Abstract
Background
Maternal diet during pregnancy may influence childhood allergy and asthma.
Objective
To examine the associations between maternal intake of common childhood food allergens during early pregnancy and childhood allergy and asthma.
Methods
We studied 1277 mother-child pairs from a United States pre-birth cohort unselected for any disease. Using food frequency questionnaires administered during the first and second trimesters, we assessed maternal intake of common childhood food allergens during pregnancy. In mid-childhood (mean age 7.9 years), we assessed food allergy, asthma, allergic rhinitis, and atopic dermatitis by questionnaire and serum specific IgE levels. We examined the associations between maternal diet during pregnancy and childhood allergy and asthma. We also examined the cross-sectional associations between specific food allergies, asthma, and atopic conditions in mid-childhood.
Results
Food allergy was common (5.6%) in mid-childhood, as was sensitization to at least one food allergen (28.0%). Higher maternal peanut intake (each additional z-score) during the first trimester was associated with 47% reduced odds of peanut allergic reaction (OR 0.53, 95%CI 0.30–0.94). Higher milk intake during the first trimester was associated with reduced asthma (OR 0.83, 95%CI 0.69–0.99) and allergic rhinitis (OR 0.85, 95%CI 0.74–0.97). Higher maternal wheat intake during the second trimester was associated with reduced atopic dermatitis (OR 0.64, 95%CI 0.46–0.90). Peanut, wheat, and soy allergy were each cross-sectionally associated with increased childhood asthma, atopic dermatitis, and allergic rhinitis (ORs 3.6 to 8.1).
Conclusion
Higher maternal intake of peanut, milk, and wheat during early pregnancy was associated with reduced odds of mid-childhood allergy and asthma.
Keywords: maternal diet, pregnancy, food allergy, sensitization, asthma, allergic rhinitis, peanut, milk, wheat, childhood
Introduction
Allergy and asthma are growing as clinical and public health problems in the United States.1, 2 Recent data suggest that approximately 5% of the US population has food allergy,1 and 8.4% has asthma.2 Intra-uterine exposures may play a role in the development of childhood allergy and asthma, as the immune system takes form during the fetal period.3, 4 Research on the effects of early-life exposures—such as maternal diet during pregnancy-- on childhood allergy and asthma development could inform primary prevention.
The American Academy of Pediatrics previously advised that “no maternal dietary restrictions during pregnancy are necessary with the possible exception of excluding peanuts.”5 Subsequent systematic reviews of the literature concluded that the current evidence is inadequate to support any dietary restrictions during pregnancy.6–9 The majority of studies on this topic have been conducted in populations selected for allergic propensity,10–14 rendering inference to the general population challenging. Further, previous studies of the potential impact of maternal diet during pregnancy have examined only the last month or trimester of pregnancy10–18 and analyzed outcomes only in the first year(s) of life.10–13, 16–18 Because many cells thought to be involved in the pathogenesis of allergy and asthma are formed during early pregnancy19, 20, an examination of exposures during the first and second trimesters could be worthwhile. Further, studying the potential effects of such exposures beyond early-childhood could help with clinical counseling.
In this article, we characterize the association between maternal diet during early pregnancy and risk of childhood allergy and asthma in mid-childhood in a US pre-birth cohort unselected for any disease. In particular, we focused on maternal intake of foods containing common childhood food allergens (peanut, milk, wheat, egg, and soy) during early pregnancy. As data on food allergen sensitization in US populations unselected for any disease are limited to a few childhood food allergens,21, 22 we also present our cross-sectional findings on food allergen sensitization and associations between specific food allergies, asthma, and atopic conditions.
Methods
Study design and subjects
Participants of the Project Viva pre-birth cohort were recruited from a large multispecialty practice in Massachusetts. The goal of this longitudinal epidemiologic cohort was to study dietary factors that could influence health in early life. Health was broadly defined to encompass diverse areas. Participants were not selected for any disease. Study details have been previously described.23
Enrollment occurred between 1999 and 2002 for women with singleton pregnancy. In-person interviews and questionnaires were administered after the initial prenatal visit, at an average of 10 weeks of gestation, and at 26–28 weeks of gestation. Interviews and questionnaires on child health were administered at 6 months, 1 year, and annually thereafter. We collected outcome data for this study at the mid-childhood in-person visit (mean age 7.9 years). Study protocols were approved by the institutional review boards of participating institutions. Of the 2128 children delivered in Project Viva, we included 1277 mother-child pairs who presented for an in-person interview at mid-childhood.
Maternal dietary assessment during pregnancy
Maternal dietary assessments at the first and second trimester visits were based on a validated 166-item semi-quantitative food frequency questionnaire (FFQ) modified for pregnancy24 and have been previously described (see Online Supplement for additional details).23, 25, 26 The total servings per day of each major food allergen (peanut, milk, wheat, egg, soy) were calculated by summing the servings per day of the foods on the FFQ containing these respective food allergens. We derived z scores for the servings per day of each major food allergen that were standardized to a mean of 0 and standard deviation of 1. We chose to use z-scores to (1) allow readers to more easily compare results across different food allergens, which had varying distributions for servings/day, and (2) to aid with interpretation of food allergens with mean servings/day < 1.
Childhood outcomes
Questions for asthma, allergic rhinitis and atopic dermatitis were from the International Study of Asthma and Childhood.27 Current asthma was defined as positive if a mother reported at the mid-childhood visit that her child had ever doctor-diagnosed asthma plus either use of asthma medication or wheezing in the past 12 months. Current allergic rhinitis was defined as positive if a mother reported that her child had a runny nose or sneezing apart from colds in the past 12 months. Current atopic dermatitis was defined as positive if a mother reported at the mid-childhood visit that her child had ever doctor-diagnosed eczema plus an itchy rash in the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears, or eyes in the past 12 months that did not go completely away for at least 6 months. Ever asthma, ever allergic rhinitis, and ever atopic dermatitis were defined as positive if a mother reported a doctor’s diagnosis of each respective condition in the child in any questionnaire since birth. Maternal and paternal asthma, allergic rhinitis, and atopic dermatitis were each considered positive if a mother reported at week 10 of gestation that she or the child’s biological father had a history of the respective condition. Maternal asthma and allergy (henceforth “maternal atopy”) was considered positive if maternal asthma, allergic rhinitis, or atopic dermatitis was positive; the analogous was used to define paternal atopy. Parental atopy was considered positive if maternal or paternal atopy was positive.
Of the 1277 children who presented for an in-person interview at mid-childhood, 702 (55.0%) agreed to have blood drawn and 616 (87.7% of those with blood samples) children had sufficient sample to measure allergen specific IgE (spIgE) levels by Phadia ImmunoCAP. Sensitization to a food allergen was considered positive if the respective allergen spIgE level was ≥ 0.35 kU/L. Prescription of an epinephrine auto-injector was assessed with the question, “Has a health care professional, such as a doctor, physician assistant or nurse practitioner, ever prescribed an EpiPen for your child?” A child was considered to have food allergy to peanut, milk, wheat, egg, and/or soy if (s)he had a spIgE level ≥ 0.35 kU/L to the particular food and EpiPen prescribed. For further details, please see the Online Supplement. We additionally assessed peanut allergy specifically given rising prevalence of peanut allergy at the inception of this cohort study28; a child was considered to have had a peanut allergic reaction if his/her mother answered yes to, “Has your child ever had an allergic reaction to peanuts,” and yes to at least one of the following categories of allergic reaction symptoms with peanut ingestion: “Skin related (e.g. hives, swelling),” “Respiratory (e.g., shortness of breath, wheezing, cough),” “Cardiovascular (e.g. low blood pressure, dizziness or fainting,” “Gastrointestinal (e.g. vomiting, diarrhea),” or “Anaphylaxis (severe, multi-system allergic reaction).” Assessment of food allergy based on report of convincing IgE-mediated reaction symptoms such those covered by our questions has been shown to be effective, with only a 7% false positive rate.29
Statistical analyses
To assess the associations between maternal diet during the first and second trimesters of pregnancy and allergy and asthma outcomes, we created multivariable logistic regression models using food allergen z-score as the unit for maternal dietary intake. Models with food sensitization or food allergy as the outcome were constrained to the 616 subjects with spIgE levels. Because the associations between specific childhood food allergies, asthma, and atopic conditions have not been well characterized, we additionally used multivariable logistic regression to assess the cross-sectional associations between food allergy and current asthma, current allergic rhinitis, and current atopic dermatitis. Given the known associations between food allergy and sex30, age30, family history of allergy,30, 31 maternal education32, and breastfeeding32, we adjusted all models for these variables (Model 1). We created secondary models additionally adjusted for race/ethnicity (Model 2). Although we anticipated power limitations, we also created secondary models stratified by parental atopy. We performed all analyses using SAS 9.3 (SAS Institute, Cary, NC).
Results
Study population
The baseline characteristics of the participants are shown in Table 1. Compared to the 851 participants excluded, the 1277 participants included showed higher proportions of maternal white race (69% vs. 62%), college or graduate education (69% vs. 58%), annual household income exceeding $70,000 (63% vs. 58%), and parental atopy (59% vs. 56%). Compared to the general US population,33 there was a higher proportion of blacks and lower proportion of Hispanics among participants. The majority of mothers were college-educated and most households were not low income. Rates of parental asthma, allergic rhinitis, and atopic dermatitis were consistent with those for the general US population.34–36 Consistent with previous observations demonstrating underdiagnosis of allergic rhinitis by physicians,37 the prevalence of current allergic rhinitis (definition based on current symptoms) was higher than the prevalence of ever allergic rhinitis (defined based on physician’s diagnosis).
Table 1.
Parental and child characteristics among participants from the Project Viva pre-birth cohort
| Characteristic | Participants with mid-childhood data (N = 1277) | Participants with mid-childhood data AND spIgE levels measured (N = 616) |
|---|---|---|
| Parental Characteristics | ||
| Maternal education ≥ college graduate | 881 (69.3%) | 407 (66.5%) |
| Household income ≥ $70,000 | 733 (63.0%) | 359 (64.2%) |
| Maternal atopy | 510 (40.1%) | 241 (39.4%) |
| Maternal asthma | 161 (12.7%) | 84 (13.7%) |
| Maternal allergic rhinitis | 376 (29.6%) | 167 (27.3%) |
| Maternal atopic dermatitis | 167 (13.1%) | 72 (11.8%) |
| Paternal atopy | 433 (34.6%) | 200 (33.2%) |
| Paternal asthma | 146 (11.8%) | 71 (12.0%) |
| Paternal allergic rhinitis | 324 (26.7%) | 154 (26.5%) |
| Paternal atopic dermatitis | 76 (6.1%) | 29 (4.9%) |
| Maternal intake during pregnancy (servings/day) | ||
| First trimester | ||
| Peanut | 0.34 (0.44) | 0.34 (0.48) |
| Milk | 1.16 (1.04) | 1.17 (1.07) |
| Wheat | 2.65 (1.48) | 2.64 (1.44) |
| Egg | 0.32 (0.30) | 0.32 (0.28) |
| Soy | 0.08 (0.27) | 0.08 (0.29) |
| Second trimester | ||
| Peanut | 0.36 (0.43) | 0.35 (0.42) |
| Milk | 1.50 (1.82) | 1.52 (1.85) |
| Wheat | 2.69 (1.44) | 2.66 (1.41) |
| Egg | 0.33 (0.30) | 0.33 (0.29) |
| Soy | 0.08 (0.28) | 0.09 (0.34) |
| Child characteristics | ||
| Sex- female | 632 (49.5%) | 302 (49.0%) |
| Age - years | 7.93 (0.82) | 7.82 (0.71) |
| Race | ||
| White | 831 (65.2%) | 383 (62.4%) |
| Black | 199 (15.6%) | 121 (19.7%) |
| Hispanic | 53 (4.2%) | 30 (4.9%) |
| Asian | 43 (3.4%) | 13 (2.1%) |
| >1 race or other | 149 (11.7%) | 67 (10.9%) |
| Breastfed ≥ 6 months | 662 (55.6%) | 319 (55.5%) |
| Child food sensitization and food allergy | ||
| Food allergen sensitization | 168 (28.0%) | |
| Peanut | 85 (13.8%) | |
| Milk | 96 (15.9%) | |
| Wheat | 77 (12.9%) | |
| Egg | 50 (8.3%) | |
| Soy | 47 (7.9%) | |
| Food allergy | 32 (5.6%) | |
| Peanut | 29 (4.9%) | |
| Milk | 13 (2.3%) | |
| Wheat | 16 (2.8%) | |
| Egg | 7 (1.2%) | |
| Soy | 18 (3.2%) | |
| EpiPen prescribed | 45 (7.6%) | |
| Peanut allergic reaction | 27 (4.6%) | |
| Child asthma and atopy | ||
| Asthma- ever | 277 (22.3%) | 137 (22.5%) |
| Asthma- current | 219 (19.6%) | 105 (19.2%) |
| Allergic rhinitis- ever | 291 (23.5%) | 145 (23.8%) |
| Allergic rhinitis - current | 389 (33.6%) | 199 (34.1%) |
| Atopic dermatitis- ever | 306 (24.7%) | 149 (24.4%) |
| Atopic dermatitis - current | 75 (7.4%) | 42 (8.3%) |
Values are number (%) or mean (SD)
Association between maternal intake during pregnancy and current outcomes at mid-childhood
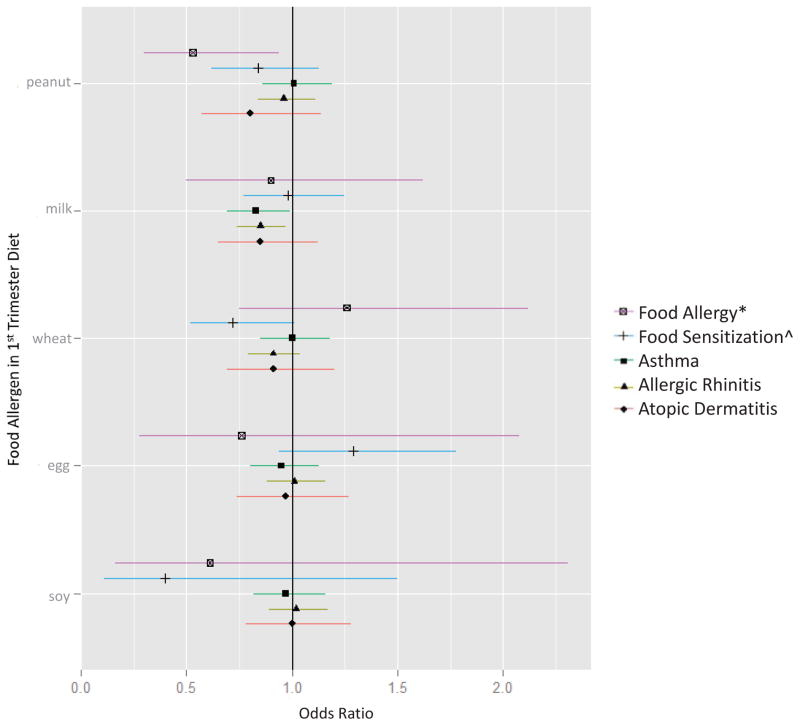
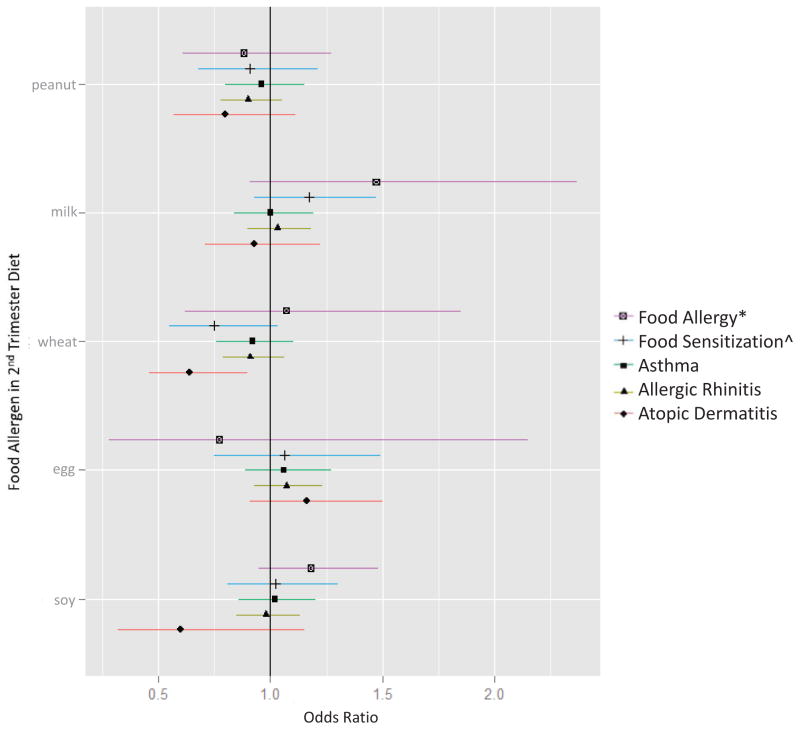
Distributions of maternal intake of each food allergen during pregnancy are in Table 1. For additional FFQ results, see Tables E1 and E2. Each additional z-score of maternal peanut intake during the first trimester was associated with a 47% reduced odds of peanut allergic reaction in childhood (OR 0.53, 95%CI 0.30–0.94) (Figure 1; Table E3). We focus on the results of Model 1 because child’s race/ethnicity was highly collinear with maternal education in our sample (χ2 187.9, P value < 0.0001), and models containing collinear terms (i.e. Model 2) yield inaccurate results for individual predictors.38 We did not observe associations between first strimester peanut intake and current asthma or other atopic outcomes, nor between second trimester peanut intake and current outcomes (Figure 2; Table E4). Each additional z-score of maternal milk intake during the first trimester was associated with reduced odds of current asthma (OR 0.83, 95%CI 0.69–0.99) and current allergic rhinitis (OR 0.85, 95%CI 0.74–0.97) (Figure 1; Table E3). We did not detect these associations with second trimester milk intake (Figure 2; Table E4). Maternal wheat intake during the second trimester was associated with reduced odds of current atopic dermatitis (OR 0.64, 95%CI 0.46–0.90) (Figure 2; Table E4). Results of the models stratified by parental atopy are shown in Table E5 and E6. Of note, maternal intakes of peanut, milk, wheat, egg, and soy during pregnancy did not differ in families with and without parental atopy (Table E7).
Figure 1. Associations between maternal intake of food allergens during the first trimester and current allergy and asthma outcomes at mid-childhood.
Models were adjusted for child age, sex, breastfeeding history, parental atopy, and maternal education. *Food allergy defined as sensitization to the respective food AND EpiPen prescribed, except for peanut allergy, which was more specifically defined by convincing symptoms of a peanut allergic reaction (history of peanut allergy AND a cutaneous, respiratory, cardiovascular, gastrointestinal and/or anaphylactic symptom following peanut ingestion). ^Food sensitization defined as spIgE ≥ 0.35 kU/L to the respective food.
Figure 2. Associations between maternal intake of food allergens during the second trimester and current allergy and asthma outcomes at mid-childhood.
Models were adjusted for child age, sex, breastfeeding history, parental atopy, and maternal education. *Food allergy defined as sensitization to the respective food AND EpiPen prescribed, except for peanut allergy, which was more specifically defined by convincing symptoms of a peanut allergic reaction (history of peanut allergy AND a cutaneous, respiratory, cardiovascular, gastrointestinal and/or anaphylactic symptom following peanut ingestion). ^Food sensitization defined as spIgE ≥ 0.35 kU/L to the respective food.
Sensitization, allergy, and asthma
The 616 participants with specific IgE levels measured at mid-childhood had similar characteristics to the 1277 study sample (Table 1). The most prevalent food allergen sensitization among them was to milk (15.9%) followed by peanut (13.8%) (Table 1). The most prevalent food allergy was to peanut (4.9%). 27 (4.6%) specifically reported a peanut allergic reaction (i.e. convincing IgE-mediated symptoms following peanut intake, specified as cutaneous, respiratory, cardiovascular, gastrointestinal, and/or anaphylactic symptoms).
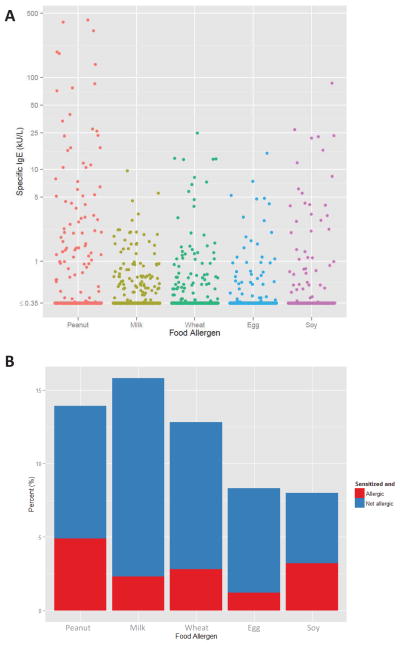
Figure 3A shows the distribution of food allergen spIgE levels among study participants. The range for peanut spIgE levels was wide, spanning <0.35 to 423 kU/L.
Figure 3. Food allergen sensitization and food allergy among participants of the Project Viva birth cohort.
A: Distribution of food allergen specific IgE levels among the 616 participants for whom spIgE levels were measured.
B: Percent of study population sensitized with and without allergy. The total height of each bar represents the prevalence of sensitization to each food allergen among the 616 participants for whom spIgE levels were measured. Each bar is then divided into two parts—the red part representing the proportion sensitized to the food allergen and food allergic, and the blue part representing the proportion sensitized to the food allergen and not food allergic.
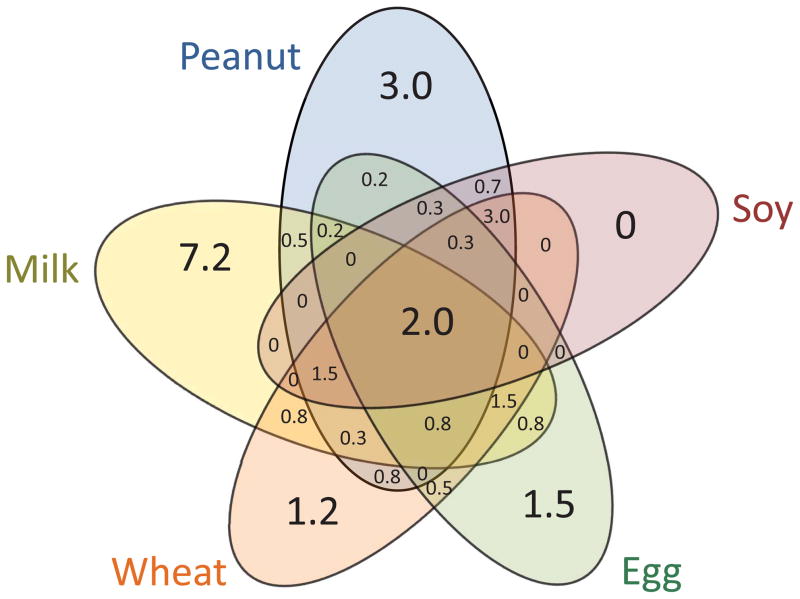
Sensitization to multiple food allergens was more prevalent than monosensitization. Figure 4 summarizes the prevalences of combination food allergen sensitizations. 77 children (13% of subjects with specific IgE levels) were sensitized to 1 food allergen only, 26 (4.4%) were sensitized to 2 food allergens, 32 (5.4%) to 3 food allergens, 16 (2.7%) to 4 food allergens, and 12 (2.0%) to all 5 food allergens. 29 (19%) of the 155 children sensitized to at least one food allergen also had food allergy (Figure 3B).
Figure 4. Prevalences of single and multiple food allergen sensitization.
Numbers are percent of study sample with mid-childhood data and spIgE levels (N = 616).
Association between food allergy, asthma, and atopic conditions
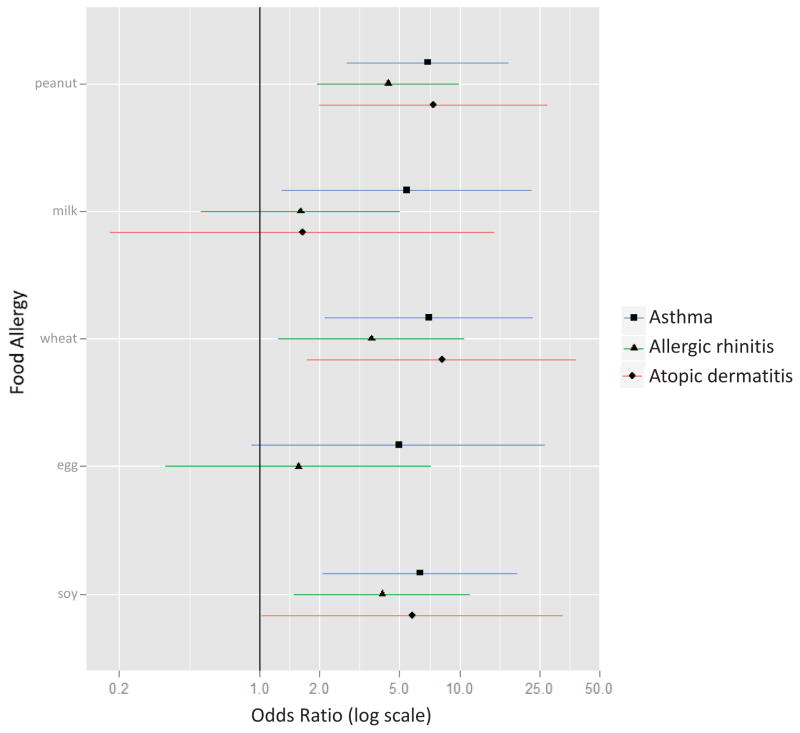
In mid-childhood, peanut, wheat, and soy allergy were each cross-sectionally associated with increased odds of current atopic dermatitis (ORs 5.8 to 8.1), current asthma (ORs 6.4 to 7.0), and current allergic rhinitis (ORs 3.6 to 4.4) (Figure 5; Table E8). Milk allergy was associated with increased odds of asthma (OR 5.4, 95% CI 1.3–23.1).
Figure 5. Cross-sectional associations between food allergy and current asthma, current allergic rhinitis, and current atopic dermatitis at mid-childhood.
Models were adjusted for child age, sex, breastfeeding history, parental atopy, and maternal education. Association for egg allergy and atopic dermatitis not shown given 0 subjects with both conditions at mid-childhood.
Discussion
In this prospective pre-birth cohort study of participants unselected for atopic propensity, higher maternal intakes of allergenic foods during early pregnancy were associated with lower risks of allergy and asthma in mid-childhood. Maternal peanut intake during the first trimester was associated with a 47% reduction in the odds of childhood peanut allergic reaction, maternal milk intake during the first trimester was associated with reduced odds of childhood asthma and allergic rhinitis, and maternal intake of wheat during the second trimester was associated with reduced childhood atopic dermatitis. We did not detect associations between maternal egg or soy intakes and childhood outcomes. We also observed that allergies to these five foods in childhood were associated with concurrent asthma and atopic disease.
Our results support the hypothesis that maternal diet during early pregnancy affects allergy and asthma outcomes in mid-childhood. Our study is distinct from previous work for several reasons. Most prior studies on this topic were conducted in European populations,10–12, 15–18, 39–41 which have different trends in atopy42 and different lifestyles.43 US-based studies addressing this issue selected subjects from atopic families,13, 31 or relied on distant recall of pregnancy diet after food allergy status was known.13 Overall, most studies examining the role of maternal diet on child atopy selected subjects from atopic families,10–14 or recruited subjects with the aim of examining asthma and allergy.15, 17, 18, 44 The topic has been examined in general birth cohort studies, but with limitations; a UK-based general birth cohort study relied on distant recall of pregnancy diet after study enrollment,39 and a Danish general birth cohort study did not assess maternal diet before the 25th week of pregnancy.40 In that study, Maslova et al. found that midpregnancy nut intake was associated with decreased odds of asthma and allergic rhinitis, although they did not examine other foods or outcomes other than asthma and allergic rhinitis.40 We found no other studies that examined maternal diet before 25 weeks, with most assessing diet for the last month or last trimester of pregnancy only,10–16, 18 and most relying on distant recall of diet after the child’s birth and after study enrollment.13, 16, 39 Thus, a strength of our study is the prospective collection of dietary information at two time points during pregnancy. Additionally, to assess the potential longer term effects of such exposures, we examined outcomes in mid-childhood. The majority of previous studies examined outcomes in the initial year(s) of life only, assessing for allergy and asthma in infancy,12, 13 at 18 months,10 2 years,17, 3 years,18 and 5 years.11, 16
The first trimester is a formative period of fetal immune system development.3 Cells with dendritic/macrophage structure are present at 4–6 weeks.45 Positive and negative selection in T cell development occur during this time, such that by 15 weeks, human thymocytes express a complete set of T cell receptors,19 and the variable domains of TCR beta chains are expressed.46 B cells with sIgM+ isotypes are detectable by 9 weeks, and sIgA, sIgG, and sIgD isotypes appear by 10 weeks.20 IgE is made by 11 weeks.47 Thus, early forms of many cells involved in allergy are formed during the first trimester. Early encounter with food allergens via maternal diet during this critical period of immune system formation could lead to tolerance rather than sensitization. Maternal dietary antigens are known to cross the placenta.48 Maternal diet may influence Th cell differentiation as well as fetal airway differentiation.4 For example, some studies report associations between maternal intake of antioxidants and wheezing, asthma, allergen sensitization, lung function, and exhaled nitric oxide.26, 49, 50 Our observation of a 47% reduced odds of peanut allergic reaction with each z-score of peanut intake in the maternal first trimester diet, and a 15% reduced odds of asthma and allergic rhinitis with each z-score of milk intake in the maternal first trimester diet, are consistent with these developmental principles. Such effects may be food-specific due to distinct nutrients in each food. For example, peanut contains high linoleic acid, an essential fatty acid that has been associated with T-cell signaling, MHCII expression, and the production of arachidonic acid and prostaglandins.51 Milk is the main food source for vitamin D, and maternal vitamin D intake has been associated with decreased childhood wheezing in this cohort.25 Although many prior studies found no association between maternal diet during pregnancy and childhood outcomes,6, 8–12, 18, 39 their null results could be because maternal diet before 25 weeks of pregnancy was not assessed. Consistent with this hypothesis, we observed strongest associations with our first trimester data.
In contrast to our findings, some studies observed a positive association between high peanut intake during pregnancy and child atopic outcomes. 13–16, 44 For example, a Dutch birth cohort study of asthma and dust mite allergy reported increased asthma in children whose mothers had daily versus rare intake of nut products during the last month of pregnancy.15 Sicherer et al. observed that maternal recall of frequent third trimester peanut ingestion was associated with peanut sensitization in children with existing egg or milk allergy.13 Frank et al. reported increased peanut allergy, atopic dermatitis, and other food sensitization in children from atopic families whose mothers recalled frequent peanut intake during pregnancy.44 Our findings may differ from these earlier findings because: (1) we targeted maternal diet during early rather than late pregnancy, (2) we administered FFQs during pregnancy, reducing recall bias, and/or (3) our participants were unselected for atopic propensity.
The 5.6% prevalence of mid-childhood food allergy in our study population is consistent with data from the National Health Interview Survey (NHIS) showing increasing rates of food allergy in the United States from 3.4% in 1997–1999 to 5.1% in 2009–2011.1 The 2005–2006 National Health and Nutrition Examination Survey (NHANES) estimated food allergy prevalence to be 3.8% among those 6–19 years old.22 Gupta et al. reported a higher rate of overall food allergy (8.0%).52 These differences likely reflect methodology,53 as NHIS defined food allergy using parental report,1 NHANES relied on spIgE level,22 and Gupta et al. used subject-reported history.52 Because food allergy is not accurately reported by patients, the NIAID-sponsored expert panel recommends that objective measurements are used to establish a true diagnosis of food allergy.7 We required both elevated food-spIgE level and prescribed EpiPen to define a food allergy, and we had specific questions targeting convincing symptoms of peanut allergic reaction.
We recognize that the rates of specific food allergies that we report are higher (e.g. peanut 4.9%) than those previously reported.22, 52 We believe our results are valid for several reasons. First, the prevalence of peanut allergic reaction (i.e. convincing IgE-mediated symptoms following peanut ingestion) was 4.6% in our study, which is similar to our peanut allergy prevalence. Assessment of food allergy based on report of convincing IgE-mediated reaction symptoms such those covered by our questions has been previously shown to have a low false positive rate.29 Therefore, our similar rates of peanut allergy and peanut allergic reaction support our strategy of using elevated spIgE and prescribed EpiPen for defining a specific food allergy. Second, the odds of food allergy are higher in the Northeast54 and in urban areas55; our subjects came from mostly urban eastern Massachusetts. Third, the estimated prevalence of clinical allergy to peanut, milk, and egg were lower among 6–19 year olds in NHANES (e.g. peanut allergy 2.7%). While the 6–19 year-old category overlaps with the mean age of our cohort (7.9 years), it includes many older individuals, and the prevalence of food allergy is generally lower in older age groups.22 Fourth, selection bias may have caused children with allergies and other pediatric diseases to present for the mid-childhood visit, as compared to healthy children. Finally, food allergy is increasingly prevalent, and our results could be consistent with this trend.1
Apart from NHANES, 21, 22 limited data are available on food sensitization in the general US population.22 Sensitization to multiple food allergens (14.5%) was more prevalent than monosensitization (13%) in our cohort. This was not observed in NHANES,22 but NHANES did not examine sensitization to soy and wheat, common childhood food allergens that we studied in addition to peanut, milk, and egg. Our study reports rates of combination cosensitizations to the common childhood food allergens in a US population unselected for atopy or any disease, which we have not seen reported. Consistent with prior observations, many sensitized subjects were not also clinically allergic to the relevant food allergen.21, 22
Food allergy, asthma, allergic rhinitis, and atopic dermatitis commonly occur in combination, which we observed and quantified. Previous studies have demonstrated concurrence of asthma and food allergy overall, suggesting a relationship due to shared mechanisms, or perhaps a causal link.21 We sought to characterize the association between specific food allergies and current atopic outcomes, choosing to do so in the same cohort to minimize variation between studies. We observed that peanut, wheat, and soy allergy were all consistently associated with higher odds of prevalent asthma, allergic rhinitis, and atopic dermatitis. The associations were not uniformly consistent for milk and egg allergies, perhaps due to their lower prevalence among our participants. Our results reinforce that clinicians treating school-age children with peanut, wheat, and soy allergy in particular should screen for comorbid asthma and allergy.
We recognize limitations to our study. While imperfect, our definition of food allergy based on both spIgE levels and EpiPen prescription status should reflect food allergy better than either parameter alone, as sensitization alone overestimates clinical food allergy,21, 22 while epinephrine is underprescribed among those with convincing symptoms of IgE-mediated food allergy.29 Although 95% predictive spIgE values for clinical food allergy have been described,56 these were derived from highly atopic patients referred to allergists for food allergy concerns. Because our study sample had markedly different baseline characteristics, the application of such predictive values to define food allergy in our cohort was deemed unwise, as it would likely lead to misclassification. Our implemented definition requiring diagnostic evaluation and EpiPen prescription following healthcare provider evaluation may in fact represent more rigor than real-world practice, where 30% of reported food allergy is not diagnosed by a physician, and 23% is not evaluated by diagnostic testing.57 We recognize the limitation that subjects sensitized to >1 food allergen and prescribed EpiPen may have been classified as having >1 food allergy when they may have had only one. While we could corroborate our definition for peanut allergy with our specific questions targeting convincing IgE-mediated symptoms that would characterize a peanut allergic reaction, we did not have analogous questions targeting reaction symptoms to milk, wheat, egg, and soy. Our results for non-peanut allergies should therefore be interpreted with caution. We plan to ask questions targeting reaction symptoms to non-peanut foods going forward as we follow this pre-birth cohort into adolescence. Another limitation is the number of children with IgE measurements. While there were no differences in the characteristics of these children with the bigger sample, we cannot fully rule out selection bias in the analysis. Additionally, we did not administer FFQs during the third trimester, although theoretically and empirically, early pregnancy is likely to be a more sensitive period for development of atopic predisposition. Our study examined multiple outcomes and we performed multiple tests, so our findings should be cautiously interpreted in this light. Finally, our results are from an observational longitudinal pre-birth cohort study; further study using a randomized, controlled interventional design will be more conclusive.
While many clinicians and researchers believed that maternal dietary restrictions during pregnancy and lactation and delayed introduction of allergenic foods to infants could prevent atopic disease,5 systematic reviews do not support these interventions.6, 8 Recent guidelines acknowledge that there are insufficient data to support such restrictions.6–9, 58 In fact, evidence is accumulating that early introduction of peanut, egg, wheat, milk, and fish to the infant diet—rather than delay or avoidance-- may be helpful in inducing tolerance rather than allergy.58–63 Our findings suggest potential benefits to including peanut, milk, and wheat in the maternal diet during pregnancy.
Supplementary Material
Key messages.
The relationship between maternal diet and childhood allergy and asthma is controversial
In a US pre-birth cohort unselected for any disease, higher maternal intake of peanut, milk, and wheat during early pregnancy was associated with reduced odds of mid-childhood allergy and asthma
Our findings do not support avoidance of specific foods during pregnancy to prevent allergy and asthma in children. Inclusion of these foods could be beneficial for allergy and asthma prevention
Acknowledgments
Funding/Support: This study was supported by the National Institutes of Health (NIH AI093538, HL61907, HL64925, HD34568, AI35786, HL68041, and HL007427)
Abbreviations
- CI
confidence interval
- FFQ
food frequency questionnaire
- MHCII
major histocompatibility complex II
- NHIS
National Health Interview Survey
- NHANES
National Health and Nutrition Examination Survey
- NIAID
National Institute of Allergy and Infectious Disease
- OR
odds ratio
- SD
standard deviation
- spIgE
specific IgE
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jackson KD, Howie LD, Akinbami LJ. Trends in Allergic Conditions Among Children: United States, 1997–2011. National Center for Health Statistics Data Brief, Centers for Disease Control and Prevention; 2013. pp. 1–7. http://www.cdc.gov/nchs/data/databriefs/db121.pdf, downloaded May 6, 2013. [PubMed] [Google Scholar]
- 2.Akinbami L, Moorman JE, Baily C, Zahran HS, King M, Johnson CA, Liu X. Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010. National Center for Health Statistics Data Brief, Centers for Disease Control and Prevention; 2012. pp. 1–8. http://www.cdc.gov/nchs/data/databriefs/db94.htm, downloaded August 30, 2013. [PubMed] [Google Scholar]
- 3.Maheshwari A, Calhoun DA. The development of immune cells in the fetus and neonate. UpToDate 2013. 2013 Apr; http://www.uptodate.com/contents/the-development-of-immune-cells-in-the-fetus-and-neonate, downloaded May 7, 2013.
- 4.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–74. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics, Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106:346–9. [PubMed] [Google Scholar]
- 6.Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133. doi: 10.1002/14651858.CD000133.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson RL, Miles LM, Lunn J, Devereux G, Dearman RJ, Strid J, et al. Peanut sensitisation and allergy: influence of early life exposure to peanuts. Br J Nutr. 2010;103:1278–86. doi: 10.1017/S000711450999376X. [DOI] [PubMed] [Google Scholar]
- 9.Greer FR, Sicherer SH, Burks AW American Academy of Pediatrics Committee on N, American Academy of Pediatrics Section on A Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 10.Falth-Magnusson K, Kjellman NI. Development of atopic disease in babies whose mothers were receiving exclusion diet during pregnancy--a randomized study. J Allergy Clin Immunol. 1987;80:868–75. doi: 10.1016/s0091-6749(87)80279-8. [DOI] [PubMed] [Google Scholar]
- 11.Falth-Magnusson K, Kjellman NI. Allergy prevention by maternal elimination diet during late pregnancy--a 5-year follow-up of a randomized study. J Allergy Clin Immunol. 1992;89:709–13. doi: 10.1016/0091-6749(92)90378-f. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann ME, Dannemann A, Gruters A, Radisch B, Dudenhausen JW, Bergmann R, et al. Prospective study of the atopy preventive effect of maternal avoidance of milk and eggs during pregnancy and lactation. Eur J Pediatr. 1996;155:770–4. doi: 10.1007/BF02002904. [DOI] [PubMed] [Google Scholar]
- 13.Sicherer SH, Wood RA, Stablein D, Lindblad R, Burks AW, Liu AH, et al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol. 2010;126:1191–7. doi: 10.1016/j.jaci.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeiger RS, Heller S, Mellon MH, Forsythe AB, O’Connor RD, Hamburger RN, et al. Effect of combined maternal and infant food-allergen avoidance on development of atopy in early infancy: a randomized study. J Allergy Clin Immunol. 1989;84:72–89. doi: 10.1016/0091-6749(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 15.Willers SM, Wijga AH, Brunekreef B, Kerkhof M, Gerritsen J, Hoekstra MO, et al. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am J Respir Crit Care Med. 2008;178:124–31. doi: 10.1164/rccm.200710-1544OC. [DOI] [PubMed] [Google Scholar]
- 16.Erkkola M, Nwaru BI, Kaila M, Kronberg-Kippila C, Ilonen J, Simell O, et al. Risk of asthma and allergic outcomes in the offspring in relation to maternal food consumption during pregnancy: a Finnish birth cohort study. Pediatr Allergy Immunol. 2012;23:186–94. doi: 10.1111/j.1399-3038.2012.01272.x. [DOI] [PubMed] [Google Scholar]
- 17.Sausenthaler S, Koletzko S, Schaaf B, Lehmann I, Borte M, Herbarth O, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530–7. doi: 10.1093/ajcn/85.2.530. [DOI] [PubMed] [Google Scholar]
- 18.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, et al. Factors associated with maternal dietary intake, feeding and weaning practices, and the development of food hypersensitivity in the infant. Pediatr Allergy Immunol. 2009;20:320–7. doi: 10.1111/j.1399-3038.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 19.George JF, Jr, Schroeder HW., Jr Developmental regulation of D beta reading frame and junctional diversity in T cell receptor-beta transcripts from human thymus. J Immunol. 1992;148:1230–9. [PubMed] [Google Scholar]
- 20.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–9. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 21.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549–55. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 22.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126:798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken E, Kleinman KP, Berland WE, Simon SR, Rich-Edwards JW, Gillman MW. Decline in fish consumption among pregnant women after a national mercury advisory. Obstet Gynecol. 2003;102:346–51. doi: 10.1016/S0029-7844(03)00484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14:754–62. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA, Jr, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84:903–11. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 28.Warner JO. Peanut allergy: a major public health issue. Pediatr Allergy Immunol. 1999;10:14–20. doi: 10.1034/j.1399-3038.1999.101011.x. [DOI] [PubMed] [Google Scholar]
- 29.Sicherer SH, Munoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J Allergy Clin Immunol. 1999;103:559–62. doi: 10.1016/s0091-6749(99)70224-1. [DOI] [PubMed] [Google Scholar]
- 30.Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2013 doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 31.Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95:1179–90. doi: 10.1016/s0091-6749(95)70074-9. [DOI] [PubMed] [Google Scholar]
- 32.Milner JD, Stein DM, McCarter R, Moon RY. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114:27–32. doi: 10.1542/peds.114.1.27. [DOI] [PubMed] [Google Scholar]
- 33.United States Census Bureau. Current Population Survey. U.S. Department of Commerce. 2013 http://www.census.gov/hhes/www/cpstables/032012/hhinc/hinc01_000.htm, downloaded May 1, 2013.
- 34.Centers for Disease Control and Prevention. Lifetime Asthma Prevalence Percents by Age, United States. [accessed November 12, 2013];2011 National Health Interview Survey (NHIS) Data. 2011 http://www.cdc.gov/asthma/nhis/2011/table2-1.htm.
- 35.Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1–84. doi: 10.1016/j.jaci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunyavanich S, Soto-Quiros ME, Avila L, Laskey D, Senter JM, Celedon JC. Risk factors for allergic rhinitis in Costa Rican children with asthma. Allergy. 2010 doi: 10.1111/j.1398-9995.2009.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belsley D, Kuh E, Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Wiley; 2004. [Google Scholar]
- 39.Lack G, Fox D, Northstone K, Golding J Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 40.Maslova E, Granstrom C, Hansen S, Petersen SB, Strom M, Willett WC, et al. Peanut and tree nut consumption during pregnancy and allergic disease in children-should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J Allergy Clin Immunol. 2012;130:724–32. doi: 10.1016/j.jaci.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Nwaru BI, Ahonen S, Kaila M, Erkkola M, Haapala AM, Kronberg-Kippila C, et al. Maternal diet during pregnancy and allergic sensitization in the offspring by 5 yrs of age: a prospective cohort study. Pediatr Allergy Immunol. 2010;21:29–37. doi: 10.1111/j.1399-3038.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 42.Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65:103–8. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 43.Hadley C. Food allergies on the rise? Determining the prevalence of food allergies, and how quickly it is increasing, is the first step in tackling the problem. EMBO Rep. 2006;7:1080–3. doi: 10.1038/sj.embor.7400846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank L, Marian A, Visser M, Weinberg E, Potter PC. Exposure to peanuts in utero and in infancy and the development of sensitization to peanut allergens in young children. Pediatr Allergy Immunol. 1999;10:27–32. doi: 10.1034/j.1399-3038.1999.101010.x. [DOI] [PubMed] [Google Scholar]
- 45.Foster CA, Holbrook KA, Farr AG. Ontogeny of Langerhans cells in human embryonic and fetal skin: expression of HLA-DR and OKT-6 determinants. J Invest Dermatol. 1986;86:240–3. doi: 10.1111/1523-1747.ep12285201. [DOI] [PubMed] [Google Scholar]
- 46.Bonati A, Zanelli P, Ferrari S, Plebani A, Starcich B, Savi M, et al. T-cell receptor beta-chain gene rearrangement and expression during human thymic ontogenesis. Blood. 1992;79:1472–83. [PubMed] [Google Scholar]
- 47.Punnonen J, Aversa GG, Vandekerckhove B, Roncarolo MG, de Vries JE. Induction of isotype switching and Ig production by CD5+ and CD10+ human fetal B cells. J Immunol. 1992;148:3398–404. [PubMed] [Google Scholar]
- 48.Loibichler C, Pichler J, Gerstmayr M, Bohle B, Kisst H, Urbanek R, et al. Materno-fetal passage of nutritive and inhalant allergens across placentas of term and pre-term deliveries perfused in vitro. Clin Exp Allergy. 2002;32:1546–51. doi: 10.1046/j.1365-2222.2002.01479.x. [DOI] [PubMed] [Google Scholar]
- 49.Martindale S, McNeill G, Devereux G, Campbell D, Russell G, Seaton A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121–8. doi: 10.1164/rccm.200402-220OC. [DOI] [PubMed] [Google Scholar]
- 50.Devereux G, Turner SW, Craig LC, McNeill G, Martindale S, Harbour PJ, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174:499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 51.Yaqoob P, Calder PC. Fatty acids and immune function: new insights into mechanisms. Br J Nutr. 2007;98 (Suppl 1):S41–5. doi: 10.1017/S0007114507832995. [DOI] [PubMed] [Google Scholar]
- 52.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 53.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 54.Rudders SA, Espinola JA, Camargo CA., Jr North-south differences in US emergency department visits for acute allergic reactions. Ann Allergy Asthma Immunol. 2010;104:413–6. doi: 10.1016/j.anai.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 55.Gupta RS, Springston EE, Smith B, Warrier MR, Pongracic J, Holl JL. Geographic variability of childhood food allergy in the United States. Clin Pediatr (Phila) 2012;51:856–61. doi: 10.1177/0009922812448526. [DOI] [PubMed] [Google Scholar]
- 56.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–6. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 57.Gupta RS, Lau CH, Sita EE, Smith B, Greenhawt MJ. Factors associated with reported food allergy tolerance among US children. Ann Allergy Asthma Immunol. 2013;111:194–8. e4. doi: 10.1016/j.anai.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 58.Fleischer D, Spergel JM, Assa’ad AH, Pongracic JA. Primary Prevention of Allergic Disease Through Nutritional Interventions. Journal of Allergy and Clinical Immunology: In Practice. 2013;1:29–36. doi: 10.1016/j.jaip.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–91. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 60.Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010;126:807–13. doi: 10.1016/j.jaci.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 61.Poole JA, Barriga K, Leung DY, Hoffman M, Eisenbarth GS, Rewers M, et al. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics. 2006;117:2175–82. doi: 10.1542/peds.2005-1803. [DOI] [PubMed] [Google Scholar]
- 62.Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol. 2010;126:77–82. e1. doi: 10.1016/j.jaci.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 63.Alm B, Aberg N, Erdes L, Mollborg P, Pettersson R, Norvenius SG, et al. Early introduction of fish decreases the risk of eczema in infants. Arch Dis Child. 2009;94:11–5. doi: 10.1136/adc.2008.140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.