Abstract
The retinoblastoma protein (RB) suppresses cell proliferation and apoptosis. We have previously shown that RB degradation is required for tumor necrosis factor alpha (TNF-α) to induce apoptosis. We show here the identification of two apoptotic effectors, i.e., c-ABL tyrosine kinase and p73, which are activated by TNF-α following RB degradation. In cells expressing a degradation-resistant RB protein (RB-MI), TNF-α does not activate c-ABL. RB-MI also inhibits TNF-α-mediated activation of p73. Genetic deletion and pharmacological inhibition of c-ABL or p73 diminish the apoptotic response to TNF-α in human cell lines and mouse fibroblasts. Thymocytes isolated from RbMI/MI, Abl−/−, or p73−/− mice are resistant to TNF-α-induced apoptosis compared to their wild-type counterparts. This is in contrast to p53−/− thymocytes, which exhibit a wild-type level of apoptosis in response to TNF-α. Thus, c-ABL and p73 contribute to apoptosis induced by TNF-α, in addition to their role in promoting DNA damage-associated cell death.
Tumor necrosis factor alpha (TNF-α) is an inflammatory cytokine that coordinates the systemic response to infection and injury. TNF-α interacts with two types of cell surface receptors (types I and II) to regulate various cell-type-specific responses (8). Binding of TNF-α to the type I receptor (TNFRI) can activate the NF-κB survival pathway (23) or caspase-dependent cell death (24, 38). Activated TNFRI recruits TRADD (TNFR-associated death domain) (25), which in turn recruits TRAFs (TNFR-associated factors) and RIP to activate NF-κB. TNFRI also stimulates the formation of a cytoplasmic TRADD complex, containing FADD (FAS-associated death domain) and pro-caspase 8, leading to the activation of caspase 8 and the initiation of an apoptotic signaling cascade (24, 25, 38). Activated caspase 8 can directly cleave and activate executioner caspases (11). Caspase 8 also cleaves a BH3-only protein, BID (32, 35). The truncated BID, in collaboration with BAX and BAK, causes the release of apoptogenic factors from mitochondria to activate the apoptosome and downstream caspases (58). Cells derived from Bid−/− mice show delayed caspase activation and reduced apoptosis in response to TNF-α (63). Thus, TNFRI-induced apoptosis is mediated through mitochondrion-independent and mitochondrion-dependent mechanisms.
It was previously shown that degradation of the retinoblastoma tumor suppressor protein (RB) is required for TNF-α to activate mitochondrion-dependent apoptosis (6). Upon the activation of TNFRI, RB is cleaved by caspase and then degraded. We have created in the mouse germ line an Rb-MI allele, which contains two substitutions at a C-terminal caspase cleavage site (DEADG to DEAAE) (6). Rb-MI is not degraded upon the activation of TNFRI; cells expressing Rb-MI maintain their mitochondrial integrity and display a protracted death response to TNF-α (6). Rb-MI is sensitive to degradation when TNFRI and TNFRII are both activated (6). As a result, cells expressing Rb-MI are sensitive to death induced by the costimulation of TNFRI and TNFRII (6). These and other results strongly suggest that RB has an apoptosis suppression function (7).
It has been previously shown that RB binds to nuclear c-ABL tyrosine kinase and inhibits its activity (59, 60). The activation of nuclear c-ABL tyrosine kinase by DNA damage is associated with the induction of apoptosis (57). In addition to DNA damage, nuclear c-ABL kinase could also be activated by TNF-α (12, 57), and STI-571, a chemical inhibitor of c-ABL kinase, reduces the apoptotic response to TNF-α (12). It was found that z-VAD, a general inhibitor of caspase, blocks TNF-α-induced activation of c-ABL (12). While caspase can cleave c-ABL protein, cleavage does not activate the tyrosine kinase activity but instead promotes the nuclear accumulation of c-ABL (3). In this study, we show that caspase-dependent removal of RB is required for TNF-α to activate the nuclear c-ABL tyrosine kinase, thus explaining the inhibitory effect of z-VAD on c-ABL activation.
c-ABL tyrosine kinase regulates the function of p53 and p73, which play essential roles in DNA damage-induced apoptosis (17). The human c-ABL protein may interact directly with p53 to activate its function (18). Overproduction of c-ABL can also interfere with MDM2-mediated degradation of p53 (19, 46). However, activated nuclear c-ABL kinase can induce apoptosis in p53-deficient cells (52, 53). By contrast, activated nuclear c-ABL kinase does not induce apoptosis in p73-deficient cells (52). The c-ABL kinase can directly phosphorylate p73 (1, 64), increase the half-life of p73 (21), and promote the acetylation of p73 by p300 (10). Coexpression of activated nuclear c-ABL kinase with p73 synergistically activates cell death (52). Thus, c-ABL and p73 cooperate to promote apoptosis. In this study, we show p73 to also be required for TNF-α-induced cell death and RB to be a suppressor of the p73 apoptotic function.
MATERIALS AND METHODS
Cell culture.
3T3 fibroblasts, mouse embryo fibroblasts (MEFs), and HCT116-3(6) colorectal carcinoma cells were cultured with high-glucose (4.5-g/liter) Dulbecco modified Eagle medium supplemented with penicillin G (100 U/ml), streptomycin (100 μg/ml), 10% fetal bovine serum, and l-glutamine (2 mM). MCF-7 cells and freshly isolated thymocytes were cultured in RPMI 1640 medium similarly supplemented with penicillin G, streptomycin, 10% fetal bovine serum, and l-glutamine.
Mice.
Abl−/−, p53−/−, and RbMI/MI mice were maintained in a pure 129 background. p73−/− mice were in a mixed BALB/c and C57BL/6 background and were the kind gift of Frank McKeon (Harvard Medical School). Thymocytes were isolated from wild-type and mutant littermates. Rb−/− Abl+/+ and Rb−/− Abl−/− littermate embryos were generated by crossing Rb+/− Abl+/− mice, and MEFs were isolated from E12.5 embryos.
In vitro kinase assays.
In vitro c-Abl kinase assays were performed as described previously with CTD-CRK as the substrate (21). The phosphorylated substrate was detected by autoradiography, and the immunoprecipitated c-Abl was detected by immunoblot analysis with the monoclonal antibody 8E9.
TNF-α and TRAIL treatment and apoptosis assays.
Recombinant human and mouse TNF-α and recombinant human TNF-α-related apoptosis-inducing ligand (TRAIL) were obtained from R & D Systems. In general, cells were treated with 10 ng of TNF-α/ml and 2.5 μg of cycloheximide (CHX; Calbiochem)/ml. At this concentration, CHX did not induce apoptosis in the cell types used in this study. Specific conditions of TNF-α-CHX treatment are indicated in the figure legends. Apoptosis was determined by several different methods. For morphological characterization, cells were fixed with 4% formaldehyde and permeabilized with 0.5% Triton X-100 followed by incubation with Hoechst 33258 (Molecular Probes). Dead cells were identified by characteristic condensed nuclear staining. For sub-G1 fluorescence-activated cell sorting (FACS) analysis, cells were harvested and fixed with ice-cold 75% ethanol and then resuspended with RNase (40 μg/ml) and propidium iodide (20 μg/ml) prior to analysis by FACS. For annexin V-propidium iodide staining, thymocytes were collected after the indicated treatment and were processed according to the manufacturer's instructions with the annexin V staining kit (BD Bioscience) prior to analysis by FACS.
Adenovirus infection.
Recombinant adenoviruses were generated in the gene therapy facility at the University of California, San Diego, with a plasmid (pAdTrack-CMV-ΔN-p73β with green fluorescent protein [GFP]) kindly provided by Christine Pozniak (McGill University). Cells were infected at a multiplicity of infection of 100 overnight prior to treatment.
Antibodies and recombinant cytokines.
Polyclonal anti-procaspase 3 antibody was a kind gift from Yuri Lazebnik (Cold Spring Harbor Laboratory). Polyclonal anti-cleaved caspase 3 antibody was obtained from Cell Signaling Technology. Monoclonal anti-cytochrome c antibody was obtained from BD Bioscience. Polyclonal anti-p73 antibody was generated against recombinant human p73 protein and affinity purified. Monoclonal anti-p53 antibody (DO-1) was obtained from Calbiochem.
siRNA-mediated down-regulation of endogenous protein.
Small interfering RNA (siRNA) duplexes (LacZ, AAC-AGU-UGC-GCA-GCC-UGA-AUG; p53-1, AAC-AGC-UUU-GAG-GUG-CGU-GUU; p53-2, AAG-CGA-GCA-CUG-CCC-AAC-AAC; p73, AAG-CUG-AAA-GAG-AGC-CUG-GAG) were obtained from Dharmacon. Proliferating cells were trypsinized and seeded at 30% confluence overnight. Cells were transfected with RNA duplexes by the use of Oligofectamine (Invitrogen) as previously described (30).
RESULTS
TNF-α activates c-Abl downstream of Rb degradation.
Treatment of primary MEFs with TNF-α plus CHX led to the degradation of wild-type Rb but not Rb-MI protein (Fig. 1A) (6). In Rb+/+ MEFs, treatment with TNF-α-CHX caused an increase in the nuclear c-Abl kinase activity, coinciding with Rb degradation (lanes 1 and 2). In MEFs derived from Rb−/− embryos, a higher basal level of nuclear c-Abl kinase activity was detected (lane 3), consistent with Rb being an inhibitor of nuclear c-Abl kinase (56, 59, 60). In the Rb−/− cells, nuclear c-Abl kinase activity was further increased following treatment with TNF-α-CHX (lane 4). In RbMI/MI MEFs, nuclear c-Abl had a basal activity similar to that of the Rb+/+ cells. However, a TNF-α-CHX-induced further increase in c-Abl kinase activity did not occur in RbMI/MI cells (lanes 5 and 6). The specificity of the c-Abl kinase assay was demonstrated by using nuclear extracts of Abl/Arg (Abl-related gene) double-knockout fibroblasts retrovirally transduced with vector (lanes 7 and 8) or Abl (lanes 9 and 10). These results show that Rb degradation is necessary for TNF-α to activate c-Abl tyrosine kinase, but Rb loss is not the only step, because TNF-α can further activate Abl kinase in Rb-deficient cells.
FIG. 1.
Rb degradation is required for c-Abl kinase activation, which contributes to TNF-α-induced apoptosis. (A) Primary MEFs with indicated Rb genotypes were treated with human TNF-α (hTNF-α)-CHX. At the indicated time points, Rb was immunoprecipitated from the whole-cell lysate and analyzed with immunoblotting (upper panel). Primary MEFs of the indicated Rb genotypes and vector (MSCV)- or c-Abl (type Ib)-reconstituted Abl−/− Arg−/− 3T3 fibroblasts were treated with human TNF-α-CHX for 24 h, c-Abl was immunoprecipitated from whole-cell lysates, and kinase activity was determined by in vitro phosphorylation of glutathione S-transferase-Crk-CTD (middle panel). The amount of immunoprecipitated c-Abl was determined by immunoblotting with anti-c-ABL antibody (8E9) (lower panel). (B and C) Rb−/− 3T3 fibroblasts were left untreated or treated with mouse TNF-α (50 ng/ml) alone, STI-571 (10 μM) alone, or a combination of mouse TNF-α and STI-571 for 48 h. Cells were fixed, and condensed nuclei were visualized by Hoechst staining and fluorescence microscopy (B); the percentages of apoptotic cells were counted and are shown in panel C. (D) Primary MEFs derived from Rb−/− Abl−/− or Rb−/− Abl+/+ embryos were treated with mouse TNF-α (50 ng/ml), and cells were fixed at indicated time points. Condensed nuclei were determined as described for panel B. (E) Whole-cell lysates from MEFs described in the legend to panel D were isolated and analyzed by immunoblotting with caspase 3 antibodies, and procaspase and cleaved (activated) caspase 3 are marked. (F) Abl−/− 3T3 cells were reconstituted with vector (MSCV)- or c-Abl-expressing retroviruses and were subjected to human TNF-α-CHX treatment. At the indicated time points, cell death was determined by propidium iodide staining and the sub-G1 population was quantified by FACS analysis.
c-Abl contributes to TNF-α-induced apoptosis.
To determine if c-Abl kinase is required for TNF-α-induced apoptosis, we disrupted its function with a chemical inhibitor, STI-571 (14), and by genetic deletion (51). To avoid the use of CHX, we experimented with the Rb−/− 3T3 fibroblasts, which underwent apoptosis when treated with TNF-α alone (Fig. 1B, panel ii). STI-571 by itself did not affect the viability of Rb−/− 3T3 cells (Fig. 1B, panel iii); however, STI-571 reduced apoptosis induced by TNF-α (Fig. 1B, panel iv, and C). We also compared early-passage MEFs derived from embryos of Rb−/− Abl−/− and Rb−/− Abl+/+ littermates for their apoptotic response to TNF-α. The knockout of the Abl gene significantly reduced TNF-α-induced apoptosis (Fig. 1D), and the cleavage of procaspase 3 was diminished in TNF-α-treated Rb−/− Abl−/− MEFs in comparison to the Rb−/− Abl+/+ MEFs (Fig. 1E). To further determine if c-Abl contributes to TNF-α-CHX-induced apoptosis, we transduced Abl−/− 3T3 fibroblasts with vector or Abl (type Ib) retrovirus and examined their response to TNF-α-CHX. Abl−/− 3T3 fibroblasts underwent apoptosis in response to TNF-α-CHX, suggesting the existence of an Abl-independent apoptotic pathway in these cells. Nonetheless, the reintroduction of c-Abl significantly enhanced the apoptotic response to TNF-α-CHX compared to that of vector-reconstituted cells (Fig. 1F). These results support a role for c-Abl in TNF-α-induced apoptosis.
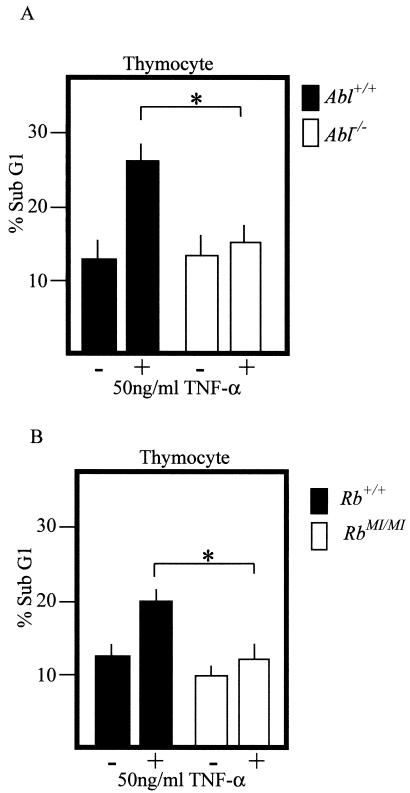
We also isolated thymocytes from Abl+/+ and Abl−/− littermate mice, exposed them to TNF-α for 8 h, and measured apoptosis by the sub-G1 DNA content (Fig. 2A). Thymocytes underwent spontaneous apoptosis during ex vivo culturing, and the knockout of Abl did not affect this death response. Treatment with TNF-α caused a twofold increase in apoptosis of Abl+/+ thymocytes but not that of the Abl−/− thymocytes (Fig. 2A). Since c-Abl was required for TNF-α to kill thymocytes, Rb-MI, which prevented c-Abl kinase activation by TNF-α, should reduce apoptosis. We therefore treated thymocytes isolated from Rb+/+ and RbMI/MI littermates with TNF-α (Fig. 2B). The apoptotic response of RbMI/MI thymocytes to TNF-α was indeed reduced in comparison with that of Rb+/+ thymocytes (Fig. 2B). Attenuation of apoptosis in RbMI/MI thymocytes was observed with different doses of TNF-α ranging from 10 to 100 ng/ml (data not shown). Taken together, results in Fig. 1 and 2 show that the c-Abl tyrosine kinase is activated by TNF-α following Rb degradation and that it contributes to TNF-α-induced apoptosis in fibroblasts and thymocytes.
FIG. 2.
Abl−/− and RbMI/MI thymocytes are resistant to TNF-α-induced apoptosis. (A) Freshly isolated thymocytes from 5- to 7-week-old Abl+/+ and Abl−/− littermates were left untreated (−) or treated with human TNF-α (50 ng/ml) (+) for 8 h. Cell death was determined by the sub-G1 DNA content determined by propidium iodide staining and FACS analysis. (B) Freshly isolated thymocytes from 5- to 7-week-old Rb+/+ and RbMI/MI littermates were left untreated (−) or treated with human TNF-α (50 ng/ml) (+) for 5 h, and the percentages of cell death were determined as described for panel A. *, the percentage of cell death was analyzed by an independent t test. The difference in cell death is statistically significant with P = 0.002 in panel A and P = 0.001 in panel B.
TNF-α activates the proapoptotic function of p73.
The p53-related transcription factor p73 is a downstream effector in c-ABL-dependent apoptosis in response to DNA damage (31). Activated nuclear c-ABL kinase kills p53−/− but not p73−/− murine fibroblasts, and the killing is restored with the ectopic expression of p73α (52). Because c-Abl contributed to TNF-α-induced apoptosis, we examined the role of p73 in this response.
During DNA damage-induced apoptosis, p73 protein is stabilized and its proapoptotic function is activated through a c-ABL-dependent mechanism (1, 10, 21, 64). To determine if TNF-α-CHX induced the accumulation of p73 protein, we experimented with p73−/− cells retrovirally transduced with vector or p73α (Fig. 3A and B). Accumulation of the reconstituted p73α protein was observed upon treatment with DNA-damaging agents, such as cisplatin or 1-β-d-arabinofuranosylcytosine, correlating with Abl kinase activation (50). We show here that doxorubicin-induced p73α accumulation was partially reduced by STI-571, the Abl kinase inhibitor (Fig. 3A, left panel). Treatment with TNF-α-CHX also caused the accumulation of p73α protein, but with a delayed kinetics (Fig. 3A, middle panel) that did not correlate with the onset of apoptosis (Fig. 3B). Treatment with STI-571 caused a small but detectable reduction in this delayed accumulation of p73α (Fig. 3A, right panel), suggesting that Abl kinase plays a minor role in this TNF-α-mediated induction in p73α protein. Although the p73α protein level did not change during the first 24 h of treatment, it had a significant effect on apoptosis (Fig. 3B). The vector murine stem cell virus (MSCV)-reconstituted p73−/− fibroblasts did not die at 24 h after TNF-α-CHX treatment (Fig. 3B). By contrast, expression of p73α significantly enhanced the apoptotic response to TNF-α-CHX (Fig. 3B). To determine if TNF-α-CHX activated the transcription function of p73, we performed luciferase reporter assays using promoters of either p21CIP1 or BAX in transient-cotransfection experiments. We detected a robust transactivation activity of p73α but did not observe any increase in this activity by TNF-α-CHX (not shown). Mihara et al. have shown that p53 can translocate from the nucleus to the mitochondria to activate apoptosis (39). The p73 protein contains a nuclear export signal and appeared to shuttle between the nucleus and the cytoplasm (26). However, we did not detect any significant change in p73 subcellular localization following TNF-α treatment (data not shown).
FIG. 3.
p73 sensitizes cells to TNF-α-induced apoptosis. (A) p73−/− 3T3 cells were reconstituted with vector (MSCV) or hemagglutinin-tagged human p73α-expressing retroviruses. (Left panel) Cells were left untreated or treated with doxorubicin (1 μM) or doxorubicin plus STI-571 (5 μM) for 20 h. (Middle panel) Cells were treated with human TNF-CHX for the indicated time. (Right panel) Cells were left untreated or treated with human TNF-CHX or human TNF-CHX plus STI-571 for 40 h. Whole-cell lysates were isolated and immunoblotted with anti-p73 antibody. (B) 3T3 fibroblasts described for panel A were treated with human TNF-CHX for 20 h followed by FACS analysis. The percentage of cells with sub-G1 DNA content was determined. (C and D) Early-passage Rb−/− 3T3 fibroblasts (C) or Rb−/− p53−/− MEFs (D) were infected with adenoviruses expressing β-galactosidase or ΔN-p73β. Following the infection, cells were treated with mouse TNF-α and fixed at indicated time points. Condensed nuclei were visualized by Hoechst staining and counted. (E) SAOS-2 cells were transfected with the indicated plasmids and enhanced GFP. At 48 h posttransfection, cells were left untreated or treated with human TNF-α-CHX for 8 h and then fixed. The percentage of transfected (GFP+) live cells was determined in the untreated and TNF-α-CHX-treated cultures (counting at least 300 total cells). The percentage of GFP+ cells in TNF-α-CHX-treated cultures was compared to that in untreated cultures for each indicated transfection. The positive scale shows that the percentage of GFP+ cells was higher in TNF-α-CHX-treated culture, and the negative scale shows that the percentage of GFP+ cells was lower in TNF-α-CHX-treated culture. WT, wild type.
The p73 gene encodes several isoforms with N-terminal and C-terminal variations. At the N terminus, two different isoforms with or without the transactivation domain (TA or ΔN) are generated by two alternative promoters (36, 43, 62). At the C terminus, alternative splicing generates α, β, γ, δ, ɛ, and ζ isoforms (31). We prepared recombinant adenovirus expressing ΔN-p73β, which has an antiapoptotic function (22, 43). Infection of Rb−/− 3T3 fibroblasts with adenovirus expressing ΔN-p73β caused a reduction in apoptotic response to TNF-α in comparison to infection with adenovirus expressing β-galactosidase (Fig. 3C). We also prepared primary MEFs from Rb−/− p53−/− embryos and infected them with these two adenoviruses followed by treatment with TNF-α (Fig. 3D). The knockout of p53 reduced but did not prevent TNF-α-induced apoptosis (compare Fig. 3C and D). In the Rb−/− p53−/− MEFs, infection with recombinant adenoviruses expressing ΔN-p73β but not β-galactosidase also reduced the apoptotic response to TNF-α (Fig. 3D). These results are consistent with the notion that the endogenous TA-p73 in these fibroblasts contributes to TNF-α-induced apoptosis.
In addition, we found that TNF-α-CHX enhanced the proapoptotic function of p73α but not p53 (Fig. 3E). Ectopic expression of either p53 or p73α can induce a low level of apoptosis in SAOS-2 cells, an osteosarcoma cell line that does not express RB, p53, or p73α (29, 52). We transfected SAOS-2 cells with GFP plus empty vector, p53, or p73α, treated the transfected cultures with or without TNF-α-CHX, and then compared the percentages of GFP+ cells. In vector- or p53-transfected cultures, the percentage of GFP+ cells after TNF-α-CHX treatment was similar to that in cultures without treatment (Fig. 3E). This indicated that TNF-α-CHX did not enhance the apoptotic function of p53. In contrast, TNF-α-CHX treatment reduced the percentage of GFP+ cells in p73α-transfected culture by 1.8-fold, suggesting that p73α promoted TNF-α-induced cell death. The increased sensitivity of p73α-expressing cells to TNF-α-CHX could be suppressed by RB or RB-MI, with RB-MI exerting better protection against cell death than that of RB (Fig. 3E). These results show that TNF-α-CHX activates, while RB inhibits, the proapoptotic function of p73α.
Differential requirement for p73 and p53 in TNF-α-induced apoptosis.
Since TNF-α-induced apoptosis was compromised in RbMI/MI and Abl−/− thymocytes, we examined the apoptotic response of p73−/− thymocytes to TNF-α (Fig. 4). Compared to thymocytes isolated from p73+/+ littermates, p73−/− thymocytes exhibited a reduced apoptotic response to TNF-α or to TNF-α-CHX (Fig. 4A and B). The p73−/− thymocytes did not have a general defect in apoptosis because they were as sensitive as the p73+/+ thymocytes were to killing by dexamethasone (Fig. 4C). Dexamethasone-induced apoptosis requires new protein synthesis, as it is blocked by CHX, and this was the case with p73+/+ and p73−/− thymocytes (Fig. 4C). Interestingly, thymocytes from p53−/− mice exhibited a similar apoptotic response to TNF-α as did thymocytes from littermate p53+/+ mice (Fig. 4D). Therefore, TNF-α-induced apoptosis in thymocytes requires Rb degradation, c-Abl, and p73 but not p53.
FIG. 4.
p73−/− but not p53−/− thymocytes were resistant to TNF-α-induced apoptosis. (A) Thymocytes were isolated from 4- to 6-week-old p73+/+ or p73−/− mice and treated with mouse TNF-α (50 ng/ml) for 8 h in the absence or presence of CHX. Cell death was determined by FACS analysis of the sub-G1 population. (B) p73+/+ or p73−/− thymocytes described for panel A were treated with the indicated doses of mouse TNF-α (50 ng/ml) for 5 h. Cell death was determined by annexin V binding and propidium iodide uptake. (C) p73+/+ or p73−/− thymocytes described for panel A were treated with dexamethasone (100 ng/ml) for 10 h. Cell death was determined by sub-G1 DNA content. (D) p53+/+ or p53−/− thymocytes were treated with (+) or without (−) human TNF-α (50 ng/ml) for 8 h.
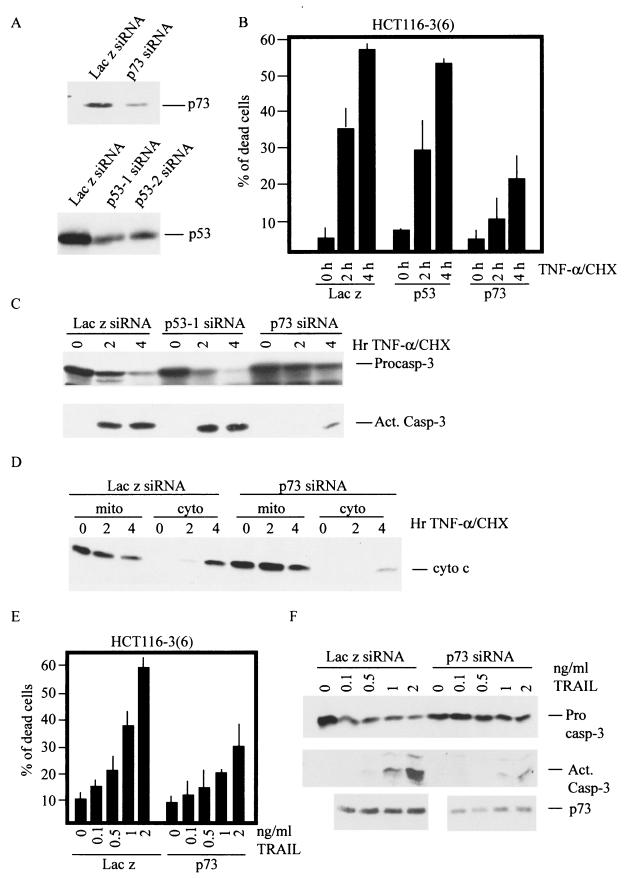
The differential role of p53 and p73 in TNF-α-induced apoptosis was also observed in two human carcinoma cell lines, HCT116-3(6) (Fig. 5) and MCF-7 (data not shown), both expressing wild-type p73α and p53. The p53 or p73 protein was down-regulated by transient transfection with the corresponding siRNA (Fig. 5A). The knockdown of p73 caused an attenuation of TNF-α-CHX-induced apoptosis, whereas knockdown with LacZ siRNA or with p53 siRNA had no effect (Fig. 5B). Correspondingly, p73 siRNA-transfected cells contained lower levels of cleaved (activated) caspase 3 than did LacZ siRNA- or p53 siRNA-transfected cells, when these cells were treated with TNF-α-CHX (Fig. 5C). TNF-α-CHX caused cytochrome c release in LacZ siRNA-transfected cells, but this was significantly reduced in p73 siRNA-transfected cells (Fig. 5D). We also found that p73 siRNA-transfected cells were less sensitive to TRAIL-induced apoptosis (Fig. 5E). Again, p73 siRNA-transfected cells showed reduced cleavage and activation of caspase 3 in response to TRAIL (Fig. 5F). Therefore, p73 plays an important role in TNF-α- and TRAIL-induced cell death and contributes to the activation of the mitochondrion-dependent apoptotic pathway.
FIG. 5.
siRNA-mediated down-regulation of p73 but not p53 suppressed apoptosis triggered by TNF-α or TRAIL. (A) HCT116-3(6) colorectal carcinoma cells were transfected with siRNA against LacZ, p53, or p73. At 48 h posttransfection, whole-cell lysates were isolated and analyzed by immunoblotting with monoclonal anti-p53 antibody or polyclonal anti-p73 antibody. (B to D) HCT116-3(6) cells transfected with the indicated siRNA were treated with human TNF-α-CHX for the indicated amounts of time. Cell death was determined by FACS analysis of sub-G1 population (B). The activation of caspase 3 was determined by immunoblotting with antibodies recognizing procaspase 3 or cleaved caspase 3 (C). Mitochondrial and cytosolic fractions were prepared, and the amount of cytochrome c in each fraction was analyzed by immunoblotting with anti-cytochrome c antibody (D). (E and F) HCT116-3(6) cells transfected with the indicated siRNA were treated with human TRAIL at the indicated doses for 4 h. Cell death was determined by FACS analysis of the sub-G1 population (E). The activation of caspase 3 was determined by immunoblotting with antibodies to procaspase 3 or cleaved caspase 3 (F).
DISCUSSION
In this report, we show RB degradation to be required for TNF-α to activate the nuclear c-Abl kinase, which contributes to apoptosis (Fig. 1). TNF-α also activates the proapoptotic function of p73 (Fig. 3). Thymocytes from Abl−/−, p73−/−, or RbMI/MI mice are resistant to TNF-α-induced apoptosis (Fig. 2 and 4). By contrast, thymocytes from p53−/− mice show a TNF-α-induced apoptotic response indistinguishable from that of p53+/+ mice (Fig. 4). Knockdown of p73, but not p53, by siRNA in human carcinoma cell lines diminishes TNF-α- and TRAIL-induced apoptosis (Fig. 5). Loss of c-Abl or p73 reduces the release of cytochrome c and the cleavage of procaspase 3 in response to TNF-α (Fig. 1 and 5). Taken together, these results are consistent with a pathway in which c-ABL and p73 are downstream of RB in TNF-α-induced apoptosis (Fig. 6). In this pathway, caspase-dependent removal of RB allows the activation of c-ABL and p73, which contribute to the mitochondrion-dependent apoptotic mechanism (Fig. 6).
FIG. 6.
Caspase-dependent elimination of RB unleashes nuclear apoptotic effectors. Stimulation of TNFRI leads to the activation of initiator caspase (i-caspase), which can directly activate executioner caspase (e-caspase). We envision the existence of amplifier caspase (a-caspase), whose function is to cleave the nuclear RB protein, leading to the activation of E2F1, c-ABL, and p73. E2F1 has been shown to induce transcription in the TA-p73 promoter (27, 33, 47). c-ABL has been shown to activate the apoptotic function of p73 (1, 10, 21, 64). Activation of c-ABL and p73 contributes to the apoptotic response to TNF-α. TNF-α also activates NF-κB (25), which induces the expression of caspase inhibitors such as IAPs and FLIP to antagonize apoptosis (37, 55). NF-κB also suppresses the expression of p73. Taken together, the present evidence suggests that nuclear events are involved in determining the apoptotic response to TNF-α.
The c-ABL tyrosine kinase induces apoptosis when it is activated in the nucleus by DNA damage or TNF-α (1, 12, 21, 57, 64). Even the oncogenic BCR-ABL tyrosine kinase of chronic myelogenous leukemia can activate apoptosis, if it is trapped in the nucleus (53). The proapoptotic function of c-ABL tyrosine kinase is regulated by a combination of signals. For example, DNA damage activates nuclear c-ABL kinase through ATM (4). However, DNA damage cannot activate c-ABL in G0/G1 cells (34), nor in fibroblasts deprived of adhesion signals (50). In this study, we show that TNF-α does not activate c-ABL unless RB is degraded. In other words, the loss of RB is a prerequisite for c-ABL activation. With Rb−/− cells, a higher basal c-Abl kinase activity is observed, and it is further increased in response to TNF-α (Fig. 1A), suggesting that RB loss is not the only mechanism of c-ABL activation. This and other observations on the regulation of c-ABL kinase have led to a model in which c-ABL is regulated by “autoinhibition” and “coinhibition” (56). In this model, RB functions as a coinhibitor to sequester ABL in its autoinhibited conformation. RB degradation liberates c-ABL, allowing it to be further released from its autoinhibited state by other mechanisms to gain catalytic function (56). Since c-ABL tyrosine kinase can be activated by growth factors and cell adhesion to regulate F-actin (61), a multilayered control of its apoptotic function is probably necessary to avoid unintended cell death.
In the DNA damage response, p73 is stabilized and is thought to trigger cell death by activating the transcription of proapoptotic genes (10). In E1A-immortalized p63-p73 double-knockout fibroblasts, p53 does not bind to promoters of proapoptotic genes (17). This result suggests collaboration between p53 family members in the transcriptional regulation of apoptosis. We have found that ΔN-p73β, a p73 isoform lacking the amino-terminal transactivating domain, reduced TNF-α-induced apoptosis (Fig. 3C and D). The simplest interpretation of this result would be that the transactivation function of p73 is required. However, apoptotic response to TNF-α occurs without new gene expression and is enhanced by CHX. Therefore, TNF-α-mediated activation of p73 is not likely to require the upregulation of proapoptotic genes. Perhaps the full-length p73 tetramer contributes to apoptotic signaling through a mechanism independent of transcription, and this alternative function also is disrupted by ΔN-p73β. It has been reported that p53 can induce apoptosis through a transcription-independent pathway (9, 13), e.g., p53 can promote cell death by binding to Bcl-xL (39). Whether p73 directly promotes the release of apoptogenic factors from the mitochondria will await further investigation. The role of p53 in TNF-α-induced apoptosis has been controversial (2, 42, 44). In this study, we have found that p53 is dispensable for TNF-α-induced apoptosis in thymocytes and carcinoma cells, although p53 might contribute to TNF-α-induced apoptosis in Rb−/− fibroblasts, because Rb−/− p53−/− shows a reduced apoptotic response compared to that of Rb−/− p53+/+ fibroblasts. By contrast, we have found p73 to be required for TNF-α-induced apoptosis in mouse fibroblasts, thymocytes, and human carcinoma cell lines. These results suggest that p53 and p73 have nonredundant functions in TNF-α-induced apoptosis.
It is well established that p73 is essential for transmitting apoptotic signals resulting from DNA damage (17, 31, 36). Here, we show p73 to be also required for transmitting an apoptotic signal downstream of death receptor (TNF-α and TRAIL) stimulation. The involvement of p73 in transmitting both intrinsic and extrinsic apoptotic signals highlights its importance in apoptosis regulation. As discussed above, the p73 gene generates proapoptotic (TA) as well as antiapoptotic (ΔN) isoforms. Previous studies have shown that TA-p73 isoforms are upregulated by E2F1 (Fig. 6) and account for E2F1-induced apoptosis (27, 36, 41). Splenic T cells from E2f1- or p73-knockout mice appeared to be defective in activation-induced cell death (27, 33), and E2f1-deficient thymocytes are more resistant to spontaneous cell death during ex vivo culturing (16). Thus, RB degradation can cause increased expression of TA-p73 through E2F1-dependent transcription and activation of TA-p73 function through c-ABL tyrosine kinase (Fig. 6). TNF-α activates NF-κB to inhibit apoptosis (49). Interestingly, activation of NF-κB has been linked to the repression of p73, through an as yet unknown mechanism (54) (Fig. 6). The NF-κB-mediated suppression of p73 expression is required for the survival of antigen-activated T cells (54). In adult sympathetic neurons, nerve growth factor maintains the expression of ΔNp73 to promote survival (43). Taken together, these results suggest that p73 isoforms play a pivotal role in regulating cell survival and apoptosis. The p73 gene is rarely mutated in sporadic human cancers; as a result, activation of p73 by cisplatin and other chemotherapeutic agents has been shown to contribute to the killing of cancer cells (5, 21, 28). We have found that the knockdown of p73 in cancer cells reduces TRAIL-induced apoptosis. Thus, the activity of p73 may be an important prognosis factor for the effectiveness of cancer therapies.
The involvement of RB degradation, nuclear c-ABL activation, and p73 in TNF-α-induced apoptosis suggests that nuclear events participate in the regulation of this death response (Fig. 6). Studies of extrinsic cell death pathways, largely based on FAS-mediated apoptosis, have led to the conclusion that the nucleus is dispensable for death receptor-induced killing (15). While this conclusion is correct, it may not apply to TNF-α-induced apoptosis. Unlike FAS, TNFRI does not recruit FADD or procaspase 8. Instead, TNFRI induces the formation of a cytoplasmic TRADD-FADD-procaspase 8 complex to initiate apoptotic signal transduction (24, 38). Interestingly, TRADD and FADD appear to undergo nucleocytoplasmic shuttling (20, 40, 45). Similarly, both c-ABL and p73 contain nuclear localization and nuclear export signals and undergo nucleocytoplasmic shuttling (26, 48). Further investigation of how signaling to and from the nucleus contributes to the regulation and the execution of TNF-α-induced apoptosis will shed light on the physiological function of this pleiotropic cytokine.
Acknowledgments
We thank members of the Wang lab for their critical comments throughout the course of this work. We are particularly indebted to Sewon Ki for reconstituting HA-p73α expression in p73−/− fibroblasts and to Irina Hunton for generating Rb−/− Abl−/− and Rb−/− p53−/− fibroblasts.
B.N.C. is a Robert Black Fellow of the Damon Runyon Cancer Research Foundation (DRG-1725-02). This work was supported by NIH grant CA58320 to J.Y.J.W. and American Cancer Society grant RSG LIB-101051 to J.D.
REFERENCES
- 1.Agami, R., G. Blandino, M. Oren, and Y. Shaul. 1999. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature 399:809-813. [DOI] [PubMed] [Google Scholar]
- 2.Ameyar, M., V. Shatrov, C. Bouquet, C. Capoulade, Z. Cai, R. Stancou, C. Badie, H. Haddada, and S. Chouaib. 1999. Adenovirus-mediated transfer of wild-type p53 gene sensitizes TNF resistant MCF7 derivatives to the cytotoxic effect of this cytokine: relationship with c-myc and Rb. Oncogene 18:5464-5472. [DOI] [PubMed] [Google Scholar]
- 3.Barila, D., A. Rufini, I. Condo, N. Ventura, K. Dorey, G. Superti-Furga, and R. Testi. 2003. Caspase-dependent cleavage of c-Abl contributes to apoptosis. Mol. Cell. Biol. 23:2790-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskaran, R., L. D. Wood, L. L. Whitaker, C. E. Canman, S. E. Morgan, Y. Xu, C. Barlow, D. Baltimore, A. Wynshaw-Boris, M. B. Kastan, and J. Y. Wang. 1997. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature 387:516-519. [DOI] [PubMed] [Google Scholar]
- 5.Bergamaschi, D., M. Gasco, L. Hiller, A. Sullivan, N. Syed, G. Trigiante, I. Yulug, M. Merlano, G. Numico, A. Comino, M. Attard, O. Reelfs, B. Gusterson, A. K. Bell, V. Heath, M. Tavassoli, P. J. Farrell, P. Smith, X. Lu, and T. Crook. 2003. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 3:387-402. [DOI] [PubMed] [Google Scholar]
- 6.Chau, B. N., H. L. Borges, T. T. Chen, A. Masselli, I. C. Hunton, and J. Y. Wang. 2002. Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat. Cell Biol. 4:757-765. [DOI] [PubMed] [Google Scholar]
- 7.Chau, B. N., and J. Y. Wang. 2003. Coordinated regulation of life and death by RB. Nat. Rev. Cancer 3:130-138. [DOI] [PubMed] [Google Scholar]
- 8.Chen, G., and D. V. Goeddel. 2002. TNF-R1 signaling: a beautiful pathway. Science 296:1634-1635. [DOI] [PubMed] [Google Scholar]
- 9.Chipuk, J. E., U. Maurer, D. R. Green, and M. Schuler. 2003. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell 4:371-381. [DOI] [PubMed] [Google Scholar]
- 10.Costanzo, A., P. Merlo, N. Pediconi, M. Fulco, V. Sartorelli, P. A. Cole, G. Fontemaggi, M. Fanciulli, L. Schiltz, G. Blandino, C. Balsano, and M. Levrero. 2002. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell 9:175-186. [DOI] [PubMed] [Google Scholar]
- 11.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551-1570. [DOI] [PubMed] [Google Scholar]
- 12.Dan, S., M. Naito, H. Seimiya, A. Kizaki, T. Mashima, and T. Tsuruo. 1999. Activation of c-Abl tyrosine kinase requires caspase activation and is not involved in JNK/SAPK activation during apoptosis of human monocytic leukemia U937 cells. Oncogene 18:1277-1283. [DOI] [PubMed] [Google Scholar]
- 13.Ding, H. F., Y. L. Lin, G. McGill, P. Juo, H. Zhu, J. Blenis, J. Yuan, and D. E. Fisher. 2000. Essential role for caspase-8 in transcription-independent apoptosis triggered by p53. J. Biol. Chem. 275:38905-38911. [DOI] [PubMed] [Google Scholar]
- 14.Druker, B. J., S. Tamura, E. Buchdunger, S. Ohno, G. M. Segal, S. Fanning, J. Zimmermann, and N. B. Lydon. 1996. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 2:561-566. [DOI] [PubMed] [Google Scholar]
- 15.Enari, M., A. Hase, and S. Nagata. 1995. Apoptosis by a cytosolic extract from Fas-activated cells. EMBO J. 14:5201-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field, S. J., F. Y. Tsai, F. Kuo, A. M. Zubiaga, W. G. Kaelin, Jr., D. M. Livingston, S. H. Orkin, and M. E. Greenberg. 1996. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell 85:549-561. [DOI] [PubMed] [Google Scholar]
- 17.Flores, E. R., K. Y. Tsai, D. Crowley, S. Sengupta, A. Yang, F. McKeon, and T. Jacks. 2002. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416:560-564. [DOI] [PubMed] [Google Scholar]
- 18.Goga, A., X. Liu, T. M. Hambuch, K. Senechal, E. Major, A. J. Berk, O. N. Witte, and C. L. Sawyers. 1995. p53 dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene 11:791-799. [PubMed] [Google Scholar]
- 19.Goldberg, Z., R. Vogt Sionov, M. Berger, Y. Zwang, R. Perets, R. A. Van Etten, M. Oren, Y. Taya, and Y. Haupt. 2002. Tyrosine phosphorylation of Mdm2 by c-Abl: implications for p53 regulation. EMBO J. 21:3715-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Angelats, M., and J. A. Cidlowski. 2003. Molecular evidence for the nuclear localization of FADD. Cell Death Differ. 10:791-797. [DOI] [PubMed] [Google Scholar]
- 21.Gong, J. G., A. Costanzo, H. Q. Yang, G. Melino, W. G. Kaelin, Jr., M. Levrero, and J. Y. Wang. 1999. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399:806-809. [DOI] [PubMed] [Google Scholar]
- 22.Grob, T. J., U. Novak, C. Maisse, D. Barcaroli, A. U. Luthi, F. Pirnia, B. Hugli, H. U. Graber, V. De Laurenzi, M. F. Fey, G. Melino, and A. Tobler. 2001. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ. 8:1213-1223. [DOI] [PubMed] [Google Scholar]
- 23.Gupta, S. 2002. A decision between life and death during TNF-alpha-induced signaling. J. Clin. Immunol. 22:185-194. [DOI] [PubMed] [Google Scholar]
- 24.Harper, N., M. Hughes, M. MacFarlane, and G. M. Cohen. 2003. Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis. J. Biol. Chem. 278:25534-25541. [DOI] [PubMed] [Google Scholar]
- 25.Hsu, H., H. B. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 26.Inoue, T., J. Stuart, R. Leno, and C. G. Maki. 2002. Nuclear import and export signals in control of the p53-related protein p73. J. Biol. Chem. 277:15053-15060. [DOI] [PubMed] [Google Scholar]
- 27.Irwin, M., M. C. Marin, A. C. Phillips, R. S. Seelan, D. I. Smith, W. Liu, E. R. Flores, K. Y. Tsai, T. Jacks, K. H. Vousden, and W. G. Kaelin, Jr. 2000. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407:645-648. [DOI] [PubMed] [Google Scholar]
- 28.Irwin, M. S., K. Kondo, M. C. Marin, L. S. Cheng, W. C. Hahn, and W. G. Kaelin. 2003. Chemosensitivity linked to p73 function. Cancer Cell 3:403-410. [DOI] [PubMed] [Google Scholar]
- 29.Jost, C. A., M. C. Marin, and W. G. Kaelin, Jr. 1997. p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 389:191-194. [DOI] [PubMed] [Google Scholar]
- 30.Lassus, P., J. Rodriguez, and Y. Lazebnik. 2002. Confirming specificity of RNAi in mammalian cells. Sci. STKE 2002:PL13. [DOI] [PubMed]
- 31.Levrero, M., V. De Laurenzi, A. Costanzo, J. Gong, J. Y. Wang, and G. Melino. 2000. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J. Cell Sci. 113:1661-1670. [DOI] [PubMed] [Google Scholar]
- 32.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 33.Lissy, N. A., P. K. Davis, M. Irwin, W. G. Kaelin, and S. F. Dowdy. 2000. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature 407:642-645. [DOI] [PubMed] [Google Scholar]
- 34.Liu, Z. G., R. Baskaran, E. T. Lea-Chou, L. D. Wood, Y. Chen, M. Karin, and J. Y. Wang. 1996. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature 384:273-276. [DOI] [PubMed] [Google Scholar]
- 35.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 36.Melino, G., V. De Laurenzi, and K. H. Vousden. 2002. p73: friend or foe in tumorigenesis. Nat. Rev. Cancer 2:605-615. [DOI] [PubMed] [Google Scholar]
- 37.Micheau, O., S. Lens, O. Gaide, K. Alevizopoulos, and J. Tschopp. 2001. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micheau, O., and J. Tschopp. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181-190. [DOI] [PubMed] [Google Scholar]
- 39.Mihara, M., S. Erster, A. Zaika, O. Petrenko, T. Chittenden, P. Pancoska, and U. M. Moll. 2003. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11:577-590. [DOI] [PubMed] [Google Scholar]
- 40.Morgan, M., J. Thorburn, P. P. Pandolfi, and A. Thorburn. 2002. Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms. J. Cell Biol. 157:975-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips, A. C., M. K. Ernst, S. Bates, N. R. Rice, and K. H. Vousden. 1999. E2F-1 potentiates cell death by blocking antiapoptotic signaling pathways. Mol. Cell 4:771-781. [DOI] [PubMed] [Google Scholar]
- 42.Piguet, P. F., C. Vesin, J. Guo, Y. Donati, and C. Barazzone. 1998. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur. J. Immunol. 28:3499-3505. [DOI] [PubMed] [Google Scholar]
- 43.Pozniak, C. D., S. Radinovic, A. Yang, F. McKeon, D. R. Kaplan, and F. D. Miller. 2000. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science 289:304-306. [DOI] [PubMed] [Google Scholar]
- 44.Ravi, R., and A. Bedi. 2002. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L). Cancer Res. 62:1583-1587. [PubMed] [Google Scholar]
- 45.Screaton, R. A., S. Kiessling, O. J. Sansom, C. B. Millar, K. Maddison, A. Bird, A. R. Clarke, and S. M. Frisch. 2003. Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: a potential link between genome surveillance and apoptosis. Proc. Natl. Acad. Sci. USA 100:5211-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sionov, R. V., E. Moallem, M. Berger, A. Kazaz, O. Gerlitz, Y. Ben-Neriah, M. Oren, and Y. Haupt. 1999. c-Abl neutralizes the inhibitory effect of Mdm2 on p53. J. Biol. Chem. 274:8371-8374. [DOI] [PubMed] [Google Scholar]
- 47.Stiewe, T., and B. M. Putzer. 2000. Role of the p53-homologue p73 in E2F1-induced apoptosis. Nat. Genet. 26:464-469. [DOI] [PubMed] [Google Scholar]
- 48.Taagepera, S., D. McDonald, J. E. Loeb, L. L. Whitaker, A. K. McElroy, J. Y. Wang, and T. J. Hope. 1998. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc. Natl. Acad. Sci. USA 95:7457-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-κB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 50.Truong, T., G. Sun, M. Doorly, J. Y. Wang, and M. A. Schwartz. 2003. Modulation of DNA damage-induced apoptosis by cell adhesion is independently mediated by p53 and c-Abl. Proc. Natl. Acad. Sci. USA 100:10281-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tybulewicz, V. L., C. E. Crawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 52.Vella, V., J. Zhu, F. Frasca, C. Y. Li, P. Vigneri, R. Vigneri, and J. Y. Wang. 2003. Exclusion of c-Abl from the nucleus restrains the p73 tumor suppression function. J. Biol. Chem. 278:25151-25157. [DOI] [PubMed] [Google Scholar]
- 53.Vigneri, P., and J. Y. Wang. 2001. Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR-ABL tyrosine kinase. Nat. Med. 7:228-234. [DOI] [PubMed] [Google Scholar]
- 54.Wan, Y. Y., and J. DeGregori. 2003. The survival of antigen-stimulated T cells requires NFκB-mediated inhibition of p73 expression. Immunity 18:331-342. [DOI] [PubMed] [Google Scholar]
- 55.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 56.Wang, J. Y. 2004. Controlling Abl: auto-inhibition and co-inhibition? Nat. Cell Biol. 6:3-7. [DOI] [PubMed] [Google Scholar]
- 57.Wang, J. Y. 2000. Regulation of cell death by the Abl tyrosine kinase. Oncogene 19:5643-5650. [DOI] [PubMed] [Google Scholar]
- 58.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933. [PubMed] [Google Scholar]
- 59.Welch, P. J., and J. Y. Wang. 1993. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell 75:779-790. [DOI] [PubMed] [Google Scholar]
- 60.Welch, P. J., and J. Y. Wang. 1995. Disruption of retinoblastoma protein function by coexpression of its C pocket fragment. Genes Dev. 9:31-46. [DOI] [PubMed] [Google Scholar]
- 61.Woodring, P. J., T. Hunter, and J. Y. Wang. 2003. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J. Cell Sci. 116:2613-2626. [DOI] [PubMed] [Google Scholar]
- 62.Yang, A., N. Walker, R. Bronson, M. Kaghad, M. Oosterwegel, J. Bonnin, C. Vagner, H. Bonnet, P. Dikkes, A. Sharpe, F. McKeon, and D. Caput. 2000. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404:99-103. [DOI] [PubMed] [Google Scholar]
- 63.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
- 64.Yuan, Z. M., H. Shioya, T. Ishiko, X. Sun, J. Gu, Y. Y. Huang, H. Lu, S. Kharbanda, R. Weichselbaum, and D. Kufe. 1999. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature 399:814-817. [DOI] [PubMed] [Google Scholar]