Abstract
Platelet gel, a fibrin network containing activated platelets, is widely used in regenerative medicine due the capacity of platelet-derived growth factors to accelerate and direct healing processes. However, limitations to this approach include poor mechanical properties, relatively rapid degradation, and the lack of control of release of growth factors at the site of injection. These issues compromise the ability of platelet gels for sustained function in regenerative medicine. In the present study, a combination of platelet gels with silk fibroin gel was studied to address the above limitations. Mixing sonicated silk gels with platelet gels extended the release of growth factors without inhibiting gel forming ability. The released growth factors were biologically active and their delivery was modified further by manipulation of the charge of the silk protein. Moreover, the silk gel augmented both the rheological properties and compressive stiffness of the platelet gel, tuned by the silk concentration and/or silk/platelet gel ratio. Silk-platelet gel injections in nude rats supported enhanced cell infiltration and blood vessel formation representing a step towards new platelet gel formulations with enhanced therapeutic impact.
Keywords: platelet gel, silk biomaterials, growth factor delivery, silk gel
1. Introduction
Platelets play a vital role in normal hemostasis and wound healing. For many years, the scientific community has recognized the role of platelets in tissue repair [1–4]. Recently, commercial entities have investigated implantable or injectable therapies for clinical use that are enriched with supra-physiological levels of platelet growth factors [5, 6]. In particular, the benefits of one-time injections of Platelet Rich Plasma (PRP) or platelet gel (PG), in the presence of autologous thrombin and fibrinogen have been explored, with fibrin hydrogel networks containing activated platelets that assemble in 10–20 minutes. PG can be exogenously applied to wound tissues, conferring benefits due to the released growth factors and the ability to localize the platelet concentrates in the site of injury, a method that was more efficient than the use of recombinant growth factors [7]. The use of PG also provides a microenvironment for the sequential process of tissue regeneration involving migration, proliferation and differentiation of osteogenic and endothelial cells [8, 9]. Because of a predictable yet transient liquid (pre-gel) status, PG can be injected alone or in combination with different bone substitutes to avoid the unwanted migration of bone particles [10]. The combination of PG with bone allografts as scaffolds, and with bone marrow stromal cells, can increase the efficacy of PG in animal studies and in clinical applications [11, 12].
Although most clinical studies report good outcomes from the use of PRP for the enhancement of healing, many questions remain, particularly with regard to the timing of the therapy and the volume and frequency of treatment [13]. One feature of PRP that can limit efficacy is the rapid and uncontrolled release of growth factors at the site of injection [14, 15]. In the absence of long-lasting growth factor (GF) availability, repeated PRP applications are required in order to achieve sustained therapeutic effects. Moreover, while the diversity and quantity of GFs derived from activated platelets are biologically unique, especially compared to factors stored in extracellular matrices (ECM) or those presented by cells at the site of tissue injury, PRP injections cannot supply specific factors in a sequence to fit the needs of true regenerative healing. Since it has been demonstrated that the outcome of healing at a wound site is influenced by fibrin structure, in terms of thickness of fibers, number of branch points, porosity, and permeability of the clot [16], a PG-based method could therefore improve outcomes via mechanical augmentation of the entrapping gel phase.
The objective of the present work was to utilize silk protein gels in combination with platelet gels in injectable formats, to improve mechanical properties and growth factor sustained release. The use of silk sonicated silk solutions modulated the release of bioactive platelet growth factors and enhanced the mechanical properties of the system.
2. Material and methods
2.1. Materials
Human peripheral blood was purchased from Research Blood Component, LLC (Boston, MA). Calcium gluconate was purchased from APP Pharmaceuticals (Lake Zurich, IL). Bombyx mori silkworm cocoons were supplied by Tajima Shoji Co., LTD (Yokohama, Japan). Histology reagents including ε-poly-L-lysine and Masson’s Trichrome were purchased from Sigma Aldrich (St. Louis, MO). Silk filtration and purification were conducted using dialysis tubing from Spectrum Laboratories Inc. (Rancho Dominguez, CA) and centrifugal filter units from Millipore (Billerica, MA). The CellTiter Cell Proliferation Assay (MTS) was purchased from Promega (Madison, WI). Cell proliferation assays were conducted using human umbilical vein endothelial cells (HUVECs; Cambrex, East Rutherford, NJ) with control media (endothelial cell basal medium-2) and complete media (endothelial cell basal medium-2, hydrocortisone, human Epidermal Growth Factor (hEGF), Fetal Bovine Serum (FBS), Vascular Endothelial Growth Factor (VEGF), basic Fibroblast Growth Factor-B (bFGF-B), human Recombinat Insulin-like Growth Factor (R3-IGF-1), ascorbic acid, heparin). All cell culture media components were obtained from Lonza (Hopkinton, MA) and used at standard concentrations. The following antibodies were used: mouse anti-phospho ERK (extracellular signal-regulated kinases), mouse anti-actin from Cell Signaling (Danvers, MA), rabbit anti-vascular endothelial (VE) cadherin from LifeSpan Bioscences (Seattle, WA) and rabbit anti-CD31 from Abcam (Cambridge, MA). UO 126 was from Calbiochem (San Diego, CA). Human VEGF, Trasforming growth factor β1 (TGF-β1), Platelet-Derived Growth Factor-AB (PDGF-AB) DuoSet and human VEGF affinity purified polyclonal antibody were purchased from R&D system (Minneapolis, MN). Recombinant-VEGF-165 was purchased from Shenandoah Biotechnology Inc. (Warwick, PA).
2.2. Platelet gel (PG) preparation
Human platelets were derived from whole blood taken from healthy volunteers under New England Institutional Review Board approval (# 04-144 “The Collection of Whole Blood for Research Purposes”) obtained by Research Blood Components (Brighton, MA). The samples were prepared in citric acid/citrate/dextrose solution and maintained sterile over the entire process of PG preparation. Whole blood was centrifuged at 120xg for 15 minutes to obtain platelet rich-plasma. PRP was subsequently centrifuged at 100xg for 15 minutes to eliminate leukocytes in the supernatant. Platelets were recovered by an additional centrifugation at 720xg for 15 minutes to obtain a pellet of platelets and a supernatant of plasma poor of platelets (PPP). Platelet count was adjusted to a final concentration of 4 × 106 platelets/μL by re-suspending platelets in PPP. Autologous thrombin was prepared by mixing (5:1, v/v) PPP with 0.22 M calcium gluconate [17]. After 15 minutes incubation at 37°C and centrifugation at 1000xg for 15 minutes, the thrombin-containing supernatant was collected. PGs were obtained by mixing PRP (final concentration 2 × 106 platelets/μL)/autologous thrombin/calcium gluconate 0.22 M (ratio 8:2:1) and incubated in a humidified chamber at 37°C until use. To evaluate growth factor release from PG, 500 μL of PBS was added to each sample after gelation, which occurred in about 20 minutes. Separately, plasma enriched in growth factors was formed by removal of the fibrin gel, diluted 1:1 with phosphate buffered saline (PBS; Invitrogen, Grand Island, NY) or 1% w/v silk solution. For growth factor studies, the samples were stored at 37°C, or room temperature or 4°C.
2.3. Silk gel preparation
Silk fibroin aqueous solution was obtained from B. mori silkworm cocoons using previously described procedures [18]. Briefly, following extraction in boiling ultra pure water containing 0.02M Na2CO3, fibroin was dissolved in 9.3M LiBr and dialyzed using a 3.5 kDa cut-off dialysis cassette (Pierce Thermo Scientific Inc., Rockford, IL). The boiling time was modified from 30–60 minutes in certain experiments, as indicated, in order to modify the molecular weight of the silk solution as previously described [19]. The resulting 6–8% (w/v) fibroin solution was diluted in ultra pure water to obtain a 4% (w/v) silk solution, or concentrated by placing the solution in the 3.5 kDa dialysis cassettes and letting the excess of water evaporate at RT for periods of time depending on the desired concentration. Solutions were then sterilized by 0.2 μm filtration (Millipore) or by standard 20 minute liquid autoclave cycle, as previously reported to sterilize solutions prior to sonication [20]. The resultant solutions were sonicated at different amplitudes and time with a Branson 450 Sonifier (Branson Ultrasonics Co., Danbury, CT) [20]. In order to investigate the impact of the charges in growth factor release, 10% w/v ε-poly-L-lysine or 0.1 % w/v silk fibroin ionomers (silk fibroin-poly-L-lysine and silk fibroin-poly-L-glutamic acid ionomers) [21] were added to the 4% silk solution prior to the sonication. The sonicated silk solution, cooled to room temperature in ice for 1 minute, was subsequently mixed in a ratio 1:1 with PRP, generating PG-Silk. A volume of 500 μL of the solution was incubated in 24 well plates in an incubator at 37°C to promote gelation. A volume of 500 μL of PBS was then added to each sample, after gelation was visually confirmed. At different time points (1 hour, 1 day, 7 days, 14 days, 21 days) the PBS from each sample was collected and frozen at −80°C, and completely replaced with fresh PBS.
At the last time point of incubation, the silk/platelet gel samples (500 μL/well) were solubilized in 500 μL urea 8M at 37°C, a process which required 30 minutes for completion based on visual confirmation. Residual salt was removed by dialysis against deionized water (by using dialysis tubing with MWCO 2kDa) for 16 hours. The solution obtained was concentrated to a final volume of 500 μL by using centrifugal filter units (3kDa cut-off).
In some experiments, PG-Silk samples mixed with silk fibroin ionomers were analyzed for their ability to swell. The weight of the samples was recorded 1 hour after preparation. After adding 500 μL of PBS, samples were allowed to swell for 24 hours and measured again, and the percentage weight gain was calculated.
2.4. Growth factor evaluation
PG-Silk was evaluated for growth factor release at 1 hour, 1 day, 7 days, 14 days and 21 days incubation. The platelet gels were removed from 37°C storage, centrifuged at 2000xg for 15 minutes at RT in order to separate the gel and the PBS supernatant rich in growth factors, which was immediately stored at −80°C. At the final 21 day collection point, the PG-Silk samples were solubilized in 500 μL urea 8M for 30 minutes at 37°C, as described above. The collected growth factor solutions were quantified through enzyme-linked immunosorbent assay (ELISA) kits for platelet-derived growth factor-AB (PDGF-AB), transforming growth factor-beta1 (TGF-β1) and vascular endothelial growth factor (VEGF) according to manufacturer’s instructions.
The theoretical net charges at neutral pH of the growth factors analyzed, including VEGF, PDGF-AB and TGF-β1, were calculated from the primary sequences, obtained from the UniProt Knowledgebase (http://uniprot.org), by using the Innovagen Peptide property calculator software (http://pepcalc.com).
2.5. Rheometer testing
In order to monitor the gelation kinetics and capture the rheological shear stiffness of the various PG-Silk formulations, several designated samples of silk solution diluted in water, or PPP, or PG (ratio 1:1) were prepared for rheology analysis and compared to platelet gel alone. Dynamic oscillatory time sweeps were performed using an ARES strain-controlled rheometer (TA Instruments, New Castle, DE) with 25-mm-diameter stainless steel cone-and-plate geometry (1° cone angle) at 0.051-mm measuring gap distance. In a typical experiment, the silk solution was applied slowly via pipette on the rheometer plate to prevent shearing of the sample immediately after sonication. The normal force applied on the sample during lowering of the top plate was limited to 20 grams. A low viscosity mineral oil was used to prevent sample evaporation from the sides of the plate, as previously described [22]. An air-driven oven was used to control temperature at 37°C for the duration of the test. Dynamic oscillatory time sweeps were collected at a low strain amplitude (γ = 1%, ω = 3.14 rad/s) to prevent possible sample damage due to applied shear during measurements. This testing protocol was applied continuously over 1 hour for PG alone, which decayed quickly after reaching its maximum, and 16 hours for all silk containing groups, which asymptotically approached a maximum threshold over that time span.
2.6. Compression testing
For unconfined compression testing, silk solutions were generated from cocoons boiled from 30- to 60-minutes and all solutions with concentration ranging from 4 w/v% to 12 w/v%. Where possible, sterilization via filtration or autoclave cycle was performed in advance of sonication. For all groups, a larger volume of sonicated silk (2mL–3mL) was mixed with either PPP or PG in a 1:1 ratio and cast into 30cm petri dishes [20, 23]. Plugs were prepared after gelation using a 6 mm inner diameter biopsy punch. The gel plugs were left in PBS prior to testing. A strain-to-failure test was used to extract an elastic modulus. A minimum of N=4 samples were evaluated and were tested on a 3366 Instron machine (Norwood, MA) equipped with unconfined compression platens and a 100 Newton (N) load transducer and sample data exported using Bluehill Software Version 2.0. Each sample was compressed at a strain rate of 0.1 %/s, beginning after nominal tare loads (0.005N) were reached and sample heights recorded. The compressive stress and strain were determined by normalizing against sample geometries and the elastic modulus was calculated as the best fit linear regression established at a 5% strain range of each stress/strain curve [20, 23].
2.7. Cell proliferation assay
HUVECs were seeded in 96 well plates at a density of 5,000 cells/well, supplemented with control medium. One day later, media was fully replaced with complete medium in positive control groups, control medium containing 25% (v/v) PG-Silk supernatant in experimental groups, or control medium alone in negative control groups. During this step the cells that did not initially attached to the plates were removed. After 3 days, the MTS assay was performed by following the manufacturer’s instructions. The absorbance was measured at 490 nm in a microplate reader (Molecular Devices, Sunnyvale, CA). In order to evaluate molecular pathway activation, cells were prepared as described above and, one day after seeding, HUVECs were supplemented with control medium containing 25% (v/v) PG-Silk supernatant with 10 μM UO 126, a pharmacological inhibitor of ERK, or 10 μg/mL anti-VEGF blocking antibody. After 48 hours the MTS ((3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium) assay was performed.
2.8. Western immunoblotting
After 2 hours of incubation with UO 126 or anti-VEGF blocking antibody, HUVECs were washed with PBS and lysed with RIPA buffer for 20 minutes in ice. The lysates were clarified by centrifugation at 15700xg at 4°C for 15 minutes. Protein concentration was measured by the bicinchoninic acid assay (Pierce, Rockford, IL), according to the manufacturer’s instructions. Samples containing equal amounts of proteins were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then immunoblotted with antibodies against pERK (1:1000) or actin (1:1000). Immunoreactive bands were detected by a horseradish peroxidase-labeled secondary antibody, using an enhanced chemiluminescence reagent.
2.9. In vivo injection of PG-Silk formulations
Animals were cared for in compliance with Tufts University Institutional Animal Care and Use Committee (IACUC) in accordance with the Office of Laboratory Animal Welfare (OLAW) at the National Institutes of Health (NIH). Athymic nude rats (RNU, Charles River) were allowed to acclimate for 1 week prior to implantation and maintained in sterile housing. The animals were anesthetized using isoflurane (4% induction, 2.5% maintenance). Each animal received 6 × 300 μL subcutaneous injections of either PG-Silk (2%, 6% w/v, diluted in ultrapure water or PG at a 1:1 ratio) or PG alone in random distribution. Given prior experience with silk alone, a pilot study was first performed to see if any PG-containing groups would be dispersed in the subcutaneous tissue following injection or if they would stay local to the site of injection. For this pilot study, we injected all PG-containing formulations (N=2 each), with sacrifice at 12 hrs. Subsequent trials included all groups listed above (N= 3 injections per material) with sacrifice at 2 and 4 weeks time points. Animals were visually analyzed daily for the first three days, and then three times a week, in order to monitor the sites of injection for changes in physical appearance.
2.10. Histology and immunohistochemistry
Harvested tissues were stored in formalin for 48 hours and then processed through a series of alcohol dehydration solvents before paraffin embedding. Samples were cut in 10 μm sections for subsequent staining with Hematoxylin and Eosin (H&E) and Masson’s Trichrome, and immunohistochemistry described below. For immunohistochemistry, the sections were blocked with serum and incubated with anti-CD31 or VE-cadherin. Sections were then washed in PBS, incubated with a secondary anti-rabbit antibody for 30 min and finally with ImmPACT DAB enzyme substrate for 5 min. After washing with water, the sections were counterstained with hematoxylin and mounted.
2.11 Statistics
Values were expressed as mean ± SD (standard deviation). Student’s t-test was performed for paired observations. Multiple comparisons were evaluated by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc tests. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Kinetic profile of growth factors released by PG-Silk
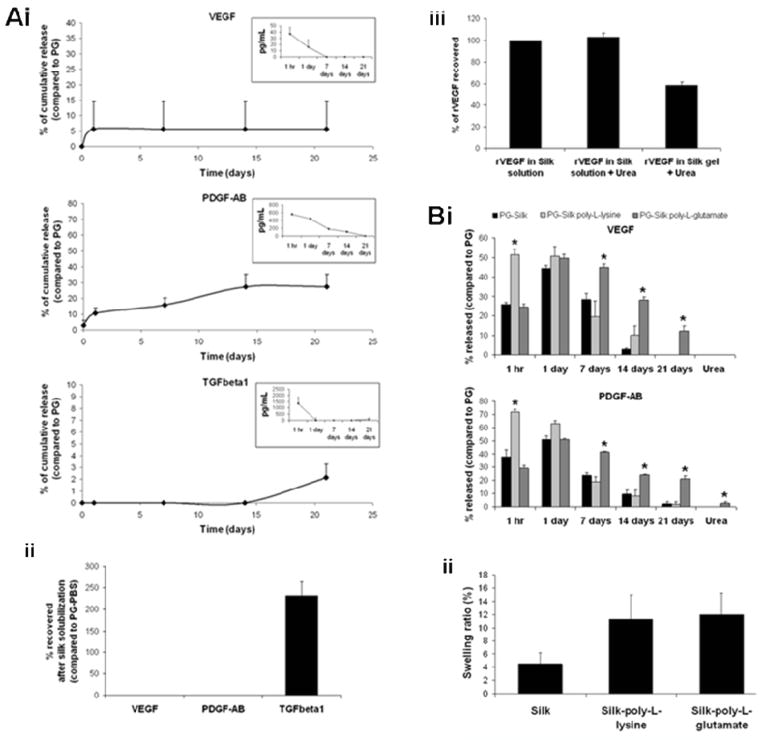
PG-Silk samples were evaluated for their ability to sustain platelet growth factor release. The kinetic profiles of VEGF, PDGF-AB and TGF-β1 were analyzed by ELISA overtime (21 days) and compared to growth factors released by PG. The initial growth factor concentration in PGs, recovered from plasma removed from the fibrin gel, 1 hr after the gelation occurred, was 90.8±5.7 pg/mL (VEGF), 928.2±4.6 pg/mL (PDGF-AB), 1670.2±974 pg/mL (TGF-β1). In PGs, as indicated in the insert of each graph (Fig. 1Ai), VEGF, PDGF-AB and TGF-β1 were released rapidly into the PBS supernatant in 1 hour, with progressively-decreasing amounts over time. In the case of growth factors released from PG-Silk, VEGF and TGF-β1 were detected at very low levels during the 21-day release study as compared to the PG groups. Moreover, while VEGF and PDGF-AB were released at higher levels at earlier time points, with PDGF-AB progressively increasing over time, TGF-β1 was detected only at the last time point (21 day).
Fig. 1.
(A) Cumulative release of platelet growth factors in PBS. (i) Values were normalized to growth factor released by PG-PBS at the first time point and expressed as %. At each time point PG-Silk supernatant (PBS) were evaluated by ELISA. The insert in each graph represents the kinetic profile of PG-PBS. (ii) At the last time point PG-Silk samples were solubilized with urea 8M and the obtained solutions were evaluated by ELISA and normalized to PG-PBS at the first time point. (iii) rVEGF recovery after urea treatment. rVEGF was loaded to 2% silk solution and subjected to dialysis, or treatment with urea 8 M. rVEGF was loaded to 2% silk solution, sonicated to allow gelation, solubilized with urea 8 M and subjected to dialysis. Values were normalized to relative control (rVEGF in silk solution). (B) Contribution of silk fibroin ionomers towards release and recovery of growth factors from PG-Silk. (i) Silk solution was mixed or not with silk fibroin-poly-L-lysine and silk fibroin-poly-L-glutamic acid ionomers (0.1% w/v) prior to adding PRP in order to obtain PG-Silk. Growth factor release was measured from PBS overtime (21 days) or after gel solubilization with urea. Values were normalized to the relative growth factor released by PG after 1 hr of gelation. (ii) Swelling of PG-Silk and PG-Silk mixed with silk fibroin ionomers after incubation in PBS for 24 hrs.
In order to differentiate between growth factors degraded while entrapped in silk vs. the non-specific binding or entrapment of the factors within the silk matrix (as has been previously observed for antibodies entrapped in silk) [24] PG-Silks were solubilized at day 21 using 8M urea and the recovered material was evaluated by ELISA. VEGF and PDGF-AB were not detected in the solubilized PG-Silks, while TGF-β1 was detected at a high level (3,091.4 ± 441.4 pg/mL). The percent of TGFβ-1 recovered from the solubilized PG-Silk complex with urea was about 200% as compared to TGFβ-1 released from PG into PBS at 1 hour (i.e. the maximum theoretical TGFβ-1 which could be released by activated platelets into PBS from within the PG clot) (Fig. 1Aii). We hypothesized that the increased TGFβ-1 obtained from PG-Silks may be ascribed to the ability of urea to dissolve the clot, which potentially entraps growth factors when compared to non-associated factors in plasma. Preliminary experiments performed with recombinant VEGF (rVEGF) showed that the presence of urea 8M did not affect rVEGF recovery, however, almost 50% was retained in the silk (Fig. 1Aiii). These results suggest that increased growth factor stores were available in the PG-Silk systems but were partially diminished in our experiments due to the urea treatment, otherwise, these stores would be available over time by degradation-mediated release from the silk gels.
3.2. Silk properties in modulating growth factor release
Considering the net charge at neutral 7.4 of the growth factors analyzed (2.2 VEGF, 14.3 PDGF-AB, 8.4 TGF-β1), and of silk (−36.1) [25], we investigated electrostatic interactions related to growth factor release from silk. The silk solution was mixed with 0.1% w/v silk fibroin ionomers (silk-poly-L-lysine or silk-poly-L-glutamate), prior to gelation through sonication and mixing with PRP-calcium gluconate and autologous thrombin. VEGF and PDGF-AB were analyzed by ELISA over 21 days (Fig. 1Bi). Because of the interference in the ELISA assay from the silk ionomers with the acidic-basic procedure used to activate the latent form of TGFβ-1 (data not shown), the contribution of silk ionomers in TGFβ-1 release was not analyzed. The positively-charged ionomer silk-poly-L-lysine promoted the release of both VEGF and PDGF-AB into PBS in 1 hour, when compared to the native, unmodified silk, suggesting a contribution of electrostatic forces or hydrophobic interactions in growth factor release[26]. In contrast, at early time points, the negatively charged silk-poly-L-glutamate ionomer-PG system released growth factors at the same rate as the unmodified silk. However, silk-poly-L-glutamate promoted sustained growth factor release at late time points, when compared to unmodified silk. We observed similar changes in growth factor release within silk upon addition of soluble poly-L-lysine prior to gelation by sonication/PG activation, confirming that the data linked to charge modification was not due solely to the method of silk modification (data not shown).
To confirm that these effects were related specifically to charge, the net charge of silk and silk-ionomers was analyzed by zeta potential. Even when diluted in plasma and calcium gluconate, following the procedure used to analyze growth factor release, the silk maintained a net negative charge similar to the silk when diluted in water, which increased when mixed with poly-L-glutamate, and decreased when mixed with poly-L-lysine (Table S1). The swelling of silk gel, known to be a critical step in drug release [24, 27], was expected to be greater in the presence of charged silks than in unmodified silks, due to the high charge density conferring higher hydrophilicity [28]. The swelling behavior of PG-Silk mixed with silk ionomers was compared to that of PG-Silk alone. An increased swelling, over 24 hours, was observed both for silk mixed with poly-L-lysine and poly-L-glutamate, when compared to silk alone (Fig. 1Bii). These data indicate that growth factor release was modulated by changing the net charge of the silk, through the use of silk ionomers. Moreover, ionomers were able to modulate the release of growth factors by a synergistic effect through the modulation of electrostatic interactions, resulting in increased release promoted by the cationic ionomer silk-poly-L-lysine, and through the increased swelling potential compared to silk alone. This swelling effect amplifies the charge effect in the case of the silk-poly-L-lysine, and overcomes the retention of the growth factors exerted by silk-poly-L-glutamate in the later time points, where the effect of charge is no longer dominant because of the dilution.
3.4. VEGF and pERK signaling in HUVEC proliferation induced by PG-Silk
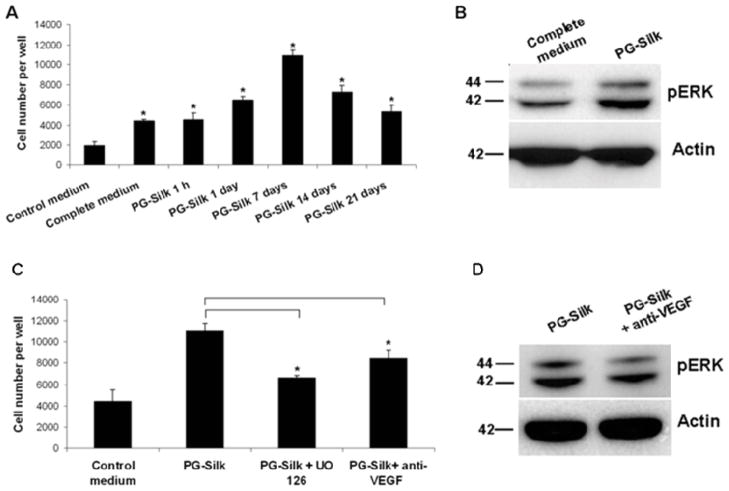
To investigate if the growth factors released by PG-Silks were biologically active, their effects on HUVEC proliferation were determined. PG-Silk supernatants collected at 1 hour, 1 day, 7, 14 and 21 days were assayed by MTS and revealed that control medium supplemented with PG-Silk supernatants collected at each time point (25%, taken from PBS added to the PG-Silk well) sustained HUVEC proliferation, when compared to control medium alone (p<0.05) (Fig. 2A). Moreover, HUVEC proliferation in the presence of the PG-silk supernatants collected at day 1, 7 and 14 resulted in significantly increased activity compared to complete medium (p<0.05).
Fig. 2. VEGF and pERK signaling in HUVEC proliferation induced by PG-Silk.
(A) PBS supernatant collected from PG-Silk (25% v/v with control media) was used to inoculate HUVEC cells. The MTS assay was conducted after 3 days. * denotes statistically significant difference compared with the control medium (p < 0.05). (B) Representative immunoblotting image showing a increased ERK1/2 phosphorylation in HUVEC cells cultured with PBS supernatant collected from PG-Silk, as compared to cells cultured with complete media. Actin was used as control of protein equal loading. (C) HUVEC cells were cultured in PBS supernatant collected from PG-Silk (25% v/v with control media) in presence of UO 126 or anti-VEGF neutralizing antibody. The MTS assay was conducted after 3 days (p < 0.05). (D) Representative immunoblotting image showing a decreased ERK1/2 phosphorylation in HUVEC cells cultured 2 hrs with PBS supernatant collected from PG-Silk in presence of anti-VEGF. Actin was used as control of protein equal loading.
VEGF, through its receptor kinase insert domain-containing receptor (KDR), is known to be a strong activator of ERK 1 and 2, resulting in HUVEC proliferation. To analyze the ability of VEGF released by PG-Silk to mediate the same outside-in signaling pathway, ERK phosphorylation in HUVECs was analyzed. PG-Silk supernatant collected at day 7 induced ERK1/2 phosphorylation greater than expansion media, as revealed by Western blot (Fig. 2B). To confirm the involvement of VEGF mediated-ERK signaling induced by PG-Silk, HUVECs were preincubated with a pharmacological inhibitor of ERK, UO 126. Fig. 2C shows that HUVEC proliferation, induced by PG-Silk collected at day 7, was attenuated when ERK was inhibited. Moreover, depletion of VEGF from PG-Silk supernatant through a neutralizing antibody against VEGF resulted in decreased HUVEC proliferation, suggesting that VEGF released by PG-Silk contributed at least partially to HUVEC proliferation. A concomitant decrease in ERK phosphorylation in HUVECs exposed to the neutralizing antibody against VEGF was demonstrated by Western blotting (Fig. 2D).
3.5. Silk properties in platelet growth factors stabilization
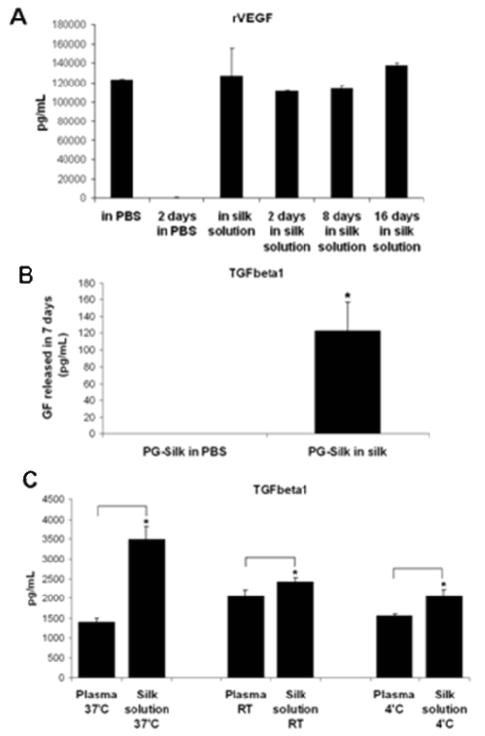
The ability of silk to stabilize platelet-derived growth factors was investigated based on the recovery of rVEGF over time. When rVEGF was added to a 2% w/v silk solution, the concentration (by ELISA) was constant over 16 days (Fig. 3A). In contrast, rVEGF diluted in PBS (or water, data not shown), was almost completely degraded in 2 days, as expected. Based on this evidence, we analyzed the ability of silk to stabilize the growth factors released by PG-Silk. In particular, since we observed a high level of TGFβ1 retained in the PG-Silk after two weeks of release, we evaluated the ability of silk to stabilize this growth factor when compared to PBS or plasma. The sonicated silk solution was mixed directly with PG; however, the resulting gel was instead re-suspended in 1% silk solution or PBS over time. While silk did not show enhanced stabilization effects on PDGF-AB, as it showed the same concentration over time as compared to plasma (data not shown), the silk stabilized TGFβ-1 over 7 days (Fig. 3B). Moreover, when the growth factors obtained from PG were diluted 1:1 in 1% silk solution, a significantly higher concentration of TGF-β1 was detected by ELISA after 2 weeks, when compared to growth factors diluted 1:1 in PBS (Fig. 3C). This increase in detectable TGF-β1 occurred during sample storage at 37°C, room temperature and 4°C. These experiments suggest that silk stabilized TGF-β1 greater than plasma, and this mechanism was temperature independent. Together these findings suggest that the silk can have a stabilizing effect on released growth factors, in solution phase, even before gelation.
Fig. 3. Ability of silk to stabilize growth factors.
(A) rVEGF stability in silk solution overtime. rVEGF was resuspended in PBS or in 2% silk solution and the concentration was assessed by Elisa overtime (16 days). (B) PG-Silk samples, upon gelation, were re-suspended in PBS or silk solution and TGFbeta1 release was measured after 7 days. (C) Growth factors in plasma, obtained from PG, were diluted 1:1 in PBS or 1:1 in 1% silk solution and maintained at 37°C, or room temperature, or 4°C. After 2 weeks the concentration of TGFbeta1 was assessed by Elisa.
3.6. Rheological silk gelation behavior
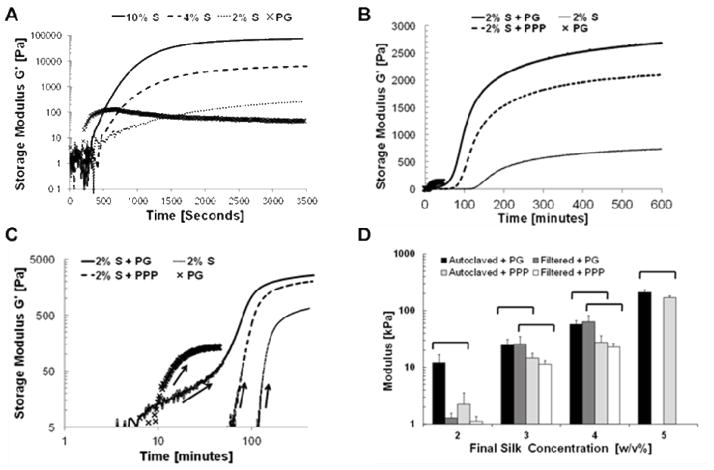
The stiffness of silk fibroin hydrogels can be tuned by controlling the solution concentration and quantified by compressive properties [20, 23]. To confirm that rheological stiffness of PG-Silk was dependent on the incorporation of silk and its concentration, a range of concentrations of silk were prepared and gelled immediately prior to a series of nondestructive time-lapse dynamic oscillatory shear tests (Fig. 4A). The equilibrium stiffness values were concentration-dependent as shown previously for vortex-induced silk gels [22]. Interestingly, we routinely observed that the PG alone rapidly formed a fibrin clot, as denoted by the storage modulus peak located ~480 seconds (~8 minutes). Following this peak, the storage modulus of PG precipitously decreased, and this was likely due to the fragile nature of the fibrin gel network and its propensity to dissociate from the plasma. Conversely, silk hydrogels continued to increase in storage modulus during oscillatory shear, and well beyond its initial point of increase until reaching a plateau at ~16 hours. This behavior is due to the relatively slow maturation of the silk gel physical crosslink network under sonication parameters employed here. Using these unique gelation signatures for the two protein components we were also able to resolve the PG gelation event in the context of a 1:1 dilution with sonicated silk gel (Fig. 4B, C), allowing us to verify that the presence of the silk gel did not inhibit normal platelet gel formation. Moreover, despite the relatively soft behavior of the PG alone compared to silk at 2% w/v concentration, each 2% silk gel reinforced with a PG network was significantly stiffer than its PPP-laden counterpart (Fig. 4B, C). These findings suggest that not only does the inclusion of added protein (in the case of PPP) contribute to the PG-Silk system’s stiffness, but also its ability to form a fibrin gel network in the context of a more slowly forming silk gel.
Fig. 4. Time-dependent rheology and compressive mechanics of silk and platelet gels.
(A) Dynamic time-dependent rheological behavior of sonicated 10% (solid line), 4% (dashed line), and 2% w/v (dotted line) silk solutions compared to platelet gel (X marker) behavior over 6 hours of oscillatory shear. Not shown are the monotonically-increasing modulus values for the silk groups for up to 16 hrs, whereas the platelet gel group begins decreasing at ~10 minutes of oscillatory shear. (B) Rheological behavior of sonicated 4% w/v silk solutions diluted 1:1 with platelet gel (+PG, solid line), Platelet Poor Plasma (+PPP, thick dotted line), or water as a control (fine dotted line). Platelet gels alone are also shown (X marker). (C) Re-plotted log-log data from (B) of the initial 100 minutes following gel initiation, showing the incidence of gel stiffening as indicated by the arrows. (D) Silk solutions were either autoclaved or sterile-filtered prior to mixing with either PG or PPP at a 1:1 dilution to generate the final silk concentration shown (therefore starting silk concentrations are 2X as listed, 10% was the maximum concentration that could be used from silk boiled for 30 minutes). Bar indicates significance between groups identified in post-hoc analysis of ANOVA (p< 0.05).
Since the various silk solution sterilization methods required prior to sonication and injection could affect the properties of the solution, and therefore the quality of the resultant hydrogel networks, we repeated the rheological assessments with variably-sterilized precursor silk solutions mixed with platelet gel components to draw comparisons to the untreated solutions. Data comparing the two sterilization methods to the virgin silk solution conditions revealed no differences in rheological stiffness of the undiluted silk at 2% (w/v) nor the ability to participate in dual gel network formation (Fig. S1A). We also sought to confirm our rheological results with quasi-static compressive tests across a wider range of silk concentrations (Fig. 4D). We were able to concentrate solutions taken from cocoons boiled for 30 minutes and subsequently sterilize them by either 0.2 μm filtration or a 20-minute autoclave cycle at up to 8 w/v% (prior to 1:1 dilution to 4 w/v% with either PPP or PG), but were unable to filter sterilize them at 10 w/v%. However, when solutions were generated from 45-minute- and 60-minute-boiled (hereafter referred to as 45MB and 60MB) silk cocoons, they could be successfully sterilized at higher concentrations using both methods (Fig. S1B). Boil time of silk solution did not significantly alter the compressive properties of the material across all concentrations, independent of PG inclusion vs. PPP, and this was consistent until the maximum concentration that could be sterile-filtered (i.e. 60MB group at 14w/v% starting silk concentration).
3.7. In vivo analysis of PG, silk gel and PG-Silk gel behavior
Nude rats were injected with the same volume of PG or 2% or 6% silk solution (with or without PG dilution) upon sonication in order to induce gelation after injection, and the in vivo degradation rate of the injected gels and cell infiltration were analyzed. No acute or chronic adverse reactions were observed in any animal group over time. The raised bumps from the injected bolus were stable for all groups immediately following injection. After 12 hrs the injection sites containing PG alone were no longer raised or palpable. However, the 2% silk groups, with and without PG, were visible over time with a progressively decreasing size starting after 2 weeks from injection. Meanwhile, the 6% silk groups, with and without PG, were visible over 1 month and continuously maintained the same size throughout the study.
Since the therapeutic effects of PG occur immediately due to the rapid release of growth factors at the site of injection, some animals injected with PG were sacrificed after 12 hrs and compared to PG mixed with silk. At 12 hours following injection, histological analysis including H&E (Fig. 5Ai, ii) and Masson’s Thricrome (Fig. S2), revealed the presence of the platelet gel with entrapped aggregated platelets. The immunohistochemistry analysis revealed that vascularization was stimulated, due to the presence of VE-cadherin and CD31 positive cells (Fig. 6Ai, Ci). At this initial time point, 2% and 6% silk-PG were the same size, and were much larger than PG alone in terms of both gross and histological analyses (Fig. 5Bi, ii). The 2% PG-Silk was more diffuse in eosin staining suggesting a softer network when compared to 6% PG-Silk, and neither contained CD31 positive cells within the gel bulk at this early time point, although positive staining was observed around the periphery (Fig. 6B, Di). After 2 weeks from injection, the PG was still present, although diminished in size (Fig. 5Aiii, iv; 5Biii, iv), with newly form vessels revealed by the presence of VE-cadherin positive cell aggregates (Fig. 6Aii). Regarding the silk constructs, after 2 weeks there was a significant decrease in size of 2% injected silk gels, with or without PG (Fig. S3). Only when mixed with PG did the 2% silk constructs start to be infiltrated with CD31 and VE-cadherin positive cells at 2 weeks, which progressively increased over 1 month. (Fig. 5B iii, iv; 6 B, D ii; Fig. S4). VE-cadherin positive staining was co-localized with the appearance of luminal structures characteristic of a capillary vascular phenotype. In contrast, after 1 month the 2% silk construct without PG showed infiltration of only sparse hemotoxylin-positive infiltrating cells, presumably fibroblasts and inflammatory macrophages (Fig. 5 Bv, vi). Meanwhile, the overall dimensions of 6% silk constructs, both with or without PG, was relatively stable over the same 1-month time frame. Histologically, these groups showed only peripheral infiltration of cells and development of vessel-like structures at 2 weeks, which showed only a small increase at 1 month (Fig. 6 Biii, Diii).
Fig. 5. In vivo histology analysis of PGs and Silk-PGs.
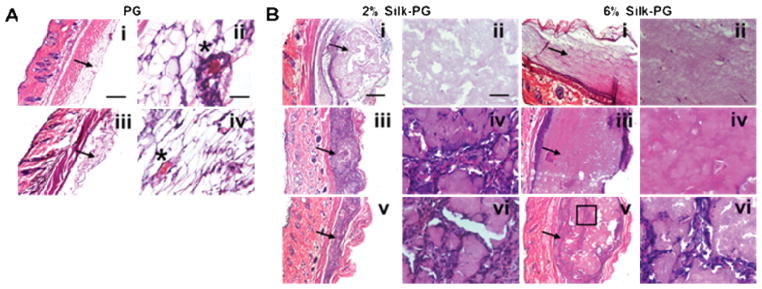
H&E staining of PG (A) and Silk-PG (B) after 12 hours from injection (i scale bar 200 μm, ii scale bar 100 μm), after 2 weeks from injection (iii scale bar 200 μm, iv scale bar 100 μm) and after 1 month from injection (v scale bar 200 μm, vi scale bar 100 μm). The arrows indicate the PG or Silk-PG constructs. The stars indicate aggregated platelets. The box indicates the area of infiltrated cells where high magnification pictures have been acquired. Images are representative of three independent experiments.
Fig. 6. In vivo immunohistochemistry analysis of PGs and Silk-PGs.
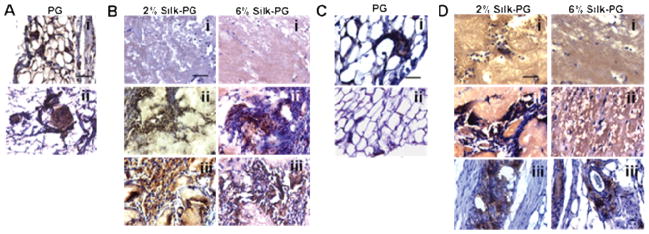
(A) Ve-cadherin immunohistochemistry of PGs, (B) Silk-PG constructs, (C) CD31 immunohistochemistry of PGs, and (D) different Silk-PG constructs injected and analyzed after 12 hours (i), 2 weeks (ii), and 1 month (iii) (scale bar 100 μm). Images are representative of three independent experiments.
4. Discussion
Autologous PG is widely used in regenerative medicine, such as in the acceleration of bone repair through the release of GFs from activated platelets [6]. Several strategies have been proposed to improve the efficacy of PG injections, by optimizing growth factor release with different activators [29] or by combining PG with bone allografts and bone marrow stromal cells [11, 12]. Another key aspect in the therapeutic efficacy of PGs is the spatial/temporal and bioavailability of the growth factors released at the site of injection. To address these limitations, we explored a new system of platelet growth factor delivery based on a combination with silk hydrogels. PGs mixed with silk gels obtained by sonication slowed the release of GFs (VEGF, PDGF-AB and TGF-β1) from activated platelets when compared to PGs alone over 21 days. Upon solubilizing the PG-loaded silk gels using chaotropic agents, such as urea, we found that additional GFs were entrapped into the PG-Silk matrix. Together, these findings suggest that embedded GFs would be available in vivo by degradation-mediated release from the silk gel and would offer a mechanism by which silk could extend the therapeutic effects of PG-derived GFs.
The charge interactions of compounds with silk are known to result in different release profiles [30, 31]. By introducing two charged derivatives of silk, silk-poly-L-lysine and silk-poly-L-glutamate [21], one of the mechanisms underlying silk-growth factor associations was determined to involve electrostatic interactions. Moreover, by using these silk ionomers, the delivery of platelet growth factors can be temporally modulated by both electrostatic interactions and the swelling properties of silk. This mechanism is consistent with a model proposed by Guziewicz et al. in which increased hydrophobicity of the high density β-sheets decreases swelling and antibody release [24, 28].
GF interactions with silk did not affect their biological activity, as they effectively promoted HUVEC proliferation via the canonical extracellular signal-regulated protein kinase activation pathway (pERK). As VEGF is one of the main GFs involved in HUVEC proliferation, by depleting VEGF from PG-Silk supernatant through a neutralizing antibody, the released VEGF contributed at least in part to HUVEC proliferation through the pERK pathway. Due to the incomplete attenuation of cell proliferation by blocking the VEGF-specific pathway, it is likely that other factors active in our PG-Silk system (FGF, IGF, etc) could have contributed to the increased HUVEC proliferation. Human mesenchymal cells responded similarly as the hUVECS when PG-Silk was used to supplement growth media formulations (data not shown), suggesting that the bioactivity of the PG-Silk system is not completely VEGF-specific. These findings demonstrate that silk is able to preserve the biological effect of platelet growth factors, which could potentially result in an in vivo therapeutic improvement of vascularization as well as bone formation due to the putative function of these well-characterized growth factors.
Another advantage conferred by silk to the platelet GFs was improved stability. rVEGF was stable over time when re-suspended in silk solution; however, when re-suspended in PBS, rVEGF was quickly degraded. We also demonstrated that silk can stabilize TGF-β1 released from PG-Silk, as we observed significantly higher values than in PBS. Moreover, we showed that silk improved TGF-β1 stability over time, independent of storage temperature, when compared to plasma diluent. Recently the stabilizing effect of silk on vaccines, antibiotics, and labile enzymes has been reported [32–34], as have the mechanisms of antibody stabilization in silk materials [28, 34]. Here we report the stabilizing effect exerted by silk in solution with respect to recombinant growth factors. We hypothesized that this enhanced liquid stability was conferred by the ability of silk to reduce molecular mobility during storage and in turn, prevent protein unfolding and aggregation. It is therefore expected that in the solution phase, before gelation event was finalized, or during silk gel degradation, the stabilizing effect preserved the initial amount of available GFs.
In addition to the biologic activity of the PG-Silk system, we also observed important mechanical benefits of silk with the PG. In particular, rheological data showed that the presence of gel-forming silk protein did not interrupt normal kinetics of PG formation and the silk acted as an additional reinforcing agent to bulk up the gel in a manner dependent on the initial silk concentration. Interestingly, we observed that PG-laden silk gels were stiffer than their PPP-laden counterparts, suggesting that a fibrin-based PG was able to form first before the physical crosslinks of the silk gel formed and that the two acted in a synergistic manner. These behaviors are typical of sequential interpenetrating polymer networks, compatibility previously observed with other silk-and-protein hybrid gel systems [35, 36]. The exact nature of the interaction between silk and the fibrin components of the PG network requires further study. Nonetheless, since demonstrating that the silk content can be increased to high protein loading in order to enhance the mechanical properties (Supplemental Figures) [20, 23], we anticipate that the final PG-Silk construct mechanical properties can be tuned in order to meet mechanical benchmarks established by native tissues, ranging from soft calcaneous fat tissue [37] to stiff fibrocartilage [38], ~8.5 kPa to ~500–1000 kPa, respectively.
The degradation of injected platelet gels in some animal models [39] has been reported to occur in about 14 days, presumably by fibrinolysis. However, dependent on the silk concentration and formulation, some of the injected silk gels demonstrated measurable degradation in several weeks to much longer [40–42]. Our results provide further confirmation of the rapid degradation of PGs alone, in addition to the retention of silk gel for weeks following injection. While the rate of silk degradation was inversely proportional to concentration, the addition of PG had a normalizing effect, with both 2% and 6% degrading at the same rate. The increased stability and retention of the PG-Silk constructs may enhance growth factor stability and subsequent cell infiltration, even with minimal silk content. Immmunohistochemistry confirmed the development of vascularized networks in all PG-Silk constructs as the majority of infiltrated cells were both CD31 and VE-cadherin positive. These experiments demonstrated that by modulating the concentration of silk, the degradation rate of silk gels and in turn, the release of functional platelet growth factors, were modulated.
5. Conclusions
A formulation of platelet gel mixed with silk gel was demonstrated which enabled control over the rate of platelet-derived growth factor release via manipulation of silk-specific properties. The mechanical properties of the resultant gel, which reflects contributions from both gelling components, can also be tuned by increasing the final silk protein concentration. Moreover, the prolonged and concentration-dependent enzymatic stability of the silk component can extend the time scale for remodeling and bioactivity of these gels in vivo. This PG-Silk system represents a step towards the development of PG formulations with enhanced therapeutic effects.
Supplementary Material
Acknowledgments
The authors thank Sezin Yigit and Dr. Zhang, from Northeastern University, Department of Chemistry and Chemical Biology, for the zeta potential measurements, Nick Guziewicz, Antonio Varone and Philipp Seib for advice, Mike Lovett for providing the HUVECs.
This work was supported by US National Institutes of Health (grant EB016041-01) and the Cariplo Foundation, Italy (2010-0807). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Prof. David L. Kaplan, Email: david.kaplan@tufts.edu.
Prof. Alessandra Balduini, Email: alessandra.balduini@tufts.edu.
References
- 1.Whitman DH, Berry RL, Green DM. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–9. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma - Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 3.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 4.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75:93–9. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 5.Kaux JF, Le Goff C, Seidel L, Peters P, Gothot A, Albert A, et al. Comparative study of five techniques of preparation of platelet-rich plasma. Pathol Biol (Paris) 2011;59:157–60. doi: 10.1016/j.patbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Simonpieri A, Del Corso M, Vervelle A, Jimbo R, Inchingolo F, Sammartino G, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone Graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012;13:1231–56. doi: 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 8.Ogino Y, Ayukawa Y, Kukita T, Koyano K. The contribution of platelet-derived growth factor, transforming growth factor-beta1, and insulin-like growth factor-I in platelet-rich plasma to the proliferation of osteoblast-like cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:724–9. doi: 10.1016/j.tripleo.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Cenni E, Ciapetti G, Granchi D, Fotia C, Perut F, Giunti A, et al. Endothelial cells incubated with platelet-rich plasma express PDGF-B and ICAM-1 and induce bone marrow stromal cell migration. J Orthop Res. 2009;27:1493–8. doi: 10.1002/jor.20896. [DOI] [PubMed] [Google Scholar]
- 10.Aghaloo TL, Moy PK, Freymiller EG. Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants. 2004;19:59–65. [PubMed] [Google Scholar]
- 11.Dallari D, Fini M, Stagni C, Torricelli P, Nicoli Aldini N, Giavaresi G, et al. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J Orthop Res. 2006;24:877–88. doi: 10.1002/jor.20112. [DOI] [PubMed] [Google Scholar]
- 12.Dallari D, Savarino L, Stagni C, Cenni E, Cenacchi A, Fornasari PM, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–20. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 13.Civinini R, Macera A, Nistri L, Redl B, Innocenti M. The use of autologous blood-derived growth factors in bone regeneration. Clin Cases Miner Bone Metab. 2011;8:25–31. [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzucco L, Balbo V, Cattana E, Borzini P. Platelet-rich plasma and platelet gel preparation using Plateltex. Vox Sang. 2008;94:202–8. doi: 10.1111/j.1423-0410.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 15.Cenni E, Savarino L, Perut F, Fotia C, Avnet S, Sabbioni G. Background and rationale of platelet gel in orthopaedic surgery. Musculoskelet Surg. 2010;94:1–8. doi: 10.1007/s12306-009-0048-9. [DOI] [PubMed] [Google Scholar]
- 16.Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost. 2006;4:932–9. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 17.Mazzucco L, Medici D, Serra M, Panizza R, Rivara G, Orecchia S, et al. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44:1013–8. doi: 10.1111/j.1537-2995.2004.03366.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–85. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Wray LS, Hu X, Gallego J, Georgakoudi I, Omenetto FG, Schmidt D, et al. Effect of processing on silk-based biomaterials: reproducibility and biocompatibility. J Biomed Mater Res B Appl Biomater. 2011;99:89–101. doi: 10.1002/jbm.b.31875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–64. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabrese R, Kaplan DL. Silk ionomers for encapsulation and differentiation of human MSCs. Biomaterials. 2012;33:7375–85. doi: 10.1016/j.biomaterials.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yucel T, Cebe P, Kaplan DL. Vortex-induced injectable silk fibroin hydrogels. Biophys J. 2009;97:2044–50. doi: 10.1016/j.bpj.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluge JA, Rosiello NC, Leisk GG, Kaplan DL, Dorfmann AL. The consolidation behavior of silk hydrogels. J Mech Behav Biomed Mater. 2010;3:278–89. doi: 10.1016/j.jmbbm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guziewicz N, Best A, Perez-Ramirez B, Kaplan DL. Lyophilized silk fibroin hydrogels for the sustained local delivery of therapeutic monoclonal antibodies. Biomaterials. 2011;32:2642–50. doi: 10.1016/j.biomaterials.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X, Tang-Schomer MD, Huang W, Xia X-X, Weiss AS, Kaplan DL. Charge-tunable autoclaved silk-tropoelastin protein alloys that control neuron cell responses. Adv Funct Mater. 2013;23:3875–84. doi: 10.1002/adfm.201202685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guziewicz NA, Massetti AJ, Perez-Ramirez BJ, Kaplan DL. Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. Biomaterials. 2013;34:7766–75. doi: 10.1016/j.biomaterials.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard EM, Kaplan DL. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin Drug Deliv. 2011;8:797–811. doi: 10.1517/17425247.2011.568936. [DOI] [PubMed] [Google Scholar]
- 28.Guziewicz NA, Massetti AJ, Perez-Ramirez BJ, Kaplan DL. Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr ME, Jr, Carr SL, Tildon T, Fisher LM, Martin EJ. Batroxobin-induced clots exhibit delayed and reduced platelet contractile force in some patients with clotting factor deficiencies. J Thromb Haemost. 2003;1:243–9. doi: 10.1046/j.1538-7836.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Yucel T, Lu Q, Hu X, Kaplan DL. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials. 2010;31:1025–35. doi: 10.1016/j.biomaterials.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lammel AS, Hu X, Park SH, Kaplan DL, Scheibel TR. Controlling silk fibroin particle features for drug delivery. Biomaterials. 2010;31:4583–91. doi: 10.1016/j.biomaterials.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Pritchard E, Hu X, Valentin T, Panilaitis B, Omenetto FG, et al. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proc Natl Acad Sci U S A. 2012;109:11981–6. doi: 10.1073/pnas.1206210109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto FG, et al. Stabilization of enzymes in silk films. Biomacromolecules. 2009;10:1032–42. doi: 10.1021/bm800956n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pritchard EM, Dennis PB, Omenetto F, Naik RR, Kaplan DL. Review physical and chemical aspects of stabilization of compounds in silk. Biopolymers. 2012;97:479–98. doi: 10.1002/bip.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gil ES, Spontak RJ, Hudson SM. Effect of beta-sheet crystals on the thermal and rheological behavior of protein-based hydrogels derived from gelatin and silk fibroin. Macromol Biosci. 2005;5:702–9. doi: 10.1002/mabi.200500076. [DOI] [PubMed] [Google Scholar]
- 36.Gil ES, Frankowski DJ, Hudson SM, Spontak RJ. Silk fibroin membranes from solvent-crystallized silk fibroin/gelatin blends: Effects of blend and solvent composition. Mater Sci Eng C. 2007;27:426–31. [Google Scholar]
- 37.Miller-Young JE, Duncan NA, Baroud G. Material properties of the human calcaneal fat pad in compression: experiment and theory. J Biomech. 2002;35:1523–31. doi: 10.1016/s0021-9290(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 38.Johns DE, Athanasiou KA. Design characteristics for temporomandibular joint disc tissue engineering: learning from tendon and articular cartilage. Proc Inst Mech Eng [H] 2007;221:509–26. doi: 10.1243/09544119JEIM158. [DOI] [PubMed] [Google Scholar]
- 39.Cheng K, Malliaras K, Shen D, Tseliou E, Ionta V, Smith J, et al. Intramyocardial injection of platelet gel promotes endogenous repair and augments cardiac function in rats with myocardial infarction. J Am Coll Cardiol. 2012;59:256–64. doi: 10.1016/j.jacc.2011.10.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etienne O, Schneider A, Kluge JA, Bellemin-Laponnaz C, Polidori C, Leisk GG, et al. Soft tissue augmentation using silk gels: an in vitro and in vivo study. J Periodontol. 2009;80:1852–8. doi: 10.1902/jop.2009.090231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diab T, Pritchard EM, Uhrig BA, Boerckel JD, Kaplan DL, Guldberg RE. A silk hydrogel-based delivery system of bone morphogenetic protein for the treatment of large bone defects. J Mech Behav Biomed Mater. 2012;11:123–31. doi: 10.1016/j.jmbbm.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Wang X, Wang S, Zhao J, Xu L, Zhu C, et al. The use of injectable sonication-induced silk hydrogel for VEGF(165) and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials. 2011;32:9415–24. doi: 10.1016/j.biomaterials.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.