Summary
It was recently shown that mouse fibroblasts could be reprogrammed into cells of a cardiac fate by forced expression of multiple transcription factors and microRNAs. To ultimately apply such reprogramming strategy for cell-based therapy or in vivo cardiac regeneration, reducing or eliminating the genetic manipulations by small molecules would be highly desirable. Here, we report the identification of a defined small-molecule cocktail that enables highly efficient conversion of mouse fibroblasts into cardiac cells with only one transcription factor, Oct4, without entering the pluripotent state. Small-molecule-induced cardiomyocytes spontaneously contract and exhibit a ventricular phenotype. Furthermore, such induced cardiomyocytes under our condition pass through a cardiac progenitor stage. This study lays the foundation for future pharmacological reprogramming approaches and provides a novel small-molecule condition to investigate the mechanisms underlying cardiac reprogramming process.
Keywords: cardiac reprogramming, small molecules, screening
Introduction
Heart failure, a leading cause of mortality, typically results in the loss or dysfunction of cardiomyocytes (Kathiresan and Srivastava, 2012). Despite biomedical advances to prevent or limit cardiovascular disease, restoring the function of a damaged heart is a formidable challenge. This is in part attributed to the limited regenerative capability of adult cardiomyocytes. However, the human heart also contains a large population of endogenous cardiac fibroblasts (Ieda et al., 2009) that could serve as a cell source for regenerative therapy if they could be directly reprogrammed into beating cardiomyocytes. Recent advances in induced pluripotent stem cell (iPSC) technology have re-galvanized research on reprogramming of one somatic cell type into another developmentally non-permissive cell type without passing through the pluripotent state, a process known as transdifferentiation (Sancho-Martinez et al., 2012). This approach offers new promise that the repair of damaged hearts can be achieved with reduced risk of tumorigenesis associated with pluripotent cells, via cell replacement therapy and/or in vivo regeneration by cellular reprogramming.
In addition to the conventional paradigm of using target cell type–specific factors (e.g., transcription factors (TFs) and miRNAs) for transdifferentiation, we previously established the Cell-Activation and Signaling-Directed (CASD) lineage conversion method. This approach employs transient overexpression of iPSC-TFs (cell activation, CA) in conjunction with lineage-specific soluble signals (signal-directed, SD) to reprogram somatic cells into diverse lineage-specific cell types without entering the pluripotent state. Using this approach, we and others have reported the generation of induced cardiac (Efe et al., 2011), neural (Han et al., 2012; Kim et al., 2011; Lu et al., 2013; Thier et al., 2012; Zhu et al., 2014), endothelial cells (Li et al., 2013a), and definitive endoderm(Ke Li, 2014) from mouse and human fibroblasts without first establishing the pluripotent state.
While the above approaches employing multiple (e.g., three to seven) genetic factors established proofs of concept to reprogram non-cardiac cells to cardiomyocytes, such genetic manipulations inevitably harbor safety, efficiency and other technical issues. Alternatively, to more effectively apply transdifferentiation to cell-based therapies and/or in vivo repair/regeneration of endogenous cells in an ultimate tissue-specific manner, it would be essential to develop methods that eliminate or reduce the dependence on genetic manipulation. Replacing the cardiac reprogramming TFs with defined small molecules would therefore represent an essential first step toward the ultimate pharmacological induction of cardiac cells. Such small molecules may also enhance reprogramming for in vitro applications, and provide chemical tools to study the underlying reprogramming mechanisms.
Toward this end, we screened for small-molecule conditions that can replace TFs to reprogram mouse fibroblasts into cardiac cells. As the general CASD lineage-conversion process depends on only transient iPSC-TF expression and not on the establishment of pluripotency (Efe et al., 2011; Kim et al., 2011), we hypothesized that ectopic expression of TFs might be more easily substituted by small molecules known to induce or enhance iPSC generation. Here, we report the identification of a defined small-molecule cocktail consisting of SB431542 (ALK4/5/7 inhibitor), CHIR99021 (GSK3 inhibitor), parnate (LSD1/KDM1 inhibitor, also called tranylcypromine) and forskolin (adenylyl cyclase activator). This cocktail enabled the conversion of mouse fibroblasts into cardiomyocytes with only one TF, Oct4, without traversing the pluripotent state. Small molecule–enabled cardiac reprogramming is very efficient, inducing the conversion of fibroblasts to spontaneously beating cardiomyocytes that exhibit a ventricular phenotype, while drastically reducing the number of genetic reprogramming factors required (i.e., from four TFs to one TF). This study lays the foundation for developing methods for 100% pharmacological reprogramming and provides a novel small-molecule condition to investigate the mechanisms underlying lineage reprogramming.
Results
Small molecule cocktails enable the cardiac conversion of mouse fibroblasts in conjunction with a single transcription factor, Oct4
To develop small-molecule conditions for inducing cardiac reprogramming from mouse fibroblasts, we envisioned a strategy based on the CASD paradigm that combines iPSC-inducing and -enhancing small molecules to induce cell activation with cardiogenic small molecules that serve to direct the cardiomyocyte fate. In previous studies, we and others identified a large number of small molecules that, either alone or in combination, enhance reprogramming efficiency and/or accelerate reprogramming speed (Nie et al., 2012; Zhu et al., 2011). Importantly, some combinations can functionally substitute one, two, or three reprogramming TFs to generate iPSCs from various mouse and human somatic cell types (Li et al., 2009; Li et al., 2011; Shi et al., 2008; Yuan et al., 2011; Zhu et al., 2010). Those small molecules include modulators of epigenetic enzymes, signaling pathways, metabolism, and transcription. In recent years, cardiogenic small molecules were also identified in studies of the pluripotent stem cell differentiation process, including modulators of the Wnt, TGFβ BMP, and Activin/Nodal signaling pathways (Hilcove, 2010; Kattman et al., 2011; Lian et al., 2012; Naito et al., 2006; Willems et al., 2012; Yang et al., 2008).
In the present study, we first tested small molecules in the presence of three TFs (Oct4, Klf4 and Sox2/OKS) to determine which combinations mediated efficient cardiac reprogramming. To better control exogenous TF expression and minimize variability in screening, we used a lentiviral construct carrying the three TFs/OKS in a single tetracycline-inducible vector (pHAGE2-TetO-STEMCCA-redlight, or TetO-OKS). To carry out the screening, mouse embryonic fibroblasts (MEFs) were infected with TetO-OKS and split into 24-well plates. The various small-molecule combinations and doxycycline (Dox) were added into the cardiac reprogramming medium (CRM, see Materials and Methods) for the first 6 days, and were followed by treatment with BMP4. The number of beating cell clusters was counted as a readout of reprogramming efficiency (Figure S1A). In control wells without any compound treatment, there were few beating clusters, while the single addition of SB431542 or CHIR99021 slightly increased reprogramming efficiency. However, exposure to both SB431542 and CHIR99021 doubled the efficiency of cardiac reprogramming. Owing to the synergism induced by Wnt signaling activation and TGFβ signaling inhibition, the combination of SB431542 and CHIR99021 (SC) became our baseline small-molecule condition in conjunction with TFs. On top of which additional iPSC inducing/enhancing and cardiogenic small molecules were screened. Primary hits that could enhance cardiac reprogramming under the basal condition containing CHIR99021 and SB431542 were shown in Figure S1B and Table S1. Then different small molecule combinations were further tested and optimized. Notably, we found that the addition of parnate (LSD1/KDM1 inhibitor) and forskolin (adenylyl cyclase activator) together further increased the reprogramming efficiency dramatically. This result was confirmed in a larger well format as shown in Figure S1C and D. Hereafter, the small molecule combination of SB431542, CHIR99021, parnate and forskolin will be referred to as SCPF.
With this remarkable efficiency of cardiac induction, we next asked whether SCPF could enable cardiac reprogramming in conjunction with a single TF, Oct4. We found that MEFs infected with Oct4 and treated with SCPF could indeed be converted to spontaneously contracting clusters (Figure 1A). The first contracting cluster was observed around day 20. At day 30, a total of 99±17 contracting clusters could be generated from the original 10,000 plated MEFs (n=6). In contrast, no beating clusters were found in control wells without compound treatment. Immunocytochemistry analysis confirmed that these beating patches were positive for cardiac-specific markers, including cTnT and cardiac myosin heavy chain (cMHC) (Figure 1B).
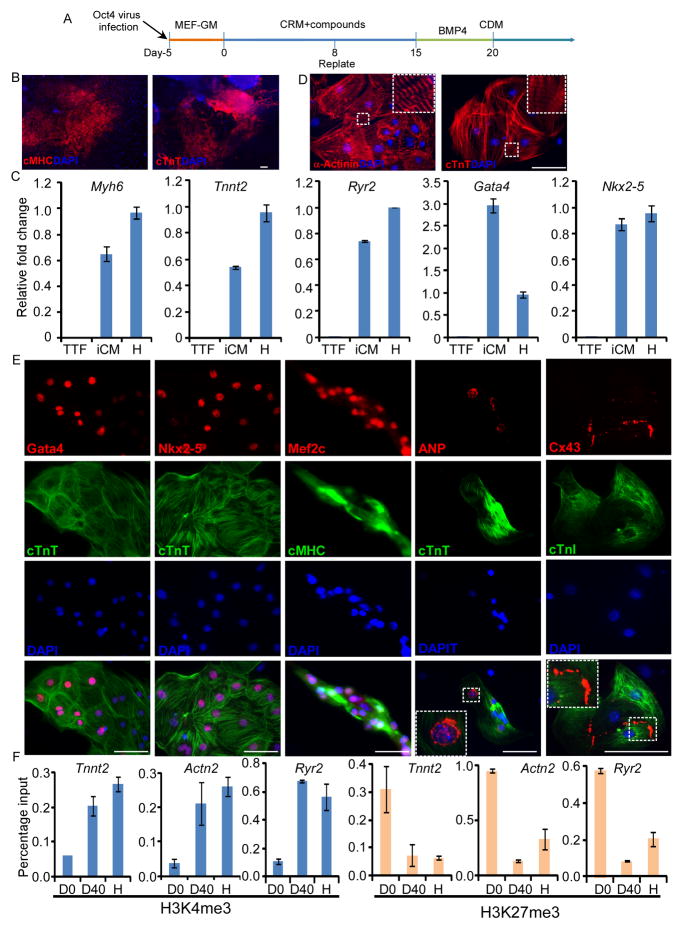
Figure 1. The small molecule cocktail, SCPF, enables cardiac reprogramming with a single factor, Oct4.
(A) The scheme of direct cardiac reprogramming with the small molecule cocktail, SCPF, and a single factor, Oct4. (B) Immunostaining of cTnT and cardiac MHC in beating clusters generated from MEFs on day 25. (C) qPCR analysis of indicated markers in the induced cardiomyocytes (iCM) from TTFs at day 35. mRNA from TTFs and neonatal heart (in short, “H” ) were used as negative or positive controls. (D, E) Immunostaining of various markers in small clumps or single cells digested from TTF-derived beating clusters. Scale bar is 100 μm. (F) ChIP assays examining H3K4me3 and H3K27me3 at three cardiac loci during reprogramming at day 0 (D0), day 40 (D40) and neonatal heart tissues (“H”). Quantitative PCR was used to assess changes in H3K4me3 (Left) and H3K27me3 (Right) levels. Scale bars, 100μm.
To exclude the possibility that beating cells might be generated from rare cardiac progenitors in MEF cells, we next tested whether, in conjunction with Oct4, SCPF could enable cardiac induction in mouse tail-tip fibroblast (TTF), a much more homogenous cell population that does not contain any cardiac lineage cells. Using the same conditions applied to MEFs (Figure 1A), we found that nearly 50 spontaneously beating colonies (n=6) could be generated from 10,000 TTFs by day 30. Quantitative RT-PCR (qRT-PCR) showed that cardiac-specific genes, including Myh6, Tnnt2, Ryr2, Gata4, and Nkx2-5, were highly expressed in these induced cardiomyocytes compared with control TTFs (Figure 1C). To further characterize these reprogrammed TTFs, large beating patches of cells were digested into small cell clusters or single cells for immunocytochemistry analysis. These induced cardiomyocytes displayed a tri- or multi-angular morphology. Immunocytochemistry analysis revealed that these cells were positive for the cardiomyocyte markers, cardiac troponin-T (cTnT) and α-Actinin, and displayed a clear cross-striated pattern (Figure 1D). Core TFs and peptides important for cardiac development and function, including Gata4, Mef2c and Nkx2-5, as well as ANP, were also highly expressed in the induced cardiomyocytes. Moreover, connexin-43 (Cx43), a gap junction protein expressed in cardiomyocytes, was also detected along the periphery of the induced cardiomyocytes, indicating the development of gap junctions for intercellular communication (Figure 1E). To investigate relevant epigenetic changes, we used ChIP-qPCR assays to examine H3K4me3 and H3K27me3 at three key cardiac loci: Actn2 (encoding α–actinin), Ryr2 (encoding the cardiac ryanodine receptor) and Tnnt2 (encoding cardiac troponin T). As shown in Figure 1F, we observed that the promoters of these three cardiac genes exhibited increased H3K4me3 and decreased H3K27me3 compared to initial MEFs (D0), consistent with cardiac reprogramming and gene expression.
Serendipitously, we also consistently observed a few twitching/contracting cells in the control wells treated only with small molecules (e.g., SCPF) without Oct4 transduction. They exhibited a long tube-like morphology different from neonatal cardiomyocytes, pluripotent stem cell–derived cardiomyocytes, or induced cardiomyocytes obtained in the presence of Oct4 and characterized above. Interestingly, they were positive for cTnT, cMHC and α-Actinin immunostaining (Figure S2A). However, further characterization revealed that core cardiac TFs, such as Nkx2-5 and Gata4 were barely detected in those cells (Figure S2B), while they modestly expressed skeletal muscle TFs like MyoD and Myogenin (Figure S2C). These findings suggest that these tube-like contracting cells are not fully reprogrammed cardiomyocytes or may instead possess certain skeletal muscle features. Therefore, we did not further investigate them in this study.
Induced cardiomyocytes exhibited typical cardiac electrophysiological features
We next sought to characterize the functional properties of these TTF-derived induced cardiomyocytes by electrophysiological analysis. In single-cell patch-clamp, action potentials (APs) were recorded from single spontaneously beating cells around day 35 of reprogramming. Based on the ratio of AP duration at 90% repolarization (APD90) to APD50 (Kuzmenkin et al., 2009), we found that most of our induced cardiomyocytes showed ventricular-like AP morphology with a mean diastolic potential (MDP) of -75.2 mV and a mean overshoot potential (OSP) of 23.5 mV (n = 16), whereas very few of the cells displayed atrial or nodal-like APs (Figure 2A, B). Consistently, immunostaining analysis confirmed expression of myosin light chain-2v (MLC2v), a ventricular specific marker, in most of the induced cardiomyocytes (Figure 2C). This is in contrast to our previous four-factor (OSKM) cardiac reprogramming condition, where only atrial-like APs were detected in the induced cardiomyocytes (Efe et al., 2011). This finding suggests that subtype specificity of induced cardiomyocytes can be modulated by small molecule conditions. Consequently, the newly developed SCPF/Oct4 condition may represent a more desirable condition for cardiac reprogramming, as ventricular cardiomyocytes are typically lost in heart failure. In addition, the maximal depolarization rate (Vmax) for ventricular-like APs was comparatively very high (Kuzmenkin et al., 2009), which is likely accounted-for by a combination of the appreciable density of tetrodotoxin (TTX) -sensitive Na current (185 +/- 32 A/F N=9 cells, Figure S3) and the relatively hyperpolarized MDP. In conclusion, these data confirm that cardiomyocytes induced by SCPF/Oct4 possess many typical cardiac electrophysiological features. Moreover, typical Ca2+ transients, which underlie the contraction and relaxation of spontaneously beating cardiomyocytes, were also detected (Figure 2D). The peak fluorescence ratio in these transients, i.e., peak fluorescence expressed relative to the resting level (F/Fo), was 2.72 ± 0.22 (N=5), and is comparable to previous measurements made using embryonic or embryonic stem cell derived myocytes (Korhonen et al., 2010; Lieu et al., 2009; Sauer et al., 2001). Calibrated intracellular free [Ca2+] peaked at 320 ± 30 nM (N=5) which is also consistent with previous estimates from intact ventricular myocytes (Takamatsu et al., 2003) indicating the development of functional excitation-contraction coupling in these cells.
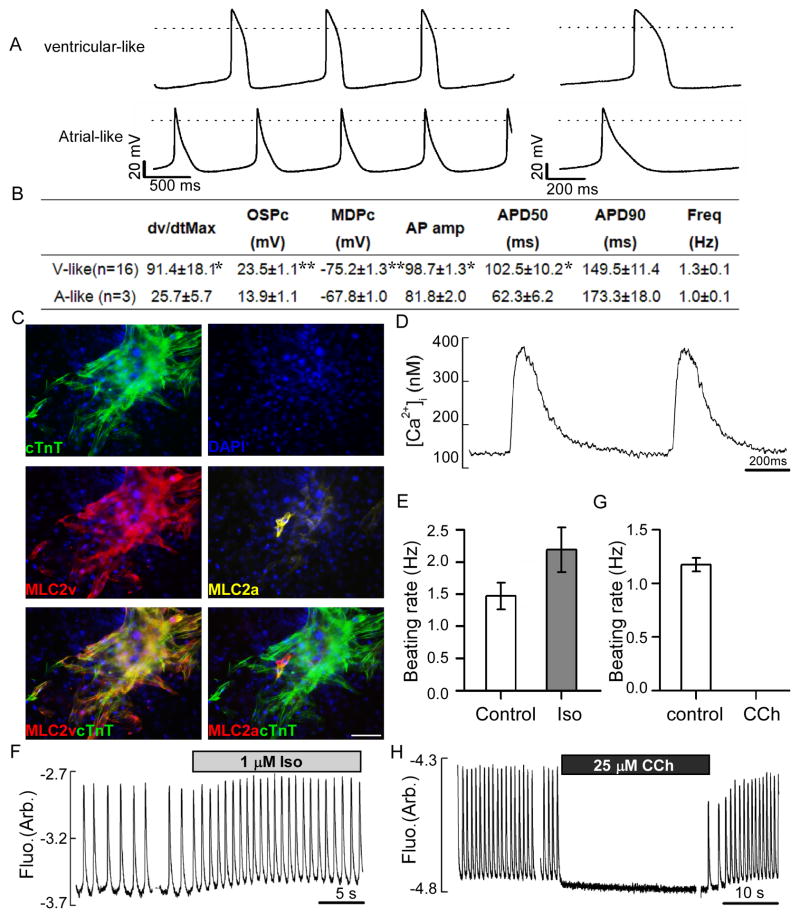
Figure 2. Contracting cardiomyocytes generated by SCPF/Oct4 exhibit typical electrophysiological features.
TTFs were converted into spontaneously contracting cardiomyocytes by SCPF/Oct4. Spontaneous action potentials (APs) and calcium transients were recorded from the individual cardiomyocytes at day 35–40. (A) Representative spontaneous APs showing characteristics of ventricular or atrial-like morphology. The waveform of a single AP is shown on the right using an expanded time scale. Dotted lines indicate zero membrane potential (0 mV). (B) Table of AP parameters measured for the induced cardiomyocytes, including maximum upstroke velocity (dV/dt max), overshoot potential (OSP), minimum diastolic potential (MDP), AP amplitude, AP durations (APDs) at the level of 90 and 50% repolarization, and the beating frequency. Significance levels are symbolized: * p<0.005, **p<0.001. (C) Triple immunostaining of cTnT, MLC2v and MLC2a antibody on induced cardiomyocytes at day 45. (D) Characteristic calcium transients ([Ca2+]i) recorded from the spontaneously contracting cardiomyocytes generated by SCPF/Oct4. (E–H) Record of calcium transients showing the effects on beating rates of treatment with 1μM isoproterenol (Iso) (E, F) and 25μM carbachol (CCh) (G, H). Scale bars, 100μm.
The ability to respond appropriately to hormones and transmitters of the autonomic nervous system is among the most critical functions of normal cardiomyocytes. We therefore sought to determine how TTF-derived induced cardiomyocytes respond to β-adrenergic and muscarinic stimulation. Addition of the β-adrenergic agonist isoproterenol (Iso) significantly increased the frequency of cell contractions and spontaneous calcium transients (Figure 2E, F), whereas carbachol (CCh), a muscarinic agonist, had the opposite effects (Figure 2G, H). After washing out carbachol, cells immediately resumed contracting at baseline frequency (Fig. 2H). The reversible modulation of the frequency of Ca2+ transients by Iso or CCh indicates that coupled β-adrenergic and muscarinic signaling cascades, as well as their associated intracellular signaling partners, are present and functional.
Induced cardiomyocytes were generated through cardiac precursor stage
As shown in Figure S1C and Figure 1B, we found that the beating clusters were clonally derived. Next we examined whether induced cardiomyocytes were generated through a cardiac precursor stage. Consistently with previous finding, robust expression of Flk1, Mesp1, Isl1 and Gata4, all of which are widely used as cardiac progenitor markers, could be detected during the early cardiac reprogramming stage by qRT-PCR (Figure 3A). Importantly, the Isl1 positive cells were also found to be Ki-67 positive by immunostaining (Figure 3B), indicating that those cells are mitotically active precursor cells. During embryic development, cardiac precursors also differentiate into smooth muscle cells and endothelial cells. Consistently, after BMP4 treatment, when we switched to different cell differentiation conditions that favor either endothelial or smooth muscle cell differentiation and maintenance, endothelial or smooth muscle cells could be generated, respectively (Figure 3C,D). Gene expression analysis of smooth muscle cell makers including ATCA2, Myh11, and Calponin 2 (Cnn2) and endothelial cell markers including CD146, VE-cadiherin, CD31 and Tie1 by qRT-PCR further confirmed the generation of those cardiovascular cell types (Figure 3E, F). Those results suggested the generation of nascent cardiac precursors during a critical reprogramming window. To further confirm this, we conducted a lineage tracing study using sorted GFP negative Isl1-Cre/ROSAmTmG MEFs (which would mark Isl1 expressing cells permanently with GFP) (Muzumdar et al., 2007; Srinivas et al., 2001), which were induced to beating cardiomyocytes under the same reprogramming condition. It was found that GFP positive cells were generated after addition of BMP4 (Figure 3G) and then they became beating clusters (Figure 3H and Movie S1). All these results collectively indicated that induced cardiomyocytes generated by our method have passed through a cardiac precursor stage.
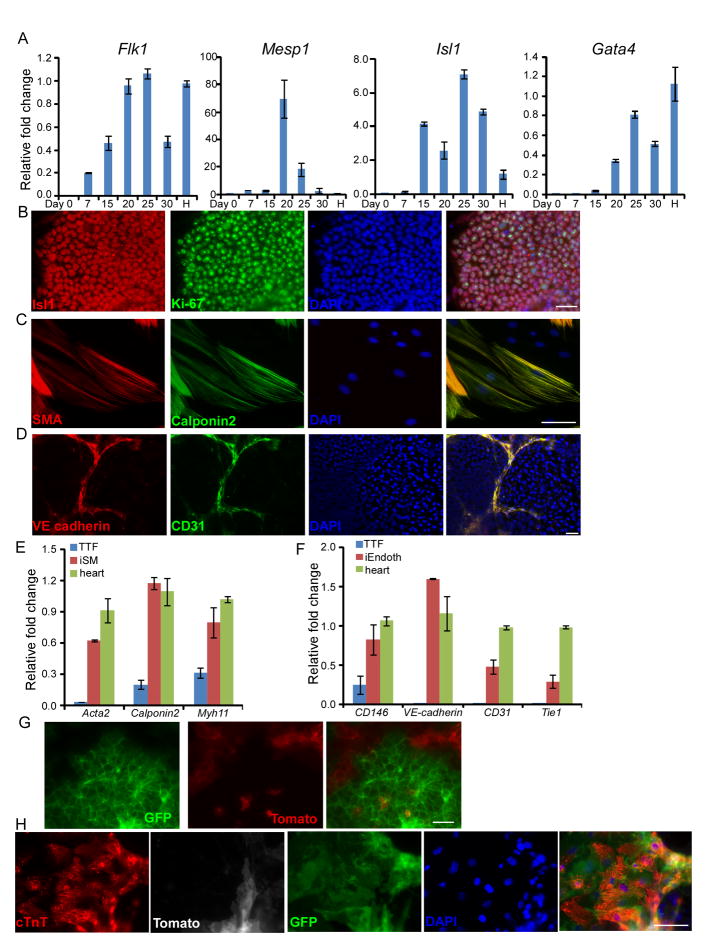
Figure 3. Induced cardiomyocytes were generated through cardiac precursor stage.
(A) Time course analysis of cardiac precursor marker expression by quantitative RT–PCR. “H” represents a positive control using mRNA from embryonic heart at E13.5 (B) At day 20, cells were fixed and immunostained with Isl1 and Ki-67 antibodies. (C-F) After BMP4 treatment, culture medium was switched to either smooth muscle cell differentiation medium (C, E) or endothelial cell differentiation medium (D, F) for another two weeks. Then cells were fixed and stained with smooth muscle actin (SMA) and Calponin 2 antibodies (C) or CD31 and VE-cadherin antibodies (D). mRNA expression of indicated markers were analyzed by qPCR. Data are mean±s.d. (E, F). (G, H) MEFs from Isl1-Cre/ROSAmTmG were used for cardiac reprogramming. At day 18, live cell images were taken to show GFP signal. (G) At day 35, cells were fixed and stained with cTnT antibody. Scale bars, 100μm.
The pluripotent state was not established during the reprogramming of fibroblasts to cardiomyocyte-like cells
The reprogramming of adult cells to iPSCs generally depends on the presence of LIF. Although LIF is not added to our transdifferentiation cocktail, we nevertheless wanted to rule out the possibility that our reprogramming paradigm induces iPSCs en route to cardiomyocytes. Therefore, to more carefully examine the reprogramming process, we performed time-lapse imaging of OG2 MEFs (Oct4-GFP reporter-expressing MEFs) (Do and Scholer, 2004) undergoing SCPF/Oct4-induced cardiac reprogramming (Figure 4A, B). Using OG2 MEFs, the presence of iPSCs can be clearly detected by GFP fluorescence (Figure 4C). We observed the first beating cluster at day 20 of a 25-day imaging period, and immunohistochemistry analysis confirmed that those beating clusters were cTnT-positive (Figure 4B, Movie S2). However, GFP+ cells were never detected throughout the whole reprogramming process (Figure 4A). This finding is consistent with our previous studies on direct cardiac, neural and endothelial cell reprogramming using the CASD paradigm (Efe et al., 2011; Kim et al., 2011; Li et al., 2013b). By qRT-PCR, the expression of pluripotency genes, such as Nanog and Rex1 remained nearly undetectable during the whole process (Figure 4D, E). In contrast, cardiac markers, including Gata4, Tnnt2, Myh6, and Ryr2, were gradually induced (Figure 4F).
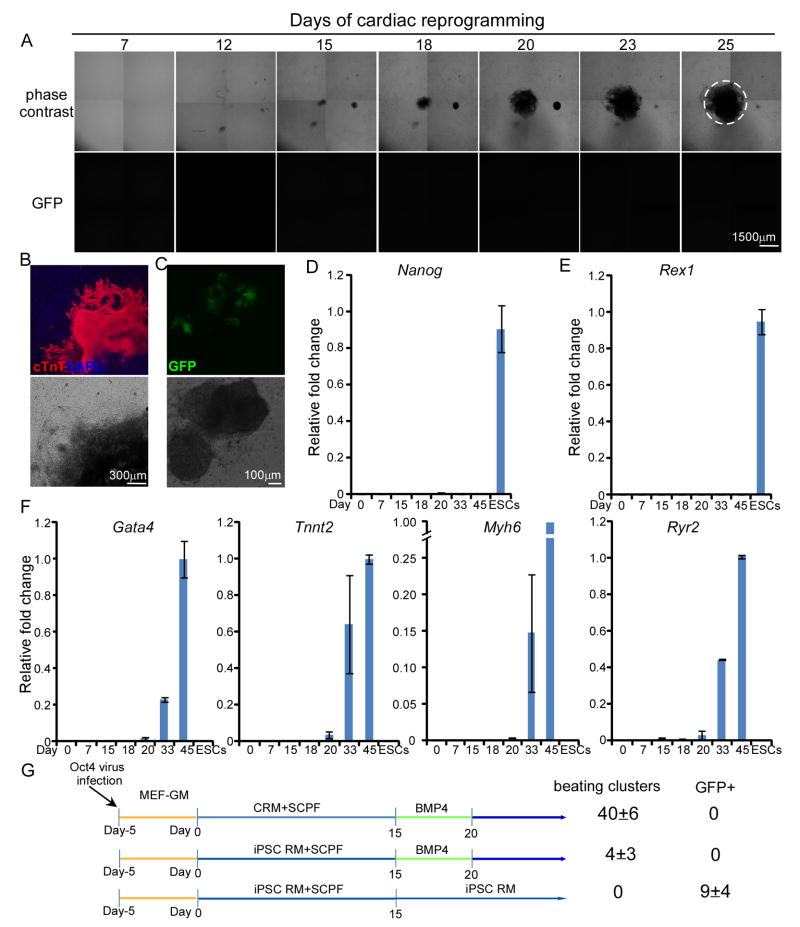
Figure 4. Pluripotent cells were not generated during direct cardiac reprogramming by SCPF/Oct4.
(A) Time-lapse imaging by phase contrast (top) and fluorescence microscopy (bottom) of induced cardiac colony formation in Oct4-GFP reporter (OG2) MEFs at the indicated days during reprogramming. The area of spontaneous contraction was encircled (see Supplementary Movie S2 for video). (B) At day 25, cells were fixed and immunostained with cTnT antibody. Nuclei were stained with DAPI. (C) Robust early activation of Oct4-GFP with OKSM transduction at day 6 using standard iPSC reprogramming medium was showed as a positive control. (D, E, F) Time course qRT-PCR analysis of pluripotency markers, Nanog (C) and Rex1 (D), and cardiac lineage markers (E). (G) Either cardiac reprogramming medium (CRM) or standard iPSC reprogramming medium (iPSC RM) was used in the first 15 days. The numbers of beating clusters and GFP+ colonies were counted at day 30. Data are mean±s.d.
In addition, if generation of pluripotent intermediates were critical, using iPSC reprogramming medium with LIF for the initiation stage (first 15 days) would yield more beating clusters. However, this substitution even resulted in 10-fold decrease in beating cluster number (Figure 4G). This result indicated that iPSC reprogramming condition is detrimental to Oct4-induced cardiac reprogramming. Clearly, all data collectively indicate that the conversion to cardiac cells occurred at a very early time point far before the reprogramming to pluripotent state, and PSC-like cells were not generated during such early stage.
Discussion
Our results provide the first proof-of-principle demonstration that effective cardiac reprogramming can be achieved from fibroblast cells using a single genetic factor enabled by small molecules. Compared with mouse cardiac reprogramming using multiple (e.g., 3–4) cardiac-specific TFs (Efe et al., 2011; Ieda et al., 2010; Song et al., 2012), our method appears to generate spontaneously contracting cardiomyocytes in an accelerated fashion, by passing through a cardiac mesoderm progenitor stage. Such a stage could be further exploited for the expansion of cardiac precursor cells for various applications in which large numbers of characterized cardiac cells are required.
Although ectopic Oct4 expression was still needed for cardiac reprogramming under the current condition, the endogenous pluripotency circuitry, consisting of Oct4, Nanog and Rex1, remains silenced. In addition, consistent with previous findings(Efe et al., 2011; Ke Li, 2014; Kim et al., 2011; Li et al., 2013a; Zhu et al., 2014), conditions specifically favoring establishment of pluripotency dramatically inhibited cardiac reprogramming during this process. While it remains elusive whether rare cells could transiently (shorter than hours) express certain pluripotency-associated genes, our data collectively and strongly support the notion that our cardiac reprogramming process passes through a cardiac precursor stage, but does not enter typical/stable pluripotent state. Because our paradigm does not produce pluripotent stem cells and reduces the culture time required to obtain cardiac cells, our approach is also faster and potentially safer than methods that have iPSCs as an intermediate step.
In addition, the generation of functional cardiomyocytes, most of which exhibit a ventricular subtype as characterized by marker expression and electrophysiological analyses, will be of great interest for both regenerative medicine and drug discovery. While these findings highlight the feasibility of using small molecules to precisely specify cell fate within a particular cell lineage, mechanistic dissection of the underlying signaling and epigenetic pathways will require further investigation.
Similarly, the precise mechanisms underlying the synergism in cardiac reprogramming induced by combined exposure to SCPF and Oct4 remain to be determined. It is tempting to postulate that TGFβ signaling inhibition by SB431542 may play a role in destabilizing the fibroblast phenotype (e.g., promoting MET) and blocking endoderm induction (Chambers et al., 2009; Li et al., 2010; Lin et al., 2009). Wnt signaling activation by CHIR99021 may promote initial reprogramming (Li et al., 2009) and cardiac mesoderm induction (Lian et al., 2012). In addition, as shown in figure S4, we found Wnt3a has a similar effect as CHIR99021. Importantly, XAV939, which selectively blocks Wnt/β-catenin-mediated transcription through tankyrase1/2 inhibition, strongly blocked the cardiac enhancing effect of CHIR99021, suggesting CHIR99021’s effect is mainly dependent on Wnt signaling downstream activity. Furthermore, increased H3K4 methylation by parnate may enhance initial epigenetic activation of the fibroblasts (Li et al., 2009), and forskolin, which activates adenylate cyclase to increase intracellular cAMP levels (Hanna et al., 2010), may further enhance cardiac reprogramming through downstream chromatin-remodeling mechanisms. Future mechanistic investigations will yield greater insight into the role of these signaling pathways and perhaps identify other targets for small-molecule intervention that further enhance cardiac reprogramming efficiency.
EXPERIMENTAL PROCEDURES
Cardiac reprogramming
MEFs and TTFs were originally passaged onto gelatin-coated tissue culture plates. Cells were incubated with lentivirus for 12h and then cultured in MEF-GM. After 3–5 days of recovery, cells were replated onto Matrigel (BD Biosciences)-coated plates at 5000 cells per cm2. The next day, infected MEFs were switched to cardiac reprogramming media (CRM) containing knockout DMEM with 5% knockout serum replacement, 15% ES cell-qualified FBS, 1% Glutamax, 1% nonessential amino acids, and 0.1 mM β-mercaptoethanol, with or without small molecules. For TetO-OKS-induced cardiac reprogramming, 2 μg/ml DOX was also added to CRM for 6 days and then the culture medium was changed to CDM containing RPMI-1640 supplemented with 0.5X N2 supplement, 1X B27 supplement (without vitamin A), 0.05% BSA (bovine serum albumin) fraction V, 0.5% Glutamax, and 0.1 mM β-mercaptoethanol (all from Invitrogen). 20 ng/ ml of BMP4 (Stemgent) was added into CDM for the first 5 days. For Oct4 induced cardiac reprogramming, cells were replated at a ratio of 1:6 at day 8 and kept cultured in CRM with small molecules until day 15, then switched to CDM and treated with BMP4 for the first 5 days. All small molecules were added to cells during culture in CRM. The following small molecules were used in this paper: 2 μM SB431542, 10 μM CHIR99021, 2 μM parnate, and 10 μM forskolin.
Supplementary Material
Highlights.
Small molecules enable cardiac reprogramming with Oct4
Induced cardiomyocytes pass through cardiac progenitor stage.
Induced cardiomyocytes exhibit cardiac specific features and spontaneously beat.
Most of induced cardiomyocytes are ventricular-like cells.
Acknowledgments
We are grateful to all the members of the DING lab for critical discussions and comments on the manuscript. We also thank Dr. Gustavo Mostoslavsky for providing the plasmid of pHAGE2-TetO-STEMCCA-redlight and Dr. Anna Lisa Lucido for editing of this manuscript. S.D. is supported by funding from California Institute for Regenerative Medicine, NICHD, NHLBI, NEI/NIH, the Roddenberry Foundation, the William K. Bowes, Jr. Foundation, and the Gladstone Institutes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JT, Scholer HR. Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells. 2004;22:941–949. doi: 10.1634/stemcells.22-6-941. [DOI] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nature Cell Biology. 2011;13:215–U261. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilcove SA. Small Molecules, Regeneration, and Cell Fate: A Thesis Presented. Scripps Research Institute; La Jolla, California: 2010. [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Srivastava D. Genetics of human cardiovascular disease. Cell. 2012;148:1242–1257. doi: 10.1016/j.cell.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Li Ke, Z S, Russ Holger A, Xu Shaohua, Xu Tao, Zhang Yu, Ma Tianhua, Hebrok Matthias, Ding Sheng. Small Molecules Facilitate the Reprogramming of Mouse Fibroblasts into Pancreatic Lineages. cell stem cell. 2014 doi: 10.1016/j.stem.2014.01.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Rapila R, Ronkainen V-P, Koivumäki JT, Tavi P. Local Ca2+ releases enable rapid heart rates in developing cardiomyocytes. The Journal of physiology. 2010;588:1407–1417. doi: 10.1113/jphysiol.2009.185173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmenkin A, Liang H, Xu G, Pfannkuche K, Eichhorn H, Fatima A, Luo H, Saric T, Wernig M, Jaenisch R, et al. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 2009;23:4168–4180. doi: 10.1096/fj.08-128546. [DOI] [PubMed] [Google Scholar]
- Li J, Huang NF, Zou J, Laurent TJ, Lee JC, Okogbaa J, Cooke JP, Ding S. Conversion of Human Fibroblasts to Functional Endothelial Cells by Defined Factors. Arterioscler Thromb Vasc Biol. 2013a;33:1366–1375. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Huang NF, Zou J, Laurent TJ, Lee JC, Okogbaa J, Cooke JP, Ding S. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013b;33:1366–1375. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Scholer HR, Hayek A, Ding S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Q, Yin X, Yang W, Du Y, Hou P, Ge J, Liu C, Zhang W, Zhang X, et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848–1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu DK, Liu J, Siu C-W, McNerney GP, Tse H-F, Abu-Khalil A, Huser T, Li RA. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem cells and development. 2009;18:1493–1500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Liu H, Huang CT-L, Chen H, Du Z, Liu Y, Sherafat MA, Zhang S-C. Generation of Integration-free and Region-Specific Neural Progenitors from Primate Fibroblasts. Cell reports. 2013 doi: 10.1016/j.celrep.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie B, Wang H, Laurent T, Ding S. Cellular reprogramming: a small molecule perspective. Curr Opin Cell Biol. 2012;24:784–792. doi: 10.1016/j.ceb.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Martinez I, Baek SH, Belmonte JCI. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol. 2012;14:892–899. doi: 10.1038/ncb2567. [DOI] [PubMed] [Google Scholar]
- Sauer H, Theben T, Hescheler J, Lindner M, Brandt MC, Wartenberg M. Characteristics of calcium sparks in cardiomyocytes derived from embryonic stem cells. American Journal of Physiology-Heart and Circulatory Physiology. 2001;281:H411–H421. doi: 10.1152/ajpheart.2001.281.1.H411. [DOI] [PubMed] [Google Scholar]
- Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu H, Nagao T, Ichijo H, Adachi-Akahane S. L-type Ca2+ channels serve as a sensor of the SR Ca2+ for tuning the efficacy of Ca2+-induced Ca2+ release in rat ventricular myocytes. The Journal of physiology. 2003;552:415–424. doi: 10.1113/jphysiol.2003.050823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nothen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Willems E, Cabral-Teixeira J, Schade D, Cai W, Reeves P, Bushway PJ, Lanier M, Walsh C, Kirchhausen T, Izpisua Belmonte JC, et al. Small molecule-mediated TGF-beta type II receptor degradation promotes cardiomyogenesis in embryonic stem cells. Cell Stem Cell. 2012;11:242–252. doi: 10.1016/j.stem.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- Yuan X, Wan H, Zhao X, Zhu S, Zhou Q, Ding S. Brief report: combined chemical treatment enables Oct4-induced reprogramming from mouse embryonic fibroblasts. Stem Cells. 2011;29:549–553. doi: 10.1002/stem.594. [DOI] [PubMed] [Google Scholar]
- Zhu S, Ambasudhan R, Sun W, Kim HJ, Talantova M, Wang X, Zhang M, Zhang Y, Laurent T, Parker J, et al. Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 2014;24:126–129. doi: 10.1038/cr.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wei W, Ding S. Chemical strategies for stem cell biology and regenerative medicine. Annu Rev Biomed Eng. 2011;13:73–90. doi: 10.1146/annurev-bioeng-071910-124715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.