beneficial for hippocampal neurogenesis and what kind of beneficial factors for hippocampal neurogenesis can be induced by pretreatment of TCMs.
POSSIBLE MECHANISMS MEDIATING THE EFFECTS OF CHINESE HERBS ON THE HIPPOCAMPAL NEUROGENESIS
Upregulation of neurotrophic factors by Chinese herbs
Neurotrophic factors, including neural growth factor (NGF), brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), etc., play a central role in cellular proliferation, migration, differentiation, and maintenance in the developing brain.[27] Their presence is crucial across the entire life span for maintenance of neuronal functions, structural integrity of neurons, and neurogenesis. Many Chinese herbs exhibited the ability to promote the secretion of neurotrophic factors and thus enhanced neurogenesis in the hippocampus. Kim Hyo Geun and Oh Myung Sook reported that extracts from Mori Fractus (森喜朗山萸 Sēn Xĭ Lăng Shān Yú) increased the level of NGF in the mouse hippocampus in a dose-dependent manner. The increased NGF significantly enhanced the neuronal differentiation and cell proliferation.[28] Yao et al. found that intragastric administration of cornel iridoid glycoside (CIG), an ingredient extracted from a traditional Chinese herb Cornus officinalis (山茱萸 Shān Zhū Yú), obviously enhanced the mRNA expression of VEGF and its receptor Flk-1 and the protein expression of VEGF, 7 and 28 days after ischemia. This enhancement of VEGF level appeared to be responsible for the neurogenesis in the MCAO rats.[29] Fuzi, kam-ondam tang, and curcumin have been reported to be able to increase the mRNA and protein levels of BDNF in the mouse hippocampus, respectively.[10,30,31] Furthermore, the beneficial effects of Fuzi on hippocampal neurogenesis were neutralized by using TrkB receptor blocker, K252a.[31] Therefore, neurotropic factor signaling pathway seemed to be one of the major targets for traditional Chinese herbs to modulate hippocampal neurogenesis.
Modulation of basic helix-loop-helix family proteins
Basic helix-loop-helix (bHLH) is a protein structural motif that characterizes a family of transcription factors.[32] bHLH transcription factors proved to be essential in mammalian neurogenesis.[33] Li et al. found that in vitro, baicalin treatment selectively upregulated the expression of Mash1 and NeuroD1 in neural progenitor cells,[34] the two members in bHLH family that previously proved to be essential for neuronal commitment in neural stem cells,[35] as well as olfactory neurogenesis.[36] The finding provided the evidence that baicalin is able to modulate the transcription factors and, thus, exerts its effects on neurogenesis in mammals.
PI3K/Akt and ERK pathways
Extracellular signal-regulated kinases (ERK) and PI3K/Akt pathways are most frequently associated with regulation of cell growth, survival, and differentiation.[37,38] Salvianolic acid B (Sal B), one of the major ingredients in the water-soluble extracts of S. miltiorrhiza Bunge, dramatically promoted the proliferation of neural progenitor cells in a dose- and time-dependent manner. This process was exclusively mediated by the PI3K/Akt pathway since the stimulation of neural progenitor cells with Sal B was abolished by Ly294002, a PI3K/Akt inhibitor, while U0126 (ERK inhibitor) or DAPT (Notch inhibitor, N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) had little impact on the Sal B–induced proliferation of neural progenitor cells.[39] On the contrary, administration of curcumin to adult mice resulted in a significant increase in the number of newly born neurons in the dentate gyrus of hippocampus. This increase could be blocked by ERK inhibitor, indicating that curcumin promoted hippocampal neurogenesis through the mitogen-activated protein kinase (MEK)/ERK pathway.[40] The above evidence shows that some Chinese herbs enhance hippocampal neurogenesis through the classic kinase pathways such as PI3K/Akt or MEK/ERK pathway.
In summary, traditional Chinese herbs provide an abundant pharmaceutical platform to modulate hippocampal neurogenesis. Exploring the effective components in Chinese herbs beneficial to hippocampal neurogenesis has improved our understanding of how the administration of Chinese herbs affects hippocampal neurogenesis. Considering health maintenance and disease prevention according to TCM theory, pretreatment of Chinese herbs might be promising to induce factors beneficial in modulating hippocampal neurogenesis. Thus, further studies should be carried out focusing on the effects of pretreatment of TCMs on modulation of hippocampal neurogenesis. Although many promising achievements have been made by using TCMs to enhance hippocampal neurogenesis in several animal models, as well as in in vitro cell cultures, few clinical trials have been conducted so far. One limitation impeding the drug clinical trials on neurogenesis is lack of an in-site method to monitor and calculate the neurogenesis in the hippocampus. Therefore, there still exist several challenging tasks to extend the application of TCMs to neurogenesis in clinical cases.
ACKNOWLEDGMENT
This work was supported by the Fundamental Research Funds for the Central Universities (21609101, K. F. So).
REFERENCES
- 1.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 2.Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–7. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–42. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin PC, Chang LF, Liu PY, Lin SZ, Wu WC, Chen WS, et al. Botanical drugs and stem cells. Cell Transplant. 2011;20:71–83. doi: 10.3727/096368910X532747. [DOI] [PubMed] [Google Scholar]
- 5.Lee KS, Lim BV, Chang HK, Yang HY, Bahn GH, Paik EK, et al. Liuweidihuang-tang improves spatial memory function and increases neurogenesis in the dentate gyrus in rats. Fitoterapia. 2005;76:514–9. doi: 10.1016/j.fitote.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Park SW, Kim YK, Lee JG, Kim SH, Kim JM, Yoon JS, et al. Antidepressant-like effects of the traditional Chinese medicine kami-shoyo-san in rats. Psychiatry Clin Neurosci. 2007;61:401–6. doi: 10.1111/j.1440-1819.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 7.Hong JG, Kim DH, Park SJ, Kim JM, Cai M, Liu X, et al. The memory-enhancing effects of Kami-ondam-tang in mice. J Ethnopharmacol. 2011;137:251–6. doi: 10.1016/j.jep.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Shen JG, XM C. in Ch. 11. Drug Discovery from Traditional Chinese Medicine for Neurogenesis: Implications for Stroke and Neurodegenerative Diseases. In: James EJ, Adams D, editors. Traditional Chinese Medicine. Hong Kong: 2013. pp. 204–37. [Google Scholar]
- 9.Tong L, Tan XH, Shen JG. Comparative study of Buyang Huanwu Decoction and the different combinations of its ingredients on neurogenesis following ischemic stroke in rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27:519–22. [PubMed] [Google Scholar]
- 10.Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci. 2013;33:2961–72. doi: 10.1523/JNEUROSCI.3878-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–20. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva R. Neurobiology of major depressive disorder. Neural Plast 2013. 2013 doi: 10.1155/2013/873278. 873278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai G, Liu B, Liu W, Tan X, Rong J, Chen X, et al. Buyang Huanwu Decoction can improve recovery of neurological function, reduce infarction volume, stimulate neural proliferation and modulate VEGF and Flk1 expressions in transient focal cerebral ischaemic rat brains. J Ethnopharmacol. 2007;113:292–9. doi: 10.1016/j.jep.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Lo CJ, Lin JG, Kuo JS, Chiang SY, Chen SC, Liao ET, et al. Effect of salvia miltiorrhiza bunge on cerebral infarct in ischemia-reperfusion injured rats. Am J Chin Med. 2003;31:191–200. doi: 10.1142/S0192415X03000916. [DOI] [PubMed] [Google Scholar]
- 15.Ye R, Yang Q, Kong X, Han J, Zhang X, Zhang Y, et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58:391–8. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Guan T, Liu Q, Qian Y, Yang H, Kong J, Kou J, et al. Ruscogenin reduces cerebral ischemic injury via NF-kappaB-mediated inflammatory pathway in the mouse model of experimental stroke. Eur J Pharmacol. 2013;714:303–11. doi: 10.1016/j.ejphar.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Deng J, Qi XL, Guan ZZ, Yan XM, Huang Y, Wang YL. Pretreatment of SH-SY5Y cells with dicaffeoylquinic acids attenuates the reduced expression of nicotinic receptors, elevated level of oxidative stress and enhanced apoptosis caused by beta-amyloid peptide. J Pharm Pharmacol. 2013;65:1736–44. doi: 10.1111/jphp.12096. [DOI] [PubMed] [Google Scholar]
- 18.Chen DL, Zhang P, Lin L, Shuai O, Zhang HM, Liu SH, et al. Protective effect of Bajijiasu against beta-amyloid-induced neurotoxicity in pc12 cells. Cell Mol Neurobiol. 2013;33:837–50. doi: 10.1007/s10571-013-9950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo T, Jiang W, Kong Y, Li S, He F, Xu J, et al. The protective effects of jatrorrhizine on beta-amyloid (25-35)-induced neurotoxicity in rat cortical neurons. CNS Neurol Disord Drug Targets. 2012;11:1030–7. doi: 10.2174/1871527311211080013. [DOI] [PubMed] [Google Scholar]
- 20.Park H, Oh MS. Houttuyniae Herba protects rat primary cortical cells from Abeta (25-35)-induced neurotoxicity via regulation of calcium influx and mitochondria-mediated apoptosis. Hum Exp Toxicol. 2012;31:698–709. doi: 10.1177/0960327111433898. [DOI] [PubMed] [Google Scholar]
- 21.Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP. Protective effect of isorhynchophylline against beta-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:353–60. doi: 10.1007/s10571-011-9763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho YS, Yu MS, Lai CS, So KF, Yuen WH, Chang RC. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on beta-amyloid peptide neurotoxicity. Brain Res. 2007;1158:123–34. doi: 10.1016/j.brainres.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 23.Yu MS, Lai CS, Ho YS, Zee SY, So KF, Yuen WH, et al. Characterization of the effects of anti-aging medicine Fructus lycii on beta-amyloid peptide neurotoxicity. Int J Mol Med. 2007;20:261–8. [PubMed] [Google Scholar]
- 24.Sun YF, Pei DS, Zhang QX, Zhang GY. Neuroprotection of GST, an extract of traditional Chinese herb, against ischemic brain injury induced by transient brain ischemia and reperfusion in rat hippocampus. Neurol Res. 2008;30:471–5. doi: 10.1179/174313208X289507. [DOI] [PubMed] [Google Scholar]
- 25.Yau SY, Lau BW, Tong JB, Wong R, Ching YP, Qiu G, et al. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS One. 2011;6:e24263. doi: 10.1371/journal.pone.0024263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang E, Yau SY, Lau BW, Ma H, Lee TM, Chang RC, et al. Synaptic plasticity, but not hippocampal neurogenesis, mediated the counteractive effect of wolfberry on depression in rats (1) Cell Transplant. 2012;21:2635–49. doi: 10.3727/096368912X655181. [DOI] [PubMed] [Google Scholar]
- 27.Dwivedi Y. Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–49. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HG, Oh MS. Memory-enhancing effect of Mori Fructus via induction of nerve growth factor. Br J Nutr. 2013;110:86–94. doi: 10.1017/S0007114512004710. [DOI] [PubMed] [Google Scholar]
- 29.Yao RQ, Zhang L, Wang W, Li L. Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res Bull. 2009;79:69–76. doi: 10.1016/j.brainresbull.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Ku B, Cui L, Li X, Barish PA, Foster TC, et al. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007;1162:9–18. doi: 10.1016/j.brainres.2007.05.071. [DOI] [PubMed] [Google Scholar]
- 31.Yan HC, Qu HD, Sun LR, Li SJ, Cao X, Fang YY, et al. Fuzi polysaccharide-1 produces antidepressant-like effects in mice. Int J Neuropsychopharmacol. 2010;13:623–33. doi: 10.1017/S1461145709990733. [DOI] [PubMed] [Google Scholar]
- 32.Massari ME, Murre C. Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–40. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kageyama R, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr Opin Genet Dev. 1997;7:659–65. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Zhuang P, Shen B, Zhang Y, Shen J. Baicalin promotes neuronal differentiation of neural stem/progenitor cells through modulating p-stat3 and bHLH family protein expression. Brain Res. 2012;1429:36–42. doi: 10.1016/j.brainres.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Katakura M, Hashimoto M, Shahdat HM, Gamoh S, Okui T, Matsuzaki K, et al. Docosahexaenoic acid promotes neuronal differentiation by regulating basic helix-loop-helix transcription factors and cell cycle in neural stem cells. Neuroscience. 2009;160:651–60. doi: 10.1016/j.neuroscience.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 36.Boutin C, Hardt O, de Chevigny A, Coré N, Goebbels S, Seidenfaden R, et al. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc Natl Acad Sci U S A. 2010;107:1201–6. doi: 10.1073/pnas.0909015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao Z, Kong Y, Yang S, Li M, Wen J, Li L. Upregulation of Flk-1 by bFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation. Cell Res. 2007;17:73–9. doi: 10.1038/sj.cr.7310126. [DOI] [PubMed] [Google Scholar]
- 38.Shioda N, Han F, Fukunaga K. Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int Rev Neurobiol. 2009;85:375–87. doi: 10.1016/S0074-7742(09)85026-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M, Zhang J, et al. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One. 2012;7:e35636. doi: 10.1371/journal.pone.0035636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang M, Feng W, Zhang Y, Zhong J, Zhang J. Salvianolic acid B improves motor function after cerebral ischemia in rats. Behav Pharmacol. 2006;17:493–8. doi: 10.1097/00008877-200609000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Zhong J, Tang MK, Zhang Y, Xu QP, Zhang JT. Effect of salvianolic acid B on neural cells damage and neurogenesis after brain ischemia-reperfusion in rats. Yao Xue Xue Bao. 2007;42:716–21. [PubMed] [Google Scholar]
- 43.Zhang Z, Wu R, Li P, Liu F, Zhang W, Zhang P, et al. Baicalin administration is effective in positive regulation of twenty-four ischemia/reperfusion-related proteins identified by a proteomic study. Neurochem Int. 2009;54:488–96. doi: 10.1016/j.neuint.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M, Zhang J, et al. Baicalin regulates neuronal fate decision in neural stem/progenitor cells and stimulates hippocampal neurogenesis in adult rats. CNS Neurosci Ther. 2013;19:154–62. doi: 10.1111/cns.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Si YC, Zhang JP, Xie CE, Zhang LJ, Jiang XN. Effects of Panax notoginseng saponins on proliferation and differentiation of rat hippocampal neural stem cells. Am J Chin Med. 2011;39:999–1013. doi: 10.1142/S0192415X11009366. [DOI] [PubMed] [Google Scholar]
- 46.Lau BW, Lee JC, Li Y, Fung SM, Sang YH, Shen J, et al. Polysaccharides from wolfberry prevents corticosterone-induced inhibition of sexual behavior and increases neurogenesis. PLoS One. 2012;7:e33374. doi: 10.1371/journal.pone.0033374. [DOI] [PMC free article] [PubMed] [Google Scholar]


