Abstract
An epidemiological study indicates higher plasma level of genistein in girls with earlier puberty. This study tests the hypothesis in C57BL/6J mice that postweaning (peripubertal) dietary genistein exposure could result in earlier puberty in females assessed by vaginal opening, estrous cyclicity, corpus luteum and mammary gland development. Newly weaned female mice were fed with 0, 5, 100, or 500 ppm genistein diets. Decreased age at vaginal opening, increased length on estrus stage, and accelerated mammary gland development were detected in 100 and 500 ppm genistein-treated groups. Increased presence of corpus luteum was found in 5 ppm genistein-treated group at 6 weeks old only. Increased expression of epithelial-specific genes but not that of ERα and ERβ was detected in 500 ppm genistein-treated mammary glands at 5 weeks old. No significant adverse effect on embryo implantation was observed. These data demonstrate causal effect of dietary genistein on earlier puberty in female mice.
Introduction
Genistein is a phytoestrogen abundant in soy [1]. High levels of genistein are found in traditional soy food, such as soy milk, tofu, miso, etc., as well as a variety of processed food, such as meatless burger, energy bar and soy yogurt, etc. [2]. The estimated daily intake of genistein in US adults is ~0.6 mg/day based on National Health and Nutrition Examination Survey 1999-2002 data [3], and ~6-19 mg/day in Asian people [4-6]. Since US FDA approved the health claims of soy diet on reducing coronary disease in 1999 [7], soy consumption in US has been steadily increasing [8].
Genistein could have different effects. The beneficial effects of genistein include relieving menopausal symptom, protecting cardiovascular system, preventing breast cancer, etc. [9-12]. Since genistein is a weak estrogen [13, 14], its potential endocrine disruptive effects have also been identified in many studies and recognized in the NTP-CERHR Expert Panel Report [15]. For example, genistein has been widely regarded as a contributing agent for a trend of earlier puberty in US and European girls [16-20]. Puberty is the physical development process of an immature body to an adult body capable of reproducing under the regulation of sexual hormones, such as estrogen [21]. A longitudinal study in UK including 1920 girls shows a positive correlation between soy formula intake during infancy and earlier menarche age [22]. Since menarche is an indicator of puberty [23] and genistein is the major phytoestrogen in the infant plasma after soy formulate consumption [24], it is most likely that genistein contributes to the puberty advancement upon infant soy formulate consumption. A case-control study of 150 6-12 years old precocious girls and 90 age-matched control girls in Korea reveals a significantly higher plasma level of genistein in the precocious group [25], implying that increased prepubertal exposure to genistein is associated with early puberty.
The majority of the human population is mainly exposed to genistein from food after infancy when non-milk food is added to the diet, equivalent to postweaning dietary exposure in rodents. We hypothesized that postweaning exposure to genistein in the diet could lead to earlier puberty in females. This hypothesis was tested in C57BL/6J female mice using human relevant exposure levels (5 ppm, 100 ppm, and 500 ppm genistein diets). It was reported that rats fed with 5 ppm and 100 ppm genistein diets could produce plasma levels of genistein similar to that in Western and Asian people, respectively [26], while 500 ppm genistein diet could be found in soy products, e.g., soy bacon [2]. These doses were also used in the multi-generational studies of genistein by the National Toxicology Programs (NTP) [27]. Vaginal opening, estrous cyclicity, ovulation initiation, and mammary gland development were monitored as indicators for puberty development in this study.
Materials and Methods
Animals
C57BL/6J is a sensitive mouse strain to endocrine disruptors [28-30] and was selected as an in vivo model in this study. C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and maintained on phytoestrogen-free AIN-93G diet (Bio-Serv, Frenchtown, NJ) in Coverdell animal facility at the University of Georgia. The mice were housed in polypropylene cages with free access to food and water on a 12 h light/dark cycle (0600–1800) at 23±1 °C with 30–50% relative humidity. All methods used in this study were approved by the University of Georgia IACUC Committee (Institutional Animal Care and Use Committee) and conform to National Institutes of Health guidelines and public law.
Treatment
The genistein diets were prepared following the similar procedure as described previously [30]. Briefly, 0 g, 0.0025 g, 0.05 g, or 0.25 g genistein were dissolved in 150 ml 70% ethanol. Each solution was well mixed with 500 g AIN-93G diet in a glass bowl to attain 0 ppm (control), 5 ppm, 100 ppm, and 500 ppm genistein diets, respectively. Food pellets were hand squeezed, air dried at room temperature, and kept at 4°C in the dark. Fresh diets were prepared every two weeks. Breeding females were on phytoestrogen-free AIN-93G diet ad libitum throughout pregnancy and lactation. Newly weaned (postnatal day 21) female pups were randomly assigned into four groups and fed with 0 ppm, 5 ppm, 100 ppm, or 500 ppm genistein diet, respectively, until sacrificed for tissue collection / determination of pregnancy status. Food and water consumption were monitored weekly. The numbers of mice included in different experiments were indicated under each experiment. Newly weaning males were sacrificed without further study.
Vaginal opening and estrous cycle
Vaginal opening was evaluated daily from weaning until detection of vaginal opening (N=35 per group). Estrous cycle was monitored daily during two periods: from the day of vaginal opening for 10 days and from 5 to 8 weeks old (N=6 per group). Some overlaps of dates were seen during these two periods, especially for 0 and 5 ppm genistein groups. Vaginal smear was collected at 0800 h. The stages of estrous cycle were determined according to the composition of nucleated, cornified cells, and leukocytes as described [30, 31].
Tissue collection
All mice were dissected at estrus stage, which was determined by vaginal smear prior to dissection, by CO2 inhalation and cervical dislocation. Only the mice on estrus stage at the selected time points (5, 6, 7, or 10 weeks old) ± 1 day were included. One side of the mammary glands was frozen, the other side of the mammary glands was used for whole mount staining or fixed for immunohistochemistry, and an ovary was fixed for histology.
Ovary histology
After fixation in formalin for 24 h, the ovaries were washed in 50%, 70%, 80%, 90%, and 95% ethanol for 30 min each, 100% ethanol for 30 min twice, and xylene for 5 min twice, then embedded in paraffin. Paraffin sections were cut at 5 μm. Serial sections of the ovaries in all four groups at 6 weeks old (N=5-6 per group), in 0 and 500 ppm genistein-treated groups at 5 weeks old (N=3 per group) and 7 weeks old (N=6 per group), and in 5 ppm and 10 ppm genistein-treated groups at 7 weeks old (N=3 per group) were evaluated. Consecutive sections were separated by 50 μm.
Mammary gland whole mount and quantification of mammary gland development
The dissected whole inguinal (the 4th) mammary gland was flattened on a slide (Fisher scientific, Pittsburgh, PA) with weight for 24 h, then fixed in Carnoy’ solution, stained by carmine alum, dehydrated through alcohol, cleared in xylene and mounted, as described [32]. Pictures were taken with an Olympus microscope BX41 with DP70 digital camera. The morphology of the mammary glands from 0 ppm, 5 ppm, 100 ppm, and 500 ppm genistein-treated groups (N=6-10 per group) at 5, 6, 7, or 10 weeks old, respectively, was analyzed by Image J (National Institutes of Health, Bethesda, MD, USA). The duct length of each mammary gland was indicated by the length of the longest duct. The occupied area of each mammary gland was approximated by a polygon area that covered all the ducts.
Realtime PCR
The whole inguinal mammary gland was dissected from 5 weeks old mice in 0 and 500 ppm genistein-treated groups (N=6-7 per group) and the lymph node was removed. Each lymph node-free mammary gland was homogenized in Trizol for total RNA isolation and cDNA synthesis using random primers (Invitrogen, Carlsbad, CA, USA) as previously described [33, 34]. Realtime PCR was performed in 384-well plates using Sybr-Green I intercalating dye on ABI 7900 (Applied Biosystems, Carlsbad, CA, USA). The mRNA expression levels of amphiregulin (Areg), cytokeratin 5 (CK5), CK8, CK14, CK18, estrogen receptor α (Esr1) and Esr2, progesterone receptor (PR), and wingless-related MMTV Integration Site 4 (Wnt4) were determined using gene-specific primers from different exons (Integrated DNA Technology, San Diego, CA, USA) The mRNA expression levels were normalized by the expression of Gapdh (glyceraldehyde-3-phosphate dehydrogenase). Hprt1 (hypoxanthine phosphoribosyltransferase 1) served as the second house-keeping gene (Suppl Table S1).
Immunohistochemistry
Paraffin sections (5 μm) of the inguinal mammary glands from 5 weeks old mice (N=3 per group) were used for immunohistochemistry. Sections from three mice each in 0 and 500 ppm genistein-treated groups were evaluated. After dewaxing, the slides were immunostained with rabbit anti-PR antibody (1:200, 6 μg/ml, Daco, Denmark), rabbit anti-ERα (ESR1) antibody (1:100, 5 μg/ml, Abcam), rabbit anti-phospho ERα (1:100, 10 μg/ml, Abcam), and anti-ERβ (ESR2) (1:50, 20 μg/ml, Abcam) as previously described [29].
Embryo implantation
Female mice from 0 and 500 ppm groups (N=8-27 per group) were mated with stud males starting at three time points: right after vaginal opening, at 5 weeks old, or at 7 weeks old, respectively. The morning of a detected copulation plug was defined as gestation day 0.5 (D0.5). All the mice were sacrificed on D4.5 to determine embryo implantation using blue dye reaction as described previously [35]. If no implantation sites were detected, the uterine horns and oviducts would be flushed with 1XPBS to determine the presence of oocyte or embryos in the reproductive tract. Pregnancy status was determined by the presence of embryos and/or implantation sites in the reproductive tract.
Statistical analysis
One way ANOVA followed by Post-hoc Tukey’s test for data with equal variance homology, or followed by Dunnett’s T3 tests for data with unequal variance homology, were used to compare the age of vaginal opening and quantitative data from whole mount mammary glands. Kruskal-Wallis equality-of-populations rank test was used to compare the % of time in estrus stage. Fisher’s exact test was used to determine the distribution of mice with estrous cyclicity and mice with extended estrus stage. Student’s t test with two tails and unequal variance was used to compare gene expression between 0 and 500 ppm genistein-treated groups. The significance level was set at p<0.05.
Results
Promoted vaginal opening
No significant differences among different groups in body weight or diet consumption were observed during the entire treatment duration (data not shown). Based on the food consumption, the estimated average doses in 0 ppm, 5 ppm, 100 ppm, or 500 ppm genistein groups were 0, 0.5, 10, or 50 mg/kg body weight per day, respectively.
Figure 1 shows the average ages at vaginal opening upon different doses of genistein treatment. Comparable ages were observed between 0 ppm control group and 5 ppm genistein-treated group (p=0.078). Compared to the control and 5 ppm genistein-treated groups, significant younger ages at vaginal opening were observed in both 100 ppm (p<0.001) and 500 ppm (p<0.001) genistein-treated groups. There was also significant difference between 100 and 500 genistein-treated groups (p<0.001). In the 500 ppm genistein-treated group, the average vaginal opening occurred within 3 days of treatment.
Figure 1.
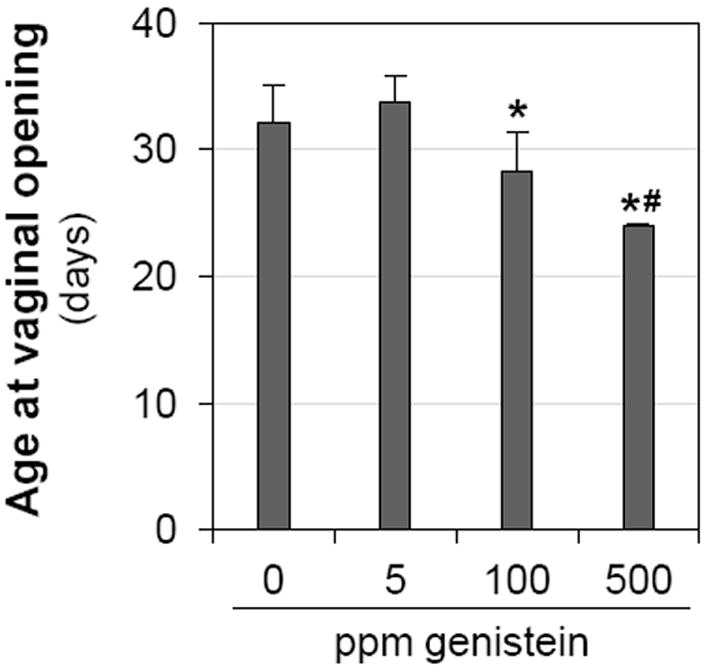
Effect of genistein on vaginal opening. N=35 per group; error bar, standard deviation; * p<0.05, compared to control (0 ppm); # p<0.05, compared to 100 ppm genistein-treated group.
Disrupted estrous cycle
Estrous cyclicity is established after puberty onset. There was a dose-dependent increase of time in estrus stage during the 10 days following vaginal opening. Compared to the control group, the average time (% of 10 days) in the estrus stage was marginally higher in 5 ppm (p=0.055) genistein-treated group, and significantly higher in 100 ppm (p<0.001) and 500 ppm (p<0.001) genistein-treated groups (Fig. 2A and Fig.S1). Significant differences were also observed in 100 ppm and 500 ppm genistein-treated groups compared to 5 ppm genistein-treated group (p<0.05). Most mice in the 0 or 5 ppm dose groups (5/6 each) demonstrated progression through their estrous cycles, however, only 50% (3/6) and 0% (0/6, p=0.015) of the animals in the 100 and 500 ppm exposure groups demonstrated normal estrous cycles, respectively (Figs. 2B, S1).
Figure 2.
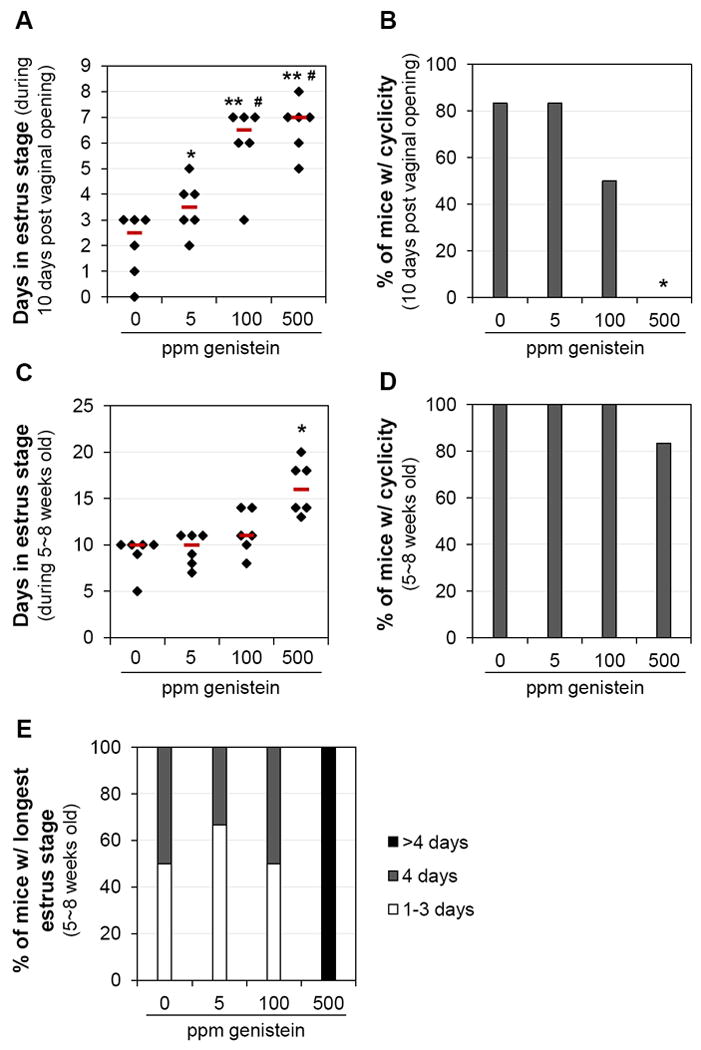
Effect of genistein on estrous cycle. A. Time (days) in estrus stage during the 10 days following vaginal opening. * p=0.055, ** p<0.001, compared to control (0 ppm); # p<0.05, compared to 5 ppm genistein-treated group. B. Percentage of mice with estrous cyclicity during the 10 days following vaginal opening. C. Time (days) in estrus stage during 5~8 weeks old. D. Percentage of mice with estrous cyclicity during 5~8 weeks old. E. Distribution of mice with the longest estrus stage during 5~8 weeks old. A~E. N=6; error bar, standard deviation; * p<0.05, compared to 0, 5, and 100 ppm genistein-treated groups. A & C. black diamonds indicating data from individual mice; red lines indicating median in each group.
Since genistein treatment accelerated vaginal opening (Fig. 1), the ages during these 10 days following vaginal opening were different and those in the 500 ppm genistein-treated group were the youngest. To eliminate age as a contributing factor for the disrupted estrous cyclicity in the genistein-treated groups (Figs. 2, S1), the same set of mice was examined for estrous cyclicity until 8 weeks old and the data during the 21 days from 5 to 8 weeks old in all four groups were analyzed. Prolonged estrus stage was still present in the 500 ppm genistein-treated group (Fig. 2C). Estrous cyclicity was observed in all mice except one in the 500 ppm genistein-treated group but those with estrous cyclicity had very irregular estrous cycle(s) during the observed period (Figs. 2D, S2). Extended length of estrus stage (>4 days) was only found in all the mice in the 500 ppm genistein-treated group (Fig. 2E), with two of them (33%) having persistent estrus stage for more than 10 days (Fig. S2). These data demonstrate that postweaning exposure to a high level of dietary genistein can disrupt estrous cyclicity.
Altered ovulation timing
Ovulation follows puberty onset and can be indicated by the presence of corpus luteum in the ovary [36, 37]. Primary, secondary, antral, and pre-ovulatory follicles were present in all the ovaries from different groups (Figs. 3A, 3B and data not shown). At 5 weeks old, none of the mice in 0 ppm (0/3) and 500 ppm (0/3) genistein-treated groups had corpus luteum (data not shown). At 6 weeks old, the percentages of mice with corpus luteum were 0% (0/6), 100% (5/5, P=0.002), 50% (3/6, P=0.182), and 33% (2/6, P=0.455) in 0 ppm, 5 ppm, 100 ppm, and 500 ppm genistein-treated groups, respectively (Fig. 3C). At 7 weeks old, 67% (4/6) of the mice in the 0 ppm group and 100% of the mice in the 5 ppm (3/3), 100 ppm (3/3), and 500 ppm (6/6) genistein-treated groups had corpus luteum (P>0.05, data not shown).
Figure 3.
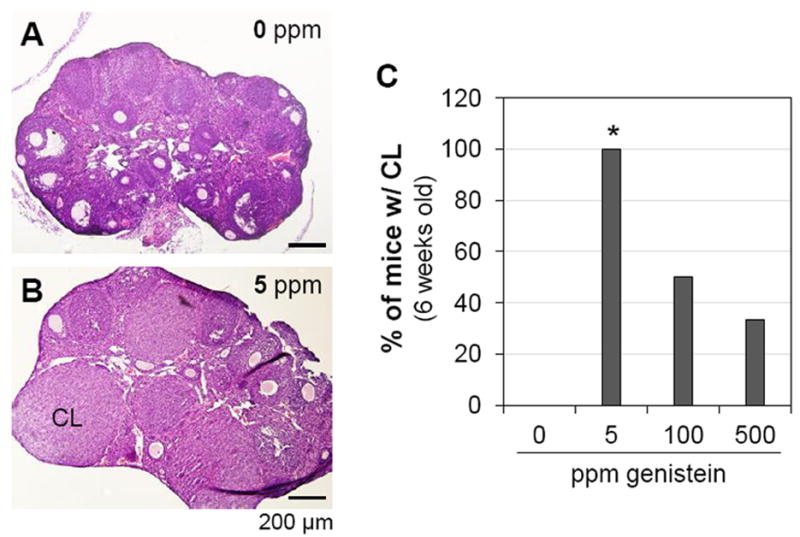
Effect of genistein on ovulation at 6 weeks old. A. A representative image of ovarian histology from control group. B. A representative image of ovarian histology from 5 ppm genistein-treated group. A & B. H & E stain; CL: corpus luteum; scale bar: 200 μm. C. Percentage of mice with corpus luteum. N=5-6; * p<0.05 compared to control (0 ppm).
Accelerated mammary gland development
During pubertal development, the mammary gland ducts grow towards the lymph node, therefore, mammary gland development can be evaluated based on the length of ducts and the area occupied by the ducts in whole mount mammary glands [38]. At 5 weeks old the frontier mammary gland ducts were halfway towards the lymph node in 0 and 5 ppm genistein-treated groups (Figs. 4A, 4B), but had reached or passed the lymph node in 100 ppm (Fig. 4C) and 500 ppm (Fig. 4D) genistein-treated groups, respectively. At 6 weeks old, the frontier mammary gland ducts had reached the lymph node in 0 and 5 ppm genistein-treated groups, had passed the lymph node in 100 ppm genistein-treated group, and had extended away from the lymph nodes in 500 ppm genistein-treated group; similar development trend of mammary gland ducts was also observed at 7 weeks and 10 weeks old (Suppl Fig. S3). Quantitative data showed no difference between 0 and 5 ppm genistein-treated groups in the duct length and the area occupied by ducts from 5 weeks old to 10 weeks old (Figs. 4E, 4F), indicating that low dose of 5 ppm genistein did not have an obvious effect on mammary gland development. However, postweaning exposure to 100 ppm and 500 ppm genistein diets significantly increased the length of mammary ducts from 5 weeks old to 10 weeks old (Figs. 4E, 4F). At 5 and 6 weeks old, significant difference in duct length and area occupied by ducts was also observed between 100 ppm and 500 ppm genistein-treated groups, indicating the dose-dependent effects of genistein on mammary gland development.
Figure 4.
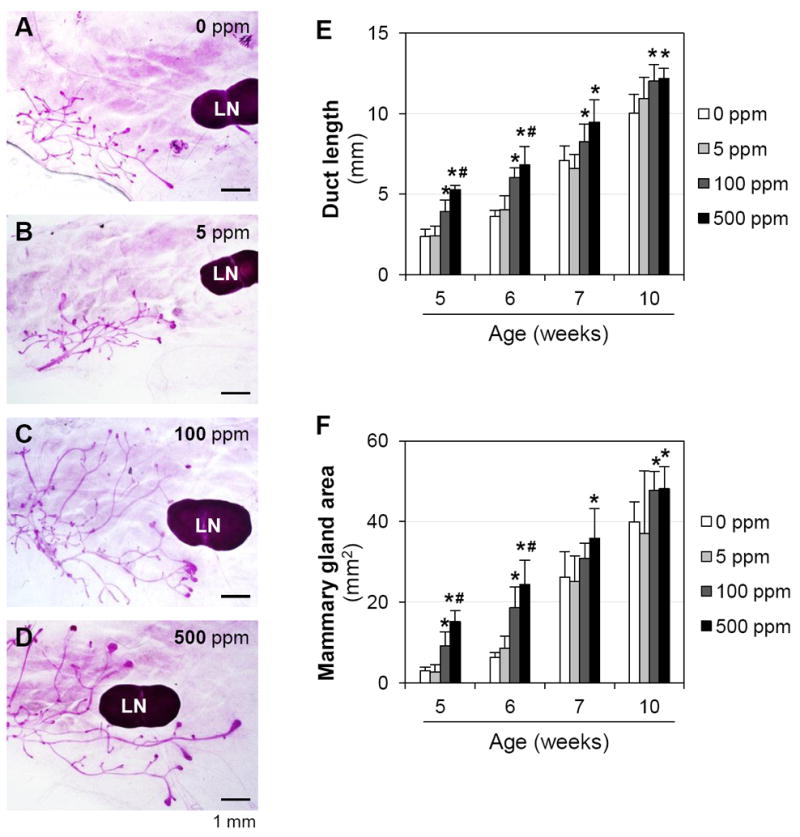
Effects of genistein on mammary gland development. A~D. Representative images of whole mount mammary glands in 0 ppm (A), 5 ppm (B), 100 ppm (C) and 500 ppm (D) genistein-treated groups at 5 weeks old. Pink lines, mammary gland ducts; LN: lymph node. E. Length of the longest mammary gland duct. F. Area occupied by mammary gland ducts. N=6-10; error bar, standard deviation; * p<0.05, compared to control (0 ppm); # p<0.05, compared to 100 ppm genistein-treated group.
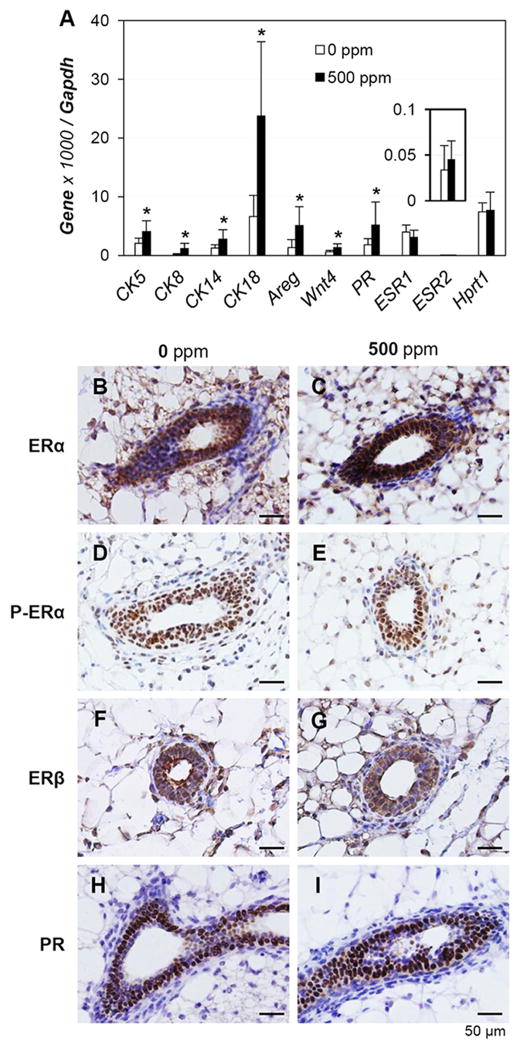
To confirm the accelerated development of mammary gland, the mRNA expression levels of several epithelial specific genes, CK5, CK8, CK14, and CK18, PR, Areg, and Wnt4, were examined in mammary glands from 5 weeks old mice treated with 0 or 500 ppm genistein diets. Significant upregulation of these genes was detected in the 500 ppm genistein-treated group (Fig. 5A). However, no significant difference was observed for Esr1 (ERα) and Esr2 (ERβ), as well as the house-keeping gene Hprt1 between these two groups (Fig. 5A). Immunohistochemistry results indicated that ERα and ERβ were also highly expressed in other cell types, e.g., adipocytes, as that in the mammary gland epithelial cells, and no obvious difference in signal intensity was observed between the two groups (Figs. 5B, 5C, 5F, 5G), which could explain why no difference was observed in Esr1 and Esr2 in the whole mammary gland (Fig. 5A). ERα and ERβ were detected in both nucleus and cytoplasm, while phosphor-ERα was mainly detected in the nucleus and no obvious difference was observed between these two groups (Figs. 5B~5G). PR was mainly detected in the nuclei of mammary gland epithelial cells and no obvious difference in signal intensity was observed between the two groups (Figs. 5H, 5I). The difference in mRNA levels for the epithelial specific genes most likely correlates with the difference in the abundance of mammary gland ducts thus epithelial cells (Figs. 4, 5A).
Figure 5.
Effects of 500 ppm genistein diet on gene expression in mammary gland at 5 weeks old. A. Real-time PCR. N=6-7; error bar, standard deviation; * p<0.05. B~I. Representative images of gene expression by immunohistochemistry in control (0 ppm) and 500 ppm genistein-treated groups. B. ERα, 0 ppm. C. ERα, 500 ppm. D. Phosphor-ERα, 0 ppm. E. Phosphor-ERα, 500 ppm. F. ERβ, 0 ppm. G. ERβ, 500 ppm. H. PR, 0 ppm. I. PR, 500 ppm. N=3; dark brown, immunostaining; purple-blue, counter staining with Harris hematoxylin; no specific immunostaining in negative controls (data not shown).
No significant adverse effects on early pregnancy
Figure 6A shows the average ages at the first copulation from each cohabitation period, right after vaginal opening, at 5 weeks old, and at 7 weeks old. Since 500 ppm genistein significantly advanced the age of vaginal opening, the average age of first copulation right after vaginal opening was significantly younger in the 500 ppm genistein-treated group. Although the average ages of first copulation after 5 weeks or 7 weeks showed no significant difference between 0 and 500 ppm genistein-treated groups, there were significant differences among the three examined times (Fig. 6A). First copulation right after vaginal opening yielded 0% implantation rate (Fig. 6B) in both groups, and 11% (1/9) and 0% (0/9) pregnancy rate in the control and 500 ppm genistein-treated groups, respectively. The first copulation after 5 weeks old yielded 40.7% (11/27) implantation rate and 55.6% (15/27) pregnancy rate in the control group, with 14.8% (4/27) of the mice having embryos but no implantation sites in the reproductive tract; as well as 48.0% (12/25) implantation rate and 64.0% (16/25) pregnancy rate in the 500 ppm genistein-treated group, with 16.0% (4/25) of the mice having embryos but no implantation sites in the reproductive tract (Figs. 6B, 6C). The first copulation after 7 weeks old resulted in 100% (8/8) implantation rate and pregnancy rate in the control group; as well as 80.0% (8/10) implantation rate and 90.0% (9/10) pregnancy rate in the 500 ppm genistein-treated group, with 10.0% (1/10) of the mice having blastocysts but no implantation sites in the uterus (Figs. 6B, 6C). For those mice with implantation sites from mating after 5 weeks old or 7 weeks old, the ages at first copulation were not significantly different between the two groups at each time point (Fig. 6A and data not shown) and the average numbers of implantation sites were comparable between the two groups at each time point (Fig. 6D). These data indicate that 500 ppm genistein diet did not have a dramatic adverse effect on early pregnancy because comparable embryo implantation reflects no significant adverse effects on ovulation, fertilization, embryo transport and preimplantation embryo development, the early pregnancy events leading to successful embryo implantation [30]. However, we noticed that in the 500 ppm genistein-treated group at 5 weeks old time point, two of the 12 mice with implantation sites had 3 faint blue bands (data not shown), an indication of delayed embryo implantation [39], and two of the 13 mice without implantation sites had swollen appearance of uterus (data not shown), an indication of estrogenic effect [30]. Since no significant difference was observed compared to the control group and no faint blue bands or distended uteri were observed at 7 weeks old time point, these observations suggested that 500 ppm genistein diet might still have some minor effects on embryo implantation before the females are fully mature.
Figure 6.
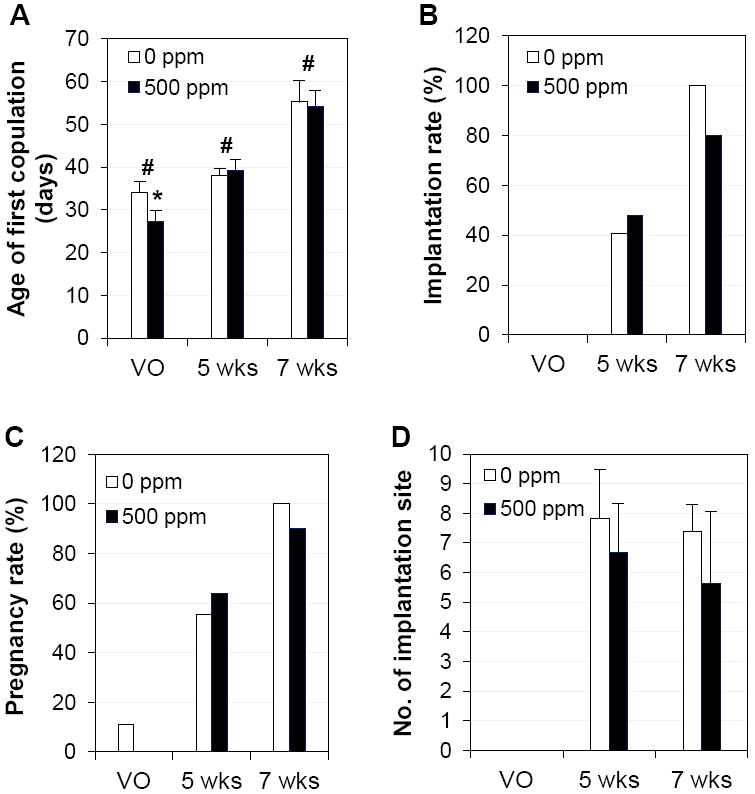
Effects of 500 ppm genistein on first copulation and early pregnancy. A. Age of first copulation upon mating at vaginal opening (VO), 5 weeks (wks) or 7 wks old. * p<0.05 compared to control at VO; # p<0.05 indicating differences among three mating periods. B. Implantation rate (% of mice with implantation sites on gestation day 4.5 / mice with a vaginal plug). C. Pregnancy rate (% of mice with implantation sites and/or embryos on gestation day 4.5 / mice with a vaginal plug). D. Number of implantation sites from mice with implantation sites. N=8-27; error bar, standard deviation.
Discussion
This study verifies our hypothesis that postweaning exposure to dietary genistein at human relevant levels can promote puberty in C57BL/6J female mice. All three doses examined have some effects on one or multiple parameters for puberty, including vaginal opening, estrous cyclicity, ovulation, and mammary gland development.
Dose-response is seen in all parameters but ovulation at 6 weeks old when significantly higher percentage of mice with corpus luteum, an indication of ovulation, is observed only in the lowest dose 5 ppm genistein-treated group (Fig. 3C). A reasonable explanation for this phenomenon is that genistein at all these three doses can promote ovulation. However, since ovulation is dependent on regular estrous cycle and estrous cyclicity is disrupted in the 100 and 500 ppm genistein-treated groups (Suppl Figs. S1, S2), the promotion effect of genistein on ovulation is counteracted by the disrupted estrous cyclicity in these two groups. None dose-response effect upon genistein treatment was also observed in other studies [40, 41]. For example, significantly increased number of oocytes from superovulation on postnatal day 22-23 was observed in CD-1 female mice subcutaneously treated with 0.5 mg/kg genistein but not 5 mg/kg or 50 mg/kg genistein during neonatal day 1-5 [40]; at 4 months old, same neonatal treatment regimen led to significantly increased number of corpora lutea in 5 mg/kg genistein group but significantly decreased number of corpora lutea in 50 mg/kg genistein group [41].
Although no corpus luteum was observed in the control group at 6 weeks old (Fig. 3C), 55.6% of mice were pregnant and 40.7% had implantation sites from the first copulation upon mating at 5 weeks old (Figs. 6B, 6C). These seemingly inconsistent observations could most likely be explained by the different experiment settings. The later, but not the former, had cohabitation with males. Male odor could accelerate the puberty onset of female mice [42] and male seminal plasma has an ovulation-inducing factor to induce the ovulation in prepubertal mice [43]. Therefore, cohabitation could induce ovulation and subsequent pregnancy at an earlier age.
Sexually immature females seem to be more sensitive to genistein treatment from the following observations. First, the extended estrus stage was observed in 100 and 500 ppm groups right after vaginal opening (~28-38 days old), but only observed in 500 ppm group from 5 to 8 weeks old; second, the difference in the percentages of mice with corpus luteum was only seen at 6 weeks old but not 7 weeks old; third, although no significant differences in implantation rate and number of implantation sites were observed between control and 500 ppm genistein-treated groups from both 5 weeks old and 7 weeks old mating ages, some minor effects, such as distended uteri and faint implantation sites were observed in a small fraction of 500 ppm genistein-treated group at 5 weeks old mating age only.
However, the treatment timing and route, and possibly different strains of mice during different sexually immature periods could also make some differences. For example, neonatal day 1-5 subcutaneous injection with 50 mg/kg (a dose equivalent to 500 ppm genistein diet in this study) in CD-1 mice led to a general delayed vaginal opening although no statistical difference was found [41]. Such treatment also led to disrupted uterine receptivity for embryo implantation, indicated by lower implantation rate and smaller implantation sites from embryo transfer of untreated embryo to treated pseudopregnant females [44]. However, postnatal treatment of 500 ppm genistein diet in this study significantly advanced vaginal opening in C57BL/6J mice (Fig. 1). The treatment in this study didn’t have significantly adverse effect on all events, including uterine receptivity, that are required for successful embryo implantation (Figs. 6B, 6D), although we did observe 3 out of total 80 implantation sites in the 5 weeks mating group showing faint blue bands, indicating delayed implantation. It is possible that the days prior to puberty onset is a sensitive window to exogenous estrogenic endocrine disruptors for pubertal development and neonatal day 1-5 is likely a more sensitive window than postweaning period for uterine development leading to the establishment of uterine receptivity for embryo implantation.
The mechanism of genistein on pubertal development is largely unknown. Previous studies on endocrine disruptors showed that both central regulation hypothalamus-pituitary-gonads (HPG) axis and peripheral target tissues could be involved [45]. Genistein seems to regulate both HPG axis and peripheral target tissues. For example, genistein could excite GnRH neurons in the hypothalamus of juvenile mice [46]; it could inhibit steroidogenesis in the follicles from immature rats and human granulosa-luteal cells [47, 48]; it could induce vaginal cornification in ovariectomized rats [49]. Although our study could not specify the mechanism of regulation by genistein, vaginal opening, estrous cyclicity, mammary gland development are estrogen dependent events, and ovulation is highly orchestrated by HPG axis, supporting the notion that genistein regulates both HPG axis and peripheral target tissues.
At molecular level, estrogen signaling is expected to play an important role in genistein-promoted puberty. Although significantly increased mammary gland duct growth was observed in the 500 ppm genistein-treated group at 5 weeks old (Fig. 4), comparable expression of ERα and ERβ was observed in the 5 weeks old mammary gland between 0 and 500 ppm genistein-treated groups (Fig. 5). First, since ERα and ERβ are highly expressed in both epithelial and stromal compartments in the mammary gland during puberty (Figs. 5B-5G) [50, 51], any local changes, e.g., at terminal end bud where duct epithelial proliferation leads mammary gland growth, could be obscured or missed in whole mammary gland realtime PCR (Fig. 5A) or immunohistochemistry (Figs. 5B-5G). Second, estrogen signaling might be regulated by ER coregulators without altered ER expression levels. Third, it is also possible that the ligand level is maintained at a higher level for longer time indicated by extended estrus stage (Suppl Figs. S1, S2) to increase the overall estrogen signaling. Fourth, it could act through ER downstream signaling, such as Areg (Fig. 5A), the only epidermal growth factor receptor ligand that is abundantly expressed in the mammary gland epithelial cells during puberty and an essential mediator for estrogen promoted mammary gland duct growth [52-54].
In summary, postweaning exposure to human relevant genistein diets accelerates pubertal development in female mice. The effect of genistein on puberty does not seem to have significant negative consequence on early pregnancy in young female mice.
Supplementary Material
Acknowledgments
The authors thank Dr. Suzanne Fenton at NIEHS for technical guidance and insightful suggestions on the manuscript, Department of Pathology at University of Georgia for the access to the imaging system, Dr. James N. Moore in the Department of Large Animal Medicine at University of Georgia for the access to the ABI 7900 Realtime PCR machine, and the Office of the Vice President for Research, Interdisciplinary Toxicology Program, Graduate School, and Department of Physiology & Pharmacology at University of Georgia, and National Institutes of Health (NIH R15HD066301 and NIH R01HD065939 to X.Y.) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rong Li, Email: lirong9@uga.edu.
Fei Zhao, Email: feizhao@uga.edu.
Honglu Diao, Email: hldiao@uga.edu.
Shuo Xiao, Email: shuoxiao@uga.edu.
References
- 1.Mortensen A, Kulling SE, Schwartz H, Rowland I, Ruefer CE, Rimbach G, et al. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res. 2009;53(Suppl 2):S266–309. doi: 10.1002/mnfr.200800478. [DOI] [PubMed] [Google Scholar]
- 2.USDA. USDA database for the isoflavone content of selected foods. 2008 http://wwwarsusdagov/nutrientdata/isoflav.
- 3.Chun OK, Song WO, Chung SJ. Urinary Isoflavones and Their Metabolites Validate the Dietary Isoflavone Intakes in US Adults. J Am Diet Assoc. 2009;109:245–54. doi: 10.1016/j.jada.2008.10.055. [electronic resource] [DOI] [PubMed] [Google Scholar]
- 4.Wakai K, Egami I, Kato K, Kawamura T, Tamakoshi A, Lin Y, et al. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. 1999;33:139–45. doi: 10.1207/S15327914NC330204. [DOI] [PubMed] [Google Scholar]
- 5.Surh J, Kim MJ, Koh E, Kim YKL, Kwon H. Estimated intakes of isoflavones and coumestrol in Korean population. Int J Food Sci Nutr. 2006;57:325–44. doi: 10.1080/09637480600802348. [DOI] [PubMed] [Google Scholar]
- 6.Seow A, Shi CY, Franke AA, Hankin JH, Lee HP, Yu MC. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7:135–40. [PubMed] [Google Scholar]
- 7.FDA. Food labeling: health claims; soy protein and coronary heart disease. Fed Regist. 1999;64:57700–33. [PubMed] [Google Scholar]
- 8.Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31:400–19. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gencel VB, Benjamin MM, Bahou SN, Khalil RA. Vascular effects of phytoestrogens and alternative menopausal hormone therapy in cardiovascular disease. Mini Rev Med Chem. 2012;12:149–74. doi: 10.2174/138955712798995020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs A, Wegewitz U, Sommerfeld C, Grossklaus R, Lampen A. Efficacy of isoflavones in relieving vasomotor menopausal symptoms - A systematic review. Mol Nutr Food Res. 2009;53:1084–97. doi: 10.1002/mnfr.200800552. [DOI] [PubMed] [Google Scholar]
- 11.Lanou AJ. Soy foods: are they useful for optimal bone health? Ther Adv Musculoskelet Dis. 2011;3:293–300. doi: 10.1177/1759720X11417749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010;140:2326S–34S. doi: 10.3945/jn.110.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 14.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–6. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 15.Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res B Dev Reprod Toxicol. 2006;77:485–638. doi: 10.1002/bdrb.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 17.Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics. 2008;121(Suppl 3):S167–71. doi: 10.1542/peds.2007-1813C. [DOI] [PubMed] [Google Scholar]
- 18.Mouritsen A, Aksglaede L, Sorensen K, Mogensen SS, Leffers H, Main KM, et al. Hypothesis: exposure to endocrine-disrupting chemicals may interfere with timing of puberty. Int J Androl. 2010;33:346–59. doi: 10.1111/j.1365-2605.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- 19.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–91. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 20.Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent Decline in Age at Breast Development: The Copenhagen Puberty Study. Pediatrics. 2009;123:E932–E9. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 21.Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–83. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- 22.Adgent MA, Daniels JL, Rogan WJ, Adair L, Edwards LJ, Westreich D, et al. Early-life soy exposure and age at menarche. Paediatr Perinat Epidemiol. 2012;26:163–75. doi: 10.1111/j.1365-3016.2011.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phytooestrogens from soy-based infant formula. Lancet. 1997;350:23–7. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 25.Yum T, Lee S, Kim Y. Association between precocious puberty and some endocrine disruptors in human plasma. J Environ Sci Health A. 2013;48:912–7. doi: 10.1080/10934529.2013.762734. [DOI] [PubMed] [Google Scholar]
- 26.Chang HC, Churchwell MI, Delclos KB, Newbold RR, Doerge DR. Mass spectrometric determination of Genistein tissue distribution in diet-exposed Sprague-Dawley rats. J Nutr. 2000;130:1963–70. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- 27.Delclos KB, Weis CC, Bucci TJ, Olson G, Mellick P, Sadovova N, et al. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod Toxicol. 2009;27:117–32. doi: 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–61. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- 29.Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod Toxicol. 2011;32:434–41. doi: 10.1016/j.reprotox.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao F, Li R, Xiao S, Diao H, Viveiros MM, Song X, et al. Postweaning exposure to dietary zearalenone, a mycotoxin, promotes premature onset of puberty and disrupts early pregnancy events in female mice. Toxicol Sci. 2013;132:431–42. doi: 10.1093/toxsci/kfs343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 33.Ye X, Herr DR, Diao H, Rivera R, Chun J. Unique uterine localization and regulation may differentiate LPA3 from other lysophospholipid receptors for its role in embryo implantation. Fertil Steril. 2011;95:2107–13 e4. doi: 10.1016/j.fertnstert.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diao H, Aplin JD, Xiao S, Chun J, Li Z, Chen S, et al. Altered spatiotemporal expression of collagen types I, III, IV, and VI in Lpar3-deficient peri-implantation mouse uterus. Biol Reprod. 2011;84:255–65. doi: 10.1095/biolreprod.110.086942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–8. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–49. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 37.Safranski TJ, Lamberson WR, Keisler DH. Correlations among three measures of puberty in mice and relationships with estradiol concentration and ovulation. Biol Reprod. 1993;48:669–73. doi: 10.1095/biolreprod48.3.669. [DOI] [PubMed] [Google Scholar]
- 38.Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–41. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- 39.Diao H, Paria BC, Xiao S, Ye X. Temporal expression pattern of progesterone receptor in the uterine luminal epithelium suggests its requirement during early events of implantation. Fertil Steril. 2011;95:2087–93. doi: 10.1016/j.fertnstert.2011.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–96. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 41.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- 42.Mucignat-Caretta C, Caretta A, Cavaggioni A. Acceleration of puberty onset in female mice by male urinary proteins. J Physiol. 1995;486(Pt 2):517–22. doi: 10.1113/jphysiol.1995.sp020830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams GP, Ratto MH. Ovulation-inducing factor in seminal plasma: a review. Anim Reprod Sci. 2013;136:148–56. doi: 10.1016/j.anireprosci.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, Williams CJ. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol Reprod. 2009;80:425–31. doi: 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasier G, Toppari J, Parent AS, Bourguignon JP. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: a review of rodent and human data. Mol Cell Endocrinol. 2006;254-255:187–201. doi: 10.1016/j.mce.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Bhattarai JP, Abraham IM, Han SK. Genistein excitation of gonadotrophin-releasing hormone neurones in juvenile female mice. J Neuroendocrinol. 2013;25:497–505. doi: 10.1111/jne.12020. [DOI] [PubMed] [Google Scholar]
- 47.Myllymaki S, Haavisto T, Vainio M, Toppari J, Paranko J. In vitro effects of diethylstilbestrol, genistein, 4-tert-butylphenol, and 4-tert-octylphenol on steroidogenic activity of isolated immature rat ovarian follicles. Toxicol Appl Pharmacol. 2005;204:69–80. doi: 10.1016/j.taap.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Rice S, Mason HD, Whitehead SA. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosaluteal cells. J Steroid Biochem Mol Biol. 2006;101:216–25. doi: 10.1016/j.jsbmb.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Diel P, Smolnikar K, Schulz T, Laudenbach-Leschowski U, Michna H, Vollmer G. Phytoestrogens and carcinogenesis-differential effects of genistein in experimental models of normal and malignant rat endometrium. Hum Reprod. 2001;16:997–1006. doi: 10.1093/humrep/16.5.997. [DOI] [PubMed] [Google Scholar]
- 50.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: How do they signal and what are their targets. Physiol Rev. 2007;87:905–31. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 51.Cheng G, Weihua Z, Warner M, Gustafsson JA. Estrogen receptors ER alpha and ER beta in proliferation in the rodent mammary gland. Proc Natl Acad Sci U S A. 2004;101:3739–46. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–50. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 53.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci U S A. 2007;104:5455–60. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sternlicht MD, Sunnarborg SW. The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2008;13:181–94. doi: 10.1007/s10911-008-9084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.