Abstract
Individuals with post-traumatic stress disorder (PTSD) show a cognitive bias for threatening information, reflecting dysregulated executive control for affective stimuli. This study examined whether comorbid mild Traumatic Brain Injury (mTBI) with PTSD exacerbates this bias. A computer-administered Affective Go/No-Go task measured reaction times (RTs) and errors of omission and commission to words with a non–combat-related positive or negative valence in 72 deployed United States service members from the wars in Iraq and Afghanistan. Incidents of military-related mTBI were measured with the Boston Assessment of Traumatic Brain Injury-Lifetime. PTSD symptoms were measured with the Clinician-Administered PTSD Scale. Participants were divided into those with (mTBI+, n =34) and without a history of military-related mTBI (mTBI−, n =38). Valence of the target stimuli differentially impacted errors of commission and decision bias (criterion) in the mTBI+ and mTBI− groups. Specifically, within the mTBI+ group, increasing severity of PTSD symptoms was associated with an increasingly liberal response pattern (defined as more commission errors to negative distractors and greater hit rate for positive stimuli) in the positive compared to the negative blocks. This association was not observed in the mTBI− group. This study underscores the importance of considering the impact of a military-related mTBI and PTSD severity upon affective executive control.
Keywords: Post-traumatic stress disorder, Attention, Brain injury, Military, Cognition, Deployment
INTRODUCTION
Cognitive bias for threatening information, reflecting executive dyscontrol, is observed among individuals with post-traumatic stress disorder (PTSD). This bias toward stimuli with negative valence may be exacerbated when traumatic brain injury (TBI) is comorbid with psychological trauma exposure, due to additional brain injury-related executive dysfunction. Prevalence estimates of PTSD for Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) veterans have ranged between 11 and 18% (Hoge et al., 2004; Vasterling et al., 2006), and projections suggest even higher rates in the future (Atkinson, Guetz, & Wein, 2009). OEF/OIF veterans are diagnosed with unprecedented levels of mild TBI (mTBI) with prevalence rates for mTBI ranging from 12 to 23% (Schneiderman, Braver, & Kang, 2008; Terrio et al., 2009). Data are limited regarding the rate at which these two conditions co-occur, but one study suggests that it might be as high as 30% (Calhoun et al., 2010). Civilian-based research studies have found that only a small minority of mTBI cases experience persistent cognitive and neurobehavioral symptoms (Iverson, 2005). Whether or not mTBI sustained during military deployment has a similar course of cognitive and behavioral symptom resolution, as observed in the civilian population, is not yet known.
A primary concern is the growing number of soldiers who experience both psychological and physical trauma during deployment, resulting in a high rate of comorbid mTBI and PTSD. PTSD is more common among veterans who sustain a TBI as compared to other injuries (Hoge et al., 2008). This has also been observed among civilians who sustained a TBI despite being matched for comparable levels of trauma exposure (Walilko et al., 2009). Furthermore, symptoms of PTSD in a non-military sample appear to persist for a longer period of time (Harvey & Bryant, 2000; Vanderploeg, Belanger, & Curtiss, 2009) and are more severe when accompanied by an mTBI (Chemtob et al., 1998). Underscoring the association between PTSD and TBI, a recent animal study demonstrated that fear conditioning and amygdala activation were greater in animals exposed to blast compared to animals not exposed to blast (Reger et al., 2012). It is possible that the occurrence of mTBI during trauma exposure may increase the risk for development of PTSD and PTSD-related cognitive deficits. Precisely how these comorbid conditions coalesce to affect cognitive/emotional function in general has yet to be examined critically, but given their overlapping symptomatology and neurobiology (Stein & McAllister, 2009), it has been proposed that the effects are additive or even synergistic, particularly with regard to cognitive functioning. The possibility that co-occurring mTBI may exacerbate cognitive symptoms specific to PTSD, such as attentional bias toward threat information, therefore, needs to be examined.
Threat-related attentional bias is a significant cognitive component of PTSD and has been proposed to be a factor in its development and maintenance (Kimble, Fleming, Bandy, Kim, & Zambetti, 2010). This bias toward information with a negative valence has significant impact on both information processing and subsequent behavior, as it effectively functions as a gating mechanism that directs attention based on the valence of the stimuli (Constans, 2005).
Using different experimental tasks, affective attentional bias has been shown to either interfere with or facilitate task performance. Demonstrating the interfering effect of attentional bias, multiple studies have shown that participants with PTSD, when performing a Stroop-type task, are slower to name the color of the ink when the stimulus words have a negative compared to neutral valence (Buckley, Blanchard, & Neill, 2000; Constans, McCloskey, Vasterling, Brailey, & Mathews, 2004; Thrasher, Dalgleish, & Yule, 1994). It is speculated that attention is drawn to or absorbed by (Pineles, Shipherd, Mostoufi, Abramovitz, & Yovel, 2009) stimuli with negative valence, which slows reaction times related to the additional effort required to inhibit the pre-potent response of naming the threatening word. In contrast, Bryant and Harvey (1997) observed that PTSD-related attentional bias facilitated performance during a visual attention task that required localization of a target immediately following visual presentation of neutral and negative word pairs. Specifically, they found that the PTSD group (relative to a control group) demonstrated faster reaction times (RTs) to targets that occurred in the location previously occupied by a negative compared to a neutral word. Under these experimental conditions, it is speculated that the facilitated behavioral response occurred in the PTSD group because visual attention was already drawn to the location of the threat word due to preferential processing of stimuli with negative valence. However, differences in these study outcomes also may be accounted for by findings of selective verbal versus visual system processing deficits in PTSD (Brewin, Kleiner, Vasterling, & Field, 2007).
In the current study, we examined whether attentional bias toward information with a negative valence is amplified in individuals who have a military-related mTBI and PTSD symptoms. MTBI could worsen PTSD-related affective attentional bias through persisting cognitive dysfunction related to the brain injury. As mentioned, while the cognitive sequelae of mTBI appear to resolve in a majority of civilian patients (Carroll et al., 2004; Iverson, 2005), in a minority, cognitive deficits persist (Pontifex, O’Connor, Broglio, & Hillman, 2009), and this may be especially true following multiple mTBI (Belanger, Kretzmer, Vanderploeg, & French, 2010). These residual deficits reflect, in part, impairments in speed of information processing and a reduction in the amount of information that can be processed simultaneously (Crawford, Knight, & Alsop, 2007; De Monte, et al., 2005; Van Zomeren, Brouwer, & Deelman, 1984). We speculate that residual mTBI-related cognitive impairments may be more apparent under conditions of high emotional and physiological distress, such as when PTSD and mTBI co-occur. This raises the possibility that individuals with both conditions may have an enhanced attentional bias for information with a negative valence. That is, the prepotent tendency to attend to negative information may be even greater among service members with PTSD and mTBI compared to those with PTSD without an mTBI.
The current study examined the relative impact of mTBI and PTSD symptoms upon affective information processing in OEF/OIF service members. It was hypothesized that individuals with military-related mTBI would show greater executive dyscontrol as PTSD symptom severity increased relative to those individuals without a history of military-related mTBI.
METHOD
Participants
The participants were 34 (33 men) individuals with a history of military-related mTBI and 38 (30 men) age- and education-matched participants without a history of military mTBI. All participants were deployed service members of Operation Enduring Freedom or Operation Iraqi Freedom (OEF/OIF) who had enrolled in the Veterans Administration Rehabilitation Research and Development supported TBI Center of Excellence at VA Boston Healthcare System: The Translational Research Center for TBI and Stress Disorders (TRACTS). TRACTS uses psychological, biological, and cognitive assessments to characterize the impact of mTBI and deployment stress upon the functioning of OEF/OIF veterans. All participants provided written informed consent before completing any of the experimental procedures. All procedures received approval by the local Veterans Administration Committee on Humans Subjects Research approval. Participants were recruited through advertisements, informational sessions provided to veterans, and active duty military events.
Materials and Procedure
Study participation primarily consisted of 1- or 2-day sessions with a standardized order of test administration. Participants first underwent comprehensive assessments for TBI, PTSD, and other Axis I diagnoses. A doctoral-level psychologist administered each assessment and each case was then reviewed by at least three doctoral-level psychologists/psychiatrists to achieve a consensus diagnoses for TBI, PTSD, and other Axis I disorders. This assessment was followed by administration of neuropsychological tests including the Go/No-Go task and other self-report measures.
Assessments
TBI diagnosis
The Boston Assessment of TBI-Lifetime (BAT-L) (Fortier et al., 2013) was used to assess potential brain injury during three lifetime periods: pre-military, military, and post-military. While this is a new measure developed by TRACTS, a preliminary validation study in a subsample of the participants (n =131) demonstrated excellent correpondence between the BAT-L and the Ohio State TBI Assessment Method (Kendall’s tau b =0.95), a validated method for TBI identification (Fortier et al., 2013). TBI criteria including altered mental state (AMS), post-traumatic amnesia (PTA), and loss of consciousness (LOC) were evaluated through open-ended questioning.
Diagnosis of mTBI is controversial when the service member sustains a blow to the head that is accompanied by only brief AMS/PTA, as such fleeting symptoms of AMS/PTA could in fact represent a psychological rather than biomechanical reaction to the injury (Brenner, Vanderploeg, & Terrio, 2009). It is very difficult to distinguish the symptoms of acute stress disorder characterized by confusion, amnesia, dissociation, numbing, detachment, and depersonalization from AMS or PTA associated with a TBI (Bryant, 2011; Hill, Mobo, & Cullen, 2009). Therefore, we adopted a conservative criteria for the diagnosis of mTBI. Participants were diagnosed with a military-related mTBI if they experienced either AMS and/or PTA for at least 15 minutes (although less than 24 h) or any episode of LOC. Participants with brief durations of PTA or AMS (less than 15 min) acquired during their military service, were excluded from the study. We used these conservative criteria to reduce the possibility of including individuals who did not sustain an mTBI in the military TBI group. In the mTBI+ group, 28 individuals had experienced an LOC, whereas 6 did not have an LOC but had at least 15 minutes of PTA or AMS.
Importantly, the BAT-L is not invulnerable to embellishment; nevertheless, all participants in this study passed a neuro-psychological task assessing effort (see below), suggesting that they were motivated to perform at their optimal level. Participants were excluded from the study if they had a history of moderate or severe TBI before joining the military. Participants with multiple TBIs were included in the study. Of note, the mTBI+ and mTBI− groups did not differ in number of mTBI acquired before or after military service (Table 2).
Table 2.
Group characteristics
| mTBI− (N =38) | mTBI+ (N =34) | |
|---|---|---|
| Age | 30.0 (6.3) | 29.3 (6.6) |
| Education | 13.5 (1.5) | 13.5 (1.6) |
| Estimated IQ | 101.0 (9.4) | 100.7 (8.0) |
| Gender | ||
| Male | 30 (78.9%) | 33 (97.1%) |
| Female | 8 (21.1%) | 1 (2.9%) |
| Ethnicity | ||
| Unknown | 1 (2.6%) | 0 (0%) |
| African American | 3 (7.9%) | 1 (2.9%) |
| Hispanic | 9 (23.7%) | 5 (14.7%) |
| Caucasian | 25 (65.8%) | 28 (82.4%) |
| mTBI Pre-military | ||
| Yes | 17 (44.7%) | 27 (79.3%) |
| No | 21 (55.3%) | 7 (20.6%) |
| SMAST 12 month | 1.3 (2.7) | 2.3 (2.8) |
| Number of mTBI pre-military | 0.58 (1.2) | 0.88 (1.7) |
| Number of mTBI post-military | 0.16 (0.37) | 0.03 (.17) |
| CAPS total score | 43.26 (28.6) | 67.3 (21.3) |
| Deployment Risk and Resilience: Combat Experiences | 9.5 (7.7) | 24.9 (12.6) |
| Duration of deployment (months) | 12.2 (6.2) | 15.7 (11.5) |
| Time Since Deployment (months) | 33.5 (29.6) | 39.8 (26.3) |
SMAST =Short Michigan Alcohol Test; Pre-military =before joining the military; Post-military =since deployment; CAPS =Clinician Administered PTSD Scale.
PTSD assessment
The presence and severity of PTSD were assessed using the Clinician-Administered PTSD Scale (CAPS). The CAPS is a well-validated and reliable semi-structured clinical interview used to evaluate the DSM-IV re-experiencing (Criterion B), avoidance (Criterion C), and hyperarousal (Criterion D) symptoms of PTSD (Blake, Weathers, & Nagy, 1993; Blake et al., 1995). Participants are queried about the intensity (0–4) and frequency (0–4) for each of the possible 17 DSM-IV-TR PTSD symptoms (min score =0; max =136), from which a total score is derived. The standard scoring rule was used, such that to count as a symptom, the participant had to score at least a 1 for frequency and a 2 for intensity.
Combat experience
The Deployment Risk and Resilience Inventory (DRRI) (King, King, Vogt, Knight, & Samper, 2006) Combat Experiences module was used to capture severity of combat experiences. The DRRI was developed specifically in relation to contemporary war-zones. Scores can range from 0 to 64. Evidence for the internal consistency reliability, criterion-related validity, and discriminative validity of the DRRI scales has been demonstrated (King et al., 2006; Vogt, Proctor, King, King, & Vasterling, 2008) in which internal consistency reliability ranged from .55 to .90.
Deployment characteristics
Participants were asked about the duration and location of each OEF/OIF deployment. Total months deployed was the sum of all OEF/OIF deployments. Time since last deployment was calculated from month of return home from the most recent deployment to study appointment date.
Effort
Participants were administered the Green Medical Symptom Validity Test (MSVT) to ensure that all participants included in this study demonstrated adequate motivation to perform at their optimal level. Adequate effort was determined by the cutoffs specified in the manual (Green, 2003). This led to the exclusion of seven participants (mTBI−: n =3, mTBI+: n =4).
Affective go/no-go
Upon completion of psychological assessments, participants performed the affective Go/No-Go task (Cambridge Cognition, Ltd.; Robbins, et al., 1998, 1994). This is a continuous performance task in which a series of stimulus words was presented on the center of a monitor for 300 ms with a 900 ms inter-stimulus interval (ISI). There were 10 blocks containing 18 words. Target word valence (positive or negative) was constant within each block and switched every two blocks. Target and distractor words with a negative valence were non-specific and did not relate to combat experiences. Similarly, positive valenced words were non-specific. Order of presentation was counterbalance across participants. The first two blocks served as practice trials, leaving eight blocks of 18 words as the critical dataset. Within each block, there were nine words that were consistent with the target valence (“Go” target words) and nine words that were inconsistent with the target valence (“No-Go” distractor words). Words were not associated with trauma content.
At the beginning of each block, the participant was visually and verbally informed of the targeted valence for that block (either positive or negative) and the task was to determine if the valence of the presented word matched (Go condition) or did not match (No-Go condition) the targeted valence. The participant was instructed to press the spacebar for words that matched the targeted valence and to withhold or inhibit the motor response when the stimulus did not match the targeted valence. Primary dependent measures were the number of target omissions, commission errors, and reaction times (RT) for correct responses. To evaluate participants’ overall accuracy within the positive and negative blocks, d′ was calculated d′ =Z(hit rate) −Z(false alarm rate). D′ ranges from 0 (no discrimination) to infinity (perfect discrimination). Criterion, which examines response bias was also calculated (Criterion =(−Z(false alarm rate) +Z(hit rate))/2). A criterion less than zero indicates a liberal response bias (resulting in more hits and also more false alarms). A criterion greater than zero indicates a more conservative response bias, with fewer hits and fewer false alarms. If criterion equals zero, then the subject’s criterion is neutral, showing no decision bias toward either response type (i.e., go or no-go).
Figure 1 displays a series of stimuli for a block of trials for which the targets were designated as having positive valence. Thus, in this example, the target words (“Go”) had a positive valence, while the words with negative valence served as distractors (“No-Go”). In this condition, an omission error reflected a failure to press the space bar when a positive (target valence) word was presented; an error of commission reflected incorrect “Go” responding to the negative valence (distractor) word. In the negative valence blocks, the opposite was the case, so that an omission error reflected a failure to press the space bar when a negative valence target word appeared, and a commission error reflected an incorrect “Go” response to a positive valence (distractor) word (see Table 1). Participants were instructed to press the button as quickly and accurately as possible. Reaction times and errors of omission and commission were recorded for each trial.
Fig. 1.
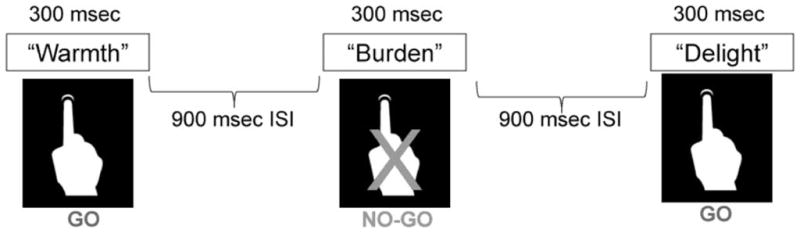
This is a schematic of the Go/No-Go task. In this representation, the target valence is positive. When a word with positive valence is displayed, the participant presses the response button (“Go” condition”). When a word with a negative valence is presented, the participant must withhold a response (“No Go”). ISI =inter-stimulus interval.
Table 1.
Error types for each experimental condition
| Error type | |
|---|---|
| Positive block | |
| Omission | Fail to respond to positive valence target |
| Commission | Respond to negative valence distractor |
| Negative block | |
| Omission | Fail to respond to negative valence target |
| Commission | Respond to positive valence distractor |
Statistical Analyses
Statistical analyses were performed using SPSS 19.0 software. Participant characteristics were compared using chi-square analyses for categorical variables and t tests or analyses of variance (ANOVA) for continuous variables.
Analysis of the Go/No-Go task data was performed using a general linear model (GLM) with repeated measures. GLM was performed for each of the dependent measures [mean RT, commission error rate, omission error rate, d’, and criterion (C)] with mTBI group as a between subject factor and block Valence (positive and negative) as the repeated within subject factor. Each GLM model included total CAPS score and a CAPS score × mTBI interaction as covariate terms. SPSS examines the effects of constant covariates and covariate interactions as between-subjects factors (http://publib.boulder.ibm.com/infocenter/spssstat/v20r0m0/index.jsp?topic=%2Fcom.ibm.spss.statistics.help%2Fidh_glmr.htm), allowing us to determine whether the relationship between PTSD severity and behavioral performance differed as a function of mTBI status. Significant CAPS × mTBI Group × Block Valence interactions were followed-up with the same GLM analysis but restricted to the mTBI+ or mTBI− group. Finally, Pearson’s correlations were conducted to examine the dynamic association between history of military mTBI and PTSD symptom severity and performance on the Go/No-Go task.
RESULTS
Participant Characteristics
The mTBI+ and mTBI− groups differed with respect to gender; however, exclusion of the single female in the mTBI+ group did not change the group’s overall pattern of performance (Table 2). The mTBI+ group endorsed more frequent combat exposures (i.e., exposure to traumatic experiences) than the mTBI− group (t =6.33; p <.001; Table 2). Consistent with their history of greater combat exposure, the mTBI+ and mTBI− groups also differed in total CAPS score, which as described above, was included as a covariate, as was the interaction term of mTBI Group × total CAPS score. The TBI groups also differed in the number of individuals diagnosed with PTSD (mTBI−, n =18 vs. mTBI+; n =29; χ2 =11.4; p =.001). In the mTBI+ Group, time since injury was 4.89 years (SD =3.4).
Affective Go/No-Go
Omissions and d′
For the repeated measures GLMs involving omissions and d’ there was no effect of Block Valence, mTBI Group, or their interaction (Block Valence × mTBI Group). Additionally, none of the covariate terms reached significance (all ps >.1).
RTs
There was a main effect of Block Valence [F(1,67) =5.04; p =.028; partial eta square =.07], such that RTs were faster to targets with positive compared to negative valence (Table 3). There was no significant effect of mTBI Group [F(1,67) <.0001; p =.98; partial eta square <.0001] or the interaction of Block Valence × mTBI Group [F(1,67) =0.30; p =.59; partial eta square =.004]. Total CAPS score [F(1,67) =0.52; p =.48; partial eta square =.008], Block Valence × CAPS score [F(1,67) =3.85; p >.05; partial eta square =.05], and Block Valence × mTBI Group × total CAPS score [F(1,67) =0.29; p =.59; partial eta square =.004] were also not significant.
Table 3.
Means and SD for affective Go/No-Go outcome measures
| TBI− (n =38) | TBI+ (n =34) | |
|---|---|---|
| RT+ | 496.6 (67.9) | 477.3 (70.0) |
| RT− | 505.2 (72.4) | 479.4 (69.2) |
| Commission errors+ | 6.1 (5.4) | 5.6 (5.2) |
| Commission errors− | 5.4 (5.0) | 6.6 (5.7) |
| Omissions+ | 3.2 (4.8) | 3.8 (4.1) |
| Omissions− | 2.6 (2.9) | 3.1 (4.0) |
| D-prime+ | 2.7 (1.1) | 2.7 (1.2) |
| D-prime− | 2.8 (1.0) | 2.7 (1.1) |
| Criterion+ | −0.22 (0.29) | −0.14 (0.24) |
| Criterion− | −0.22 (0.30) | −0.27 (0.26) |
+= Positive Block, −= Negative blocks
Commission errors
There was no main effect of Block Valence [F(1,68) =2.19; p =.14; partial eta square =.03] or mTBI Group [F(1,68) = 0.38; p =.54; partial eta square =.006]. There was a significant interaction between Block Valence × mTBI Group [F(1,68) = 8.29; p =.005; partial eta square =.11]. Total CAPS score [F(1,68) =0.69; p =.41; partial eta square =.010] and the interaction of Block Valence × CAPS score [F(1,68) =5.14; p =.29; partial eta square <.02] were not significant.
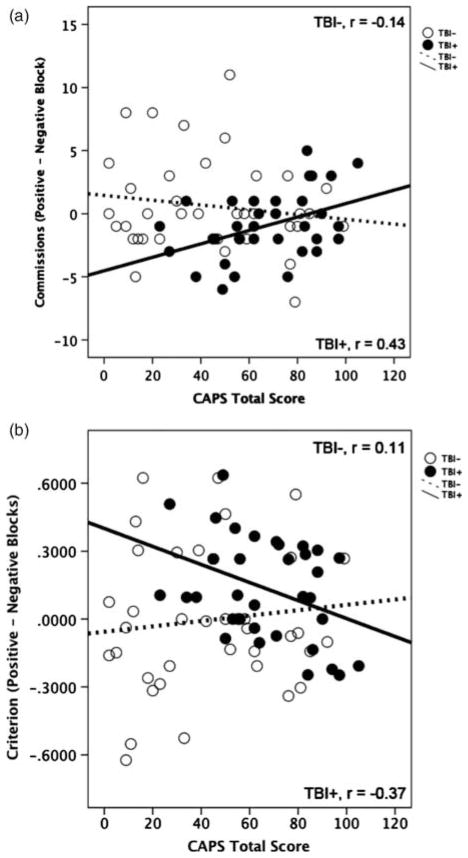
The Block Valence × mTBI Group × total CAPS score interaction [F(1,68) =5.08; p =.03; partial eta square =.07) was significant. Within the mTBI+ group analyses also revealed a significant Block Valence × CAPS score interaction [F(1,32) =7.13; p =.01; partial eta square =.8]. This interaction was not significant within the mTBI− group [F(1,36) =0.77; p =.39; partial eta square =.02]. To further examine this interaction a commission errors difference score was created (positive–negative errors) and separate correlations within the mTBI groups were performed between the commission errors difference score and total CAPS score. Within the mTBI+ group errors in the positive block (to negative valence distractors) increased with increasing CAPS score (r =.43; p =.01; Figure 2a). By contrast, in the mTBI− group there was no significant association between CAPS total score and the commission errors difference score (r =−.14; p =.39). Comparison of the two correlation coefficients revealed a significant difference between groups (p =< .02).
Fig. 2.
Scatter plots reflecting the association between Clinician-Administered PTSD Scale (CAPS) total score depicting the association between (a) CAPS total symptom scores and commission errors difference score (positive block-errors negative block) and (b) CAPS total symptom scores and criterion difference score (positive block-errors negative block). TBI =traumatic brain injury.
Criterion
The main effect of Block Valence approached significance [F(1,68) =3.82; p =.06; partial eta square =.05]. Across groups, response bias was more conservative in the positive compared to the negative blocks. The main effect of mTBI Group [F(1,68) =0.30; p =.59; partial eta square =.004] was not significant. There was a significant interaction between Block Valence × mTBI Group [F(1,68) =6.7; p =.01; partial eta square =.09, See Table 3]. Total CAPS score [F(1,68) =2.17; p =.15; partial eta square =.03] and the interaction of Block Valence × CAPS score [F(1,68) =1.1; p =.31; partial eta square <.02] were not significant.
The three-way interaction of Block Valence × mTBI Group × total CAPS score approached significance [F(1,68) = 3.6; p =.06; partial eta square =.05). Within the mTBI+group analyses revealed a significant Block Valence × CAPS score interaction [F(1,32) =5.19; p =.03; partial eta square =.14]; this interaction was not significant within the mTBI− group [F(1,36) =.43; p =.52; partial eta square =.01].
A criterion difference score was created (criterion in positive–negative blocks) to examine whether affective attentional bias increased with increasing PTSD symptoms among participants with mTBI compared to those without mTBI. Difference scores that are negative reflect more liberal responding in the positive compared to the negative blocks, whereas difference scores that are positive reflect more conservative responding in the negative blocks compared to the positive blocks. Within the mTBI+ group, responding became more liberal in positive blocks (more commission errors to negative distractors and more hits to positive targets) relative to the negative block (fewer commission errors to positive and fewer hits to negative targets) with increasing CAPS score (r =−.37; p =.03; Figure 2b). By contrast, in the mTBI− group, there was no significant association between CAPS total score and the criterion difference score (r =.11; p =.52). Comparison of the two correlation coefficients revealed a significant difference between groups (p <.04).
DISCUSSION
Attentional Bias in Comorbid mTBI and PTSD
Examination of the interplay between mTBI, PTSD symptom severity, and affective executive control was suggestive of dysregulation for processing stimuli with an affective valence, among individuals with a history of military-related mTBI and PTSD. That is, with worsening PTSD symptom severity, the mTBI+ group showed an increasingly liberal response bias (e.g., difficulty inhibiting responses/increased errors of commission to negative valence distractors, with negative compared to positive valence and greater hits to positive stimuli) in the positive compared to the negative blocks. These findings are not an artifact of a speed accuracy trade off. Among the mTBI+group, RTs were not associated with worsening PTSD symptoms regardless of the valence of the target stimulus (all ps >.29).
Valence of the stimuli appeared to interfere with task performance. Individuals with mTBI+ and higher levels of PTSD symptoms increased their hit rate and errors of commission during the task blocks in which the target valence was positive, which may reflect over arousal in the positive blocks and also possibly, difficulty with executive control when presented with distractors of a negative valence. The increased hit rate coupled with the exaggerated commission errors observed in the positive blocks may reflect the unique negative impact of mTBI upon the cognitive symptoms of PTSD. Thus, our novel findings suggest that it is the combination of elevated PTSD symptoms and mTBI+ that produced the observed pattern of errors in the presence of affectively positive targets and negative distractors.
A very different pattern of performance was observed in those individuals without a history of military-related mTBI. Among this group, there was no association between PTSD symptoms and attentional bias. That is, across omission, commission, and error analyses there was no modulation of performance based on the valence of the stimuli. The absence of an attentional bias in this group is in contrast to multiple other studies (Cisler, Olatunji, Feldner, & Forsyth, 2010; Constans et al., 2004). The Go/No-Go task used in this study, however, with its specific demands upon inhibitory processes, in addition to affective information processing, may be more sensitive to the subtle but persisting effects of mTBI upon attention to information with a negative valence.
Of note, altered inhibitory control in response to negative valence stimuli might result from pre-military training, as this is a skill taught to services members and likely has adaptive qualities in the combat zone. While we do not have information to determine if our mTBI− and mTBI+ groups differed in pre-deployment attention training experiences, we do not think this would account for our findings regardless. As reported, these groups did differ on the DRRI Combat Experiences Module. In the context of greater rates of PTSD and higher PTSD symptom scores on the CAPS in the mTBI+ compared to the mTBI− group, it may be more likely that the differences in DRRI scores reflect exposure to deployment-related combat trauma (i.e., mTBI may itself reflect traumatic exposure), rather than pre-deployment training.
Neurobiological Mechanisms
Response inhibition required for successful Go/No-Go performance appears to be mediated by the lateral and medial prefrontal cortex as well as the oribitofrontal cortex (Aupperle, Allard, et al., 2012; Esterman, Noonan, Rosenberg, & Degutis, 2012; Falconer et al., 2008; Swick, Honzel, Larsen, Ashley, & Justus, 2012). The anterior cingulate cortex is also implicated in tasks requiring executive control (Aupperle, Melrose, Stein, & Paulus, 2011; Falconer et al., 2008). Although this is a relatively unexplored area, there is emerging evidence to suggest that the pre-frontal structures, which underlie executive control and response inhibition, may be affected even by mTBI. Several studies using diffusion tensor imaging (DTI), which is sensitive to traumatic axonal injury, have demonstrated abnormalities in long white matter tracts that connect anterior and posterior regions of the brain in individuals with persistent symptoms (Huang et al., 2009; Lo, Shifteh, Gold, Bello, & Lipton, 2009; Sponheim, et al., 2011). Mac Donald and colleagues (2011) reported persistent abnormalities in the cingulum bundles and right oribitofrontal cortex among veterans with blast-related TBI compared to a control group. These axonal tearing or shearing effects are thought to particularly affect deep frontal and subcortical white matter (Mac Donald et al., 2011). In addition, they may cause vascular injury leading to microlesions in frontal regions (Bigler, 2004). Studies using functional magnetic resonance imaging (fMRI) have also shown that mTBI patients relative to a control group evidence attention network hypoactivation (i.e., bilateral dorsal prefrontal cortex) on tasks requiring inhibitory skills and attention (Mayer et al., 2009; McAllister, 2009).
Furthermore, comorbid PTSD may additionally tax these already vulnerable structures. Meta-analyses and recent reviews highlight the dynamic interaction between hyper-activation of limbic structures to threat-related information (amygdala, hippocampus, and insula), coupled with hypoactive pre-frontal regions and the anterior cingulate, especially during response inhibition (Aupperle, Allard, et al., 2012; Aupperle, Melrose, et al., 2012; Falconer et al., 2008; Pannu Hayes, Labar, Petty, McCarthy, & Morey, 2009), likely mediated in part by noradrenergic hyperarousal (Pitman et al., 2012). If mTBI affects the very same brain regions that are necessary for the inhibitory control required for successful performance on the affective Go/No-Go task, it stands to reason that these individuals may show enhanced arousal-related hit rates to positive stimuli as well as impaired frontal lobe inhibition of response to non-target stimuli as co-morbid PTSD severity increases.
Clinical Utility
Our current findings may have implications for the treatment of PTSD that co-occurs with mTBI, a common condition for returning veterans from OEF/OIF. Considering that hyper-vigilance has been proposed as a maintaining factor in PTSD (Kimble et al., 2010), our observation that OEF/OIF veterans with PTSD and mTBI might be differentially distracted by perceived negative or threat information suggests that PTSD treatment among individuals with comorbid mTBI and PTSD could present unique challenges. Consequently, PTSD symptoms may be more severe in this population and possibly more resistant to treatment, as extinction learning must occur across a wider range of situations. It is also possible that novel treatments, such as those that involve cognitive remediation, may be especially useful for veterans with PTSD and mTBI. In particular, dysfunction of the attentional control systems are amenable to cognitive rehabilitation (DeGutis & D’Esposito, 2009; DeGutis & Van Vleet, 2010) and attention training has been found to be helpful in treating other anxiety disorders (Amir et al., 2009; Eldar & Bar-Haim, 2010). Adjunctive attention training could augment standard empirically validated PTSD treatment of PTSD that co-occurs with mTBI.
CONCLUSIONS
Mild TBI sustained during military experience was associated with dysregulation for processing stimuli with an affective valence. By contrast, among individuals without a history of military-related mTBI, performance was not modulated by the valence of distracter as PTSD symptoms increased. Based on these differing patterns of performance, we suggest that these findings implicate an interactive effect of comorbid mTBI and PTSD upon inhibitory regulation to threat, a critical capacity for daily functioning.
Acknowledgments
This work was supported by the RR&D TBI Center of Excellence at VA Boston Healthcare System, The Translational Research Center for TBI and Stress Disorders. We thank all of the individuals who participated in this study. We thank Dr. Gheorghe Doros for his advisement in our statistical approach. We are grateful to all members of the Human Characterization Core of TRACTS: Marge Alhquist, BS, Brad Brummett, PhD, Racheal Dayton, BS, Andrea Levine, MA, Kathleen Moriarty, BS, Lindsay Morra, BA, Roxanne Moyer, BS, Erica Scioli, PhD, Jonathan Venne, BS, Ruby Ward, BA, David Zade PhD for their expert assessment skills. We would also like to thank Walter Musto for his extraordinary recruitment efforts and Joe Degutis, PhD, Jennifer Vasterling, PhD, and Mieke Verfaellie PhD, for valuable feedback in the interpretation of our results.
This research was supported by a VA Rehabilitation Research and Development Center Grant B6796-C awarded to Dr. Regina McGlinchey.
Footnotes
These findings were presented in part at the annual meetings of the International Neuropsychological Society, 2011, the International Trauma and Stress Society, 2011, the Federal Interagency Conference on TBI, 2011, and the American Psychological Association, 2012.
The authors report no conflicts of interest.
References
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, Chen X. Attention training in individuals with generalized social phobia: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77(5):961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MP, Guetz A, Wein LM. A dynamic model for post traumatic stress disorder among US troops in Operation Iraqi Freedom. Management Science. 2009;55(9):1454–1468. [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, Stein MB. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Archives of General Psychiatry. 2012;69(4):360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: Disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger HG, Kretzmer T, Vanderploeg RD, French LM. Symptom complaints following combat-related traumatic brain injury: Relationship to traumatic brain injury severity and posttraumatic stress disorder. Journal of International Neuropsychological Society. 2010;16(1):194–199. doi: 10.1017/S1355617709990841. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. Journal of International Neuropsychological Society. 2004;10(5):794–806. doi: 10.1017/S1355617704105146. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM. A clinician rating scale for assessing current and lifetime PTSD: the CAPS-I. Behavior Therapy. 1993;18:187–188. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Vanderploeg RD, Terrio H. Assessment and diagnosis of mild traumatic brain injury, posttraumatic stress disorder, and other polytrauma conditions: Burden of adversity hypothesis. Rehabilitation Psychology. 2009;54(3):239–246. doi: 10.1037/a0016908. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Kleiner JS, Vasterling JJ, Field AP. Memory for emotionally neutral information in posttraumatic stress disorder: A meta-analytic investigation. 2007;116(3):448–463. doi: 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- Bryant R. Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clinical Neuroscience. 2011;13(3):251–262. doi: 10.31887/DCNS.2011.13.2/rbryant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Acute stress disorder: A critical review of diagnostic issues. Clinical Psychology Review. 1997;17(7):757–773. doi: 10.1016/s0272-7358(97)00052-4. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: A review of the empirical literature. Clinical Psychology Review. 2000;20(8):1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Calhoun PS, McDonald SD, Guerra VS, Eggleston AM, Beckham JC, Straits-Troster K. Clinical utility of the Primary Care–PTSD Screen among U.S. veterans who served since September 11, 2001. Psychiatry Research. 2010;178(2):330–335. doi: 10.1016/j.psychres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Pépin M. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of Rehabiliation Medicine. 2004;43(Suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- Chemtob CM, Muraoka MY, Wu-Holt P, Fairbank JA, Hamada RS, Keane TM. Head injury and combat-related posttraumatic stress disorder. The Journal of Nervous & Mental Disease. 1998;186(11):701–708. doi: 10.1097/00005053-199811000-00007. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, Feldner MT, Forsyth JP. Emotion regulation and the anxiety disorders: An integrative review. Journal of Psychopathoology & Behavioral Assessment. 2010;32(1):68–82. doi: 10.1007/s10862-009-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans JI. Information-processing biases in PTSD. In: Vasterling JJ, editor. Neuropsychology of PTSD: Biological, cognitive and clinical perspectives. New York: Guildford Press; 2005. pp. 105–130. [Google Scholar]
- Constans JI, McCloskey MS, Vasterling JJ, Brailey K, Mathews A. Suppression of attentional bias in PTSD. Journal of Abnormal Psychology. 2004;113(2):315–323. doi: 10.1037/0021-843X.113.2.315. [DOI] [PubMed] [Google Scholar]
- Crawford MA, Knight RG, Alsop BL. Speed of word retrieval in postconcussion syndrome. Journal of International Neuropsychological Society. 2007;13(1):178–182. doi: 10.1017/S135561770707021X. [DOI] [PubMed] [Google Scholar]
- De Monte VE, Geffen GM, May CR, McFarland K, Heath P, Neralic M. The acute effects of mild traumatic brain injury on finger tapping with and without word repetition. Journal of Clinical & Experimental Neuropsychology. 2005;27(2):224–239. doi: 10.1080/13803390490515766. [DOI] [PubMed] [Google Scholar]
- DeGutis J, D’Esposito M. Network changes in the transition from initial learning to well-practiced visual categorization. Frontiers of Human Neuroscience. 2009;3:44. doi: 10.3389/neuro.09.044.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGutis J, Van Vleet TM. Tonic and phasic alertness training: A novel behavioral therapy to improve spatial non-spatial attention in patients with hemispatial neglect. Frontiers in Human Neuroscience. 2010;4:60. doi: 10.3389/fnhum.2010.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychological Medicine. 2010;40(4):667–677. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg M, Degutis J. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs261. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, Williams LM. The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatric Neuroscience. 2008;33(5):413–422. [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Amick MM, Grande L, McGlynn S, Kenna A, Morra L, McGlinchey RM. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: Evidence of research utility and validity. Journal of Head Trauma Rehabilitation. 2013 doi: 10.1097/HTR.0b013e3182865859. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. Green’s Medical Symptom Validity Test (MSVT) for Windows: User’s manual. Edmonton, Canada: Green’s Publishing; 2003. [Google Scholar]
- Harvey AG, Bryant RA. Two-year prospective evaluation of the relationship between acute stress disorder and posttraumatic stress disorder following mild traumatic brain injury. American Journal of Psychiatry. 2000;157(4):626–628. doi: 10.1176/appi.ajp.157.4.626. [DOI] [PubMed] [Google Scholar]
- Hill JJ, III, Mobo BH, Jr, Cullen MR. Separating deployment-related traumatic brain injury and posttraumatic stress disorder in veterans: Preliminary findings from the Veterans Affairs traumatic brain injury screening program. American Journal of Physical Medicine and Rehabilitation. 2009;88(8):605–614. doi: 10.1097/PHM.0b013e3181ae0f83. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. New England Journal of Medicine. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Huang MX, Theilmann RJ, Robb A, Angeles A, Nichols S, Drake A, Lee RR. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. Journal of Neurotrauma. 2009;26(8):1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Outcome from mild traumatic brain injury. Current Opinion in Psychiatry. 2005;18(3):301–317. doi: 10.1097/01.yco.0000165601.29047.ae. [DOI] [PubMed] [Google Scholar]
- Kimble MO, Fleming K, Bandy C, Kim J, Zambetti A. Eye tracking and visual attention to threating stimuli in veterans of the Iraq war. Journal of Anxiety Disorders. 2010;24(3):293–299. doi: 10.1016/j.janxdis.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DW, King LA, Vogt DS, Knight J, Samper R. Deployment Risk and Resilience Inventory: A collection of measures for studying deployment -related experiences of military personnel and veterans. Military Psychology. 2006;18(2):89–120. [Google Scholar]
- Lo C, Shifteh K, Gold T, Bello JA, Lipton ML. Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. Journal of Computer Assisted Tomography. 2009;33(2):293–297. doi: 10.1097/RCT.0b013e31817579d1. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Brody DL. Detection of blast-related traumatic brain injury in U. S. military personnel. New England Journal of Medicine. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Elgie R, Gasparovic C, Phillips JP, Yeo RA. Auditory orienting and inhibition of return in mild traumatic brain injury: A FMRI study. Human Brain Mapping. 2009;30(12):4152–4166. doi: 10.1002/hbm.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW. Psychopharmacological issues in the treatment of TBI and PTSD. The Clinical Neuropsychologist. 2009;23(8):1338–1367. doi: 10.1080/13854040903277289. [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J, Labar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Research. 2009;172(1):7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Shipherd JC, Mostoufi SM, Abramovitz SM, Yovel I. Attentional biases in PTSD: More evidence for interference. Behavior Research and Therapy. 2009;47(12):1050–1057. doi: 10.1016/j.brat.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, O’Connor PM, Broglio SP, Hillman CH. The association between mild traumatic brain injury history and cognitive control. Neuropsychologia. 2009;47(14):3210–3216. doi: 10.1016/j.neuropsychologia.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger ML, Poulos AM, Buen F, Giza CC, Hovda DA, Fanselow MS. Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biological Psychiatry. 2012;71(4):335–343. doi: 10.1016/j.biopsych.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbit PM. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. Journal of International Neuropsychological Society. 1998;4(5):474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: Persistent postconcussive symptoms and posttraumatic stress disorder. American Journal of Epidemiology. 2008;167(12):1446–1452. doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, Kang SS, Davenport ND, Aviyente S, Bernat EM, Lim KO. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. Neuroimage. 2011;54(Suppl):S21–S29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. American Journal of Psychiatry. 2009;166(7):768–776. doi: 10.1176/appi.ajp.2009.08101604. [DOI] [PubMed] [Google Scholar]
- Swick D, Honzel N, Larsen J, Ashley V, Justus T. Impaired response inhibition in veterans with post-traumatic stress disorder and mild traumatic brain injury. Journal of International Neuropsychological Society. 2012;18(5):917–926. doi: 10.1017/S1355617712000458. [DOI] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Warden D. Traumatic brain injury screening: Preliminary findings in a US Army brigade combat team. Journal of Head Trauma Rehabilitation. 2009;24(1):14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- Thrasher SM, Dalgleish T, Yule W. Information processing in post-traumatic stress disorder. Behavior Research Therapy. 1994;32(2):247–254. doi: 10.1016/0005-7967(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Van Zomeren AH, Brouwer WH, Deelman BG. Attentional deficits: The riddles of selectivity, speed and alertness. Oxford: Oxford University Press; 1984. [Google Scholar]
- Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Archives of Physical Medicine and Rehabilitation. 2009;90(7):1084–1093. doi: 10.1016/j.apmr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Proctor SP, Amoroso P, Kane R, Heeren T, White RF. Neuropsychological outcomes of army personnel following deployment to the Iraq war. Journal of American Medical Association. 2006;296(5):519–529. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- Vogt DS, Proctor SP, King DW, King LA, Vasterling JJ. Validation of scales from the Deployment Risk and Resilience Inventory in a sample of Operation Iraqi Freedom veterans. Assessment. 2008;15(4):391–403. doi: 10.1177/1073191108316030. [DOI] [PubMed] [Google Scholar]
- Walilko T, North C, Young LA, Lux WE, Warden DL, Jaffee MS, Moore DF. Head injury as a PTSD predictor among Oklahoma City bombing survivors. Journal of Trauma. 2009;67(6):1311–1319. doi: 10.1097/TA.0b013e31819adc36. [DOI] [PubMed] [Google Scholar]