Abstract
The tyrosine kinase Janus kinase 2 (JAK2) binds to the majority of the known members of the cytokine family of receptors. Ligand-receptor binding leads to activation of the associated JAK2 molecules, resulting in rapid autophosphorylation of multiple tyrosines within JAK2. Phosphotyrosines can then serve as docking sites for downstream JAK2 signaling molecules. Despite the importance of these phosphotyrosines in JAK2 function, only a few sites and binding partners have been identified. Using two-dimensional phosphopeptide mapping and a phosphospecific antibody, we identified tyrosine 813 as a site of JAK2 autophosphorylation of overexpressed JAK2 and endogenous JAK2 activated by growth hormone. Tyrosine 813 is contained within a YXXL sequence motif associated with several other identified JAK2 phosphorylation sites. We show that phosphorylation of tyrosine 813 is required for the SH2 domain-containing adapter protein SH2-Bβ to bind JAK2 and to enhance the activity of JAK2 and STAT5B. The homologous tyrosine in JAK3, tyrosine 785, is autophosphorylated in response to interleukin-2 stimulation and is required for SH2-Bβ to bind JAK3. Taken together these data strongly suggest that tyrosine 813 is a site of autophosphorylation in JAK2 and is the SH2-Bβ-binding site within JAK2 that is required for SH2-Bβ to enhance activation of JAK2.
The Janus kinase family of tyrosine kinases (JAK1, JAK2, JAK3, and Tyk2) plays an essential role in the signaling by all members of the cytokine receptor superfamily. The JAKs promote growth, proliferation, and/or differentiation of many cell types (1, 15). Activation of the JAKs occurs upon ligand binding to its receptor. Activation is thought to occur as a consequence of two JAK molecules being brought into close enough proximity to allow for rapid trans-phosphorylation of the activation loop of each kinase (15). The activated JAK molecules then phosphorylate multiple targets, including the JAKs themselves, the associated receptors, and multiple signaling molecules such as the signal transducers and activators of transcription (STATs) (4, 10, 16, 17). Dysregulation of JAKs can lead to a host of physiological problems, including diseases of the immune system (6) and cancer (22, 29, 40).
Among the JAKs, JAK2 is activated by more than two-thirds of the known cytokine receptor ligands, including growth hormone (GH), prolactin, erythropoietin, and leptin, making it the most studied of the JAK family members (14). Autophosphorylation of JAK2 is an important step in regulating signaling as it leads to activation of the kinase. Autophosphorylation also results in the production of potential docking sites for downstream signaling molecules containing phosphotyrosine binding Src homology 2 (SH2) domains, such as the adapter protein, SH2-B, and the JAK2 inhibitor suppressor of cytokine signaling 1 (SOCS-1). Identification of the autophosphorylation sites on JAK2, therefore, is critical for advancing our understanding of how these kinases signal, and could provide potential targets for pharmaceutical intervention. To date, while JAK2 has 49 tyrosines and is highly phosphorylated (3, 36, 39), only two tyrosines have been identified as binding sites for other proteins. Tyrosine 1007, which is located in the activation loop of the kinase domain, is critical for full activation of JAK2 and has been shown to bind SOCS-1 (43) and to serve as a substrate for protein tyrosine phosphatase-1B (PTP-1B) (26). Tyrosine 966 has been shown to bind p70, an SH3 domain containing protein of unknown function (7). To gain additional insight into the function and/or regulation of JAK2, we sought both to identify additional tyrosines present in JAK2 that undergo autophosphorylation and to establish a function for these phosphorylated tyrosines.
Here we show that tyrosine 813 of JAK2 is a site of autophosphorylation. In contrast to most of the previously identified phosphorylated tyrosines—including 221, 570, 1007, and 1008, which regulate kinase activity—phosphorylation of tyrosine 813 seems not to affect the intrinsic activity of JAK2. Rather, we show that tyrosine 813 is required for JAK2 to bind the β splicing variant of SH2-B, and for the ability of SH2-Bβ to enhance JAK2 activation as well as increase JAK2-mediated phosphorylation of STAT5B.
MATERIALS AND METHODS
Reagents.
The QuikChange mutagenesis kit was from Stratagene. [γ-32P]ATP (6,000 Ci/mmol) was from ICN. Methylated trypsin was from Promega. Thin-layer chromatography plates were from EM Science. Bovine serum albumin (CRG-7) was from Intergen. Dulbecco's Modified Eagle Medium (DMEM) and phosphate-free DMEM was from Invitrogen. Recombinant protein A-agarose was from Repligen. Aprotinin, leupeptin, and Triton X-100 were from Roche. The enhanced chemiluminescence detection system, nitrocellulose paper, and horseradish peroxidase-conjugated protein A (used at a dilution of 1:7,500) were from Amersham Pharmacia Biotech. Protein molecular weight standards and horseradish peroxidase-conjugated anti-mouse immunoglobulin G (used at a dilution of 1:7,500) and anti-rabbit immunoglobulin G (used at a dilution of 1:7,500) were from Santa Cruz. AlexaFluor680 anti-rabbit and IR800 anti-mouse antibodies were obtained from Molecular Probes and used at a dilution of 1:20,000. Polyvinylpyrrolidone and phosphoamino acid standards were from Sigma. X-ray film was from Kodak. Anti-JAK2 antiserum was raised in rabbits against a synthetic peptide corresponding to amino acids 758 to 776. The anti-JAK2 used for immunoprecipitation was prepared in conjunction with Pel-Freez Biologicals (3) and was used at a dilution of 1:250. Antibody to JAK2 used for immunoblotting was from Upstate Biotechnology, Inc., and used at a dilution of 1:10,000. Antibody recognizing a peptide containing phosphorylated tyrosines 1007 and 1008 of JAK2 [anti-P(Y1007)-JAK2] was kindly provided by Martin Myers (Harvard, Boston, Mass.). An antibody that recognizes the peptide CLNSLFTPD[pY]EL, containing phosphorylated tyrosine 813 [anti-P(Y813)-JAK2], was developed in conjunction with Upstate USA, Inc. Anti-JAK3 antibody was raised in rabbits against a synthetic peptide corresponding to amino acids 1104 to 1124 of human JAK3 (19) and was used at a dilution of 1:3,000 for immunoblotting. Anti-phospho-JAK3 antibody (anti-P-JAK3) was raised in rabbits against the peptide SLISSD[pY]ELLSDP, containing phosphorylated tyrosine 785 and used for immunoblotting at a dilution of 1:1,000. Monoclonal antibody against myc-tag (9E10; anti-myc) and polyclonal anti-STAT5B antibody raised against amino acids 711 to 727 of murine STAT5B (anti-STAT5B) were obtained from Santa Cruz Biotechnology, Inc. Anti-myc was used at dilutions of 1:100 for immunoprecipitation and 1:10,000 for immunoblotting. Anti-STAT5B was used at a dilution of 1:5,000 for immunoblotting. Monoclonal antibody to phosphorylated tyrosine 699 of STAT5B (anti-P-STAT5) was obtained from Zymed Laboratories Inc. and used at a 1:7,500 dilution in immunoblotting. Antiphosphotyrosine antibody 4G10 (anti-PY) was obtained from Upstate USA, Inc., and was used at 1:7,500 for immunoblotting. Anti-FLAG M2 Affinity gel (product number A1205) was from Sigma.
Plasmids.
The mammalian expression vector prk5 encoding wild-type murine JAK2 or kinase-inactive murine JAK2(K882E), in which the critical lysine in the ATP binding domain is mutated to glutamate, were generously provided by J. Ihle and B. Witthuhn (St. Jude Children's Research Hospital). The above JAK2 plasmid was used to create JAK2(Y570F) and JAK2(Y813F) via site-directed mutagenesis using the QuikChange mutagenesis kit from Stratagene. DNAs encoding FLAG-tagged JAK2(797-1129), containing amino acids 797 to 1129 of JAK2, and FLAG-tagged JAK2(830-1129), containing amino acids 830 to 1129, were created by first mutating base pairs in the JAK2 coding sequence from GCTTTC to GgaTcC (mutated bases lowercase) for FLAG-JAK2(797-1129) and from GGTGCC to GGatCC for FLAG-JAK2(830-1129) and then subcloning the JAK2 fragment into a PCMV-tag2B expression vector. JAK2 amino acid numbers are numbered according to NCBI accession number NP_032439. Plasmid encoding rat STAT5B was from L. Yu-Lee (Baylor College of Medicine). Construction of the vector encoding SH2-Bβ with a myc tag at the N terminus (32) and the vector encoding myc-SH2-Bβ(504-670) (33) have been described previously. SH2-Bβ amino acid numbers are numbered according to accession number AAC04575.
Cell culture and transfection.
The stock of murine 3T3-F442A fibroblasts was kindly provided by H. Green (Harvard University). 3T3-F442A cells and 293T cells were grown in DMEM supplemented with 1 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 0.25 μg of amphotericin per ml, and 8% calf serum. COS7 cells were grown in the same medium supplemented with 8% fetal bovine serum rather than 8% calf serum. 3T3-F442A cells were incubated overnight in serum-free medium containing 1% bovine serum albumin before adding GH. Both 293T cells and COS7 cells were transiently transfected using calcium phosphate precipitation (8). At 6 h (293T cells) or 16 h (COS7 cells) after transfection, cells were washed three times and incubated with their respective medium. 293T cells were used 24 h posttransfection, while COS7 cells were used 48 h posttransfection.
Immunoprecipitation and immunoblotting.
At 48 h after transfection, COS7 cells were washed three times in chilled PBSV (10 mM sodium phosphate, 137 mM NaCl, 1 mM Na3VO4 [pH 7.4]) and solubilized in lysis buffer (50 mM Tris [pH 7.5], 0.1% Triton X-100, 150 mM NaCl, 2 mM EGTA, 1 mM Na3VO4, [pH 7.5]), containing 1 mM phenylmethylsulfonyl fluoride, aprotinin (10 μg/ml), and leupeptin (10 μg/ml). Cell lysates were centrifuged at 16,750 × g for 10 min. The supernatant was incubated with the indicated antibody on ice for 2 h. The immune complexes were collected on protein A-agarose (40 μl) during 1 h of incubation at 4°C. The beads were washed three times with lysis buffer and boiled for 5 min in a mixture (80:20) of lysis buffer and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (250 mM Tris-HCl [pH 6.8], 10% SDS, 10% β-mercaptoethanol, 40% glycerol, 0.01% bromophenol blue). The solubilized proteins were separated by SDS-PAGE (5-to-12% gradient gels). Proteins in the gel were transferred to a nitrocellulose membrane and detected by immunoblotting with the indicated antibody using enhanced chemiluminescence or by using ODYSSEY Infrared Imaging System (LI-COR Biosciences) software (see Fig. 4B and 6B only). In some experiments, membranes were incubated in a solution of 100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl (pH 6.8) at 50° for 20 min and then reprobed with a separate antibody.
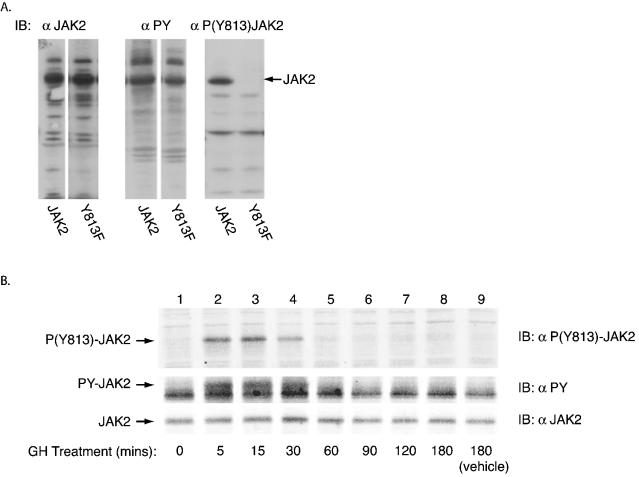
FIG. 4.
Autophosphorylation of JAK2 at tyrosine 813 in 3T3-F442A cells in response to GH. (A) 293T cells were transfected with cDNAs encoding either JAK2 or JAK2 with tyrosine 813 mutated to phenylalanine (Y813F). Cell lysates were immunoblotted with either anti-JAK2, anti-PY, or anti-P(Y813)JAK2. (B) 3T3-F442A cells were treated either with vehicle for 0 (lane 1) or 180 (lane 9) min or with GH (500 ng/ml) for 5, 15, 30, 60, 90, 120, or 180 min (lanes 2 through 8, respectively). Cell lysates were immunoblotted with either anti-P(Y813)-JAK2 (top panel), anti-PY (middle panel), or anti-JAK2 (bottom panel).
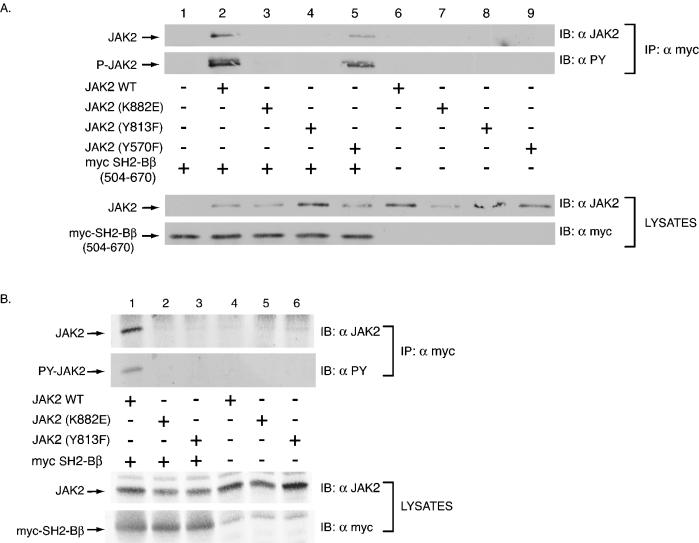
FIG. 6.
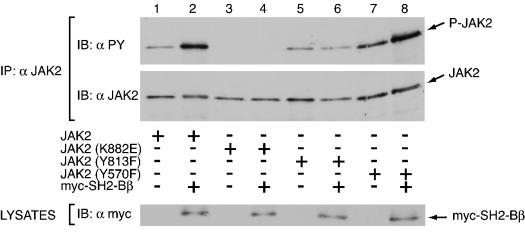
Tyrosine 813 is required for JAK2 to bind to SH2-Bβ. (A) COS7 cells were transfected with empty vector (lane 1) or 1.0 μg of cDNA encoding either JAK2 (lanes 2 and 6), JAK2(K882E) (lanes 3 and 7), JAK2(Y813F) (lanes 4 and 8), or JAK2(Y570F) (lanes 5 and 9) along with (lanes 1 to 5) or without (lanes 6 to 9) 2.0 μg of cDNA encoding myc-SH2-Bβ(504-670). myc-SH2-Bβ(504-670) was immunoprecipitated using anti-myc and immunoblotted using anti-JAK2 (first panel) and anti-PY (second panel). Lysates of the above transfected cells were immunoblotted with anti-JAK2 (third panel) and anti-myc (fourth panel) to assess levels of expression of JAK2 and myc-SH2-Bβ(504-670), respectively. (B) COS7 cells were transfected with 1.0 μg of cDNA encoding either JAK2 (lanes 1 and 4), JAK2(K882E) (lanes 2 and 5), or JAK2(Y813F) (lanes 3 and 6) along with (lanes 1 to 3) or without (lanes 4 to 6) 2.0 μg cDNA encoding myc-SH2-Bβ. Myc-SH2-Bβ was immunoprecipitated using anti-myc and immunoblotted using anti-JAK2 (first panel) and anti-PY (second panel). Lysates of the above transfected cells were immunoblotted with anti-JAK2 (third panel) and anti-myc (fourth panel) to assess levels of expression of JAK2 and myc-SH2-Bβ, respectively.
In vitro kinase assay.
For JAK2, in vitro kinase assays were performed as described previously (3, 36). Briefly, cells were washed with phosphate-buffered saline and solubilized in lysis buffer containing 1 mM phenylmethylsulfonyl fluoride, aprotinin (10 μg/ml), and leupeptin (10 μg/ml). Cell lysates were incubated with anti-FLAG affinity gel or anti-JAK2 as appropriate. The anti-JAK2 was precipitated using protein A-agarose. The immune complexes were washed with lysis buffer and then with kinase buffer (50 mM HEPES, 100 mM NaCl, 5 mM MnCl2, 0.5 mM dithiothreitol, 1 mM Na3VO4 [pH 7.6]). The immobilized JAK2 was incubated in kinase buffer containing 0.5 mCi of [γ-32P]ATP, aprotinin (40 μg/ml), and leupeptin (40 μg/ml) at 30°C for 30 min, washed five times with lysis buffer, and eluted by boiling in a mixture (80:20) of lysis buffer and SDS-PAGE sample buffer. Proteins were resolved by SDS-PAGE (5-to-12% gradient), transferred to nitrocellulose, and visualized by autoradiography. For JAK3, in vitro kinase assays were performed as described previously (45). Briefly, cell lysates were incubated with anti-JAK3, and the JAK3 immunoprecipitates were washed once with 100 mM NaCl and 10 mM HEPES, pH 7.5, and resuspended in JAK3 kinase reaction buffer (20 mM Tris [pH 7.5], 5 mM MgCl2, 5 mM MnCl2, 1 μM ATP) containing 10 μCi of [γ-32P]ATP (Amersham). The reactions were performed at room temperature for 10 min. The reactions were terminated by addition of lysis buffer containing 100 mM EDTA. The JAK3 immunoprecipitates were also washed once with ice-cold lysis buffer. Beads were boiled in sample buffer, and the proteins were separated by SDS-PAGE and transferred to nitrocellulose (Schleicher & Schuell), after which phosphopeptide mapping was performed.
Phosphopeptide mapping and phosphoamino acid analysis.
For JAK2, two-dimensional (2-D) phosphopeptide mapping and phosphoamino acid analysis were performed as previously described (5). Briefly, 32P-labeled JAK2 was cut from the nitrocellulose, digested with 5 μg of sequencing grade methylated trypsin at 37°C for 4 h, and oxidized with performic acid. Peptides were separated by thin-layer electrophoresis at pH 1.9 followed by thin-layer chromatography using phosphochromatography buffer (5). The 32P-labeled spots were visualized using a phosphorimager (Bio-Rad model 505). For phosphoamino acid analysis, 32P-labeled peptides were scraped from the cellulose plate and eluted with pH 1.9 buffer (5). Eluted peptides were mixed with phosphoamino acid standards, subjected to acid hydrolysis in 6 N HCl at 110°C for 60 min and resolved by thin-layer electrophoresis at 1,000 V at pH 3.5. Phosphoamino acid standards were visualized by ninhydrin and 32P-labeled spots were visualized with a phosphorimager.
For JAK3, reagents for peptide mapping were purchased from J. T. Baker (Phillipsburg, N.J.) and peptide mapping was performed as described previously (45). Briefly, samples were electrophoresed on polyacrylamide gels, transferred to nitrocellulose, and exposed to X-ray film. The band containing JAK3 was excised and blocked with 1% polyvinylpyrrolidone in 100 mM acidic acid for 1 h at 37°C, washed three times with digestion buffer (1% NH4CO3, pH 8.4), and digested with 0.5 μg of trypsin overnight at 37°C. Peptides were recovered from the supernatant and dried in a vacuum centrifuge. The dried precipitates were washed and resuspended in 5 μl of H2O then subjected to high-voltage electrophoresis and thin-layer chromatography (TLC). Tryptic peptides were separated in the first dimension with pH 4.72 buffer by high-voltage electrophoresis by using a HTLE-7000 electrophoretic apparatus (C.B.S. Scientific, Del Mar, Calif.) for 1 h at 1.0 kV. Separation in the second dimension was performed by TLC using butanol (5%)-pyridine (2.5%)-acetic acid (2.5%)-deionized water (90%) for 6 h. 32P-labeled peptides were visualized by autoradiography.
RESULTS
Tyrosine 813 in JAK2 is phosphorylated in vitro.
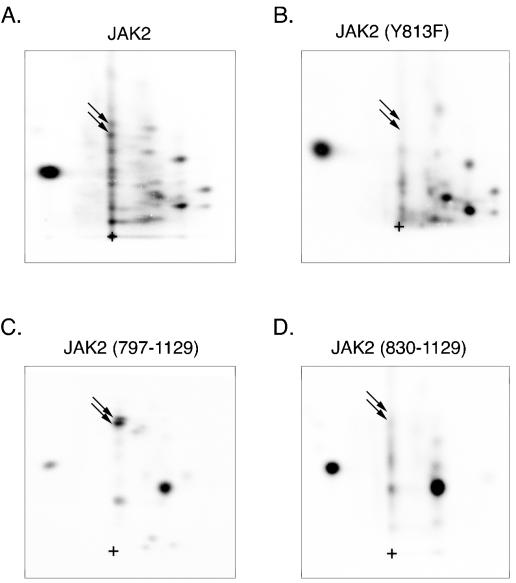
We have used mass spectroscopy and phosphopeptide mapping to identify two tyrosines (Tyr 221 and Tyr 570) that lie in YXXL motifs as autophosphorylation sites in JAK2 (3a). That finding, and an analysis of identified and hypothesized JAK2 target sites, suggested that other YXXL motifs might be sites of autophosphorylation. We therefore examined whether tyrosine 813 (YELL), lying within the pseudokinase domain (JAK homology domain 2 [JH2]) of JAK2 might be a site of autophosphorylation. Wild type JAK2 and JAK2(Y813F) were overexpressed in human epithelial kidney 293T cells, highly purified by immunoprecipitation using anti-JAK2, phosphorylated in vitro in the presence of [γ-32P]ATP, and subjected to 2-D peptide mapping. When tyrosine 813 in JAK2 was mutated to phenylalanine, two spots that were present in the map of wild-type JAK2 (indicated by the arrows in Fig. 1A) were absent in the 2-D peptide map of JAK2(Y813F) (Fig. 1B), suggesting that tyrosine 813 in JAK2 is autophosphorylated. To substantiate further that tyrosine 813 in JAK2 is phosphorylated, FLAG-tagged JAK2(797-1129) and FLAG-tagged JAK2(830-1129) were subjected to 2-D peptide mapping. The only tyrosine present in JAK2(797-1129) and absent in JAK2(830-1129) is tyrosine 813. Two closely migrating spots are present in the 2-D peptide map of FLAG-tagged JAK2(797-1129) (Fig. 1C) whose migrations correspond to the migrations of the peptides containing tyrosine 813 in the map of wild-type JAK2 (Fig. 1A). As would be expected if tyrosine 813 were a site of autophosphorylation, when the region containing tyrosine 813 is deleted (Fig. 1D), the spots corresponding to the peptides containing tyrosine 813 disappear [see the peptide map of FLAG-tagged JAK2(797-1129)]. To rule out the possibility that the 32P incorporated into the spots associated with tyrosine 813 is due to Ser/Thr phosphorylation by a contaminating Ser/Thr kinase, phosphoamino acid analysis was performed to substantiate that the spots associated with tyrosine 813 in both full-length JAK2 (Fig. 2, lane 1) and JAK2(797-1129) (Fig. 2, lanes 2 and 3) are primarily phosphorylated on tyrosine. Taken together these data provide strong evidence that JAK2 autophosphorylates tyrosine 813.
FIG. 1.
JAK2 is autophosphorylated on tyrosine 813 in vitro. 293T cells expressing the cDNA for JAK2 (A), JAK2 Y813F (B), FLAG-JAK2(797-1129) (C), or FLAG-JAK2(830-1129) (D) were lysed and immunoprecipitated with either anti-JAK2 (A and B) or anti-FLAG (C and D). The immobilized JAK2 was incubated in the presence of [γ-32P]ATP at 30°C for 30 min. The 32P-labeled JAK2 was cut from the nitrocellulose and subjected to 2-D peptide mapping with the thin-layer electrophoresis step performed at pH 1.9 (5). The arrows in the 2-D peptide maps indicate the two spots present in JAK2 (A) and FLAG-JAK2(797-1129) (C) that disappear when tyrosine 813 in JAK2 is mutated to phenylalanine in JAK2(Y813F) (B) and when the region between amino acid 797 and 830 of FLAG-JAK2(797-1129), which contains tyrosine 813, is deleted in FLAG-JAK2(813-1129) (D). The origin (+) is indicated.
FIG. 2.
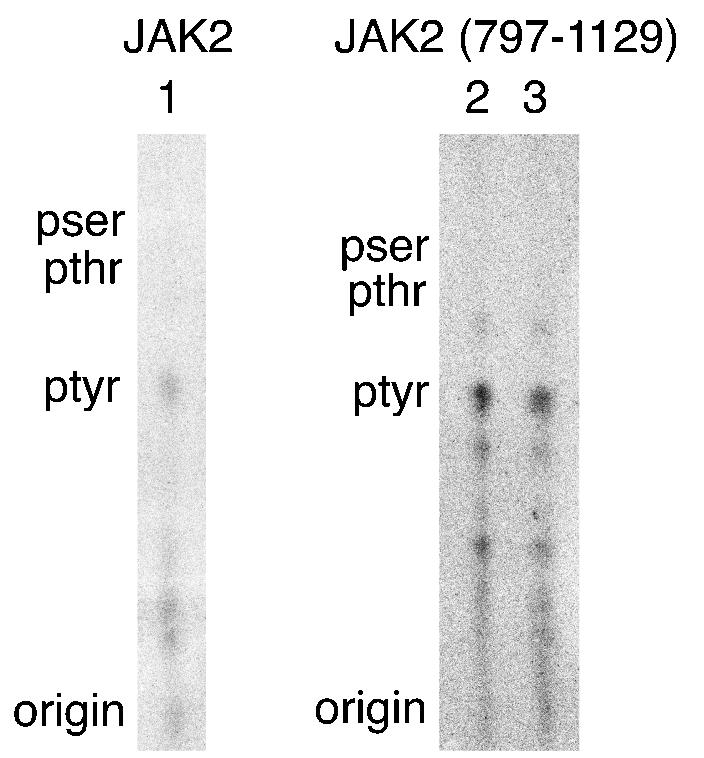
The peptide containing tyrosine 813 is autophosphorylated on tyrosine. 32P-labeled peptides in 2-D peptide maps corresponding to the lower of the two spots associated with tyrosine 813 of JAK2 (lane 1), and the upper (lane 2) and lower (lane3) spot associated with tyrosine 813 in JAK2(797-1129) were scraped from the cellulose plates and subjected to phosphoamino acid analysis. The origin and the migration of phosphotyrosine (ptyr), phosphothreonine (pthr), and phosphoserine (pser) standards are indicated. Spots seen below ptyr are due to incomplete acid hydrolysis of the peptide.
Two spots associated with tyrosine 813 are detected in the 2-D peptide maps of JAK2, one above the other. Boyle et al. (5) indicate that the presence of two spots, one above the other, suggests incomplete oxidation of the methionines in the peptide during the preparation of the samples. For the spots associated with tyrosine 813 to migrate in the column of peptides above the origin in the 2-D peptide maps, the peptide must be uncharged. Trypsin digestion yields a theoretical peptide, DLNSLFTPDpY813ELLTENDMLPNMR, whose theoretical charge at pH 1.9 is +1. Each additional phosphorylation changes the charge by −1. Thus, the finding that the two spots (one above the other) migrate as uncharged peptides suggests that the peptide containing phosphorylated tyrosine 813 is also phosphorylated at either the serine or one of the threonines. When the peptide containing tyrosine 813 was isolated from the 2-D peptide maps and subjected to phosphoamino acid analysis, no 32P-labeled serine or threonine was detected. The inability to detect 32P-labeled serine/threonine phosphorylation in the in vitro labeled JAK2 is not unexpected because the JAK2 was highly purified prior to the in vitro kinase assay. Therefore, the phosphorylation of JAK2 on serine/threonine must have occurred in vivo, prior to the isolation of JAK2 from the cells, and indicates that JAK2 is a target for phosphorylation by cellular serine/threonine kinases.
Tyrosine 813 is autophosphorylated in GH-activated JAK2.
GH binding to GH receptor activates JAK2 (3). Because GH-dependent phosphorylation of JAK2 can be detected in 3T3-F442A cells that endogenously express GH receptor and JAK2, we chose this cell line to assess whether JAK2 autophosphorylation of tyrosine 813 occurs upon activation of JAK2 by GH. We stimulated 3T3-F442A cells with a 30-ng/ml concentration (1.4 nM) of GH or vehicle for 15 min. Treated cells were then solubilized and anti-JAK2 was used to immunoprecipitate JAK2. The isolated JAK2 was then subjected to an in vitro kinase assay in the presence of [γ-32P]ATP. We observed a 4.5-fold increase in the phosphorylation of JAK2 as a result of GH treatment (Fig. 3A, compare lanes 2 and 1). The 2-D map obtained using JAK2 isolated from GH-treated 3T3-F442A cells (Fig. 3B) is very similar to the 2-D peptide map of JAK2 immunoprecipitated from 293T cells (Fig. 1A). Spots in 2-D peptide maps of GH-activated JAK2 that are analogous to those in Fig. 1A that contain tyrosine 813 are indicated by the arrows in Fig. 3B. Because Fig. 3B is a 2-D peptide map of JAK2 isolated from GH-treated cells that endogenously express JAK2 and GH receptor, these results are consistent with tyrosine 813 in JAK2 being phosphorylated in GH-activated JAK2.
FIG. 3.
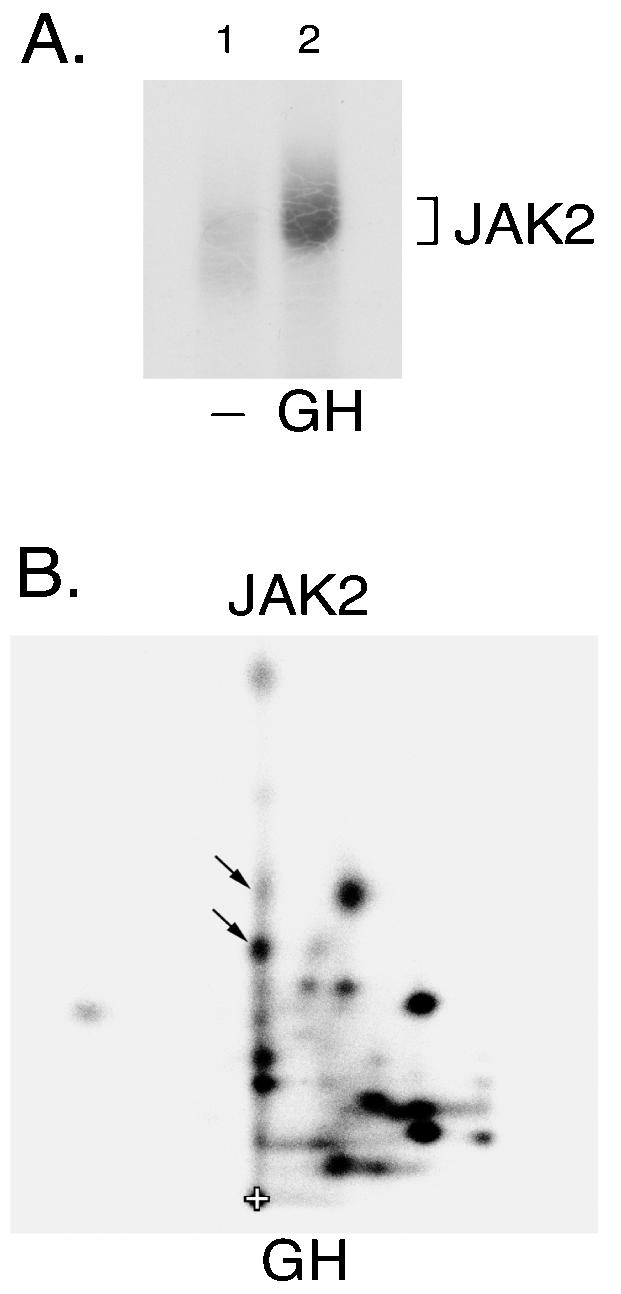
JAK2 activated in response to GH autophosphorylates tyrosine 813. (A) 3T3-F442A cells were incubated in the absence (lane 1) or presence of 30 ng of GH/ml (lane 2) for 15 min. The cells were lysed and JAK2 immunoprecipitated using anti-JAK2. JAK2 was immobilized and incubated in the presence of [γ-32P]ATP at 30°C for 30 min. The JAK2 was resolved by SDS-PAGE, transferred to nitrocellulose and visualized by autoradiography. (B) 32P-labeled JAK2 was cut from the nitrocellulose and subjected to 2-D peptide mapping with the thin-layer electrophoresis step performed at pH 1.9 (5). The origin (+), and spots whose migration are similar to spots identified in Fig. 1A are indicated.
JAK2 autophosphorylates at tyrosine 813 in response to GH.
To verify further that JAK2 autophosphorylates at tyrosine 813, when overexpressed and when stimulated by GH, an antibody specific to phosphotyrosine 813 [anti-P(Y813)-JAK2] was developed. Blotting lysates of 293T cells overexpressing either JAK2 or JAK2(Y813F) with anti-P(Y813)-JAK2 demonstrates that anti-P(Y813)-JAK2 is specific for phosphotyrosine 813 and verifies that overexpressed JAK2 is indeed phosphorylated at tyrosine 813 (Fig. 4A). To show that phosphorylation of JAK2 at tyrosine 813 in 3T3-F442A cells is a direct result of GH stimulation, 3T3-F442A cells were treated with vehicle alone for 0 or 180 min or with GH (500 ng/ml) for 5, 15, 30, 60, 90, 120, and 180 min. Immunoblotting proteins in the harvested cell lysates with anti-P(Y813)-JAK2 demonstrated that tyrosine 813 in JAK2 was phosphorylated after 5, 15, and 30 min of GH treatment; phosphorylation of tyrosine 813 returned to basal levels by 60 min, where it remained for the remainder of GH treatment. These data demonstrate that rapid and transient in vivo phosphorylation of JAK2 at tyrosine 813 occurs as a direct result of GH treatment (Fig. 4B, top panel). Immunoblotting the lysates with anti-PY demonstrated that the phosphorylation of tyrosine 813 mimics the general tyrosine phosphorylation of JAK2 upon GH stimulation (Fig. 4B, middle panel). Immunoblotting with anti-JAK2 demonstrated that these differences in JAK2 phosphorylation were not a consequence of differences in endogenous JAK2 expression (Fig. 4B, bottom panel).
Mutation of tyrosine 813 to phenylalanine does not disrupt JAK2-mediated phosphorylation of STAT5B.
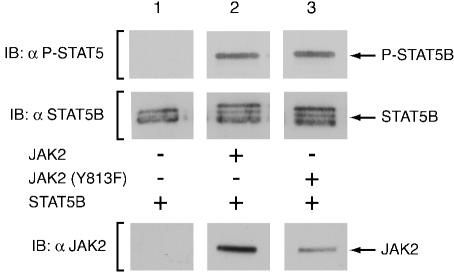
Upon establishing tyrosine 813 as a site of GH-stimulated JAK2 autophosphorylation, we sought to identify at least one of its roles in JAK2 signaling. We sought first to determine if tyrosine 813 is required for the ability of JAK2 to phosphorylate the JAK2 substrate STAT5B. Cytoplasmic STAT5B is a latent transcription factor that is recruited to phosphotyrosines on cytokine receptor/JAK complexes. Phosphorylation of STAT5B on tyrosine 699 by JAK2 enables it to dimerize via the SH2 domain of one STAT molecule and the phosphotyrosine of another. STAT5B dimers then translocate to the nucleus, bind to regulatory elements within the DNA, and regulate gene transcription (4). STAT5B was expressed alone or coexpressed with JAK2 or JAK2(Y813F) and cell lysates were prepared. As illustrated in Fig. 5, immunoblotting the cell lysates with antibody specific to phosphotyrosine 699 of STAT5B (anti-P-STAT5) demonstrated that mutation of tyrosine 813 of JAK2 to phenylalanine did not interfere with the phosphorylation of STAT5B by JAK2 (top panel). Blotting with anti-STAT5B (Fig. 5, middle panel) demonstrated that STAT5B was expressed at similar levels in each condition. Consistent with the data obtained using anti-P-STAT5, Fig. 5 reveals an additional slower-migrating band of similar intensity, in the presence of either JAK2 or JAK2(Y813F), that comigrates with P-STAT5B. The results suggest that tyrosine 813 is not essential for JAK2 to phosphorylate STAT5B.
FIG. 5.
JAK2(Y813F) is capable of phosphorylating STAT5B. Lysates were prepared of COS7 cells cotransfected with 2.0 μg of cDNA encoding STAT5B and either 0.25 μg of empty vector (lane 1) or cDNA encoding JAK2 (lane 2) or JAK2(Y813F) (lane 3). The upper panel demonstrates the degree of phosphorylation of tyrosine 699 of STAT5B as assessed by immunoblotting with anti-P-STAT5. The middle panel exhibits the expression level of STAT5B as assessed by immunoblotting with anti-STAT5B. The lower panel shows the expression level of JAK2 as assessed by immunoblotting with anti-JAK2.
Tyrosine 813 of JAK2 is important for binding of SH2-Bβ to JAK2.
SH2-Bβ was previously cloned using a yeast two-hybrid screen, and shown to be both a binding partner and substrate of active JAK2 (34). Believed to be an adapter protein, SH2-Bβ possesses three proline-rich regions and a pleckstrin homology (PH) domain in addition to its SH2 domain. In accord with its role as an adapter protein, SH2-Bβ has been shown to bind the small GTPase, Rac, in a manner dependent upon its N-terminal proline rich region, the same region required for SH2-B facilitation of GH-induced cellular motility (11). SH2-Bβ also enhances tyrosyl phosphorylation and activation of JAK2 as well as of downstream JAK2 targets such as STAT5B (32). Consequently, we were curious to determine if tyrosine 813 serves as a regulatory site within JAK2 via mediating the stimulatory effects of SH2-Bβ.
We first sought to determine if tyrosine 813 is necessary for JAK2 to interact with SH2-Bβ. SH2-Bβ has been shown to bind active JAK2 primarily via its SH2 domain (33, 34). However, full-length SH2-Bβ is capable of binding kinase-inactive JAK2 in a phosphotyrosine-independent manner via a lower-affinity JAK2-binding site that contains the PH domain of SH2-Bβ (33). To determine if the SH2 domain of SH2-Bβ binds phosphorylated tyrosine 813 of JAK2, myc-SH2-Bβ(504-670), which contains the SH2 domain but lacks the lower-affinity phosphotyrosine-independent JAK2-binding domain, was coexpressed in COS7 cells with JAK2, kinase-inactive JAK2(K882E), JAK2(Y813F), or JAK2(Y570F). JAK2(K882E)is catalytically inactive due to mutation of the critical lysine in the ATP binding site to aspartate. Binding of the SH2 domain of SH2-Bβ to JAK2 is dependent upon JAK2 autophosphorylation, thus JAK2(K882E) serves as a negative control. We chose to use tyrosine 570 as a representative of other tyrosines in JAK2 we have tested because, like tyrosine 813, tyrosine 570 is known to be a site of phosphorylation and resides within the same sequence motif, YXXL, as tyrosine 813. Myc-SH2-Bβ(504-670) was immunoprecipitated with anti-myc and the precipitated proteins were immunoblotted with anti-JAK2 (Fig. 6A, top panel) to determine if JAK2 or if the JAK2 mutants coimmunoprecipitate with SH2-Bβ(504-670). As seen in Fig. 6A, both JAK2 (lane 2) and JAK2(Y570F) (lane 5) coimmunoprecipitated with SH2-Bβ(504-670) to a similar extent. Thus, mutation of a known phosphotyrosine in JAK2, such as tyrosine 570, does not a priori disrupt the ability of JAK2 to coimmunoprecipitate with the SH2 domain of SH2-Bβ. In contrast, JAK2(Y813F) did not coimmunoprecipitate with SH2-Bβ(504-670) (Fig. 6A, lane 4), strongly implicating tyrosine 813 as being required for the interaction between JAK2 and the SH2 domain of SH2-Bβ. Failure of JAK2(Y813F) to coprecipitate with myc-SH2-Bβ(504-670) is not believed to be due to a general loss of tyrosine phosphorylation of JAK2, as JAK2(Y813F) phosphorylation under these conditions is similar to that of wild-type JAK2 (see Fig. 8 and 9). The negative control JAK2(K882E), which is not phosphorylated on tyrosines, also did not coimmunoprecipitate with myc-SH2-Bβ(504-670) (Fig. 6A, lane 3), indicating the phosphotyrosine dependence of JAK2 to coimmunoprecipitate with myc-SH2-Bβ(504-670). Expression of SH2-Bβ(504-670) without JAK2 (Fig. 6A, lane 1), or of various forms of JAK2 without SH2-Bβ(504-670) (Fig. 6A, lanes 6 through 9) show that coimmunoprecipitation of JAK2 with the SH2 domain of SH2-Bβ required expression of both the SH2-Bβ mutant and JAK2. Reprobing the membrane with anti-PY (Fig. 6A, second panel) further demonstrated that tyrosyl phosphorylated JAK2 and JAK2(Y570F) but not tyrosyl phosphorylated JAK2(Y813F) coimmunoprecipitated with myc-SH2-Bβ(504-670). Blotting the cell lysates with anti-JAK2 and anti-myc to detect myc-SH2-Bβ(504-670) (Fig. 6A, third and fourth panels, respectively) revealed that the inability of SH2-Bβ to coprecipitate JAK2(Y813F) was not due to a decreased level of expression of JAK2(Y813F) compared to JAK2, or to a decreased expression level of myc-SH2-Bβ(504-670) with JAK2(Y813F) compared to the level expressed with JAK2. To date, out of thirteen tyrosines in JAK2 tested, tyrosine 813 is the only tyrosine we have identified as a potential binding site for SH2-Bβ (data not shown).
FIG. 8.
Tyrosine 813 is required for SH2-Bβ to enhance phosphorylation of JAK2. COS7 cells were transfected with 1.0 μg of cDNA encoding JAK2 (lanes 1 and 2), JAK2(K882E) (lanes 3 and 4), JAK2(Y813F) (lanes 5 and 6), or JAK2(Y570F) (lanes 7 and 8), with (lanes 2, 4, 6, and 8) or without (lanes 1, 3, 5, and 7) 2.0 μg of cDNA encoding myc-SH2-Bβ. In the absence of myc-SH2-Bβ, cells were transfected with 2.0 μg of empty prk5 myc vector. JAK2 was immunoprecipitated (IP) with anti-JAK2 and immunoblotted with anti-PY (upper panel) and anti-JAK2 (middle panel). For the lower panel, cell lysates of the above transfected cells were immunoblotted with anti-myc to assess levels of expression of myc-SH2-Bβ.
FIG. 9.
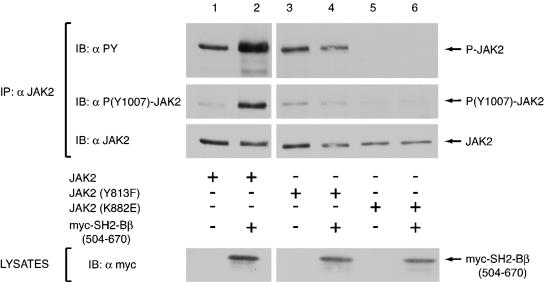
Tyrosine 813 is required for myc-SH2-Bβ(504-670) to increase the number of JAK2 molecules phosphorylated at Tyr 1007/1008. COS7 cells were transfected with 1.0 μg of cDNA for JAK2 (lanes 1 and 2), JAK2(Y813F) (lanes 3 and 4), or JAK2(K882E) (lanes 5 and 6), plus (lanes 2, 4, and 6) or minus (lanes 1, 3, and 5) 2.0 μg of cDNA encoding myc-SH2-Bβ(504-670). In the absence of myc-SH2-Bβ(504-670), cells were transfected with 2.0 μg of empty prk5 myc vector. JAK2 was immunoprecipitated with anti-JAK2 and immunoblotted with anti-PY (first panel), anti-P(Y1007)-JAK2 (second panel), and anti-JAK2 (third panel). The fourth panel shows the expression levels of myc-SH2-Bβ(504-670) as assessed by immunoblotting lysates with anti-myc.
We further sought to determine if mutation of tyrosine 813 to phenylalanine could disrupt the binding of JAK2 to full-length SH2-Bβ to confirm that tyrosine 813 is the primary binding site within JAK2 for SH2-Bβ. We expressed myc-SH2-Bβ with either JAK2, JAK2(K882E), or JAK2(Y813F) in COS7 cells; immunoprecipitated myc-SH2-Bβ from the cell lysates with anti-myc; and then immunoblotted with anti-JAK2 to detect the presence of any JAK2 that would coprecipitate with myc-SH2-Bβ (Fig. 6B). As expected, myc-SH2-Bβ was found to precipitate JAK2 to a significantly larger degree than JAK2(K882E) or JAK2(Y813F) (Fig. 6B, top panel, lanes 1 to 3), supporting the conclusion that tyrosine 813 is the major binding site in JAK2 for SH2-Bβ. Indeed, the amount of JAK2(K882E) and JAK2(Y813F) that coprecipitated with myc-SH2-Bβ appeared similar to the levels of each form of JAK2 that precipitated with anti-myc in the absence of myc-SH2-Bβ (Fig. 6B, top panel, lanes 4 to 6), suggesting that most of the JAK2(K882E) and JAK2(Y813F) seen to coimmunoprecipitate with myc-SH2-Bβ was due to nonspecific binding to either anti-myc or the protein A-agarose. Immunoblotting the cell lysates with anti-JAK2 and anti-myc (Fig. 6B, third and fourth panels, respectively) revealed that the inability of SH2-Bβ to coprecipitate JAK2(Y813F) was not due to differences in expression of the JAK2 proteins or myc-SH2-Bβ.
Tyrosine 785 of JAK3 is important for binding of SH2-Bβ.
Among the JAK family members, JAK3 is the only other JAK that possesses a YELL sequence motif. We therefore examined whether tyrosine 785 in JAK3 that corresponds to tyrosine 813 in JAK2 binds SH2-Bβ. 2-D phosphopeptide mapping was used to determine if tyrosine 785 was a site of autophosphorylation of JAK3. To this end, JAK3 or JAK3 (Y785F) were expressed in COS-7 cells, immunoprecipitated with anti-JAK3, subjected to in vitro kinase assays, and analyzed via 2-D phosphopeptide mapping. The seven most prominent spots of JAK3 have been labeled a through g (Fig. 7A). Analysis of the 2-D peptide map of JAK3 (Y785F) reveals the disappearance of spot a, implicating tyrosine 785 as a major site of phosphorylation within JAK3 (Fig. 7A). While, mutation of tyrosine 785 to phenylalanine reduces the anti-PY signal of JAK3 by about one half, consistent with tyrosine 785 being a major site of phosphorylation (data not shown) spots b through g remain present in the 2-D map of JAK3 (Y785F), indicating that mutation of tyrosine 785 does not alter the global tyrosine phosphorylation pattern of JAK3. Moreover, mutation of tyrosine 785 in JAK3 does not significantly diminish the kinase activity of JAK3 (data not shown). To verify in vivo phosphorylation at tyrosine 785 in JAK3 within COS7 cells, a phosphospecific antibody, anti-P-JAK3, was developed. Cell lysates from COS7 cells transiently transfected with cDNA for JAK3 or JAK3 (Y785F) were then immunoblotted using anti-P-JAK3. This antibody recognized JAK3 (Fig. 7B, lane 1), indicating that overexpressed JAK3 is phosphorylated at tyrosine 785 in COS7 cells. As expected, mutation of tyrosine 785 to phenylalanine dramatically reduced the signal of anti-P-JAK3 (Fig. 7B, lane 2), demonstrating the specificity of the antibody. To show that tyrosine 785 in JAK3 is phosphorylated in vivo in response to interleukin-2 (IL-2), NK 3.3 cells were deprived overnight; stimulated with IL-2 (1,000 U/ml) for 1, 5, 15, or 30 min; and then immunoprecipitated with anti-JAK3 (Fig. 7C). As shown previously (44) immunoblotting the immunoprecipitated proteins with anti-PY shows that JAK3 is heavily phosphorylated in response to treatment with IL-2 (Fig. 7C, top panel). Blotting with anti-P-JAK3 reveals that JAK3 was phosphorylated at tyrosine 785 after 5 min of IL-2 treatment, and remained phosphorylated for the remainder of the assay (Fig. 7C, middle panel). Immunoblotting the immunoprecipitated proteins with anti-JAK3 verified equal loading of proteins (Fig. 7C, bottom panel).
FIG. 7.
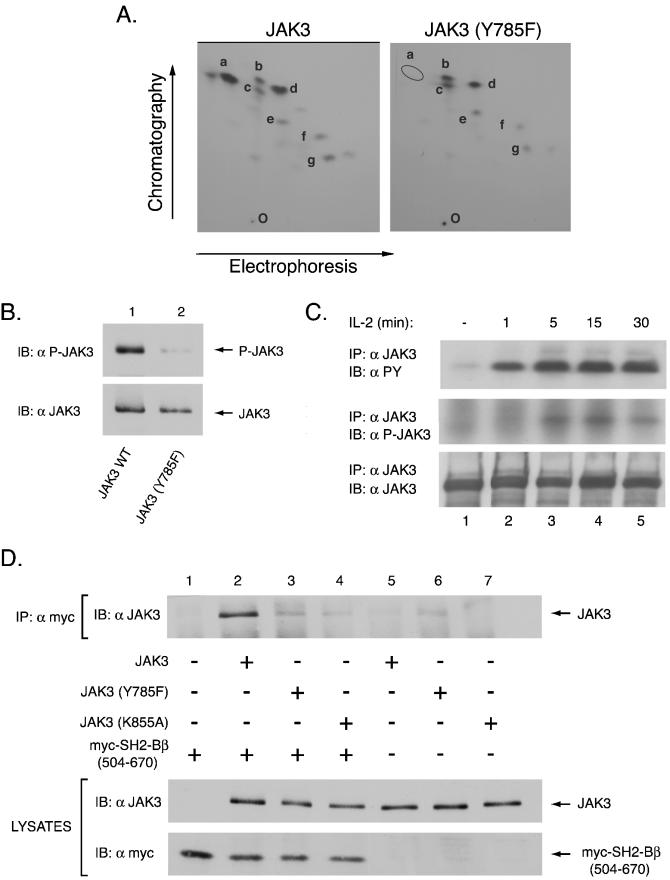
Tyrosine 785 of JAK3 is autophosphorylated and binds the SH2 domain of SH2-Bβ. (A) cDNAs encoding JAK3 or the mutant Y785F were transfected into COS-7 cells, and the expressed protein was immunoprecipitated with anti-JAK3, followed by subjection to in vitro kinase assays. The phosphorylated proteins were subjected to SDS-PAGE, transferred to nitrocellulose, and subjected to autoradiography. The portions of the membrane containing wild-type and mutant JAK3 proteins were excised and digested in situ with trypsin. Eluted tryptic phosphopeptides were analyzed by 2-D peptide mapping. The 32P-labeled phosphopeptides were visualized by autoradiography, and the most prominent spots were designated a to g. The orientation of the positive and negative electrodes during electrophoresis is indicated along with the direction of chromatography; the origin is indicated by the letter O. (B) COS7 cells were transfected with 1.0 μg of cDNA encoding either JAK3 (lane 1) or JAK3 with a phenylalanine substitution at tyrosine 785 (lane 2). Lysates of the transfected cells were immunoblotted with anti-P-JAK3 (top panel) which is specific for phosphorylation of JAK3 at tyrosine 785 and anti-JAK3 (lower panel) to assess protein expression. (C) NK 3.3 cells were starved overnight and incubated with IL-2 (1,000 U/ml) at indicated time points (lanes 2 to 5). Cell lysates were immunoprecipitated with anti-JAK3. The immunoprecipitated proteins were analyzed by SDS-PAGE, transferred to membrane and immunoblotted with anti-PY (top panel), with anti-P-JAK3 (middle panel) or with anti-JAK3 to assess protein loading (bottom panel). (D) Aliquots (1.0 μg) of empty vector (lane 1) or cDNA encoding either JAK3 (lanes 2 and 5), JAK3 (Y785F) (lanes 3 and 6), or JAK3 (K855A) (lanes 4 and 7) were transfected into COS7 cells with (lanes 1 to 4) or without (lanes 5 to 7) 2.0 μg of cDNA encodes myc-SH2-Bβ(504-670). Myc-SH2-Bβ(504-670) was immunoprecipitated using anti-myc and immunoblotted with anti-JAK3 (top panel). Cell lysates were immunoblotted with anti-JAK3 (middle panel) and anti-myc (bottom panel) to assess levels of expression of JAK3 and myc-SH2-Bβ(504-670), respectively.
To determine if SH2-Bβ binds to tyrosine 785 of JAK3, SH2-Bβ(504-670) was expressed with JAK3, JAK3 (Y785F), or kinase-inactive JAK3 (K855A). Myc-SH2-Bβ(504-670) was immunoprecipitated with anti-myc, and immunoprecipitated proteins were immunoblotted with anti-JAK3 to detect any JAK3 that coimmunoprecipitated with myc-SH2-Bβ(504-670) (Fig. 7D, top panel). JAK3 clearly coimmunoprecipitated with SH2-Bβ(504-670) (Fig. 7D, compare lanes 2 and 5). The non-tyrosyl-phosphorylated kinase-inactive JAK3 (K855A) was found to coprecipitate only poorly with SH2-Bβ(504-670) (Fig. 7D, lane 4), suggesting that the interaction between JAK3 and SH2-Bβ is primarily mediated via a phosphotyrosine/SH2 domain interaction. A small amount of JAK3 (Y785F) coprecipitated with myc-SH2-Bβ(504-670) (Fig. 7D, lane 3). However, the relative intensity of the observed band for coprecipitated JAK3 (Y785F) was comparable to the intensity of the band for JAK3(Y785F) precipitated in the absence of myc-SH2-Bβ(504-670) (Fig. 7D, lane 6), suggesting it was nonspecifically precipitated by either the myc antibody or protein A-agarose (Fig. 7D, compare lane 3 to lanes 5 to 7). Blotting the cell lysates with anti-JAK3 and anti-myc (Fig. 7D, middle and bottom panels, respectively) revealed that the inability of myc-SH2-Bβ(504-670) to coprecipitate JAK3 (Y785F) but not JAK3 was not due to differences in levels of expression of either JAK3 or myc-SH2-Bβ(504-670). Thus, these findings strongly support phosphotyrosine 785 in JAK3 as the primary binding site for the SH2 domain of SH2-Bβ.
SH2-Bβ-mediated enhancement of JAK2 autophosphorylation requires tyrosine 813 of JAK2.
Having obtained evidence that tyrosine 813 is the primary binding site of SH2-Bβ in JAK2, and having supported this conclusion with data obtained with tyrosine 785 of JAK3, we next sought to determine if mutation of tyrosine 813 would disrupt the stimulatory effect of SH2-Bβ on JAK2. To test whether SH2-Bβ requires tyrosine 813 to enhance JAK2 activity, we tested whether mutation of tyrosine 813 to phenylalanine would disrupt the enhanced activation of JAK2 reported previously for SH2-Bβ (32). COS7 cells were transiently transfected with cDNA encoding JAK2, JAK2(Y570F), JAK2(Y813F), or JAK2(K882E) with or without cDNA encoding myc-SH2-Bβ. Cell lysates were blotted with anti-myc to verify equal protein expression of myc-SH2-Bβ in the various conditions (Fig. 8, bottom panel). JAK2 was immunoprecipitated using anti-JAK2, and immunoblotted with anti-JAK2(Fig. 8, middle panel) to verify equal expression and immunoprecipitation of the various JAKs. The membrane was then stripped and reprobed with anti-PY, to determine the level of tyrosyl phosphorylation of JAK2 in the presence and absence of myc-SH2-Bβ (Fig. 8, top panel). Tyrosyl phosphorylation of JAK2 has been shown to correlate well with JAK2 kinase activity (39). As reported previously (34), JAK2 was constitutively phosphorylated on tyrosines when overexpressed in COS7 cells (Fig. 8, lane 1). Kinase-inactive JAK2(K882E) displayed no tyrosyl phosphorylation (Fig. 8, lane 3). As reported elsewhere (Argetsinger et al., submitted), JAK2(Y570F) exhibited an elevated level of tyrosyl phosphorylation (Fig. 8, compare lane 7 to l). In contrast to tyrosine 570, mutation of JAK2 at tyrosine 813 did not alter its constitutive level of tyrosyl phosphorylation (Fig. 8, compare lane 5 to lane 1), indicating that tyrosine 813 is not a site of intrinsic regulation within JAK2. The unaltered tyrosyl phosphorylation also suggested that this mutation does not lead to global structural changes within the protein. As reported previously (32), SH2-Bβ enhanced the tyrosyl phosphorylation of JAK2 (Fig. 8, lanes 1 and 2). Similarly, myc-SH2-Bβ enhanced the tyrosyl phosphorylation of JAK2 (Y570F) (Fig. 8, lanes 7 and 8). In contrast, myc-SH2-Bβ did not enhance the tyrosyl phosphorylation of JAK2(Y813F) (Fig. 8, lanes 5 and 6), implicating tyrosine 813 as critical for SH2-Bβ to enhance JAK2 activity. In addition, myc-SH2-Bβ did not enhance the tyrosyl phosphorylation of JAK2(K882E), verifying the dependence of SH2-Bβ on functionally active JAK2 to enhance JAK2 activity. To date, we have tested the effects of SH2-Bβ on twelve different JAK2 tyrosine point mutants, and tyrosine 813 is the only tyrosine we have identified whose mutation disrupts the ability of SH2-Bβ to enhance JAK2 activity (data not shown).
Tyrosine 813 is required for the SH2 domain of SH2-Bβ to increase the number of active JAK2 molecules.
Because the SH2 domain has been shown to be both necessary and sufficient for increasing JAK2 activity (32, 33), we examined whether mutation of tyrosine 813 to phenylalanine would prevent the increased activation of JAK2 by the SH2 domain of SH2-Bβ. COS7 cells were transfected with cDNA encoding JAK2, JAK2(Y813F), or JAK2(K882E) with or without cDNA encoding an SH2-Bβ truncation mutant, myc-SH2-Bβ(504-670), consisting of the SH2 domain and the C terminus of SH2-Bβ. Cell lysates were blotted with anti-myc to verify equal protein expression of myc-SH2-Bβ(504-670) for the various conditions (Fig. 9, bottom panel). JAK2 was immunoprecipitated with anti-JAK2, and immunoblotted with anti-JAK2 to verify equal protein expression, immunoprecipitation, and loading between conditions (Fig. 9, third panel). To analyze JAK2 phosphorylation, membranes were stripped and reprobed with anti-PY (Fig. 9, first panel). Similar to the effects observed with SH2-Bβ, SH2-Bβ(504-670) was found to enhance the overall tyrosyl phosphorylation of JAK2 (Fig. 9, lanes 1 and 2), as shown previously (33). In contrast, SH2-Bβ(504-670) did not enhance the phosphorylation of JAK2(Y813F) (Fig. 9, lanes 3 and 4), suggesting that tyrosine 813 is required for SH2-Bβ(504-670)-mediated enhancement of JAK2 activity. The membrane was also stripped and reprobed with anti-P(Y1007)-JAK2(Fig. 9, second panel). anti-P(Y1007)-JAK2 recognizes phosphotyrosine(s) 1007 and/or 1008 in the activation loop of JAK2. Because phosphorylation of tyrosine 1007 is thought to be required for activation of the kinase (12), an increase in signal observed from anti-P(Y1007)-JAK2 reflects an increase in the relative number of activated JAK2 molecules present. Comparison of JAK2 expressed alone (Fig. 9, lane 1) to JAK2 expressed with myc-SH2-Bβ(504-670) (Fig. 9, lane 2) shows that the presence of the SH2-Bβ(504-670) increases the number of JAK2 molecules with phosphorylated Tyr 1007/1008. As expected, because JAK2(K882E) is inactive, coexpression of JAK2(K882E) with SH2-Bβ(504-670) did not enhance overall tyrosyl phosphorylation of JAK2(K882E) (Fig. 9, lanes 5 and 6) or phosphorylation of tyrosine 1007 and/or 1008. Importantly, the presence of myc-SH2-Bβ(504-670) did not increase the number of JAK2(Y813F) molecules recognized by anti-P(Y1007)-JAK2(Fig. 9, lanes 3 and 4). These results support the conclusion that tyrosine 813 is required for the SH2 domain of SH2-Bβ to mediate an increase in the number of activated JAK2 molecules.
Tyrosine 813 of JAK2 is required for SH2-Bβ to enhance the phosphorylation of STAT5B at tyrosine 699.
Enhancement of JAK2 activity by SH2-Bβ increases the overall phosphorylation of JAK2 target proteins such as STAT5B (32). It is therefore expected that mutation of tyrosine 813 of JAK2 would not only disrupt the ability of SH2-Bβ to enhance JAK2 activity, but also preclude any enhancement of tyrosyl phosphorylation of tyrosine 699 on STAT5B. To detect maximally SH2-Bβ-mediated enhancement of JAK2 phosphorylation of STAT5B, STAT5B was expressed with or without myc-SH2-Bβ(504-670) in the presence of a lower level of JAK2 or JAK2(Y813F) than used in Fig. 5. Immunoblotting the lysates with anti-myc (Fig. 10, third panel) demonstrates equal protein expression of myc-SH2-Bβ(504-670) among the different conditions. Immunoblotting cell lysates with anti-STAT5B shows similar levels of STAT5B were coexpressed with JAK2 and JAK2(Y813F) (Fig. 10, second panel). Immunoprecipitation of the cell lysates with anti-JAK2, followed by immunoblotting with anti-JAK2 (Fig. 10, fourth panel), demonstrates equal protein expression, immunoprecipitation, and loading of JAK2. Reprobing the immunoprecipitated JAK2 with anti-PY (Fig. 10, fifth panel) verifies that myc-SH2-Bβ(504-670) enhanced the tyrosyl phosphorylation of JAK2 but not of JAK2(Y813F). Immunoblotting with antibody to phosphotyrosine 699 in STAT5B illustrates that overexpression of STAT5B alone in COS7 cells did not lead to detectable phosphorylation of STAT5B on tyrosine 699 (Fig. 10, lane 1, top panel), nor did coexpression of STAT5B with SH2-Bβ(504-670) (Fig. 10, lane 2, top panel). Expression of STAT5B with the low levels of JAK2 used in this experiment also did not lead to detectable STAT5B tyrosyl phosphorylation (Fig. 10, lane 3, top panel). However, as reported previously (32), coexpression of SH2-Bβ(504-670) with STAT5B and JAK2 led to a dramatic increase in phosphorylation of tyrosine 699 in STAT5B (Fig. 10, lanes 3 and 4, top panel). In contrast, coexpression of SH2-Bβ(504-670) with JAK2(Y813F) did not enhance the level of phosphorylation of tyrosine 699 in STAT5B over that detected in the presence of JAK2(Y813F) alone (Fig. 10, top panel, lanes 5 and 6), further implicating tyrosine 813 as being important for SH2-Bβ to mediate its stimulatory effects on JAK2.
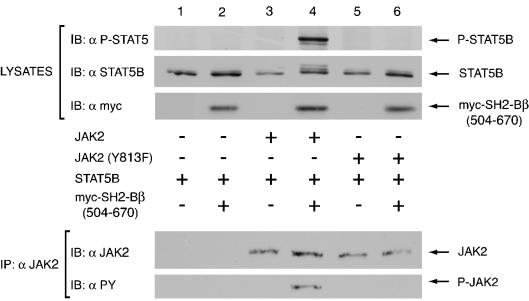
FIG. 10.
Tyrosine 813 is required for myc-SH2-Bβ(504-670) to enhance JAK2-mediated phosphorylation of STAT5B. COS7 cells were cotransfected with 2.0 μg of cDNA encoding STAT5B and 2.0 μg of either empty vector (lane 1) or cDNA encoding myc SH2-Bβ(504-670) (lanes 2, 4, and 6), without (lanes 1 and 2) or with 0.25 μg of cDNA encodes either JAK2 (lanes 3 and 4) or JAK2 (Y813F) (lanes 5 and 6). Cell lysates were immunoblotted with anti-P-STAT5 (first panel), anti-STAT5B (second panel), and anti-myc (third panel). Cell lysates were also immunoprecipitated with anti-JAK2 and the precipitated proteins were immunoblotted with anti-JAK2 (fourth panel) and with anti-PY (fifth panel). The nitrocellulose membrane was stripped between blots as outlined in Materials and Methods.
DISCUSSION
Our laboratory has initiated studies designed to identify which of the 49 tyrosines within JAK2 are phosphorylated. Previously, we identified tyrosines 221 and 570, as well as tyrosine 1007, as phosphotyrosines in active JAK2 that regulate kinase activity (Argetsinger et al., submitted). Here we show using 2-D peptide mapping of overexpressed JAK2 from 293T cells, and phosphoamino acid analysis that tyrosine 813 of JAK2 is also a site of autophosphorylation. Additionally, we have observed spots that comigrate similarly to the peptides containing tyrosine 813 in 2-D maps from JAK2 labeled in vivo (data not shown). Finally, we made an antibody that specifically recognizes the phosphorylation of tyrosine 813. Use of this antibody confirms that tyrosine 813 is phosphorylated in JAK2 overexpressed in 293T cells and in endogenous JAK2 that is activated in response to GH in 3T3-F442A cells. Phosphorylation of tyrosine 813 in response to GH was rapid and transient, following a time course similar to that of 221, 570, and 1007 (Argetsinger et al., submitted). Interestingly, tyrosine 813 falls within the amino acid sequence YELL, which conforms to the consensus motif, YXXL, shared by both tyrosines 221 and 570 in JAK2. Moreover, JAK2 has been shown to phosphorylate the JAK2 binding protein, SH2-Bβ, on tyrosines 439 and 494, both of which are contained within YXXL motifs (27). Thus, identification of tyrosine 813 as a JAK2 autophosphorylation site further implicates YXXL as a common motif that is recognized and tyrosyl phosphorylated by JAK2.
Mutation of tyrosine 813 to phenylalanine does not substantially alter phosphorylation of other tyrosines in JAK2 (Fig. 1A), the activity of JAK2 (Fig. 8 and 9), or the ability of JAK2 to phosphorylate substrates such as STAT5B (Fig. 5). Thus, mutation of tyrosine 813 does not appear to have any substantial effect on the structural conformation of JAK2. However, when we investigated the signaling of JAK2 with tyrosine 813 mutated to phenylalanine in the presence of the JAK2 activator, SH2-Bβ, major changes were detected. We observed that mutation of tyrosine 813 in JAK2 to phenylalanine prevents the coimmunoprecipitation of JAK2 with both full-length SH2-Bβ and SH2-Bβ(504-670). Additionally, we observed that the activity of JAK2 lacking tyrosine 813 could not be enhanced by either SH2-Bβ(504-670) or full-length SH2-Bβ. Additionally, SH2-Bβ(504-670) does not enhance JAK2(Y813F)-mediated phosphorylation of STAT5B on tyrosine 699. In contrast, mutation of 17 other tyrosines identified as phosphorylation sites within JAK2 or as potential phosphorylation sites, did not disrupt SH2-Bβ's ability to bind to JAK2 (13 tested) and/or to enhance JAK2 activation (12 tested) (data not shown). Together, these data strongly suggest that phosphorylated tyrosine 813 is the primary SH2-Bβ-binding site in JAK2.
Due to technical difficulties, we have been unable to demonstrate that tyrosine 813 is required for SH2-Bβ to enhance the activation of JAK2 in response to GH. Nevertheless, evidence exists to support the conclusion that tyrosine 813 is important for SH2-Bβ to enhance GH activation of JAK2. Specifically, it has been shown previously that SH2-Bβ can enhance the GH-stimulated phosphorylation of endogenous JAK2 in COS7 cells (32) and 293T cells (28). In addition, 2-D phosphopeptide mapping of overexpressed JAK2 demonstrates that tyrosines that are autophosphorylated in vitro are also phosphorylated in vivo (Argetsinger et al., submitted). Tyrosines 1007,1008, 570, 221, and 813 have all been identified as sites of JAK2 autophosphorylation by 2-D phosphopeptide mapping following an in vitro kinase assay. Phosphospecific antibodies have confirmed that tyrosines 221, 570, 813, and 1007 and/or 1008 are phosphorylated in vivo in overexpressed JAK2 and in endogenous JAK2 activated by GH (Argetsinger et al., submitted; this work) (data not shown), suggesting that activation of JAK2, whether via overexpression or via stimulation by GH, results in similar sites of tyrosyl phosphorylation. Finally, a variety of findings presented here suggest that tyrosine 813 is the primary site in JAK2 for binding SH2-Bβ and is required for SH2-Bβ enhancement of JAK2 activity. We therefore think it highly likely that GH-mediated phosphorylation of tyrosine 813 in JAK2 leads to recruitment of SH2-Bβ to GHR-JAK2 complexes, just as phosphorylation of tyrosine 813 recruits SH2-Bβ to constitutively activated JAK2 complexes.
Both tyrosine 813 of JAK2 and the corresponding tyrosine 785 of JAK3 are contained within the amino acid sequence YELL, which conforms to the motif YXXL. It is of interest that SH2-Bβ is reported to bind YXXL motifs in other receptors, including the fibroblast growth factor receptor at tyrosine 760 (YLDL) (20) and the erythropoietin receptor at tyrosines 343 (YLVL), 401 (YTIL), and 429 (YLYL) (36a). APS, a member of the SH2-Bβ family of proteins, is reported to bind the erythropoietin receptor at tyrosine 343 (YLVL) (37). Nevertheless, the YXXL motif is not sufficient for protein binding, as SH2-Bβ does not bind to other JAK2 tyrosines contained in YXXL motifs (Fig. 8 and data not shown). In addition, SH2-Bβ has been reported to bind other phosphotyrosine containing motifs, including motifs containing tyrosine 724 (YMIM) of the fibroblast growth factor receptor (20), tyrosine 740 (YMDM) in the platelet-derived growth factor receptor (31), and tyrosines 1158 (YETD), 1162 (YYRK), and 1163 (YRKG) in the catalytic region and tyrosines 960 (YLSA) and 1322 (YTHM) in the juxtamembrane and C-terminal regions of the insulin receptor, respectively (21, 38). While work remains to determine the exact SH2-Bβ binding requirements, tyrosines contained within YXXL and YXXM motifs are clearly useful starting points when attempting to determine the binding site for SH2-Bβ within an SH2-Bβ binding partner.
As mentioned above, tyrosine 813 of JAK2 is homologous to tyrosine 785 of JAK3, with both tyrosines found within the sequence YELL. Because SH2-Bβ also binds JAK3 (28), we analyzed whether YELL is a site of phosphorylation in JAK3. 2-D phosphopeptide mapping (Fig. 4A) revealed that tyrosine 785 is a major site of phosphorylation within JAK3, which is phosphorylated both when overexpressed (Fig. 4B) and when stimulated by IL-2 (Fig. 4C). As we showed for JAK2 lacking tyrosine 813, JAK3 lacking the corresponding tyrosine 785 does not coprecipitate with SH2-Bβ, supporting the conclusion that SH2-Bβ binds to phosphorylated tyrosine 785 of JAK3. These experiments also further refined the interaction of SH2-Bβ with JAK3 to amino acids 504-670 of SH2-Bβ. SH2-Bβ(504-670) contains primarily the SH2 domain and the C terminus of SH2-Bβ, suggesting that like JAK2, JAK3 interacts with the SH2 domain of SH2-Bβ. The finding that myc-SH2-Bβ(504-670) binds to kinase-active JAK3 but not to kinase-inactive JAK3, which is not tyrosyl phosphorylated, further supports the conclusion that the SH2 domain of SH2-Bβ binds one or more phosphotyrosines in JAK3. These results provide additional evidence that the primary binding sites in JAK2 and JAK3 for the SH2 domain of SH2-Bβ is pYELL.
Surprisingly, while SH2-Bβ binds to both JAK2 and JAK3 at phosphorylated tyrosines within a YELL motif, SH2-Bβ enhances the activity of only JAK2. Because the SH2-Bβ-binding site within JAK3 is analogous to the site within JAK2, it is not outwardly apparent why SH2-Bβ has a differential effect on JAK2 and JAK3. Nevertheless, such discrepancies between analogous tyrosines within the JAKs are not novel. Indeed, mutating a conserved tyrosine within the activation loop of the different JAKs (Y1007 in JAK2, Y980 in JAK3, Y1054 in Tyk2) eliminates kinase activity in JAK2 but does not abolish activity in JAK3 and Tyk2, suggesting that catalytic regulation may be quite different between members of the JAK family (23).
A better understanding for the differential effects of SH2-Bβ on JAK2 and JAK3 may come once it is determined how SH2-Bβ enhances JAK2 activity. As such, the identification of tyrosine 813 as the SH2-Bβ binding site in JAK2 should greatly help elucidate SH2-Bβ's mechanism of action. As further groundwork for determining how SH2-Bβ enhances JAK2 activity, it is important to define the exact nature of the augmented activity of JAK2 that occurs with SH2-Bβ. The increased tyrosyl phosphorylation of JAK2 seen with coexpression of SH2-Bβ could theoretically be the result of an increase in the number of phosphotyrosines on JAK2, an increase in the number of phosphorylated JAK2 molecules, or both. Using antibody to phosphorylated tyrosines 1007 and/or 1008 in the activation loop of JAK2 we show here that SH2-Bβ increases the number of active JAK2 molecules (Fig. 9). No additional major spots are observed upon 2D phosphopeptide mapping of JAK2 coexpressed with SH2-Bβ (Argetsinger et al., submitted), supporting the conclusion that SH2-Bβ does not alter the conformation of JAK2 in such a way that additional tyrosines are accessible for phosphorylation. Thus, both of these results suggest that binding of SH2-Bβ promotes the activation of JAK2.
Currently, there exist several possibilities for how SH2-Bβ might increase JAK2 function. For example, SH2-Bβ may disrupt binding of JAK2 inhibitors, including the SOCS proteins and various phosphatases, such as PTP-1B. SOCS-1 has been shown to bind JAK2 within the kinase activation loop, at tyrosine 1007 (43). That tyrosine 813 is required for SH2-Bβ to activate JAK2 suggests that SH2-Bβ does not competitively inhibit the binding of SOCS-1 to JAK2. Furthermore, preliminary results indicate that SH2-Bβ does not bind to a phosphopeptide containing phosphorylated tyrosine 1007 (data not shown). In addition, if SH2-Bβ were to compete with a JAK2 inhibitor for binding at tyrosine 813, it would be expected that mutation of tyrosine 813 would prevent binding of the inhibitor, and therefore increase the basal activity level of JAK2. As demonstrated in Fig. 8 and 9, mutation of tyrosine 813 to phenylalanine does not lead to a significant increase in JAK2 phosphorylation, suggesting that SH2-Bβ does not directly compete with a JAK2-inhibitor for binding at tyrosine 813. Nevertheless, it remains possible that the binding of SH2-Bβ to JAK2 allosterically inhibits SOCS-1 binding at the active site, or affects binding of other SOCS proteins to JAK2. Similarly, PTP-1B has been shown to bind phosphorylated JAK2 in cells treated with leptin, gamma interferon, or GH (9, 26) and appears to dephosphorylate phosphotyrosine 1007 in the activation loop of JAK2 (26). Thus, it remains plausible that the enhanced phosphorylation of JAK2 seen in the presence of SH2-Bβ results from a decreased affinity of PTP-1B for JAK2.
Another possibility is that binding of SH2-Bβ may recruit positive regulators to JAK2. Cross talk between signaling pathways, particularly the phosphorylation of tyrosine kinases by other kinases, has been shown to occur. For instance, GH has been shown to promote the phosphorylation of the epidermal growth factor receptor by JAK2, thus enabling the docking of Grb2 to the epidermal growth factor receptor and subsequent activation of MAP kinase (42). Similarly, JAK2 has been shown to be phosphorylated at tyrosine 1007 by the chimeric oncogene Bcr-Abl in M3.16 cells (41), and Bcr-Abl has been shown to be in a complex with JAK2 and SH2-Bβ in 32D cells expressing Bcr-Abl (40). In this vein, it is possible that SH2-Bβ serves as an adapter protein to recruit other tyrosine kinases, which subsequently phosphorylate and activate JAK2.
JAK dimerization has been proposed to be required for kinase activation (18, 24). Moreover, an N-terminal multimerization domain has been identified within SH2-B, and has been implicated in SH2-B-mediated potentiation of TRKA signaling (30). Thus, it remains possible that multimerization of SH2-Bβ may stabilize JAK2 multimers, thereby increasing the overall kinase activity of the enzyme. However, as shown here and previously, SH2-Bβ(504-670), which lacks the N-terminal multimerization domain, is sufficient for the enhancement of JAK2 activity. Thus, while binding of SH2-Bβ to JAK2 may promote a conformational change within JAK2 that increases the affinity of JAK2-JAK2 interactions or facilitates JAK2-JAK2 transphosphorylation and activation, multimerization of SH2-Bβ via an N-terminal dimerization domain appears unnecessary.
Finally, binding of SH2-Bβ to JAK2 may cause a conformational change that maintains JAK2 in an active conformation. Interestingly, tyrosine 813 resides in JH2 of JAK2. The JH1 domain, or catalytic domain, possesses the kinase activity of the protein, whereas the JH2 domain, termed the pseudokinase domain, is similar to the kinase domain but contains no intrinsic activity. It has been shown that deletion of the JH2 domain can lead to hyper-activation of the kinase, suggesting that the JH2 domain may negatively regulate the JH1 domain (13, 35). An intriguing possibility, therefore, is that binding of SH2-Bβ at tyrosine 813 relaxes the inhibition of JH2 on JH1, thus enhancing kinase activity. Alternatively, binding of SH2-Bβ to JAK2 may be sufficient to stabilize the kinase domain in an active conformation. In this regard, it is interesting that both APS and SH2-Bβ have been shown to bind to phosphotyrosines within the active site of the insulin receptor (2, 21, 25). However tyrosine 813 is not in the active site of JAK2 and the present work provides no evidence that the SH2 domain of SH2-Bβ binds to tyrosines in the active site of JAK2.
Summary.
We have used 2-D peptide mapping, phosphoamino acid analysis, and a phosphospecific antibody to demonstrate that tyrosine 813 is a site of autophosphorylation in JAK2. Intriguingly, while mutation of tyrosine 813 in JAK2 does not appear to affect the intrinsic activity of JAK2, it does disrupt the enhanced activation of JAK2 observed when either SH2-Bβ or SH2-Bβ(504-670) is present. Furthermore, because SH2-Bβ does not precipitate JAK2(Y813F) or enhance the ability of JAK2(Y813F) to phosphorylate STAT5B at tyrosine 699, we conclude that tyrosine 813 is the primary SH2-Bβ-binding site within JAK2 and is required to enhance JAK2 activation. This conclusion is further supported by the finding that mutation of the corresponding tyrosine in JAK3, Y785, also disrupts the coimmunoprecipitation of JAK3 with SH2-Bβ. The binding of SH2-Bβ to phosphorylated tyrosine 813 in JAK2 and phosphorylated tyrosine 785 in JAK3, both of which are found within a YELL sequence, provides additional evidence that the SH2 domain of SH2-Bβ shows some binding preference for phosphorylated YXXL motifs.
Acknowledgments
This work was supported by NIH grants DK34171 and DK54222. J.H.K. was supported by a Predoctoral Fellowship of the Cellular and Molecular Approaches to Systems and Integrative Biology Training Grant T32-GM08322 and the University of Michigan Medical Scientist Training Program (NIH T32 GM07863). Oligonucleotides were synthesized by the Biomedical Research Core Facility at the University of Michigan with support from the Michigan Diabetes Research and Training Center (P60-DK20572), the University of Michigan Multipurpose Arthritis Center (P60-AR20557), and the University of Michigan Comprehensive Cancer Center (NIH P30 CA46592). cDNA sequencing was supported by the Cellular and Molecular Biology Core of the Michigan Diabetes Research and Training Center.
We thank Xiaqing Wang for support and assistance with experiments and Barbara Hawkins for assistance with the manuscript.
REFERENCES
- 1.Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who know JAK-STAT. Science 296:1653-1655. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, Z., B. J. Smith, K. Kotani, P. Wilden, and T. S. Pillay. 1999. APS, an adapter protein with a PH and SH2 domain, is a substrate for the insulin receptor kinase. Biochem. J. 341:665-668. [PMC free article] [PubMed] [Google Scholar]
- 3.Argetsinger, L. S., G. S. Campbell, X. Yang, B. A. Witthuhn, O. Silvennoinen, J. N. Ihle, and C. Carter-Su. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244. [DOI] [PubMed] [Google Scholar]
- 3a.Argetsinger, L. S., J.-L. K. Kouadio, H. Steen, A. Stensballe, O. N. Jensen, and C. Carter-Su. Autophosphorylation on tyrosines 221 and 570 regulates its activity, Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed]
- 4.Benekli, M., M. R. Baer, H. Baumann, and M. Wetzler. 2003. Signal transducer and activator of transcription proteins in leukemias. Blood 101:2940-2954. [DOI] [PubMed] [Google Scholar]
- 5.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-148. [DOI] [PubMed] [Google Scholar]
- 6.Candotti, F., L. Notarangelo, R. Visconti, and J. O'Shea. 2002. Molecular aspects of primary immunodeficiencies: lessons from cytokine and other signaling pathways. J. Clin. Investig. 109:1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpino, N., R. Kobayashi, H. Zang, Y. Takahashi, S. T. Jou, J. Feng, H. Nakajima, and J. N. Ihle. 2002. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol. Cell. Biol. 22:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, A., N. Uetani, P. D. Simoncic, V. P. Chaubey, A. Lee-Loy, C. J. McGlade, B. P. Kennedy, and M. L. Tremblay. 2002. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2:497-503. [DOI] [PubMed] [Google Scholar]
- 10.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 11.Diakonova, M., D. R. Gunter, J. Herrington, and C. Carter-Su. 2002. SH2-Bβ is a Rac-binding protein that regulates cell motility. J. Biol. Chem. 277:10669-10677. [DOI] [PubMed] [Google Scholar]
- 12.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank, S. J., W. Yi, Y. Zhao, J. F. Goldsmith, G. Gilliland, J. Jiang, I. Sakai, and A. S. Kraft. 1995. Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J. Biol. Chem. 270:14776-14785. [DOI] [PubMed] [Google Scholar]
- 14.Herrington, J., L. S. Smit, J. Schwartz, and C. Carter-Su. 2000. The role of STAT proteins in growth hormone signaling. Oncogene 19:2585-2597. [DOI] [PubMed] [Google Scholar]
- 15.Hou, S. X., Z. Zheng, X. Chen, and N. Perrimon. 2002. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev. Cell 3:765-778. [DOI] [PubMed] [Google Scholar]
- 16.Ihle, J. N. 1996. Janus kinases in cytokine signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351:159-166. [DOI] [PubMed] [Google Scholar]
- 17.Ihle, J. N. 1996. STATs: signal transducers and activators of transcription. Cell 84:331-334. [DOI] [PubMed] [Google Scholar]
- 18.Ihle, J. N., and I. M. Kerr. 1995. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 11:69-74. [DOI] [PubMed] [Google Scholar]
- 19.Johnston, J. A., M. Kawamura, R. A. Kirken, Y.-Q. Chen, T. B. Blake, K. Shibuya, J. R. Ortaldo, D. W. McVicar, and J. J. O'Shea. 1994. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature 370:151-153. [DOI] [PubMed] [Google Scholar]
- 20.Kong, M., C. S. Wang, and D. J. Donoghue. 2002. Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B. J. Biol. Chem. 277:15962-15970. [DOI] [PubMed] [Google Scholar]
- 21.Kotani, K., P. Wilden, and T. S. Pillay. 1998. SH2-Bα is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem. J. 335:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacronique, V., A. Boureux, V. D. Valle, H. Poirel, C. T. Quang, M. Mauchauffe, C. Berthou, M. Lessard, R. Berger, J. Ghysdael, and O. A. Bernard. 1997. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278:1309-1312. [DOI] [PubMed] [Google Scholar]
- 23.Leonard, W. J., and J. J. O'Shea. 1998. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16:293-322. [DOI] [PubMed] [Google Scholar]
- 24.Mizuguchi, R., and M. Hatakeyama. 1998. Conditional activation of Janus kinase (JAK) confers factor independence upon interleukin-3-dependent cells. Essential role of Ras in JAK-triggered mitogenesis. J. Biol. Chem. 273:32297-32303. [DOI] [PubMed] [Google Scholar]
- 25.Moodie, S. A., J. Alleman-Sposeto, and T. A. Gustafson. 1999. Identification of the APS protein as a novel insulin receptor substrate. J. Biol. Chem. 274:11186-11193. [DOI] [PubMed] [Google Scholar]
- 26.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276:47771-47774. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien, K. B., L. S. Argetsinger, M. Diakonova, and C. Carter-Su. 2003. YXXL motifs in SH2-Bβ are phosphorylated by JAK2, JAK1, and platelet-derived growth factor receptor and are required for membrane ruffling. J. Biol. Chem. 278:11970-11978. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien, K. B., J. J. O'Shea, and C. Carter-Su. 2002. SH2-B family members differentially regulate JAK family tyrosine kinases. J. Biol. Chem. 277:8673-8681. [DOI] [PubMed] [Google Scholar]
- 29.Peeters, P., S. D. Raynaud, J. Cools, I. Wlodarska, J. Grosgeorge, P. Philip, F. Monpoux, L. Van Rompaey, M. Baens, H. Van den Berghe, and P. Marynen. 1997. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood 90:2535-2540. [PubMed] [Google Scholar]
- 30.Qian, X., and D. D. Ginty. 2001. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol. Cell. Biol. 21:1613-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riedel, H., N. Yousaf, Y. Zhao, H. Dai, Y. Deng, and J. Wang. 2000. PSM, a mediator of PDGF-BB-, IGF-I-, and insulin-stimulated mitogenesis. Oncogene 19:39-50. [DOI] [PubMed] [Google Scholar]
- 32.Rui, L., and C. Carter-Su. 1999. Identification of SH2-Bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc. Natl. Acad. Sci. USA 96:7172-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rui, L., D. R. Gunter, J. Herrington, and C. Carter-Su. 2000. Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-Bβ. Mol. Cell. Biol. 20:3168-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rui, L., L. S. Mathews, K. Hotta, T. A. Gustafson, and C. Carter-Su. 1997. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 17:6633-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saharinen, P., K. Takaluoma, and O. Silvennoinen. 2000. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell. Biol. 20:3387-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvennoinen, O., B. Witthuhn, F. W. Quelle, J. L. Cleveland, T. Yi, and J. N. Ihle. 1993. Structure of the murine JAK2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc. Natl. Acad. Sci. USA 90:8429-8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Tschirch, E., B. K. Beattie, N. Dowman, C. Carter-Su, and D. L. Barber. 2000. The erythropoietin receptor recruits the adaptor protein SH2-Bβ to a region necessary for proliferation and differentiation. Blood 96:568a (Abstract.)
- 37.Wakioka, T., A. Sasaki, K. Mitsui, M. Yokouchi, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing pleckstrin homology (PH) and Src homology-2 (SH2) domains inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia 13:760-767. [DOI] [PubMed] [Google Scholar]
- 38.Wang, J., and H. Riedel. 1998. Insulin-like growth factor-I receptor and insulin receptor association with a Src homology-2 domain-containing putative adapter. J. Biol. Chem. 273:3136-3139. [DOI] [PubMed] [Google Scholar]
- 39.Witthuhn, B. A., F. W. Quelle, O. Silvennoinen, T. Yi, B. Tang, O. Miura, and J. N. Ihle. 1993. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 74:227-236. [DOI] [PubMed] [Google Scholar]
- 40.Xie, S., H. Lin, T. Sun, and R. B. Arlinghaus. 2002. Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene 21:7137-7146. [DOI] [PubMed] [Google Scholar]
- 41.Xie, S., Y. Wang, J. Liu, T. Sun, M. B. Wilson, T. E. Smithgall, and R. B. Arlinghaus. 2001. Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene 20:6188-6195. [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi, T., K. Ueki, K. Tobe, H. Tamemoto, N. Sekine, M. Wada, M. Honjo, M. Takahashi, T. Takahashi, H. Hirai, T. Tsushima, Y. Akanuma, T. Fujita, I. Komuro, Y. Yazaki, and T. Kadowaki. 1998. Growth hormone-induced tyrosine phosphorylation of EGF receptor as an essential element leading to MAP kinase activation and gene expression. Endocr. J. 45:S27-S31. [DOI] [PubMed] [Google Scholar]
- 43.Yasukawa, H., H. Misawa, H. Sakamoto, M. Masuhara, A. Sasaki, T. Wakioka, S. Ohtsuka, T. Imaizumi, T. Matsuda, J. N. Ihle, and A. Yoshimura. 1999. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou, Y. J., M. Chen, N. A. Cusack, L. H. Kimmel, K. S. Magnuson, J. G. Boyd, W. Lin, J. L. Roberts, A. Lengi, R. H. Buckley, R. L. Geahlen, F. Candotti, M. Gadina, P. S. Changelian, and J. J. O'Shea. 2001. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8:959-969. [DOI] [PubMed] [Google Scholar]
- 45.Zhou, Y. J., E. P. Hanson, Y. Q. Chen, K. Magnuson, M. Chen, P. G. Swann, R. L. Wange, P. S. Changelian, and J. J. O'Shea. 1997. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. USA 94:13850-13855. [DOI] [PMC free article] [PubMed] [Google Scholar]