Abstract
Objective:
To describe the prevalence and association of sexual risk behaviours and viral suppression among HIV-infected adults in the United States.
Design:
Cross-sectional analysis of weighted data from a probability sample of HIV-infected adults receiving outpatient medical care. The facility and patient response rates were 76 and 51%, respectively.
Methods:
We analysed 2009 interview and medical record data. Sexual behaviours were self-reported in the past 12 months. Viral suppression was defined as all viral load measurements in the medical record during the past 12 months less than 200 copies/ml.
Results:
An estimated 98 022 (24%) HIV-infected adults engaged in unprotected vaginal or anal sex; 50 953 (12%) engaged in unprotected vaginal or anal sex with at least one partner of negative or unknown HIV status; 23 933 (6%) did so while not virally suppressed. Persons who were virally suppressed were less likely than persons who were not suppressed to engage in vaginal or anal sex [prevalence ratio, 0.88; 95% confidence interval (CI), 0.82–0.93]; unprotected vaginal or anal sex (prevalence ratio, 0.85; 95% CI, 0.73–0.98); and unprotected vaginal or anal sex with a partner of negative or unknown HIV status (prevalence ratio, 0.79; 95% CI, 0.64–0.99).
Conclusion:
The majority of HIV-infected adults receiving medical care in the U.S. did not engage in sexual risk behaviours that have the potential to transmit HIV, and of the 12% who did, approximately half were not virally suppressed. Persons who were virally suppressed were less likely than persons who were not suppressed to engage in sexual risk behaviours.
Keywords: antiretroviral therapy, HIV-infected persons, sexual behaviour, unsafe sex, viral load
Introduction
The number of persons aged 13 years and older newly infected with HIV in 2010 in the United States was estimated to be 47 500, and 88% of all new HIV infections were attributed to sexual transmission [1]. Recent estimates suggest that approximately four transmissions occur per 100 HIV-infected persons annually [2]; however, nationally representative data on sexual risk behaviours have not been available since the late 1990s [3]. Thus, national data on the sexual risk behaviour of HIV-infected persons are needed to inform prevention efforts to reduce sexual transmission of HIV and the acquisition of other sexually transmitted infections (STIs).
Providing antiretroviral therapy (ART) improves individual health outcomes and observational evidence and a randomized clinical trial have demonstrated that taking ART substantially reduces the risk of heterosexual HIV transmission [4,5]. Although this finding has profound implications for HIV prevention, access to ART does not eliminate all the barriers that may interfere with the elimination of HIV transmission, including delayed diagnosis, lack of continuous care, suboptimal adherence, drug resistance and subsequent increases in risky sexual behaviour (i.e. risk compensation) [6–10]. Findings from prior investigations of the association between ART provision and increased sexual risk behaviour are mixed [11–14]. Soon after effective therapy became widely available in developed countries, evidence suggested a resurgence of HIV and other STIs, especially syphilis, among MSM [13,15]. Some data, including meta-analyses, have indicated no increases in sexual risk behaviour among persons who received ART or were virally suppressed, but the prevalence of unprotected sex was higher among HIV-infected adults who believed that ART or viral suppression protects against transmission [12,14].
In this report, we present data (overall and stratified by viral suppression) to describe sexual risk behaviours in a national sample of HIV-infected adults receiving medical care in the United States. Given the increased probability of HIV transmission when individuals are not virally suppressed, we also assess the association between sexual risk behaviour and viral suppression.
Materials and methods
The Medical Monitoring Project (MMP) uses a three-stage sampling design to obtain nationally representative, annual cross-sectional samples of HIV-infected adults receiving outpatient medical care for HIV [16]. For the 2009 data collection cycle, U.S. states and territories were sampled, followed by outpatient facilities providing HIV care, and finally by HIV-infected adults aged 18 years and older who reported at least one medical care visit in a participating facility during January–April 2009. For state and territory samples, probability of selection was proportionate to AIDS prevalence; for provider samples, probability of selection was proportionate to HIV-infected patient census. Data were collected via face-to-face interviews and medical record abstractions during June 2009–May 2010. All sampled states and territories participated in MMP [California (including Los Angeles County and San Francisco), Delaware, Florida, Georgia, Illinois (including Chicago), Indiana, Michigan, Mississippi, New Jersey, New York (including New York City), North Carolina, Oregon, Pennsylvania (including Philadelphia), Puerto Rico, Texas (including Houston), Virginia, and Washington]. Of 603 sampled facilities, 461 participated in MMP (facility response rate, 76%); of 9338 sampled persons, 51% (n = 4127) were interviewed and had their medical records abstracted, resulting in a combined facility-patient response rate of 39% [17]. Data were weighted on the basis of known probabilities of selection at state or territory, facility and patient levels [18]. In addition, using information collected on all sampled facilities and 88% of sampled patients, we conducted an analysis to compare respondents and nonrespondents. Data were then weighted to adjust for nonresponse by using predictors of response, including facility size, facility type (public or private), patient race and ethnicity, time since HIV diagnosis and age group [18,19]. We analysed data on 4094 participants whose diagnosis was made at least 12 months earlier and weighted the data to represent 408 092 HIV-infected adults in care in the United States during January–April 2009. The Centers for Disease Control and Prevention (CDC) has determined that MMP is a public health surveillance activity [20]. Because MMP is not considered research, it is not subject to human subjects regulations including federal institutional review board (IRB) [21]. Despite this designation, voluntary informed consent was obtained from all participants who completed an interview. Several participating states, territories and facilities obtained local IRB approval to conduct MMP and also obtained informed consent to abstract information from patients’ medical records.
Measures
All measures reflect self-reported behaviours or experiences in the 12 months before the interview unless otherwise noted. Men were classified as MSM if they reported oral or anal sex with other men, whether or not they also reported sex with women. Men were classified as men who have sex with women (MSW) if they reported oral, anal or vaginal sex exclusively with women. Women were classified as women who have sex with men (WSM) if they reported oral, anal or vaginal sex with men, whether or not they also reported sex with women. Women were classified as women who have sex with women (WSW) if they reported oral or vaginal sex exclusively reported sex with women. Participants were considered transgender if their sex at birth did not match their current sexual identity or if they self-identified as transgender. Participants who reported no anal, vaginal or oral sex in the past 12 months were categorized according to self-reported sexual orientation. Covariates obtained from interview data included demographic characteristics (e.g. age, race/ethnicity, education) and behaviours (e.g. number of sex partners, self-reported syphilis, gonorrhoea, chlamydia, herpes, genital warts or other sexually transmitted diseases, and alcohol, stimulant, poppers, methamphetamines or any other drug use before sex).
Viral load, the prescription of ART and HIV staging data were abstracted from medical records. Because sexual risk behaviours could have occurred at any time during the 12-month period prior to the interview, we defined durable viral suppression, hereafter referred to as viral suppression, as an HIV-1 RNA level that was documented as undetectable or less than 200 copies/ml at every measurement (minimum 1) in the past 12 months.
Data analysis
We considered three separate measures of sexual behaviour that took place in the past 12 months: vaginal or anal sex; ‘unprotected vaginal or anal sex’ during which a condom was not used or was used inconsistently; and unprotected vaginal or anal sex with a partner of negative or unknown HIV status. For each outcome, we generated weighted population estimates, weighted percentages and 95% confidence intervals (95% CIs) for all persons whose HIV diagnosis had been made at least 12 months earlier and separately for MSM, MSW and WSM. We used modified Rao-Scott chi-square tests and 95% CIs to assess sociodemographic, behavioural and clinical differences among MSM, MSW and WSM [22,23]. To examine the association between viral suppression and each measure of sexual behaviour as a dependent variable, we calculated crude prevalence ratios and 95% CIs by using logistic regression with predicted marginal means [24]. Data were weighted for nonresponse [18] and all analyses accounted for the complex sample design and unequal selection probabilities by using the survey procedures in SAS 9.3 [25] and SUDAAN 10.0.1 [26].
Results
Among HIV-infected persons in care whose diagnosis occurred at least 12 months earlier, 46% (95% CI, 42–51) were MSM, 24% (95% CI, 21–26) were MSW, 27% (95% CI, 23–30) were WSM, 2% (95% CI, 1–2) were transgender and less than 1% (95% CI, 0.4–1.1) were WSW. Approximately 40% of persons were aged 40–49 years, 41% were non-Hispanic blacks and 50% had more than a high school education (Table 1). In the past 12 months, 89% had been prescribed ART, and 59% had been virally suppressed at all measurements; 68% had ever received an AIDS diagnosis. We found statistically significant differences in the sociodemographic and clinical characteristics of MSM, MSW and WSM except for continuous healthcare coverage in the past 12 months, length of time since HIV diagnosis and number of viral load measurements in the past 12 months.
Table 1.
Sociodemographic and clinical characteristics of adults with HIV diagnosis for at least 12 months and receiving medical care in the United States, Medical Monitoring Project, 2009.
| Characteristic | Totala (n = 4094) | MSM (n = 1897) | MSW (n = 1016) | WSM (n = 1093) | P for modified Rao-Scott chi-square test |
| Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) | ||
| Age at the time of interview (years) | |||||
| 18–29 | 7 (6–8) | 8 (6–9) | 4 (2–5) | 9 (7–11) | <0.001 |
| 30–39 | 17 (15–18) | 16 (14–18) | 12 (10–15) | 21 (18–24) | |
| 40–49 | 40 (38–42) | 41 (39–44) | 39 (36–42) | 39 (35–43) | |
| ≥50 | 36 (35–38) | 35 (33–38) | 45 (42–49) | 31 (28–34) | |
| Race/ethnicity | |||||
| Black, non-Hispanic | 41 (33–49) | 22 (16–28) | 56 (46–67) | 62 (54–70) | <0.001 |
| White, non-Hispanic | 35 (28–41) | 54 (48–61) | 17 (11–23) | 18 (14–22) | |
| Hispanic | 19 (14–24) | 19 (16–22) | 22 (13–32) | 17 (10–24) | |
| Multiracial/other | 5 (4–6) | 5 (4–6) | 5 (3–7) | 3 (2–5) | |
| Education | |||||
| <High school | 23 (20–25) | 9 (7–11) | 36 (33–40) | 34 (31–38) | <0.001 |
| High school | 27 (24–30) | 22 (19–24) | 34 (31–37) | 30 (26–34) | |
| >High school | 50 (46–55) | 69 (65–74) | 30 (25–34) | 36 (32–40) | |
| At or below the poverty levelb | 44 (40–48) | 26 (23–29) | 58 (54–62) | 62 (57–67) | <0.001 |
| Experienced homelessnessb | 9 (8–10) | 6 (5–8) | 14 (11–16) | 8 (7–10) | <0.001 |
| In jail >24 hb | 6 (5–7) | 3 (3–4) | 11 (8–14) | 5 (4–7) | <0.001 |
| Continuous health insurance or coverageb | 72 (67–76) | 72 (66–78) | 71 (65–76) | 74 (69–79) | 0.238 |
| HIV diagnosis ≥5 years earlier | 78 (76–80) | 78 (75–80) | 77 (74–81) | 80 (77–83) | 0.444 |
| Prescribed antiretroviral therapy | 89 (88–91) | 90 (89–92) | 91 (89–93) | 86 (83–89) | 0.006 |
| ≥3 viral load measurementsb | 62 (60–65) | 61 (59–64) | 66 (62–71) | 60 (56–64) | 0.236 |
| All viral load measures in the past year <200 copies/ml | 59 (56–62) | 63 (60–66) | 58 (54–62) | 53 (49–57) | <0.001 |
| Clinical status | |||||
| AIDS or CD4+ cell count 0–199 cells/μl (nadir) | 68 (66–70) | 65 (63–68) | 76 (73–80) | 65 (62–68) | <0.001 |
| No AIDS and CD4+ cell count 200–499 cells/μl (nadir) | 25 (23–27) | 28 (25–31) | 18 (15–21) | 27 (24–29) | |
| No AIDS and CD4+ cell count ≥500 cells/μl (nadir) | 7 (6–8) | 7(5–9) | 5 (4–7) | 8 (6–10) | |
CI, confidence interval; MSM, men have sex with men; MSW, men who have sex with women; WSM, women who have sex with men.
aExcludes persons who reported no sexual behaviour and did not report their sexual orientation as heterosexual, homosexual or bisexual. Includes transgender participants (n = 64) and women who have sex with women (n = 26) because sample sizes were too small to provide valid estimates for separate analysis.
bRefers to the 12 months before interview (June 2008–May 2009).
Overall, 62% reported oral, anal or vaginal sex with one or more partners in the past 12 months; 13% reported an STI; and 56% reported not having been tested for STIs. In addition, 24% drank alcohol and 11% used drugs before or during sex (Table 2). MSM were significantly more likely than MSW or WSM to have had two or more partners and to have drunk alcohol or used drugs before or during sex; MSM, compared with MSW, were significantly more likely to report an STI. MSW, compared with MSM or WSM, were significantly more likely to report that they had not been tested for an STI; compared with WSM, MSW were more likely to have used noninjection drugs and report exchanging sex for money or goods. WSM, compared with MSM, were significantly more likely to report no sex partners, and they were more likely than MSW to report an STI.
Table 2.
Sexual and drug-use behaviours of men who have sex with men, men who have sex with women and women who have sex with men with HIV diagnosis for at least 12 months receiving medical care in the United States, Medical Monitoring Project, 2009.
| Characteristicsa | Totalb (n = 4094) | MSM (n = 1897) | MSW (n = 1016) | WSM (n = 1093) | P for modified Rao-Scott chi-square test |
| Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) | ||
| Number of sex partners | |||||
| None | 38 (35–40) | 31 (28–34) | 43 (38–47) | 46 (43–48) | <0.001 |
| 1 | 38 (36–41) | 31 (28–33) | 44 (41–48) | 47 (44–50) | |
| ≥ 2 | 24 (21–27) | 39 (34–42) | 13 (11–15) | 8 (5–10) | |
| Self-reported sexually transmitted infection | |||||
| Yes | 13 (11–15) | 16 (13–19) | 6 (5–8) | 12 (8–16) | <0.001 |
| No | 31 (29–33) | 32 (29–34) | 26 (23–30) | 35 (31–39) | |
| Not tested | 56 (54–58) | 52 (49–56) | 68 (64–72) | 53 (49–57) | |
| Used noninjection drugs | 27 (26–29) | 35 (32–37) | 24 (21–27) | 17 (15–19) | <0.001 |
| Drank alcohol before/during sex | 24 (22–26) | 32 (29–35) | 19 (16–32) | 15 (13–18) | <0.001 |
| Drug use before or during sex | |||||
| Any drug | 11 (10–13) | 16 (14–18) | 7 (5–9) | 7 (5–8) | <0.001 |
| Stimulants | 5 (4–6) | 7 (6–9) | 2 (1–3) | 3 (2–4) | <0.001 |
| Poppers | 3 (2–4) | 6 (4–7) | — | — | |
| Methamphetamines | 2 (2–3) | 5 (3–6) | — | — | |
| Exchanged sex for money/goods | 4 (3–4) | 3 (2–4) | 5 (3–7) | 2 (1–3) | 0.009 |
CI, confidence interval; MSM, men who have sex with men; MSW, men who have sex with women; WSM, women who have sex with men; dash, coefficient of variation was >0.30 (sample size too small to produce valid estimates).
aRefers to the 12 months before interview (June 2008–May 2009)
bExcludes persons who reported no sexual behaviour and did not report their sexual orientation as heterosexual, homosexual or bisexual. Includes transgender participants (n = 64) and women who have sex with women (n = 26) because sample sizes were too small to provide valid estimates for separate analysis.
Viral suppression and sexual risk behaviour
Of the estimated 408 092 (95% CI, 366 524–449 660) HIV-infected adults diagnosed for at least 12 months and receiving care, 224 112 (95% CI, 203 005–245 219) (56%) engaged in vaginal or anal sex; 98 022 (95% CI, 83 019–113 025) (24%) engaged in unprotected vaginal or anal sex; 50 953 (95% CI, 44 791–57 115) (12%) engaged in unprotected vaginal or anal sex with a partner of negative or unknown HIV status (Fig. 1). Notably, 23 933 (95% CI, 20 155–27 712) (6%) engaged in unprotected vaginal or anal sex with a partner of negative or unknown HIV status while not virally suppressed. In other words, of those who engaged in unprotected vaginal or anal sex with a partner of negative or unknown HIV status, approximately half were not virally suppressed. As shown in a supplemental table, MSM were significantly more likely than MSW or WSM to engage in unprotected sex, and WSM were significantly more likely than MSW to engage in unprotected sex (available at www.AIDSonline.com). In addition, WSM were significantly more likely than MSW to engage in unprotected sex with a partner of negative or unknown HIV status. Approximately 51%, or an estimated 3212 (95% CI, 1675–4749) transgender persons engaged in vaginal or anal sex, and 65%, or an estimated 1986 (95% CI, 967–3005) WSW reported sex with a woman in the past 12 months (data not shown).
Fig. 1.
Prevalence of vaginal or anal sex and viral suppressiona among adults with HIV diagnosis for at least 12 months and receiving medical care in the United States, by sexual behaviour, Medical Monitoring Project, 2009.
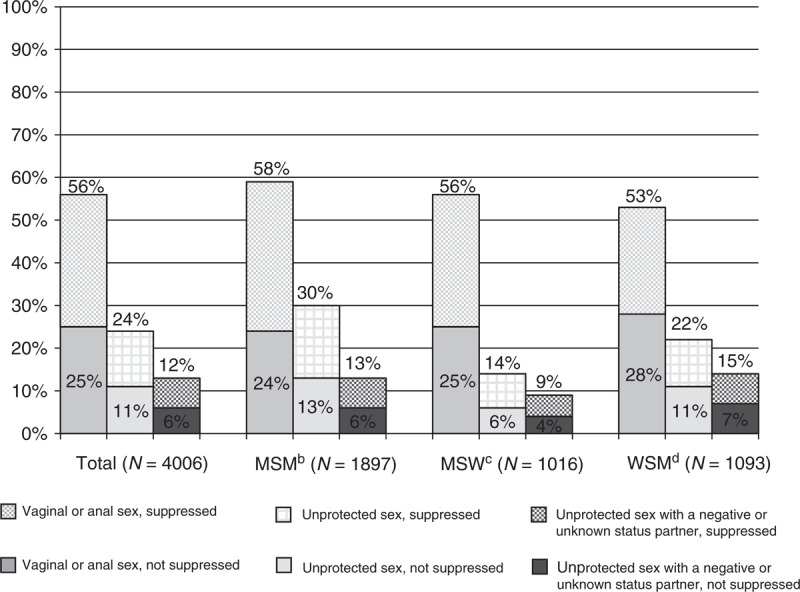
There were no statistically significant differences between MSM, MSW and WSM in engaging in anal or vaginal sex. More MSM (30%) had unprotected sex than MSW (14%) and WSM (22%). More WSM (22%) had unprotected sex than MSW (14%). More WSM (15%) had unprotected sex with an HIV-negative or unknown stats partner than MSW (9%). aAll viral load measurements in the 12 months before interview undetectable or <200 copies/ml; bMSM, men who have sex with men; cMSW, men who have sex with women; dWSM, women who have sex with men.
Relationship between viral suppression, prescription of antiretroviral therapy and sexual risk behaviour
HIV-infected adults who were virally suppressed were significantly less likely than those who were not virally suppressed to engage in vaginal or anal sex (prevalence ratio, 0.88; 95% CI, 0.82–0.93) and unprotected vaginal or anal sex (prevalence ratio, 0.85; 95% CI, 0.73–0.98). Persons who were virally suppressed were also less likely to engage in unprotected vaginal or anal sex with a partner of negative or unknown HIV status (prevalence ratio, 0.79; 95% CI, 0.64–0.99) (Table 3). MSM who achieved viral suppression were significantly less likely than MSM who were not virally suppressed to engage in vaginal or anal sex (prevalence ratio, 0.87; 95% CI, 0.80–0.96), unprotected vaginal or anal sex (prevalence ratio, 0.78; 95% CI, 0.66–0.93), and unprotected vaginal or anal sex with a partner of negative or unknown HIV status (prevalence ratio, 0.76; 95% CI, 0.60–0.97). WSM who achieved viral suppression were significantly less likely to engage in vaginal or anal sex (prevalence ratio, 0.81; 95% CI, 0.71–0.93), but viral suppression was not significantly associated with unprotected vaginal or anal sex or unprotected sex with a partner of negative or unknown HIV status. There were no statistically significant associations between viral suppression and each of the three measures of sexual risk behaviours for MSW.
Table 3.
Weighted percentage of sexual risk behaviours and associations with viral suppressiona among adults with HIV diagnosis for at least 12 months and receiving medical care in the United States, Medical Monitoring Project, 2009.
| Totalb | MSM | MSW | WSM | |||||||||
| % | PR (95% CI) | P for modified Rao-Scott chi-square test | % | PR (95% CI) | P for modified Rao-Scott chi-square test | % | PR (95% CI) | P for modified Rao-Scott chi-square test | % | PR (95% CI) | P for modified Rao-Scott chi-square test | |
| Vaginal/anal sex | ||||||||||||
| Suppressed | 53 | 0.88 (0.82–0.93) | <0.01 | 55 | 0.87 (0.80–0.96) | <0.01 | 54 | 0.93 (0.84–1.04) | 0.21 | 48 | 0.81 (0.71–0.93) | <0.01 |
| Not suppressed | 60 | Ref | 63 | Ref | 58 | Ref | 59 | Ref | ||||
| Unprotected vaginal or anal sex | ||||||||||||
| Suppressed | 22 | 0.85 (0.73–0.98) | 0.03 | 28 | 0.78 (0.66–0.93) | <0.01 | 14 | 1.00 (0.66–1.51) | 0.99 | 21 | 0.85 (0.67–1.07) | 0.16 |
| Not suppressed | 26 | Ref | 35 | Ref | 14 | Ref | 24 | Ref | ||||
| Unprotected vaginal or anal sex with a partner of negative or unknown HIV status | ||||||||||||
| Suppressed | 11 | 0.79 (0.64–0.99) | 0.04 | 12 | 0.76 (0.60–0.97) | 0.03 | 8 | 0.82 (0.44–1.53) | 0.52 | 14 | 0.87 (0.63–1.20) | 0.41 |
| Not suppressed | 14 | Ref | 15 | Ref | 10 | Ref | 16 | Ref | ||||
CI, confidence interval; MSM, men who have sex with men; MSW, men who have sex with women; PR, prevalence ratio; WSM, women who have sex with men.
aNote: viral suppression is defined as all viral load measurements in the 12 months before interview undetectable or <200 copies/ml.
bExcludes persons who reported no sexual behaviour and did not report their sexual orientation as heterosexual, homosexual or bisexual. Includes transgender participants (n = 64) and women who have sex with women (n = 26) because sample sizes were too small to provide valid estimates for separate analysis.
To establish whether the association between viral suppression and sexual risk behaviours was mediated by ART use, we used a logistic regression model of persons who were virally suppressed, persons who were prescribed ART but were not virally suppressed and persons who were not prescribed ART and were not virally suppressed. Given that most persons were prescribed ART, sample sizes were too small for a separate model for each group; thus, we present a single model. The model indicated that a significantly higher percentage of persons who were not prescribed ART, compared with persons prescribed ART and suppressed and persons prescribed ART and not suppressed, engaged in each of the three sexual risk behaviours considered (Table 4). Thus, these models suggest that ART use mediated the relationship between viral suppression and sexual risk behaviours.
Table 4.
Weighted percentage of sexual risk behaviours and associations with viral suppressiona and antiretroviral therapy use among adults with HIV diagnosis for at least 12 months and receiving medical care in the United States, Medical Monitoring Project, 2009.
| Totalb | |||
| % (95% CI) | PR (95% CI) | P for modified Rao-Scott chi-square test | |
| Vaginal/anal sex | |||
| Suppressed | 52 (49–55) | 0.78 (0.70–0.86) | <0.01 |
| Prescribed ART, not suppressed | 58 (55–61) | 0.87 (0.79–0.95) | <0.01 |
| Not prescribed ART, not suppressed | 67 (62–72) | Ref | |
| Unprotected vaginal or anal sex | |||
| Suppressed | 22 (19–25) | 0.67 (0.52–0.85) | <0.01 |
| Prescribed ART, not suppressed | 25 (21–29) | 0.75 (0.62–0.91) | <0.01 |
| Not prescribed ART, not suppressed | 33 (26–40) | Ref | |
| Unprotected vaginal or anal sex with a partner of negative or unknown HIV status | |||
| Suppressed | 11 (9–13) | 0.60 (0.42–0.85) | 0.01 |
| Prescribed ART, not suppressed | 13 (11–16) | 0.74 (0.56–0.98) | 0.04 |
| Not prescribed ART, not suppressed | 18 (14–23) | Ref | |
ART, antiretroviral therapy; CI, confidence interval; PR, prevalence ratio.
aNote: viral suppression is defined as all viral load measurements in the 12 months before interview undetectable or <200 copies/ml.
bExcludes persons who reported no sexual behavior and did not report their sexual orientation as heterosexual, homosexual or bisexual. Includes transgender participants (n = 64) and women who have sex with women (n = 26) because sample sizes were too small to provide valid estimates for separate analysis.
Discussion
We found that the majority of HIV-infected adults receiving care in the U.S. did not engage in sexual risk behaviours that have the potential to transmit HIV. However, of the 12% of persons who engaged in unprotected vaginal or anal sex with at least one partner of negative or unknown HIV status, approximately half did so while not virally suppressed. This analysis provides the first nationally representative estimates of sexual risk behaviour since the HIV Cost and Utilization Study, which was conducted in 1996, in the pre-ART era [3]. Our estimates – that 30% of HIV-infected MSM engaged in unprotected vaginal and anal sex and that 13% did so with a partner of negative or unknown HIV status – are lower than estimates from a recent meta-analysis indicating that 43% of HIV-infected MSM engaged in unprotected anal sex and that 26% did so with a partner of unknown or negative status [14]. However, a study that sampled HIV-infected persons from clinics and community organizations in four large U.S. cities found that 16% of MSM, 13% of MSW and 19% of WSM had unprotected sex with partners of negative or unknown HIV status, findings that are more consistent with our estimates of 13, 9 and 15%, respectively [27].
Since ART became widely available, concerns have been raised that the perceived reduction in sexual transmission risk secondary to viral suppression might lead to increased sexual risk behaviour. The 2012 recommendation by the U.S. Department of Health and Human Services [28] to provide ART regardless of CD4+ T-lymphocyte count further emphasized the importance of understanding the relationship between viral suppression and sexual risk behaviour. In the HIV Prevention Trials Network (HPTN) 052 study [5], which demonstrated that early initiation of ART reduces the likelihood of heterosexual HIV-1 transmission, condom use was extremely high among participants. However, condoms and behavioural-risk reduction interventions were provided to all participants; therefore, this level of behavioural risk reduction may not be achieved in real-world settings [10]. Most previous studies evaluating the association between ART use and sexual risk behaviour in the United States, among both MSM and heterosexual men and women, have not found ART use to be associated with increased sexual risk behaviour [12,14]. As our data are cross-sectional, we were unable to evaluate changes in risk behaviour before and after ART initiation. However, we found no evidence that people receiving ART were more likely to engage sexual risk behaviour than those who were not. In fact, we found that persons who were virally suppressed were less likely to engage in vaginal or anal sex, unprotected vaginal or anal sex, and unprotected vaginal or anal sex with a partner of negative or unknown HIV status. Furthermore, those persons who were prescribed ART were less likely to engage in risk behaviours, regardless of viral suppression, which suggests that ART use mediated the relationship between viral suppression and sexual behaviours. This finding may be a result of prevention interventions delivered in the care setting, possible ART side effects that could decrease libido (e.g. depression or anxiety) or an indication that persons who are prescribed ART differ from those who are not. Although our analysis was not designed to address the causal relationship between ART use, viral suppression and sexual risk behaviour, it indicates that virally suppressed persons were less likely to engage in risky sexual behaviours. In other words, those individuals who were not virally suppressed, and therefore at a greatest risk of transmitting HIV [5], were also more likely to engage in sexual behaviours that have the potential to transmit HIV. This suggests that persons not on ART and not virally suppressed may be in need of targeted prevention interventions.
The association between viral suppression and sexual behaviours was not statistically significant for all groups. MSM who were virally suppressed were significantly less likely to engage in all three measures of sexual risk behaviour than HIV-infected MSM who were not virally suppressed. For MSW and WSM, the trend was the same in regard to vaginal or anal sex, but none of the differences were statistically significant for MSW. The only statistically significant association for WSM was engagement in vaginal or anal sex. Although this null result could, in part, be explained by the smaller sample size of these groups, our findings are consistent with those of other studies that found no association between viral suppression and risk behaviour [29,30].
Compared with MSW, MSM in our analysis were more likely to report high-risk sexual behaviour, which is consistent with previous studies [3,29,31]. This increased prevalence of risk behaviours, combined with the increased per-act transmissibility of HIV through anal intercourse (both insertive and receptive) [31], highlights the pressing need for effective prevention efforts for MSM. Implementation of prevention efforts will require careful consideration of biological, network, social and structural factors (e.g. stigma, discrimination and homophobia) among MSM [32].
Combination prevention approaches, including behavioural risk reduction counselling, and access to ART and medical care should be available to all HIV-infected adults to maximize the individual and public health benefit. However, recent data on the HIV care continuum indicate that additional work is needed at every step in the continuum: expanded HIV testing, increased numbers of HIV-infected adults linked to and retained in care, and increased numbers of persons who are prescribed ART and ultimately achieve viral suppression [33]. The fact that only 25% of HIV-infected persons in the United States are virally suppressed highlights the need for action [34]. In addition, the delivery of behavioural risk reduction interventions in the clinical setting is essential. Medical visits provide an opportunity to discuss risk behaviours, reinforce prevention messages, diagnose and treat STIs, and emphasize medication adherence. Effective evidence-based interventions that can be delivered by providers as brief messages during clinic visits are available [35], but too few providers discuss HIV prevention with their patients [33]. STI screening and treatment, when indicated, is recommended by guidelines and is likely to reduce HIV transmission [36], but data indicate that too few patients are being screened by their providers [37]. Combination prevention approaches will require additional efforts to engage and retain HIV-infected persons in care and improve the delivery of risk reduction interventions in clinical settings.
Our analysis has limitations. First, MMP data reflect the experiences of HIV-infected adults receiving medical care in the United States; thus, our results cannot be generalized to persons unaware of their HIV infection or to those not receiving medical care. Sexual behaviours and viral suppression status are likely to differ among persons in care and persons not in care [38]. Also, we did not collect information on whether persons were aware of their current viral load status, which could influence subsequent risk behaviour. In addition, given less than optimal response rates, nonresponse bias is possible. Furthermore, because compliant patients may have been more likely to participate in MMP, it is possible that results may differ for less compliant patients. However, we collected information on sampled patients (sex, age, race, length of time since diagnosis) and facilities (HIV patient case load) and were able to compare the characteristics of respondents and nonrespondents. Our estimates were then weighted to minimize nonresponse bias. In addition, our probabilistic sampling frame was rigorously constructed, geographically diverse, included both urban and rural clinics, public and private facilities, providers who see many and few HIV-infected patients and jurisdictions that have high, medium and low HIV prevalence. Furthermore, empirical research indicates that low response rates are not necessarily indicative of nonresponse bias, particularly when probabilistic samples are drawn from rigorously constructed frames [39]. Next, because this is a cross-sectional analysis and we did not collect dates for the sexual behaviour data (beyond knowing they occurred in the 12 months before the interview), we cannot determine the exact timing of sexual risk behaviour with respect to viral suppression. Finally, sexual risk behaviour data were collected during face-to-face interviews, so social desirability bias may have led to underreporting of some behaviours [40].
Conclusion
Of an estimated 408 092 HIV-infected adults receiving medical care in the United States, the majority of persons did not engage in sexual risk behaviours that have the potential to transmit HIV. Of the 12% of persons who engaged in unprotected vaginal or anal sex with a partner of unknown or negative HIV status, approximately half were not virally suppressed, which highlights an unmet need for effective prevention interventions. We found no evidence that people on ART engaged in higher levels of sexual risk behaviour than those who were not. In fact, persons on ART and virally suppressed were less likely than those not on ART to engage in sexual risk behaviours. Behavioural risk reduction will continue to be a necessary component of HIV prevention efforts; clinicians play a vital role in HIV prevention and should use medical visits to discuss sexual risk behaviour, reinforce prevention messages, diagnose and treat STIs, and emphasize medication adherence.
Acknowledgements
The following are the contributions of the authors to this analysis: C.L.M, M.F. and J.S.: analysis conception, data analysis, wrote the article; E.F., P.H., C.J. provided statistical and data analysis support; J.F., L.B., E.E.V, C.S., A.D.M, P.S., A.L., J.M. and J.H. were involved in conception, design and/or implementation of the MMP. All authors contributed to data interpretation, article writing and/or review and editing. The authors thank the MMP patients, facilities and Community and Provider Advisory Board members. They also thank the 2009 MMP staff: http://www.cdc.gov/hiv/pdf/research_mmp_studygroupmembers_2009.pdf. The authors are grateful to Ms. Marie Morgan for her editorial assistance and the reviewers at AIDS for their valuable input on the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Funding for the MMP is provided by the CDC.
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Material
Contributor Information
Collaborators: for the Medical Monitoring Project
References
- 1.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012. 2012; 17 (No. 4). http://www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental [Accessed 21 December 2012] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data: United States and 6 U.S. dependent areas – 2010. HIV Surveillance Supplemental Report. 2012. 17 (No. 3, part A). http://www.cdc.gov/hiv/topics/surveillance/resources/reports/ [Google Scholar]
- 3.Ciccarone DH, Kanouse DE, Collins RL, Chen JL, Morton SC, Stall R. Sex without disclosure of positive HIV serostatus in a US probability sample of persons receiving medical care for HIV infection. Am J Public Health 2003; 93:949–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404 [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipou MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forsyth AD, Valdiserri RO. Reaping the prevention benefits of highly active antiretroviral treatment: policy implications of HIV Prevention Trials Network 052. Curr Opin HIV AIDS 2012; 7:111–116 [DOI] [PubMed] [Google Scholar]
- 7.Garnett GP, Becker S, Bertozzi S. Treatment as prevention: translating efficacy trial results to population effectiveness. Curr Opin HIV AIDS 2012; 7:157–163 [DOI] [PubMed] [Google Scholar]
- 8.Heijman T, Geskus RB, Davidovich U, Coutinho RA, Prins M, Stolte IG. Less decrease in risk behaviour from pre-HIV to post-HIV seroconversion among MSM in the combination antiretroviral therapy era compared with the precombination antiretroviral therapy era. AIDS 2012; 26:489–495 [DOI] [PubMed] [Google Scholar]
- 9.Mayer KH. Antiretrovirals for HIV prevention: translating promise into praxis. Lancet 2011; 378:206–208 [DOI] [PubMed] [Google Scholar]
- 10.Shelton JD. HIV/AIDS. ARVs as HIV prevention: a tough road to wide impact [Policy Forum]. Science 2011; 334:1645–1646 [DOI] [PubMed] [Google Scholar]
- 11.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science 2000; 287:650–654 [DOI] [PubMed] [Google Scholar]
- 12.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA 2004; 292:224–236 [DOI] [PubMed] [Google Scholar]
- 13.Katz MH, Schwarcz SK, Kellogg TA, Klausner JD, Dilley JW, Gibson S, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health 2002; 92:388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crepaz N, Marks G, Liau A, Mullins MM, Aupont LW, Marshall KJ, et al. Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: a meta-analysis. AIDS 2009; 23:1617–1629 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Trends in primary and secondary syphilis in men who have sex with men: San Francisco and Los Angeles, California, 1998-2002. MMWR Morb Mortal Wkly Rep 2004; 53:575–578 [PMC free article] [PubMed] [Google Scholar]
- 16.Frankel M, McNaghten A, Shapiro M, Sullivan PS, Berry SH, Johnson CH, et al. A probability sample for monitoring the HIV-infected population in care in the U.S. and in selected states. Open AIDS J 2012; 6 Suppl 1:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The American Association for Public Opinion Research. 2011. Standard definitions: final dispositions of case codes and outcome rates for surveys. 7th ed AAPOR; http://www.aapor.org/AM/Template.cfm?Section=Standard_Definitions2&Template=/CM/ContentDisplay.cfm&ContentID=3156 [Google Scholar]
- 18.Heeringa SG, West BT, Berglund PA. Applied survey data analysis. London:Chapman and Hall; 2010 [Google Scholar]
- 19.Särndal C-E, Lundström S. Estimation in surveys with nonresponse. Chichester:John Wiley & Sons; 2005 [Google Scholar]
- 20.Center for Disease Control and Prevention. Distinguishing public health research and public health nonresearch. 2010. http://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf [Accessed 12 July 2013] [Google Scholar]
- 21.Protection of Human Subjects, US Federal Code Title 45 Part 46. 2009. http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html [Accessed 12 July 2013] [Google Scholar]
- 22.Rao JNK, Scott AJ. The analysis of categorical data from complex sample surveys: Chi-squared tests for goodness of fit and independence in two-way tables. J Am Stat Assoc 1981; 76:221–230 [Google Scholar]
- 23.Lohr SL. Sampling: design and analysis. Duxbury Press, Imprint of Brooks/Cole Publishing Company, Pacific Grove, California; 1999 [Google Scholar]
- 24.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol 2010; 171:618–623 [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute Inc Base SAS 9.3 utilities: reference. Cary, NC:SAS Institute Inc; 2011 [Google Scholar]
- 26.SUDAAN 10.0.1. Research Triangle Park, NC: RTI International; 2009 [Google Scholar]
- 27.Weinhardt L, Kelly JA, Brondino M, Rotheram-Borus MJ, Kirshenbaum SB, Chesney MA, et al. HIV transmission risk behavior among men and women living with HIV in 4 cities in the United States. J Acquir Immune Defic Syndr 2004; 36:1057–1066 [DOI] [PubMed] [Google Scholar]
- 28.HHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents. 2012. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Accessed 1 May 2013] [Google Scholar]
- 29.Golin C, Marks G, Wright J, Gerkovich Mary, Tien Hsiao-Chuan, Patel SN, et al. Psychosocial characteristics and sexual behaviors of people in care for HIV infection: an examination of men who have sex with men, heterosexual men and women. AIDS Behav 2009; 13:1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morin SF, Myers JJ, Shade SB, Koester K, Maiorana A, Rose CD. Predicting HIV transmission risk among HIV-infected patients seen in clinical settings. AIDS Behav 2007; 11 Suppl 1:6–16 [DOI] [PubMed] [Google Scholar]
- 31.Varghese B, Maher J, Peterman T, Branson B, Steketee R. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43 [DOI] [PubMed] [Google Scholar]
- 32.Beyrer C, Sullivan PS, Sanchez J, Dowdy D, Altman D, Trapence G, et al. A call to action for comprehensive HIV services for men who have sex with men. Lancet 2012; 380:424–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention Vital signs: HIV prevention through care and treatment: United States. MMWR Morb Mortal Wkly Rep 2011; 60:1618–1623 [PubMed] [Google Scholar]
- 34.Hall HI, Frazier E, Rhodes P, Holtgrave DR, Furlow-Parmely C, Tang T, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013; 173:1337–1344 [DOI] [PubMed] [Google Scholar]
- 35.Fisher JD, Fisher WA, Cornman DH, Amico KR, Bryan A, Friedland GH. Clinician-delivered intervention during routine clinical care reduces unprotected sexual behavior among HIV-infected patients. J Acquir Immune Defic Syndr 2006; 41:44–52 [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention Incorporating HIV prevention into the medical care of persons living with HIV: recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2003; 52:1–24 [PubMed] [Google Scholar]
- 37.Flagg E. Syphilis testing among HIV-infected adults receiving medical care: national estimates from the Medical Monitoring Project, 2009 Data Collection Cycle. IDSA IDWeek 2012; 17–21 October 2012; San Diego, CA [Google Scholar]
- 38.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450 [DOI] [PubMed] [Google Scholar]
- 39.Groves RM, Peytchiva E. The impact of nonresponse rates on nonresponse bias. Public Opin Q 2008; 72:167–189 [Google Scholar]
- 40.Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior, II: accuracy of self-reports. Ann Behav Med 2003; 26:104–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


