Abstract
Background
Bioethanol fermentations follow traditional beverage fermentations where the yeast is exposed to adverse conditions such as oxidative stress. Lignocellulosic bioethanol fermentations involve the conversion of pentose and hexose sugars into ethanol. Environmental stress conditions such as osmotic stress and ethanol stress may affect the fermentation performance; however, oxidative stress as a consequence of metabolic output can also occur. However, the effect of oxidative stress on yeast with pentose utilising capabilities has yet to be investigated.
Results
Assaying for the effect of hydrogen peroxide-induced oxidative stress on Candida, Pichia and Scheffersomyces spp. has demonstrated that these yeast tolerate hydrogen peroxide-induced oxidative stress in a manner consistent with that demonstrated by Saccharomyces cerevisiae. Pichia guillermondii appears to be more tolerant to hydrogen peroxide-induced oxidative stress when compared to Candida shehatae, Candida succiphila or Scheffersomyces stipitis.
Conclusions
Sensitivity to hydrogen peroxide-induced oxidative stress increased in the presence of minimal media; however, addition of amino acids and nucleobases was observed to increase tolerance. In particular adenine increased tolerance and methionine reduced tolerance to hydrogen peroxide-induced oxidative stress.
Background
Yeasts may be confronted with a combination of different physical, biological, or chemical stresses during bio-ethanol fermentations. At the beginning of fermentation, yeast are subjected to osmotic stress due to high sugar levels. Yeast encounter osmotic shock when propagated into the wort, a complex media containing high concentrations of sugars [1]. Use of high gravity worts has been shown to reduce yeast viability, growth and fermentation profiles [2].
In addition, during fermentation other stress conditions become relevant such as ethanol accumulation, pH downshift, a shift to anaerobic growth and nutrient limitation [3,4]. During fermentation, accumulation of ethanol has been shown to inhibit yeast growth and viability [5-7].
Oxygen is an essential component of the brewing process and is required for the synthesis of unsaturated fatty acid and sterols [8]. The yeast cell is continuously exposed to oxygen during brewery propagation, a process whereby yeast cells are grown from stock cultures to a sufficient quantity for transfer into fermentation vessels. Oxygen is typically depleted within the first 12 hrs of fermentation which continues under anaerobic conditions.
Reactive oxygen species (ROS) free radicals such as superoxide anion (O2−), hydrogen peroxide (H2O2) and the hydroxyl radical (.HO) are generated as by-products of cellular metabolism [9]. Under some conditions ROS can disrupt a diverse array of biological processes [10]. ROS can damage a variety of cellular components, including DNA, proteins and unsaturated lipids [11]. ROS can damage the mitochondria causing the formation of respiratory-deficient petites [12-14]. ROS has been shown to have a direct role in cellular aging [15], replicant life-span has been related to anti-oxidant potential of the cell and the number of re-pitches into a fermentation [16,17]. Oxidative stress induced by free radical produced by aerobic metabolism may play a role in replicative aging of the yeast causing progressive deterioration of the cells as post fermentation harvesting potentially selects a higher proportion of aging yeast [18].
Toxicity arises from lipid peroxidation, production of alkoxyl/peroxyl radicals and aldehyde compounds, which are products of free radical lipid reactions triggered by ROS [19-21]. Yeast response to free radicals is diverse, enzymatic (thioredoxins, glutaredoxins, catalases, superoxide dismutasis) and non-enzymatic (glutathione, D-erythroascorbic acid, and trehalose) responses are employed by the yeast to overcome oxidative stress [22-26]. These responses differ during the course of fermentation for example, cellular superoxide dismutase and catalase activities rapidly decline during fermentation in semi-defined wort medium as the oxygen levels in the medium decline [27].
Lignocellulosic material is a cheap raw material for ethanol production and is composed of lignin, hemicelluloses, and cellulose. Cellulose is composed of long chains of glucose molecules and it is encapsulated by lignin. Conversion of cellulose derived monomeric sugars to ethanol has been shown in S. cerevisiae fermentations [28], however, this yeast is unable to utilise pentose sugars liberated from hemicellulose, the pentose polysaccharide component of plant cell walls without genetic modification [29].
Candida, Pichia and Scheffersomyces (previously named Pichia stipitis) currently represent the best natural yeast for efficient utilisation of pentose sugars into ethanol or other refined products [30]. Although much work has been done on the conversion of pentoses to ethanol, little is known about their response to the stresses posed by fermentation. Understanding the response of pentose utilising yeast to oxidative stress (induced by hydrogen peroxide in this study) will lead to an improvement in yeast strains for the production of ethanol from all available sugars post deconstruction of lignocellulosic material (LCM).
Methods
Yeast strains and growth conditions
Saccharomyces cerevisiae NCYC 2592, Candida shehatae NCYC 2389 and 3781, Candida succiphila NCYC 1403, Pichia guillermondii NCYC 441, 443 and 3063 and Scheffersomyces stipitis NCYC 1541 and 1542 were utilised in this study. All strains were obtained from the National Centre of Yeast collections (NCYC, Bristol, UK) and maintained on 10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose (YPD).
Sensitivity to hydrogen peroxide-induced oxidative stress
Sensitivity to hydrogen peroxide-induced oxidative stress was determined by growing cells to stationary phase in YPD at 30°C. Optical density (OD600) was measured and adjusted to an OD600 of 1.0 corresponding to 107 cells/ml. Cells were then serially ten-fold diluted and 5 μl was spotted onto YPD agar plates containing hydrogen peroxide (0-5 mM) as appropriate. Sensitivity to hydrogen peroxide was also measured using yeast nitrogen base (YNB) as a carbon source, as above, but the yeast were grown in 6% glucose, and 0.67% YNB, stressed and viable cells were counted after incubation on YPD plates.
Viability assays
Viability was determined by growing cells in 50 mL YPD broth at 30°C until an OD600 reading of between 0.4 and 0.6 was reached indicating cells were at mid-exponential phase of growth. Cells were then treated with hydrogen peroxide at different concentrations for 15 minutes at 30°C, and shaken at 180 rpm in an orbital shaker, in an aerobic environment. After incubation, OD was re-assessed, cells were then diluted in fresh medium to a concentration of 1 × 104 cells/mL and 10 μL plated in triplicate on YPD plates. Viable yeast counts were performed after incubation for 3 days at 30°C on YNB. All spot tests and viability studies were performed in triplicate.
Acquired hydrogen peroxide-induced oxidative stress tolerance
Aliquots (50 mL) of yeast cultures were treated with 0.5 mM H2O2 at 30°C for 1 hr and immediately challenged with 7.5 mM H2O2. Ten μL aliquots were removed from cultures at 0, 30, and 60 min after the challenge; cells were pelleted and washed before plating on YPD agar. Viability was determined by counting colonies after incubation at 30°C for 24 hrs. Survival was normalised to control samples with control samples defined as being 100% viable.
Phenotypic microarray analysis
Biolog (Hayward, CA, US) growth medium was prepared using 6 g/L (w/v) glucose, 0.67 g/L (w/v) yeast nitrogen base YNB, 0.2 μL of dye D (Biolog, USA), and supplemented with a nutrient mixture (NSx48- 24 mM Adenine-HCl, 4.8 mM L-histidine HCl monohydrate, 48 mM L-leucine, 24 mM L-lysine-HCl, 12 mM L-methionine, 12 mM L-tryptophan and 14.4 mM uracil). Assays looking at the effect of individual amino acids on hydrogen peroxide-induced stress used amino acids at the same concentration as in the complete NS mixture. For assays using xylose, 6 g/L xylose replaced 6 g/L glucose. Final volume was made up to 30 μl using reverse osmosis (RO) sterile distilled water and aliquoted to individual wells with varying concentrations of appropriate inhibitors.
Strains were prepared for inoculation into the PM assay plates (Biolog, Hayward, CA, US) as follows. Glycerol stocks stored at -80°C were streaked on to YPD plates and incubated at 30°C for approximately 48 h. Two to three colonies from each strain were re-streaked to one section of a fresh YPD plate and incubated overnight at 30°C. Cells were then inoculated into sterile water in 20 × 100 mm test tubes and adjusted to a transmittance of 62% (~5×106 cells/ml).
The cell suspension for inoculation was prepared by mixing 125 μL of cells with 2.65 mL of IFY buffer (Biolog) and adjusted to a final volume of 3 mL by the addition of sterile deionised water. Ninety μL of the above mix was inoculated to each well in a Biolog 96- well plate. Anaerobic conditions were created using Oxygen absorbing packs (Mitsubishi AnaeroPak™System) with an anaerobic indicator (Oxoid, UK) and the plates were placed inside PM gas bags (Biolog). The plates were then placed in the OmniLog reader and incubated for 96 h at 30°C without shaking.
The OmniLog reader (Biology, Hayward, CA) photographs the plates at 15 min intervals, converting the pixel density in each well to a signal value reflecting cell growth and dye conversion. After completion of the run, the signal data was compiled and exported from the Biolog software into Microsoft® Excel for further analysis. In all cases, a minimum of three replicate PM assay runs were conducted, and the average of the signal values was plotted.
Measurement of yeast growth
Cells were grown to exponential phase in YPD media as monitored by OD600 readings, cells were diluted to a starting OD600 of 0.2 using YPD broth. H2O2 (0-5 mM) was added to wells on a 96-well microtitire plate and the volume of each well was adjusted to 100 μL using RO water. Yeast growth (OD600) was measured every 15 minutes using a Tecan Infinite M200 Pro plate reader (Mannedorf, Switzerland), at 30°C for 72 hrs. The assay was performed in triplicate and the average reading was plotted.
Determination of glutathione levels
Glutathione levels, both oxidised and reduced, were determined using a glutathione assay kit (Cayman Chemical Company, US). Samples were prepared for the kit with the cells harvested by centrifugation, washed with phosphate-buffered saline (pH 7.4) to remove any traces of growth medium, and resuspended in ice-cold 8 mM HCl, 1.3% (w/v) 5-sulfosalicyclic acid. Cells were broken with glass beads using a MagNalyser (Roche, Burges Hill, UK) bead beater for 30 s at 4°C, before incubating on ice for 15 min to precipitate proteins. Cell debris and proteins were pelleted in a microcentrifuge for 15 min (17,000 g at 4°C) and the supernatant used for the determination of free glutathione. For quantification of oxidized glutathione (GSSG), samples were pretreated with 5% (v/v) 2-vinyl-pyridine for 1 hr at room temperature before analysis.
Peroxidase and catalase activity
Peroxidase activity (GPX) was measured using a glutathione peroxidase assay kit (Calbiochem, Merck); glutathione peroxidase activity was determined indirectly by measuring NADPH consumption upon recycling of oxidised glutathione by glutathione reductase. NADPH consumption was measured by a decrease in absorbance (340 nm). Crude cell extracts from cells grown to mid-exponential phase (OD600 nm 0.5) were used, cells were harvested and broken in 50 mM Tris-HCl, pH 7.5, 5 mM EDTA and 1 mM dithiothreitol (DTT) using a MagNalyser instrument (Roche Applied Science). Samples were then centrifuged at 13200 g in a microcentrifuge at 4°C to remove unbroken cells. Specific activity was defined as nanomoles of NADPH oxidised per minute per milligram of protein.
Catalase activity (CAT) was measured using a catalase activity kit (Calbiochem, Merck); crude cell extracts from cells grown to mid-exponential phase (OD600 nm 0.5) were used, cells were harvested and broken in 50 mM potassium phosphate, pH 7.0 containing 1 mM EDTA using a MagNalyser instrument (Roche Applied Science). Samples were then centrifuged at 13,200 g in a microcentrifuge at 4°C to remove unbroken cells. The assay utilises formaldehyde as a standard for the catalase reactions. Assay buffer containing hydrogen peroxide was added to each well on the plate containing cell-free extracts and incubated on a shaker for 20 minutes. Reactions were stopped by the addition of potassium hydroxide and Purplald. Plates were read at 540 nm. Catalase activity was determined by comparing samples with a formaldehyde reference curve and defined as nmol/min/ml.
Results
Sensitive to hydrogen peroxide-induced oxidative stress increased under nutrient deficient growth conditions
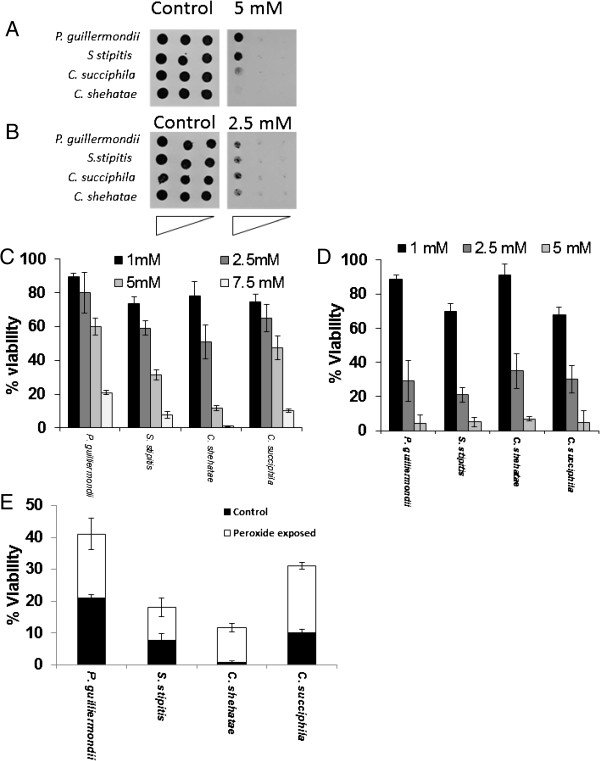
On YPD all yeast in this study were inhibited by 5 mM hydrogen peroxide, 5 mM hydrogen peroxide has been shown to inhibit Saccharomyces cerevisiae[31] (Figure 1A). P. guillermondii and S. stipitis exhibited higher tolerance to hydrogen peroxide-induced oxidative stress when compared with Candida spp when grown on YPD containing hydrogen peroxide (Figure 1A). On YNB all yeast in this study exhibited sensitivity to 2.5 mM hydrogen peroxide (Figure 1B). However, viability assays showed that only P. guillermondii exhibited higher tolerance to hydrogen peroxide-induced oxidative stress when compared with the other yeast species assayed for in this study (Figure 1C). There was no difference in viability in the presence of hydrogen peroxide when strains were grown in YNB (Figure 1D). Assays with P. guillermondii NCYC 441, 3063, S. stipitis NCYC 1542 and C. shehatae NCYC 3781 confirmed that tolerance to hydrogen peroxide-induced oxidative stress was not within this study strain specific, though further testing on other strains would be desirable (Additional file 1: Figure S1A-S1C).
Figure 1.
Candida and Pichia spp are sensitive to hydrogen peroxide-induced oxidative stress. (A) Cultures of P. guilliermondii, S. stipitis, C. succiphila, and C. shehatae were grown to stationary phase and the OD600 adjusted to 1.0, 0.1 or 0.01 before strains were spotted onto plates containing various concentrations of hydrogen peroxide. Growth was monitored after 3 days of incubation at 30°C. Results are shown for plates containing no oxidant (YPD) and 5 mM hydrogen peroxide. (B) Growth on restrictive media (YNB) makes yeast more sensitive to oxidative stress. Yeast cultures prepared as above were exposed to hydrogen peroxide and grown on restrictive media (YNB). Results are shown for plates containing no oxidant (YNB) and 2.5 mM hydrogen peroxide. (C) Yeast spp show a reduced ability to survive oxidative stress. Yeast spp were grown to exponential phase in YPD and treated with 1, 2.5, 5 or 7.5 mM hydrogen peroxide for 1 hour. Percent survival is expressed relative to that of untreated cultures. (D) Restrictive growth conditions make yeast more sensitive to oxidative stress. Yeast cultures prepared as above were exposed to hydrogen peroxide in restrictive media (YNB). (E) Exposure to hydrogen peroxide stress makes yeast spp hydrogen peroxide tolerant. Yeast spp were maintained under 0.5 mM hydrogen peroxide stress and exposed to 7.5 mM hydrogen peroxide in YPD and viable counts performed as above.
There was no difference in sensitivity to hydrogen peroxide on YNB between yeast spp (Figure 1D). Yeast pre-incubation with 0.5 mM hydrogen peroxide increased tolerance to 7.5 mM hydrogen peroxide for all yeast in this study (Figure 1E). Increased tolerance to hydrogen peroxide-induced oxidative stress under constant exposure has been reported previously for S. cerevisiae[32].
Hydrogen peroxide-induced oxidative stress inhibits metabolic outout in Candida, Pichia and Scheffersomyces spp
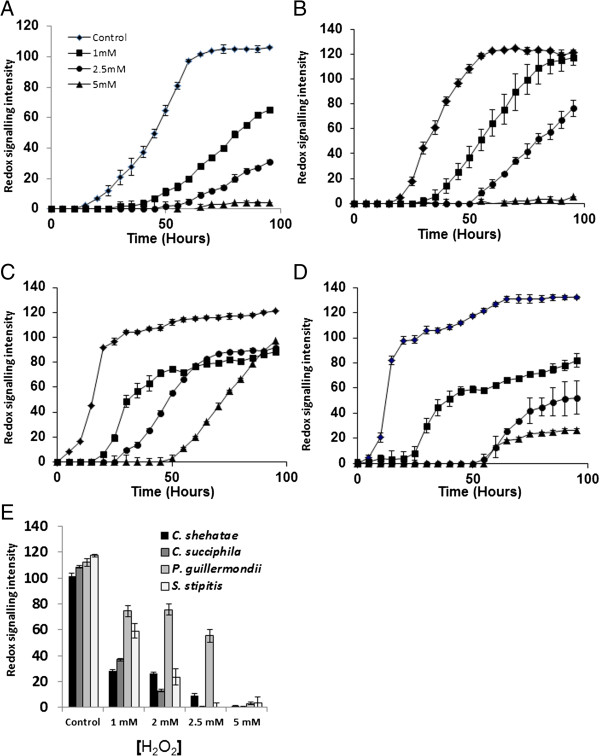
The effect of hydrogen peroxide-induced oxidative stress on metabolic outout was measured using bespoke phenotypic microarray plates for all strains assayed in this study (Figure 2A-D). Presence of hydrogen peroxide was compared with unstressed conditions on yeast metabolic output as measured by a redox sensitive dye. Addition of 5 mM hydrogen peroxide inhibited metabolic output in Candida spp (Figure 2A and B) when compared with P. guillermondii and S. stipitis (Figure 2C and D). We observed that P. guillermondii was the most tolerant to hydrogen peroxide-induced oxidative stress when assessed after 50 hours exposure (Figure 2E). This phenotype was confirmed in assays with P. guillermondii NCYC 441 in response to hydrogen peroxide-induced oxidative stress (data not shown).
Figure 2.
Oxidative stress prevents metabolic output in yeast spp assayed in this study. The effect of hydrogen peroxide on metabolic output expressed as redox signal intensity units on yeast spp. (A)C. shehatae(B)C. succiphila(C)P. guilliermondii(D)S. stipitis(E) Redox signal intensity units for yeast spp under hydrogen peroxide stress after 50 hrs for C. succiphila, C. shehatae, P. guillermondii and S. stipitis. The assay was performed in triplicate and the average reading was plotted.
Hydrogen peroxide-oxidative induced stress inhibits growth in Candida, Pichia and Scheffersomyces spp
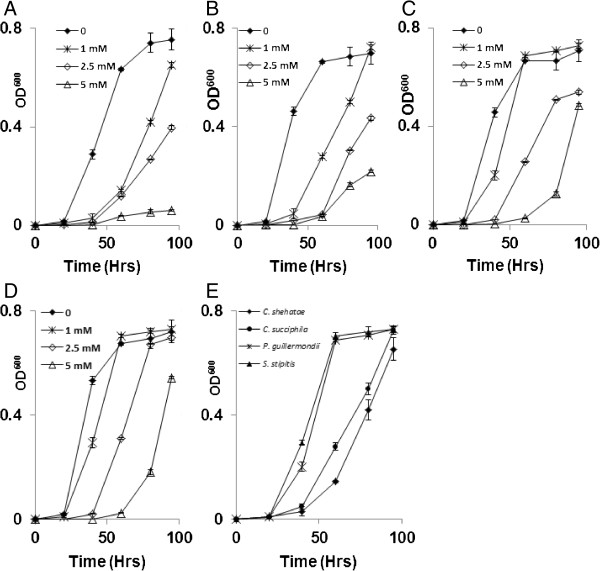
Hydrogen peroxide-induced oxidative stress was characterised by extended lag phases for all yeast assayed in this study (Figure 3A-D). P. guillermondii exhibited the highest tolerance to hydrogen peroxide and C. shehatae was the least tolerant in terms of growth when compared with other yeast assayed in this study (Figure 3E).
Figure 3.
Effect of hydrogen peroxide on the growth of ethanologenic yeast spp. Growth in the presence of 0-5 mM hydrogen peroxide was monitored on a TECAN plate reader at OD600 for 96 hrs with readings taken every 15 minutes for (A)C. shehatae, (B)C. succiphila, (C)P. guillermondii, and (D)S. stipitis and (E) Comparison of inhibition of growth by 1 mM hydrogen peroxide for yeast assayed in this study. The assay was performed in triplicate and the average reading was plotted.
Addition of amino acids and nucleobases increased tolerance to hydrogen peroxide-induced oxidative stress
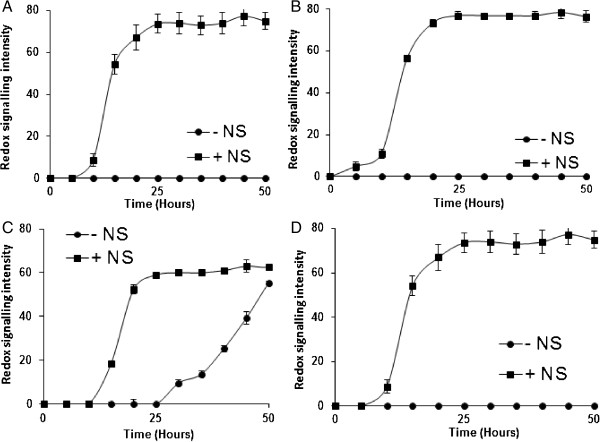
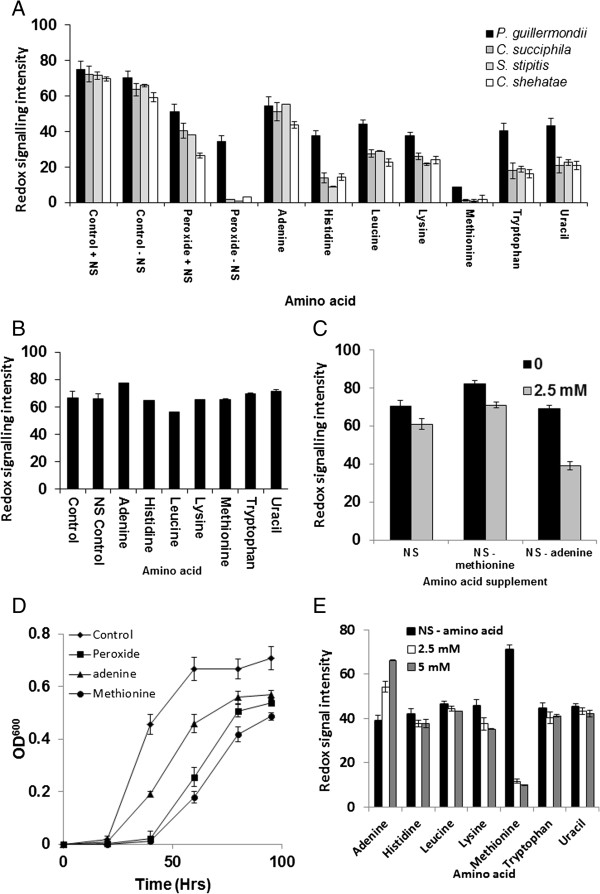
Yeast strains were more sensitive to hydrogen peroxide-induced oxidative stress when grown on YNB when compared with YPD; Biolog recommend the addition of amino acids and nucelobases for microarray assays with yeast, we investigated the impact of these amino acids and nucleobases (NS) on hydrogen peroxide- induced oxidative stressed yeast. Addition of amino acids and nucleobases elicited an increase in metabolic output in yeast under hydrogen peroxide-induced oxidative stress when compared with assays without amino acids (Figure 4A-D). P. guillermondii yeast were observed to be metabolically active under hydrogen peroxide-induced oxidative stress in the absence of NS, however, addition of NS improved tolerance (Figure 4C). We did observe this effect on P. guillermondii strain NCYC 441; more observations with strains of P. guillermondii are required before this response could be denoted as not strain specific (data not shown). This study was restricted to the amino acids recommended by Biolog, the impact of other amino acids on hydrogen peroxide oxidatively stressed yeast cells was not investigated.
Figure 4.
Effect of nutrient supplementation on hydrogen peroxide sensitivity. Effect of 2.5 mM hydrogen peroxide was assessed +/- a yeast specific nutrient supplementation containing amino acids on metabolic output, (A)C. shehatae, (B)C. succiphila, (C)P. guillermondii, and (D)S. stipitis. The assay was performed in triplicate and the average reading was plotted.
Addition of adenine and methionine affects metabolic output and cellular growth under hydrogen peroxide-induced oxidative stress
Addition of adenine (2.4 mM) improved metabolic output compared with controls, presence of leucine, lysine, tryptophan, histidine and uracil also had a slight beneficial effect on observed yeast metabolic output as measured by the conversion of a redox sensitive dye. Addition of methionine had a negative effect on metabolic output for cells under hydrogen peroxide-induced oxidative stress (Figure 5A).
Figure 5.
Effect of individual amino acids on metabolic outout for P. guillermondii. (A) Metabolic output under 2.5 mM hydrogen peroxide stress was measured in the presence of a range of amino acids, (B) Effect of amino acids on metabolic output in unstressed yeast cells, (C) Effect of NS, NS minus methionine and NS minus adenine on metabolic output under peroxide stress, (D) Effect of adenine and methionine on growth under hydrogen peroxide stress and (E) Effect of 2.5 mM or 5 mM amino acids on metabolic output of P. guillermondii under 2.5 mM hydrogen peroxide-induced oxidative stress. The assays were performed in triplicate and the average reading was plotted.
In unstressed cells, addition of amino acids had little effect on metabolic output compared with controls (Figure 5B). Metabolic output was reduced in cells under hydrogen peroxide-induced oxidative stress incubated with methionine and increased with assays using NS minus methionine when compared with NS containing methionine; similarly removal of adenine from NS reduced metabolic output compared with NS containing adenine (Figure 5C). Presence of adenine improved yeast growth when under hydrogen peroxide-induced oxidative stress, whereas addition of methionine was deleterious (Figure 5D). Under control conditions, addition of adenine or methionine had little effect on yeast growth (data not shown). Assays of P. guillermondii with 2.5 mM hydrogen peroxide and 2.5 mM and 5 mM of amino acids screened in this study showed that addition of adenine improved metabolic output compared with the other amino acids (Figure 5E). The improvement appeared to be concentration dependent as there was an increase in metabolic output in the presence of 5 mM adenine when compared with assays containing 2.5 mM adenine (Figure 5E).
The redox state under hydrogen peroxide-induced oxidative stress did not differ between yeast assayed in this study
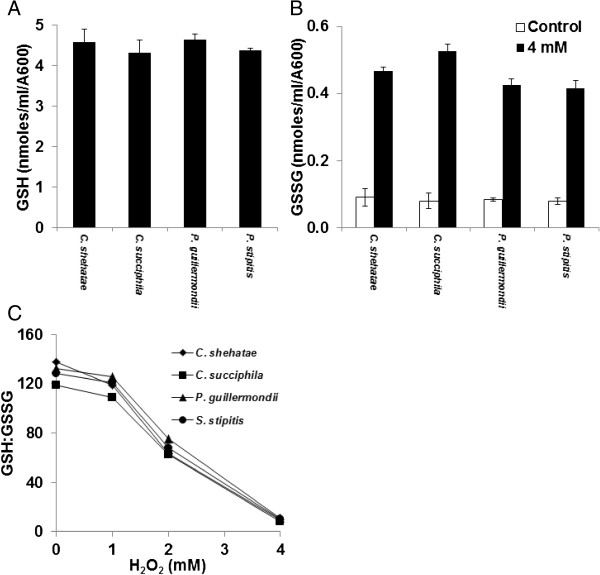
Yeast assayed in this study had total glutathione concentrations of approximately 4.0 nmols/ml/OD (Figure 6A); there were very low levels of oxidised glutathione (GSSG) in unstressed cells. Unstressed cells had a 120-fold ratio of reduced to oxidised glutathione (Figure 6B), under hydrogen peroxide-induced oxidative stress, the ratio shifted to a more oxidised state, this was observed for all yeast assayed in this study (Figure 6C). However, there was little difference between yeast in the redox status under hydrogen peroxide-induced oxidative stress.
Figure 6.
Effect of hydrogen peroxide-induced oxidative stress on redox status of yeast spp. (A) Total GSH (nmoles/ml/OD600) for yeast spp, (B) Effect of hydrogen peroxide-induced oxidative stresson reduced GSSP levels in yeast spp, and (C) Effect of hydrogen peroxide-induced oxidative stress on redox balance for yeast spp. The assay was performed in triplicate and the average reading was plotted.
C. shehatae has reduced peroxidase and catalase activity when compared with other yeast assayed in this study
We assayed for peroxidase and catalase activity under hydrogen peroxide-induced oxidative stress for the yeast in this study, along with S. cerevisiae NCYC 2592 as a reference strain. C. shehatae had significantly lower peroxidase and catalase activity when compared with the other yeast assayed in this study. Catalase activity in C. succiphila was lower when compared with other yeast assayed in this study; however, peroxidase activity was not significantly different. However, this response maybe strain specific as other C. succiphila strains have yet to be tested for oxidative tolerance or catalase activity. Peroxidase and catalase activity for S. cerevisiae is similar to published data for yeast [33,34]. There was no significant difference in peroxidase and catalase activity for P. guilliermondii or S. stipitis when compared with S. cerevisiae (Table 1).
Table 1.
Peroxidase (GPX) and catalase activity of yeast using either a GPX or catalase assay kit
| Yeast | GPX activity nmol/min −1 mg −1 | CAT activity nmol/min −1 mg −1 |
|---|---|---|
|
S. cerevisiae |
520.981 ± 2.32 |
89.154 ± 2.42 |
|
C. shehatae |
494.304 ± 4.23 |
81.566 ± 3.12 |
|
C. succiphila |
509.383 ± 5.73 |
84.651 ± 2.63 |
|
P. guillermondii |
513.141 ± 3.01 |
90.491 ± 1.71 |
| S. stipitis | 520.415 ± 8.43 | 88.911 ± 2.53 |
C. shehatae had significantly lower concentrations of peroxidase or catalase activity when compared with other yeast assayed for in this study.
Data expressed as nmol of NADPH oxidised per minute per mg of protein for peroxidase activity and nmol formaldehyde produced in the presence of hydrogen peroxide for catalase activity.
Utilisation of pentose sugars increases sensitivity to hydrogen peroxide-induced oxidative stress
Hydrolysates derived from LCM’s will contain hexose and pentose sugars [35], it has been shown in S. cerevisiae that utilisation of xylose induces expression of transcription factors which control response to oxidative stress [36] we measured the impact of hydrogen peroxide-induced oxidative stress on yeast utilising xylose as a sole carbon source. P. guillermondii exhibited sensitivity to 1 mM hydrogen peroxide when utilising 6% xylose compared with sensitivity to 5 mM hydrogen peroxide when utilising 6% glucose (Figure 7A). Plotting percentage inhibition of redox signal intensity demonstrated that yeast display greater sensitivity to hydrogen peroxide-induced oxidative stress when utilising xylose compared with glucose (Figure 7B). Increased sensitivity to hydrogen peroxide-induced oxidative stress when utilising xylose was observed for all yeast strains assayed in this study (data not shown).
Figure 7.
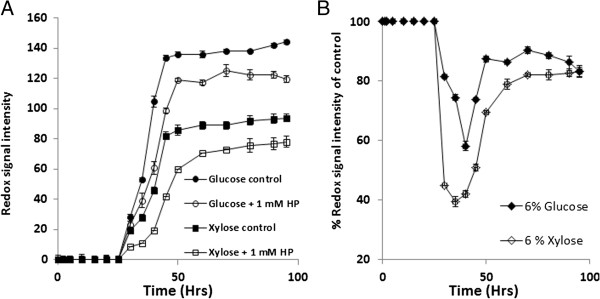
Effect of altering growth conditions on hydrogen peroxide tolerance for P. guillermondii. (A) Effect of growth on glucose or xylose on hydrogen peroxide tolerance, and (B) Effect of carbon source on hydrogen peroxide tolerance expressed as a percentage of unstressed control. The assay was performed in triplicate and the average reading was plotted.
Discussion
Utilisation of xylose is an important factor in making second generation biofuels a viable option for the conversion of LCM’s into useable refined products such as ethanol or butanol. Approaches which yield glucose rich and xylose rich streams have been considered for converting all available sugars into high value products. Candida, Pichia and Scheffersomyces (previously named Pichia stipitis) currently represent the best natural yeast for efficient utilisation of pentose sugars [30]. Conversion of pentose sugars such as arabinose into the carotenoid astaxanthin by the yeast Phaffia rhodozyma rather than conversion of ethanol in poor ethanol producing yeast is of particular interest to the bioethanol industry [37]. Oxidative stress induced by free radical production by metabolising cells is a consequence of the industrial fermentation processes, where yeast are serially sedimented out of the media and reused in subsequent fermentations, during these stages yeast are exposed to oxygen, and oxidative stress has been observed to reduce viability, induce glycerol accumulation and reduce ethanol production [38].
We assayed for the tolerance to hydrogen peroxide-induced oxidative stress in pentose utilising yeast using a be-spoke phenotypic microarray plates in which the effect of hydrogen peroxide can be compared against unstressed conditions. We measured the effect of hydrogen peroxide and assessed when each yeast was affected (i.e., an extended lag phase when compared with control) and when complete inhibition of metabolic output was observed. We confirmed the data using traditional spot plates, viability and effect on growth using a plate reader.
All yeast assayed in this study exhibited a similar tolerance to hydrogen peroxide-induced oxidative stress as those reported for S. cerevisiae[31]. However, P. guillermondii was more tolerant to hydrogen peroxide-induced oxidative stress when compared to C. shehatae, C. succiphila and S. stipitis based on metabolic output, growth and viability assays. This tolerance appeared to be yeast specific rather than a characteristic of that strain of the yeast as assays with other P. guillermondii confirmed the phenotypic response to hydrogen peroxide-induced oxidative stress. Though strain variation in response to an oxidant is always possible in any population, variation in response has been observed with the same strain when obtained from different geographic sources [39,40].
All of the yeasts were more sensitive to hydrogen peroxide when grown on YNB as opposed to YPD, however, addition of adenine improved tolerance to hydrogen peroxide-induced oxidative stress. Yeast incubated with NS minus adenine were more sensitive to hydrogen peroxide-induced oxidative stress when compared with NS containing adenine, and incubation with NS minus methionine improved oxidative tolerance compared with NS containing methionine. Adenine has been shown to induce adenosine triphosphate (ATP) production, ATP has been shown to buffer cells against oxidative stress, and is required for the activity of a mitochondrial transporter which is essential for yeast cells undergoing oxidative phosphorylation, additionally adenine is a key component of co-factors and enhancing NADPH supply has been suggested as improving xylose fermentations [41-43]. We have yet to determine the precise nature of the protective role of adenine on hydrogen peroxide-induced oxidative stressed yeast.
Exposure to high concentrations of methionine has been shown to induce apoptotic markers and lipid peroxidation in rat liver cells [44], methionine also induces ROS in rat liver mitochondria with associated mitochondria DNA damage [45]. However, yeast such as S. cerevisiae does not require polyunsaturated fatty acids that are the primary targets of such peroxidation induced by ROS and normally contain mainly saturated or monounsaturated fatty acids [46]. An adverse effect of methionine on yeast cells under hydrogen peroxide-induced oxidative stress has not been reported previously.
Glutathione (GSH) concentrations in Candida, Pichia and Scheffersomyces cells were found to be similar to levels quoted for exponentially growing S. cerevisiae cells [24], there was no difference in GSH levels between the yeast spp. Hydrogen peroxide-induced oxidative stressed cells are characterised by an increase in oxidised glutathione (GSSG) and a shift in redox balance. There was no difference in GSSG concentrations or redox balance between the yeast spp assayed in this study. The increased sensitivity of Candida spp to hydrogen peroxide is not due to differences in the glutathione maintained redox balance of the cell.
Peroxidase and catalase activity in C. shehatae was significantly reduced when compared with other yeast assayed in this study. Catalase activity for C. shehatae and C. succiphila was significantly reduced when compared with S. cerevisiae, P. guillermondii or S. stipitis. All yeast assayed in this study exhibited increased sensitivity to hydrogen peroxide-induced oxidative stress when utilising xylose as a sole carbon source when compared with glucose. Hydrolysates from LCM’s will contain variable concentrations of glucose and pentose sugars, thus this study suggests that yeast metabolising pentose sugars will be more sensitive to oxidative stress compared to those metabolising hexose sugars.
Conclusions
This study has determined that Candida, Pichia and Scheffersomyces exhibit tolerance to hydrogen peroxide-induced oxidative stress at concentrations (1- 5 mM) similar to S. cerevisiae. However, P. guillermondii was more tolerant to hydrogen peroxide-induced oxidative stress when compared to C. shehatae, C. succiphila or S. stipitis based on metabolic output, growth and viability assays. We failed to observe differences in the redox status of the yeast under hydrogen peroxide-induced oxidative stress but there were differences in key antioxidant response enzymes to hydrogen peroxide-induced oxidative stress. We observed that addition of adenine improved tolerance to hydrogen peroxide-induced oxidative stress whereas addition of methionine was deleterious.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JS: Performed the phenotypic microarray experiments, viability and growth assessments. TGP: Helped with the writing of this manuscript and helped guide the research. KAS: Helped with the writing of this manuscript and helped guide the research. DG: Performed the glutathione/GSSG assays, catalase + peroxidase assays, wrote the paper and guided JS’s research. All authors read and approved the final manuscript.
Supplementary Material
Oxidative stress prevents metabolic outout in yeast spp assayed in this study. The effect of hydrogen peroxide on metabolic output expressed as redox signal intensity units on yeast spp. (A)P. guillermondii NCYC 441 and NCYC 443 (B)S. stipitis NCYC 1541 and NCYC 1542 (C)C. shehatae NCYC 2389 and NCYC 3781 The assay was performed in triplicate and the average reading was plotted.
Contributor Information
Jennifer Spencer, Email: jenniferspencer1@hotmail.co.uk.
Trevor G Phister, Email: trevor.phister@nottingham.ac.uk.
Katherine A Smart, Email: katherine.smart@nottingham.ac.uk.
Darren Greetham, Email: Darren.greetham@nottingham.ac.uk.
Acknowledgements
The research reported here was supported (in full or in part) by the Biotechnology and Biological Sciences Research Council (BBSRC) Sustainable Bioenergy Centre (BSBEC), under the programme for ‘Lignocellulosic Conversion To Ethanol’ (LACE) [Grant Ref: BB/G01616X/1]. This is a large interdisciplinary programme and the views expressed in this paper are those of the authors alone, and do not necessarily reflect the views of the collaborators or the policies of the funding bodies. This project is part financed by the European Regional Development Fund project EMX05568. Katherine Smart was the SABMiller Professor in Brewing Science and gratefully acknowledges the support provided by SABMiller.
References
- Briggs DE, Boulton C, Brookes PA, Stevens R. In Brewing: science and practice. Oxford, UK: Taylor and Francis; 2004. Brewing: science and practice. [Google Scholar]
- D’Amore T, Panchal CJ, Russell I, Stewart GG. A study of ethanol tolerance in yeast. Crit Rev Biotechnol. 1990;9(4):287–304. doi: 10.3109/07388558909036740. [DOI] [PubMed] [Google Scholar]
- Quain CBD. Brewing Yeast and Fermentation. Oxford, UK: Blackwell publishing, Wiley-Blackwell; 2006. [Google Scholar]
- Cardona F, Carrasco P, Perez-Ortin JE, del Olmo MI, Aranda A. A novel approach for the improvement of stress resistance in wine yeasts. Int J Food Microbiol. 2007;114(1):83–91. doi: 10.1016/j.ijfoodmicro.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Casey GP, Ingledew WM. Ethanol tolerance in yeasts. Crit Rev Microbiol. 1986;13(3):219–280. doi: 10.3109/10408418609108739. [DOI] [PubMed] [Google Scholar]
- Petrov VV, Okorokov LA. Increase of the anion and proton permeability of Saccharomyces carlsbergensis plasmalemma by n-alcohols as a possible cause of its de-energization. Yeast. 1990;6(4):311–318. doi: 10.1002/yea.320060404. [DOI] [PubMed] [Google Scholar]
- Aguilera F, Peinado RA, Millan C, Ortega JM, Mauricio JC. Relationship between ethanol tolerance, H + -ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int J Food Microbiol. 2006;110(1):34–42. doi: 10.1016/j.ijfoodmicro.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lorenz RT, Parks LW. Involvement of heme components in sterol metabolism of Saccharomyces cerevisiae. Lipids. 1991;26(8):598–603. doi: 10.1007/BF02536423. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990;15(4):129–135. doi: 10.1016/0968-0004(90)90206-Q. [DOI] [PubMed] [Google Scholar]
- Vivancos AP, Jara M, Zuin A, Sanso M, Hidalgo E. Oxidative stress in Schizosaccharomyces pombe: different H2O2 levels, different response pathways. Mol Genet Genomics. 2006;276(6):495–502. doi: 10.1007/s00438-006-0175-z. [DOI] [PubMed] [Google Scholar]
- Yang MH, Schaich KM. Factors affecting DNA damage caused by lipid hydroperoxides and aldehydes. Free Radic Biol Med. 1996;20(2):225–236. doi: 10.1016/0891-5849(95)02039-X. [DOI] [PubMed] [Google Scholar]
- O’Rourke TW, Doudican NA, Mackereth MD, Doetsch PW, Shadel GS. Mitochondrial dysfunction due to oxidative mitochondrial DNA damage is reduced through cooperative actions of diverse proteins. Mol Cell Biol. 2002;22(12):4086–4093. doi: 10.1128/MCB.22.12.4086-4093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudican NA, Song B, Shadel GS, Doetch PW. Oxidative DNA damage causes mitochondrial genomic instability in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25(12):5196–5204. doi: 10.1128/MCB.25.12.5196-5204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BR, Prescott KA, Smart KA. Petite mutation in aged and oxidatively stressed ale and lager brewing yeast. Lett Appl Microbiol. 2008;46(6):636–642. doi: 10.1111/j.1472-765X.2008.02360.x. [DOI] [PubMed] [Google Scholar]
- Drakulic T, Temple MD, Guido R, Jarolim S, Breitenbach M, Attfield PV, Dawes IW. Involvement of oxidative stress response genes in redox homeostasis, the level of reactive oxygen species, and ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 2005;5(12):1215–1228. doi: 10.1016/j.femsyr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Barker MG, Walmsley RM. Replicative ageing in the fission yeast Schizosaccharomyces pombe. Yeast. 1999;15(14):1511–1518. doi: 10.1002/(SICI)1097-0061(199910)15:14<1511::AID-YEA482>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Van Zandycke SM, Sohier PJ, Smart KA. The impact of catalase expression on the replicative lifespan of Saccharomyces cerevisiae. Mech Ageing Dev. 2002;123(4):365–373. doi: 10.1016/S0047-6374(01)00382-7. [DOI] [PubMed] [Google Scholar]
- Powell CD, Van Zandycke SM, Quain DE, Swart KA. Replicative ageing and senescence in Saccharomyces cerevisiae and the impact on brewing fermentations. Microbiology. 2000;146(Pt 5):1023–1034. doi: 10.1099/00221287-146-5-1023. [DOI] [PubMed] [Google Scholar]
- Iwai K, Naganuma A, Kuge S. Peroxiredoxin Ahp1 acts as a receptor for alkylhydroperoxides to induce disulfide bond formation in the Cad1 transcription factor. J Biol Chem. 2010;285(14):10597–10604. doi: 10.1074/jbc.M109.090142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr. 1993;57(5 Suppl):779S–785S. doi: 10.1093/ajcn/57.5.779S. discussion 785S-786S. [DOI] [PubMed] [Google Scholar]
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39(8):1529–1542. [PubMed] [Google Scholar]
- Lee J, Godon C, Lagniel G, Spector D, Garin J, Labarre J, Toledano MB. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem. 1999;274(23):16040–16046. doi: 10.1074/jbc.274.23.16040. [DOI] [PubMed] [Google Scholar]
- Costa V, Amorim MA, Resi E, Quintanilha A, Moradas-Ferreira P. Mitochondrial superoxide dismutase is essential for ethanol tolerance of Saccharomyces cerevisiae in the post-diauxic phase. Microbiology. 1997;143(Pt 5):1649–1656. doi: 10.1099/00221287-143-5-1649. [DOI] [PubMed] [Google Scholar]
- Trotter EW, Grant CM. Overlapping roles of the cytoplasmic and mitochondrial redox regulatory systems in the yeast Saccharomyces cerevisiae. Eukaryot Cell. 2005;4(2):392–400. doi: 10.1128/EC.4.2.392-400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CM, MacIver FH, Dawes IW. Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr Genet. 1996;29(6):511–515. doi: 10.1007/BF02426954. [DOI] [PubMed] [Google Scholar]
- Bronzetti G, Cini M, Andreoli E, Caltavuturo L, Panunzio M, Croce CD. Protective effects of vitamins and selenium compounds in yeast. Mutat Res. 2001;496(1–2):105–115. doi: 10.1016/s1383-5718(01)00213-3. [DOI] [PubMed] [Google Scholar]
- Clarkson SP, Large PJ, Boulton CA, Bamforth CW. Synthesis of superoxide dismutase, catalase and other enzymes and oxygen and superoxide toxicity during changes in oxygen concentration in cultures of brewing yeast. Yeast. 1991;7(2):91–103. doi: 10.1002/yea.320070203. [DOI] [Google Scholar]
- Madhavan A, Srivastava A, Kondo A, Bisaria VS. Bioconversion of lignocellulose-derived sugars to ethanol by engineered Saccharomyces cerevisiae. Crit Rev Biotechnol. 2012;32(1):22–48. doi: 10.3109/07388551.2010.539551. [DOI] [PubMed] [Google Scholar]
- Attfield PV, Bell PJ. Use of population genetics to derive nonrecombinant Saccharomyces cerevisiae strains that grow using xylose as a sole carbon source. FEMS Yeast Res. 2006;6(6):862–868. doi: 10.1111/j.1567-1364.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- Toivola A, Yarrow D, van den Bosche E, van Dijken JP, Scheffers WA. Alcoholic Fermentation of d-Xylose by Yeasts. Appl Environ Microbiol. 1984;47(6):1221–1223. doi: 10.1128/aem.47.6.1221-1223.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CM, Perrone G, Dawes IW. Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1998;253(3):893–898. doi: 10.1006/bbrc.1998.9864. [DOI] [PubMed] [Google Scholar]
- Poljak A, Dawes IW, Ingelse BA, Duncan MW, Smythe GA, Grant CM. Oxidative damage to proteins in yeast cells exposed to adaptive levels of H(2)O(2) Redox Rep. 2003;8(6):371–377. doi: 10.1179/135100003225003401. [DOI] [PubMed] [Google Scholar]
- Evans MV, Turton HE, Grant CM, Dawes IW. Toxicity of linoleic acid hydroperoxide to Saccharomyces cerevisiae: involvement of a respiration-related process for maximal sensitivity and adaptive response. J Bacteriol. 1998;180(3):483–490. doi: 10.1128/jb.180.3.483-490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes AJ, Hamilton AL, de M Guesdes HL, Nosanchuk JD, Zancope-Oliveira RM. Biological function and molecular mapping of M antigen in yeast phase of Histoplasma capsulatum. PLoS One. 2008;3(10):e3449. doi: 10.1371/journal.pone.0003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FB, Guimaraes PM, Gomes DG, Mira NP, Teixeira MC, Sa-Correja I, Domingues L. Identification of candidate genes for yeast engineering to improve bioethanol production in very high gravity and lignocellulosic biomass industrial fermentations. Biotechnol Biofuels. 2011;4(1):57. doi: 10.1186/1754-6834-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Zhao H. Investigating host dependence of xylose utilization in recombinant Saccharomyces cerevisiae strains using RNA-seq analysis. Biotechnol Biofuels. 2013;6(1):96. doi: 10.1186/1754-6834-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanti J, Nghiem NP, Johnston DB. Production of astaxanthin from cellulosic biomass sugars by mutants of the yeast Phaffia rhodozyma. Appl Biochem Biotechnol. 2011;164(5):655–665. doi: 10.1007/s12010-011-9165-7. [DOI] [PubMed] [Google Scholar]
- Gibson BR, Lawrence SJ, Leclaire JP, Powell CD, Smart KA. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol Rev. 2007;31(5):535–569. doi: 10.1111/j.1574-6976.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- Bayliak M, Semchyshyn H, Lushchak V. Effect of hydrogen peroxide on antioxidant enzyme activities in Saccharomyces cerevisiae is strain-specific. Biochemistry (Mosc) 2006;71(9):1013–1020. doi: 10.1134/S0006297906090100. [DOI] [PubMed] [Google Scholar]
- Semchyshyn H, Bagnyukova T, Lushchak V. Involvement of soxRS regulon in response of Escherichia coli to oxidative stress induced by hydrogen peroxide. Biochemistry (Mosc) 2005;70(11):1238–1244. doi: 10.1007/s10541-005-0253-6. [DOI] [PubMed] [Google Scholar]
- Pereira C, Camougrand N, Manon S, Sousa MJ, Corte-Real M. ADP/ATP carrier is required for mitochondrial outer membrane permeabilization and cytochrome c release in yeast apoptosis. Mol Microbiol. 2007;66(3):571–582. doi: 10.1111/j.1365-2958.2007.05926.x. [DOI] [PubMed] [Google Scholar]
- Laco J, Zeman I, Pevala V, Polcic P, Kolorov J. Adenine nucleotide transport via Sal1 carrier compensates for the essential function of the mitochondrial ADP/ATP carrier. FEMS Yeast Res. 2010;10(3):290–296. doi: 10.1111/j.1567-1364.2010.00606.x. [DOI] [PubMed] [Google Scholar]
- Zha J, Shen M, Hu M, Song H, Yuan Y. Enhanced expression of genes involved in initial xylose metabolism and the oxidative pentose phosphate pathway in the improved xylose-utilizing Saccharomyces cerevisiae through evolutionary engineering. J Ind Microbiol Biotechnol. 2014;41(1):27–39. doi: 10.1007/s10295-013-1350-y. [DOI] [PubMed] [Google Scholar]
- Yalcinkaya S, Unlucerci Y, Giris M, Olgac V, Dogru-Abbasoglu S, Ulysal M. Oxidative and nitrosative stress and apoptosis in the liver of rats fed on high methionine diet: protective effect of taurine. Nutrition. 2009;25(4):436–444. doi: 10.1016/j.nut.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Gomez J, Caro P, Sanchez I, Naudi A, Jove M, Portero-Otin M, Lopez-Torres M, Pamplona R, Barja G. Effect of methionine dietary supplementation on mitochondrial oxygen radical generation and oxidative DNA damage in rat liver and heart. J Bioenerg Biomembr. 2009;41(3):309–321. doi: 10.1007/s10863-009-9229-3. [DOI] [PubMed] [Google Scholar]
- Avery SV, Howlett NG, Radice S. Copper toxicity towards Saccharomyces cerevisiae: dependence on plasma membrane fatty acid composition. Appl Environ Microbiol. 1996;62(11):3960–3966. doi: 10.1128/aem.62.11.3960-3966.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oxidative stress prevents metabolic outout in yeast spp assayed in this study. The effect of hydrogen peroxide on metabolic output expressed as redox signal intensity units on yeast spp. (A)P. guillermondii NCYC 441 and NCYC 443 (B)S. stipitis NCYC 1541 and NCYC 1542 (C)C. shehatae NCYC 2389 and NCYC 3781 The assay was performed in triplicate and the average reading was plotted.