Abstract
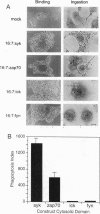
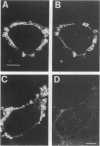
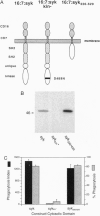
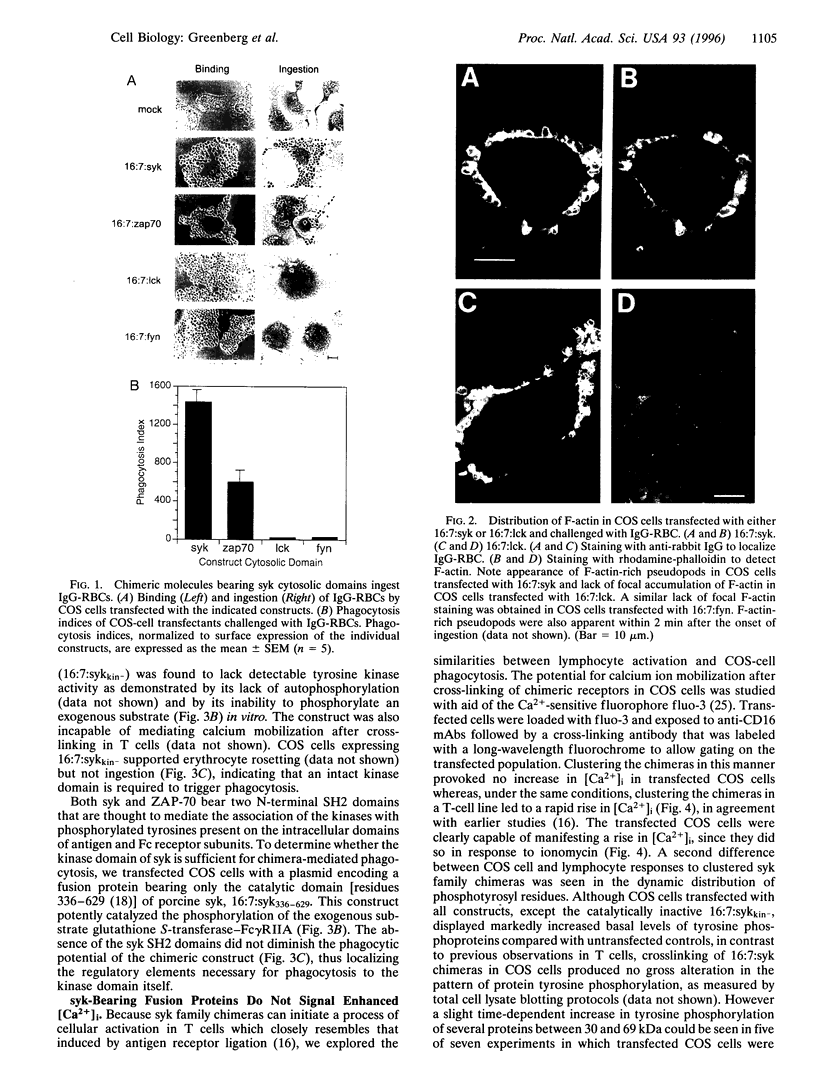
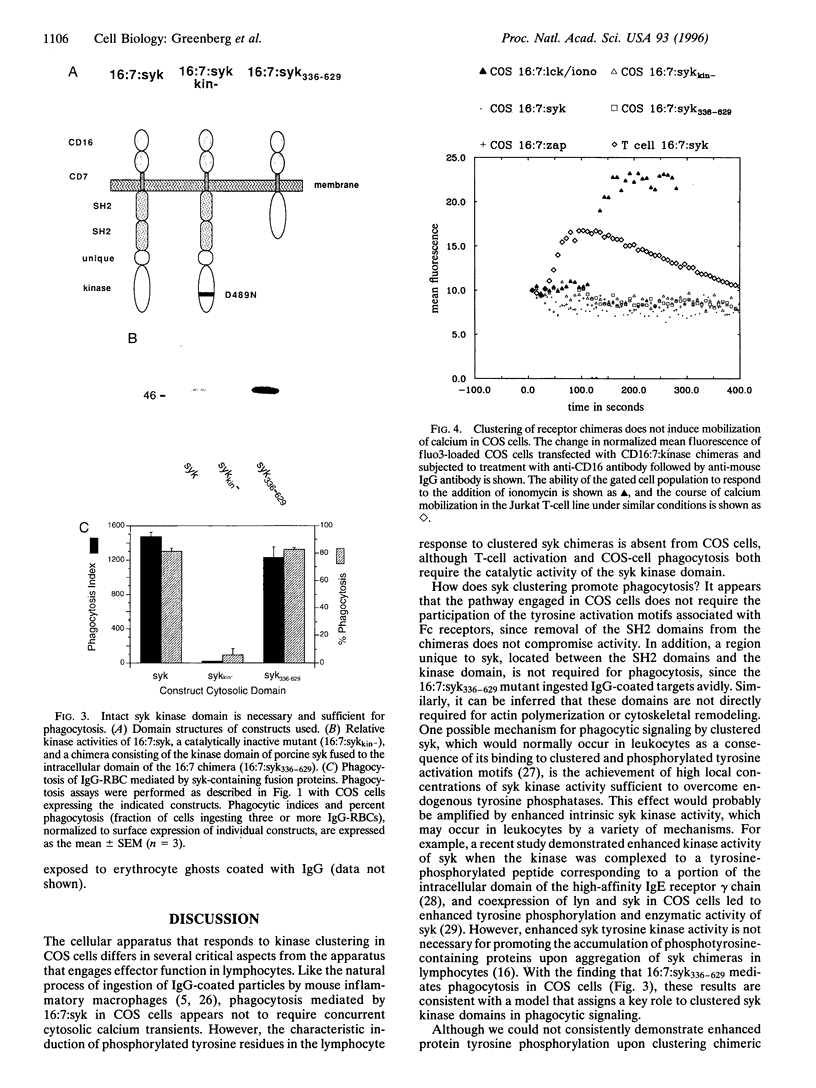
Phagocytosis is a phylogenetically primitive mechanism adapted by specialized cells of the immune system to ingest particulate pathogens. Recent evidence suggests that the program of specific cytoskeletal rearrangements that underlies phagocytosis may share elements with the antigen receptor signaling pathway in lymphocytes. Tyrosine phosphorylation, necessary for both lymphocyte effector function and phagocytosis, is thought to allow cytoskeletal elements to couple to the intracellular domains of antigen and Fc receptor subunits. We show here that the intracellular domains of the receptors are not inherently required for cytoskeletal coupling. Chimeric transmembrane proteins bearing syk but not src family tyrosine kinase domains are capable of autonomously triggering phagocytosis and redistribution of filamentous actin in COS cells. These responses cannot be initiated by a receptor chimera bearing a point mutation in the syk catalytic domain, and the kinase domain alone is sufficient for initiating cytoskeletal coupling.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Sagi D., Rotin D., Batzer A., Mandiyan V., Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993 Jul 16;74(1):83–91. doi: 10.1016/0092-8674(93)90296-3. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Meyer B. C., Greenberg S., Silverstein S. C. Fc receptor-mediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J Cell Biol. 1988 Mar;106(3):657–666. doi: 10.1083/jcb.106.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sastry K., Bailly P., Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990 Dec 1;172(6):1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Williams D. J., Koziel H., Armstrong M. Y., Warner A., Richards F. F., Rose R. M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991 May 9;351(6322):155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- Greenberg S., Chang P., Silverstein S. C. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993 Feb 1;177(2):529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Chang P., Silverstein S. C. Tyrosine phosphorylation of the gamma subunit of Fc gamma receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J Biol Chem. 1994 Feb 4;269(5):3897–3902. [PubMed] [Google Scholar]
- Greenberg S., el Khoury J., di Virgilio F., Kaplan E. M., Silverstein S. C. Ca(2+)-independent F-actin assembly and disassembly during Fc receptor-mediated phagocytosis in mouse macrophages. J Cell Biol. 1991 May;113(4):757–767. doi: 10.1083/jcb.113.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indik Z., Kelly C., Chien P., Levinson A. I., Schreiber A. D. Human Fc gamma RII, in the absence of other Fc gamma receptors, mediates a phagocytic signal. J Clin Invest. 1991 Nov;88(5):1766–1771. doi: 10.1172/JCI115496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanus W., Romeo C., Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993 Jul 16;74(1):171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989 Nov 1;170(5):1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Takata M., Yamanashi Y., Inazu T., Taniguchi T., Yamamoto T., Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994 May 1;179(5):1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A., Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994 Nov;19(11):459–463. doi: 10.1016/0968-0004(94)90130-9. [DOI] [PubMed] [Google Scholar]
- McWhirter J. R., Wang J. Y. An actin-binding function contributes to transformation by the Bcr-Abl oncoprotein of Philadelphia chromosome-positive human leukemias. EMBO J. 1993 Apr;12(4):1533–1546. doi: 10.1002/j.1460-2075.1993.tb05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed I., Downey G. P., Aktories K., Roifman C. M. Microfilament assembly is required for antigen-receptor-mediated activation of human B lymphocytes. J Immunol. 1991 Aug 15;147(4):1139–1146. [PubMed] [Google Scholar]
- Moran M. F., Koch C. A., Sadowski I., Pawson T. Mutational analysis of a phosphotransfer motif essential for v-fps tyrosine kinase activity. Oncogene. 1988 Dec;3(6):665–672. [PubMed] [Google Scholar]
- Pardi R., Inverardi L., Rugarli C., Bender J. R. Antigen-receptor complex stimulation triggers protein kinase C-dependent CD11a/CD18-cytoskeleton association in T lymphocytes. J Cell Biol. 1992 Mar;116(5):1211–1220. doi: 10.1083/jcb.116.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey M. V., Lewis G. K. Actin polymerization and pseudopod reorganization accompany anti-CD3-induced growth arrest in Jurkat T cells. J Immunol. 1993 Aug 15;151(4):1881–1893. [PubMed] [Google Scholar]
- Pfeiffer J. R., Seagrave J. C., Davis B. H., Deanin G. G., Oliver J. M. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 1985 Dec;101(6):2145–2155. doi: 10.1083/jcb.101.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Brugge J. S. Clustering of Syk is sufficient to induce tyrosine phosphorylation and release of allergic mediators from rat basophilic leukemia cells. Mol Cell Biol. 1995 Mar;15(3):1582–1590. doi: 10.1128/mcb.15.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. E., Brogle N. L., Edberg J. C., Kimberly R. P. Fc gamma receptor III induces actin polymerization in human neutrophils and primes phagocytosis mediated by Fc gamma receptor II. J Immunol. 1991 Feb 1;146(3):997–1004. [PubMed] [Google Scholar]
- Sheterline P., Rickard J. E., Richards R. C. Fc receptor-directed phagocytic stimuli induce transient actin assembly at an early stage of phagocytosis in neutrophil leukocytes. Eur J Cell Biol. 1984 May;34(1):80–87. [PubMed] [Google Scholar]
- Shiue L., Zoller M. J., Brugge J. S. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J Biol Chem. 1995 May 5;270(18):10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994 Jun 3;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Tan J. C., Nocka K., Ray P., Traktman P., Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990 Jan 12;247(4939):209–212. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Kobayashi T., Kondo J., Takahashi K., Nakamura H., Suzuki J., Nagai K., Yamada T., Nakamura S., Yamamura H. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991 Aug 25;266(24):15790–15796. [PubMed] [Google Scholar]
- Valitutti S., Dessing M., Aktories K., Gallati H., Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995 Feb 1;181(2):577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P. K., Baltimore D., Sanders M. C., Matsudaira P. T., Janmey P. A. The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J Cell Biol. 1994 Feb;124(3):325–340. doi: 10.1083/jcb.124.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe P. A., Ceuppens J. L. Flow cytometric measurement of cytoplasmic free calcium in human peripheral blood T lymphocytes with fluo-3, a new fluorescent calcium indicator. J Immunol Methods. 1990 Mar 9;127(2):197–205. doi: 10.1016/0022-1759(90)90069-8. [DOI] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Yon J., Fried M. Precise gene fusion by PCR. Nucleic Acids Res. 1989 Jun 26;17(12):4895–4895. doi: 10.1093/nar/17.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]