Abstract
Mutations in PROP1 account for up to half of the cases of combined pituitary hormone deficiency that result from known causes. Despite this, few signaling molecules and pathways that influence PROP1 expression have been identified. Notch signaling has been linked to Prop1 expression, but the developmental periods during which Notch signaling influences Prop1 and overall pituitary development remain unclear. To test the requirement for Notch signaling in establishing the normal pituitary hormone milieu, we generated mice with early embryonic conditional loss of Notch2 (conditional knockout) and examined the consequences of chemical Notch inhibition during early postnatal pituitary maturation. We show that loss of Notch2 has little influence on early embryonic pituitary proliferation but is crucial for postnatal progenitor maintenance and proliferation. In addition, we show that Notch signaling is necessary embryonically and postnatally for Prop1 expression and robust Pit1 lineage hormone cell expansion, as well as repression of the corticotrope lineage. Taken together, our studies identify temporal and cell type–specific roles for Notch signaling and highlight the importance of this pathway throughout pituitary development.
Hypopituitarism, including combined pituitary hormone deficiency (CPHD), has a prevalence of 45.5 per 100,000 individuals (1). Approximately 50% of known causes of CPHD result from mutations in the transcription factors PROP1 and PIT1 (2–6). PROP1 is necessary for the transcription of PIT1, which is required for GH, TSHβ, and prolactin (PRL) expression. Therefore, patients with mutations in PROP1 have loss of these hormones. Although PROP1 is not necessary for gonadotrope or corticotrope cell differentiation, many patients also present with reductions in gonadotropin (LH and FSH) levels and acquired ACTH deficiency (7–9). Mouse models of Prop1 deficiency have been fundamental to our understanding of the etiology of CPHD. Ames Dwarf (Prop1df) mice, which harbor a spontaneous point mutation in Prop1, and genetically engineered Prop1null mice both recapitulate the phenotype of humans with PROP1 mutations (10–12).
Although the importance of PROP1 in pituitary development and disease is well understood, few genes that regulate PROP1 expression have been elucidated. Identifying these factors is important, because mutations in upstream regulators may be responsible for additional genetic causes of CPHD. One pathway that has been implicated in the control of Prop1 is the Notch signaling pathway. In a mouse model of pituitary-specific loss of the essential Notch cofactor Rbpj, a reduction in Prop1 transcript, detected by in situ hybridization, is observed. This finding correlates with reduced expression of Pou1f1 (Pit1), Gh, and Tshb. Furthermore, it was shown that RBPJ can bind to a canonical element within the first intron of the Prop1 gene, indicating that control of Prop1 by the Notch signaling pathway may be direct (13). Interestingly, Ames Dwarf mice have reduced levels of the receptor Notch2 but increased levels of the Dll1 ligand and target Hey1, suggesting that PROP1 may also regulate Notch signaling (14, 15).
The Notch signaling pathway is an evolutionarily conserved pathway that is critical to the development of many organs, where its most characterized role is in progenitor maintenance. This pathway requires cell-to-cell contact between one cell containing the ligand and the other containing the Notch receptor. Activation of the cascade in the receptor-containing cell results in cleavage of the intracellular domain (NICD) of the receptor. The NICD can then translocate into the nucleus and, in complex with Mastermind-like and RBPJ, drive the transcription of downstream targets. These targets are basic helix-loop-helix transcriptional repressors that inhibit the expression of prodifferentiation genes, thus maintaining the progenitor state of the cell (for a review, see Ref. 16). Notch2 and Notch3 are expressed in the embryonic pituitary and global loss of the Notch effector gene, Hes1, results in reduced proliferation, pituitary hypoplasia, and loss of α-melanocyte-stimulating hormone cells in the intermediate lobe (IL) (13, 14, 17, 18). In contrast, overexpression of Notch receptors can inhibit the differentiation of gonadotropes, melanotropes, and corticotropes (19, 20). However, the contribution of individual Notch receptors during pituitary development, as well as the effects of pituitary-specific loss of these receptors, remains unclear.
In addition to its expression during embryonic development, Notch signaling components are present in the adult pituitary. Expression of these factors is restricted to the cells lining the pituitary cleft, which correlates with the localization of adult pituitary progenitors (21). In vitro treatment of side population progenitor cells from the pituitary of adult mice or dissociated whole pituitary cells from adult rats with the Notch inhibitor N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT) results in a decrease in the numbers and proliferation of progenitor cells. Conversely, in vitro treatment with a soluble Notch ligand, using the same experimental systems, results in increased progenitor cell number and proliferation (21, 22). It is not clear whether similar effects would be observed in vivo, because cell-to-cell contact is an important aspect of the Notch signaling cascade.
Studies show that the pituitary undergoes a second wave of proliferation and cell differentiation during the first 3 weeks after birth in rodents, during which it expands greatly in size (23). Some of this expansion is due to increases in cell volume, but progenitor and differentiated cells also proliferate during this period (24–28). In addition, the number of progenitor cells greatly decreases after postnatal day (p) 21 in mice, indicating that these cells may play an important role in this phase of expansion (29). The constellation of Notch signaling components expressed and their importance for proliferation and differentiation during this time are unclear.
To further dissect the role of Notch signaling during these critical windows of development, we used both genetic and chemical inhibition of Notch signaling throughout early development and specifically during postnatal gland expansion. To examine the contribution of Notch2 during embryonic pituitary development, we generated a mouse with conditional loss of Notch2 (conditional knockout [cKO]), beginning at embryonic day (e) 8.5 in Foxg1-expressing cells (30). Loss of Notch2 does not appear to greatly affect overall embryonic pituitary development but does result in decreased expression of Prop1. Rather, we show that Notch signaling components are highly expressed during the early postnatal period and that both acute chemical Notch inhibition and Notch2 cKO mice display decreases in pituitary proliferation and alterations in hormone cell proportions. These changes parallel a substantial decrease in Prop1 expression. Taken together, the results of these studies further solidify Prop1 as a bona fide Notch target and identify a critical role for Notch signaling in postnatal pituitary development. Further studies will be necessary to identify whether altered Notch signaling could be a contributing factor in CPHD.
Materials and Methods
Mice
For Notch signaling pathway component analysis, wild-type mice of mixed genetic background were used. For in vitro and in vivo DAPT treatment studies, CD-1 mice (Charles River) from a breeding colony maintained in our laboratory, were used. For all studies, p1 is considered to be the day of birth. All animals were housed in a facility with a 12-hour light-dark cycle and were maintained in accordance with the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee.
To generate Notch2 cKO mice, Notch2fl/fl mice (31) were obtained from The Jackson Laboratory and bred to Foxg1+/cre mice (32) maintained on a 129 background, also purchased from The Jackson Laboratory. The resulting Notch2+/fl,Foxg1+/cre mice were bred to Notch2+/fl,Foxg+/+ littermates to obtain cKO animals. To genotype the mice, tail biopsies were performed, and DNA was extracted using a hot shot or salt-out method. PCR for the Cre and Notch2 alleles was performed as described previously (33) using published primer sequences (31, 32).
DAPT injections
Beginning at p2, CD1 mice were injected daily for 3 days with 100 mg/kg DAPT (γ-secretase inhibitor IX; Millipore) diluted in dimethylsulfoxide (DMSO, Sigma-Aldrich) or an equivalent volume of DMSO alone. Pituitaries and whole heads were harvested 24 hours after the last injection. For studies examining hormone cell fate choice, mice were injected with 100 mg/kg DAPT or an equivalent volume of DMSO for 5 days beginning on p3. Pituitaries were harvested on p12, 5 days after the last injection. For bromodeoxyuridine (BrdU) studies, mice were injected subcutaneously with 0.1 mg/g body weight BrdU (Sigma-Aldrich) diluted in water, 30 minutes before harvest.
Pituitary explant culture and DAPT washout assay
Pituitaries were dissected from p3 CD1 mice, rinsed with PBS, and transferred to a 96-well plate. Culture medium was made by adding 1% penicillin-streptomycin (Fisher) and 10% fetal bovine serum (HyClone) to DMEM/F-12 medium (Cellgro). Then 10 μM DAPT dissolved in DMSO (Cambridge Isotope Laboratories) or an equivalent amount of DMSO (Cambridge Isotope Laboratories) was added to the culture medium from the beginning of culture. After 24 hours in culture, medium was spiked with DAPT or DMSO (an equivalent amount to what was added at the beginning of culture) and pituitaries were cultured for an additional 24 hours. For washout assays, medium was removed from pituitaries, pituitaries were rinsed, and DAPT-free, DMSO-containing medium or 10 μM DAPT-containing media was added. Pituitaries were harvested 6 hours later.
Immunohistochemistry
Whole e12.5 embryos, heads of postnatal mice, and pituitaries were fixed in 3.7% formaldehyde (Sigma Aldrich) diluted in PBS and embedded in paraffin for sectioning. Slides were deparaffinized and rehydrated. For immunohistochemical detection of SOX2, SOX9, Ki67, phospho-histone H3 (PH3), BrdU, and Pit1, samples were boiled in 10 mM citrate solution (pH 6) for 10 minutes and cooled for 15 minutes. Next, all samples were blocked in a solution containing 5% normal donkey serum (Jackson ImmunoResearch) diluted in immunohistochemical block, which contains 3% bovine serum albumin (Jackson ImmunoResearch) and 0.5% Triton X-100 (Sigma Aldrich) diluted in PBS. Primary antibodies used were raised against the following peptides: SOX2 (1:2000 Millipore), PH3 (1:500; Upstate Cell Signaling Solutions), Ki67 (1:500; BD Biosciences) SOX9 (1:500; Millipore), Pit1 (1:4000; kind gift of Dr Simon Rhodes), proopiomelanocortin (POMC) (1:1000; DAKO), GH, TSHβ, and LHβ (1:1000; Dr. A. F. Parlow and the National Hormone and Pituitary Program, University of California, Los Angeles), and BrdU (1:200; AbD Serotec). Slides were then incubated with biotin-conjugated rat (BrdU), mouse (Ki67), or rabbit (Pit1, GH, TSHβ, POMC, LHβ, SOX2, SOX9, and PH3) secondary antibody. This was followed by incubation in a streptavidin-conjugated cy3 fluorophore. Biotin and streptavidin-conjugated antibodies were purchased from Jackson ImmunoResearch and were used at a concentration of 1:500. Slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (1:1000; Life Technologies) and visualized at ×50, ×100, ×200, or ×400 magnification using a Leica DM2560 microscope. Photographs were taken using a Retiga 2000R color camera (QImaging) and acquired using QCapture Pro software (QImaging). Images were processed using Adobe Photoshop CS4.
Pit1, POMC, and LHβ BrdU quantification
To quantify cells incorporating BrdU, 3 equally spaced slides containing 3 sections each were immunohistochemically stained for BrdU. For Pit1, POMC, and LHβ cell number quantification, 5 to 6 equally spaced slides containing 2 sections each were immunohistochemically stained using their respective antibodies. For BrdU, the numbers of positive cells in the anterior lobe (AL) and IL were counted and the areas of the AL and IL were measured. For Pit1, POMC, and LHβ quantification, the number of positive cells in the AL was counted and the area of the AL was measured. In addition, for all cell counts, a ratio of cells per area was determined for each group by counting the number of DAPI-stained cells per area. This ratio was used to calculate the total cells in the each pituitary section. The number of BrdU-, Pit1-, POMC-, or LHβ-positive cells was divided by the calculated total number of cells in each pituitary, giving the percentage of immunopositive cells. Statistical significance was determined using the Student t test.
AL volumetric approximation
For volumetric approximation, every third slide, containing 2 sections each, of p6 control and cKO mice was stained with DAPI. The area of the AL was measured for each stained section using ImageJ (National Institutes of Health). To obtain an approximation of the AL volume, this area was multiplied by the thickness of the section and by 3 to account for slides between stained slides.
In situ hybridization
Slides were deparaffinized, rehydrated, and incubated in PBS containing 0.3% Triton X-100 (Sigma-Aldrich) for 10 minutes and permeabilized using proteinase K (Life Technologies) for 15 minutes at 37°C. Samples were then postfixed using 3.7% formaldehyde (Sigma-Aldrich) and acetylated for 10 minutes in a solution containing 0.25% acetic anhydride (Sigma-Aldrich) diluted in triethanolamine (Sigma-Aldrich) buffer. After acetylation, sections were incubated in hybridization solution at 55 to 58°C for a minimum of 2 hours. DNA digoxigenin-UTP (DIG)–labeled probes for Prop1 (34), Notch2 (kind gift of Dr Shelley Ross) (14), Hes1 (kind gift of Dr Ryoichiro Kageyama), Hey1 (kind gift of Dr Mannfred Gessler), or Ghrh (kind gift of Drs Iain Robinson and Paul LeTissier) were denatured and applied to slides for overnight incubation at the hybridization temperature. Next, samples were treated with a solution of 50% formamide (Sigma-Aldrich) for 1 hour at the hybridization temperature, followed by blocking and then application of anti-DIG Fab fragments for 1 to 2 hours at room temperature. Slides were then washed, treated with Chromagen buffer solutions of increasing pH, and developed at room temperature overnight in Chromagen buffer containing nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. DIG-labeled nucleotides, anti-DIG antibody, and nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate were purchased from Roche.
Quantitative RT-PCR (qRT-PCR)
RNA was isolated from embryonic and postnatal whole pituitaries using an RNAqueous micro kit (Ambion) as per the manufacturer's protocol. For p21 and adult pituitaries, 0.5 μg of RNA was synthesized into cDNA using the ProtoScript M-MuLV First Strand cDNA Synthesis Kit (New England Biolabs). For e16.5 and p1, p5, and p10 and p12 pituitaries, the total amount of RNA from each pituitary was converted into cDNA. A no RT enzyme control was also prepared and used as a negative control. For qRT-PCR, 0.1 to 0.33 μL of cDNA was amplified using gene-specific primers and SYBR Green mix (Bio-Rad Laboratories) on a Bio-Rad MyiQ real-time PCR machine. Data were analyzed with the change in cycle threshold (ΔCt) value method (20). Statistical significance was determined using a Student t test. For a list of gene-specific primers, see Supplemental Table 1.
Results
Loss of Notch2 does not affect proliferation or corticotrope differentiation at e12.5
At e12.5, the morphology of the developing pituitary appears similar between control (Figure 1A) and Notch2 cKO mice (Figure 1B). In addition, proliferation appears to be largely unchanged between control (Figure 1C) and cKO (Figure 1D) pituitaries. POMC immunostaining reveals few positive cells in control (Figure 1E) and cKO mice (Figure 1F), indicating that there is no apparent alteration in corticotrope numbers when Notch2 is lost, contrary to what is observed in the Prop1,Hes1 double knockout (33). Because no striking phenotype is observed in Notch2 cKO mice, we performed in situ hybridization for Hey1 in control and cKO mice to determine whether this canonical Notch signaling target is reduced as expected. In control mice, at e12.5, Hey1 is expressed throughout the developing pituitary (Figure 1G). In cKO mice, there is a marked reduction, although not complete loss, of Hey1 expression throughout the developing pituitary (Figure 1H). Because Notch signaling has been shown to potentially regulate Prop1, we examined Prop1 expression in control and cKO developing pituitaries. In control mice, Prop1 is expressed throughout Rathke's pouch and is restricted away from the ventral portion of the developing pituitary where differentiated cells are housed (Figure 1I). In cKO mice, Prop1 appears to be reduced throughout Rathke's pouch (Figure 1J). Taken together, these data suggest that Notch2 is not necessary for proliferation during early development but is necessary for Prop1 expression.
Figure 1.
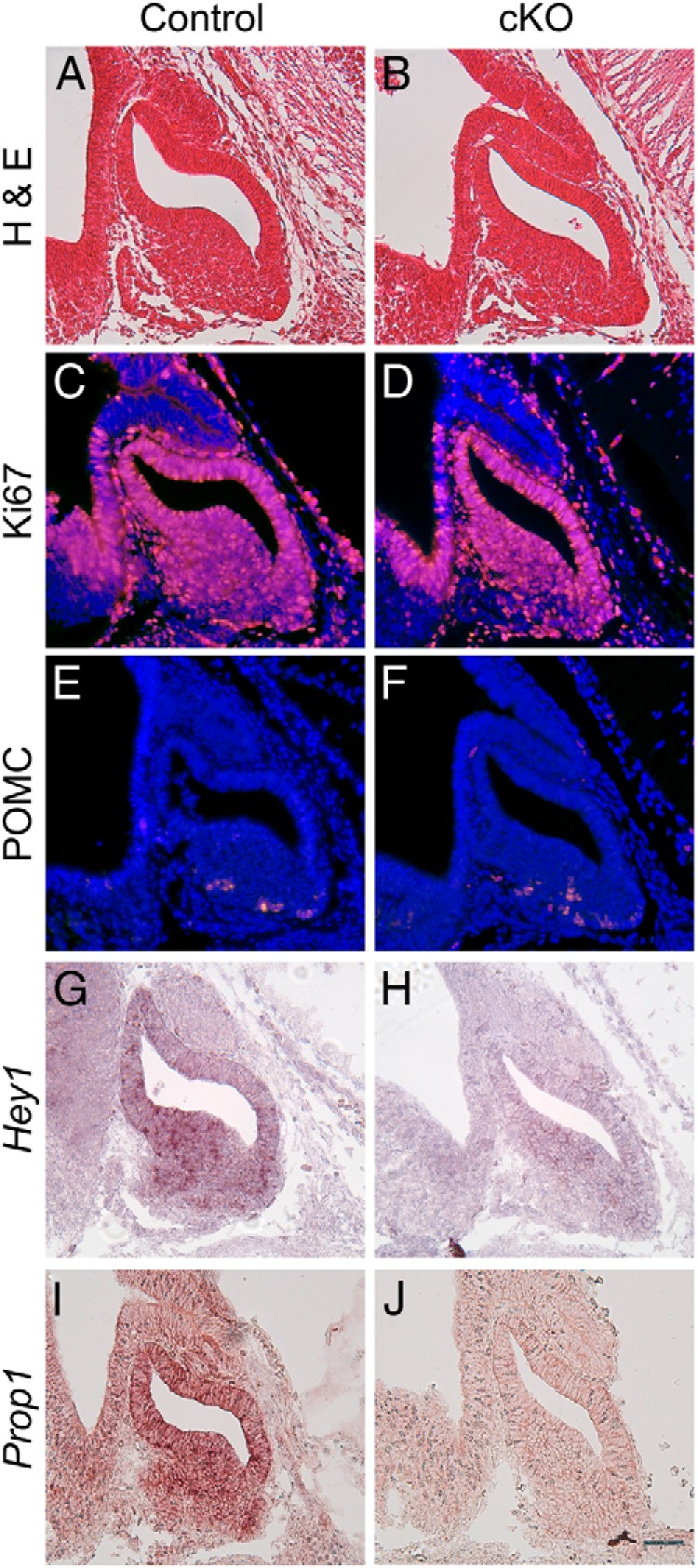
Conditional loss of Notch2 results in decreased Prop1 mRNA at e12.5. Sagittal sections of control (A) and cKO (B) e12.5 pituitaries were stained with hematoxylin and eosin (H & E) and developing pituitaries appear to be roughly the same shape and size. Ki67 immunostaining is similar between control (C) and cKO (D) pituitaries. POMC is expressed in few cells in the pituitaries of control (E) and cKO (F) mice. The canonical Notch target Hey1 is expressed in the developing pituitary in control mice (G) and appears reduced in cKO mice (H). Prop1 is expressed in control mice (I) and appears reduced in cKO mice (J). Magnification, ×200. Bar, 50 μm. n = 4 to 6.
Characterization of Notch signaling components during postnatal expansion phase of pituitary development
The pituitary undergoes two distinct waves of cell proliferation and differentiation, one occurring during embryogenesis and a second taking place during the first 3 postnatal weeks in the mouse (29, 35). Although it appears that Notch2 is not necessary for early pituitary development to proceed normally, the role of Notch signaling during the second wave of proliferation and differentiation has yet to be elucidated. To address this question, we first investigated the spatial and temporal expression patterns of Notch signaling components during this critical window to determine whether this pathway is active during this time. We used qRT-PCR to determine the relative levels of Notch family members at e16.5, p1, p5, p10, and p21 and compared these levels with those observed in the adult pituitary (Supplemental Figure 1). These data show that Notch signaling components are present during this wave of pituitary expansion and their mRNA levels are, in general, higher than those found in adult pituitaries.
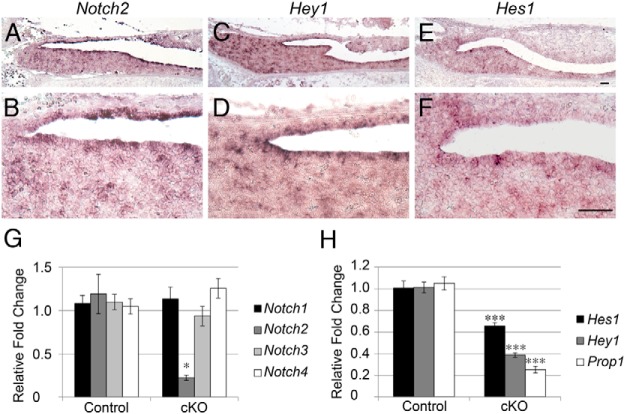
To determine the mRNA localization of Notch targets and receptors, we performed in situ hybridization for Notch2, Hey1, and Hes1 in p5 coronal sections. We found that Notch2 mRNA is mainly restricted to some but not all cells surrounding the pituitary cleft, particularly in the area where the ILs and ALs intersect. There are also Notch2-positive cells scattered throughout the AL (Figure 2, A and B). This expression pattern correlates with the localization of the population of pituitary progenitors containing SOX2 (36). A similar expression pattern is observed for Hey1 (Figure 2, C and D), with positive cells seen lining the cleft and in scattered cells in the AL. Hes1 appears to be in fewer cells than Notch2 and is restricted to the AL side of the pituitary cleft (Figure 2, E and F). Recent studies have shown that PROP1 is also present in cleft cells after birth (21, 37).
Figure 2.
Localization of Notch family members in the pituitary at p5. In situ hybridization for Notch2 (A and B), Hey1 (C and D), and Hes1 (E and F) reveals that Notch signaling components are mainly localized to cells surrounding the pituitary cleft. qRT-PCR comparing control and Notch2 cKO pituitaries at p1 shows specific and significant reductions in mRNA levels of Notch2 (G) and Hes1, Hey1, and Prop1 (H). *, P < .05; ***, P < .001. Bars, 50 μm. n = 3 to 4 (in situ hybridization) and n = 4 (qRT-PCR).
These findings indicate that Notch signaling components are present during early postnatal pituitary development and are dynamic, warranting further investigation of the role of this signaling pathway during this period. We examined the expression of Notch receptors in Notch2 cKO mice at p1 and found that Notch2 is specifically reduced, although not completely lost (Figure 2G). In addition, we found significant reductions in the mRNA levels of Notch targets, especially Prop1 (Figure 2H), making the cKO mouse a good model to investigate the effects of loss of Notch2 at this age.
Notch2 is necessary for the maintenance of pituitary progenitors after birth
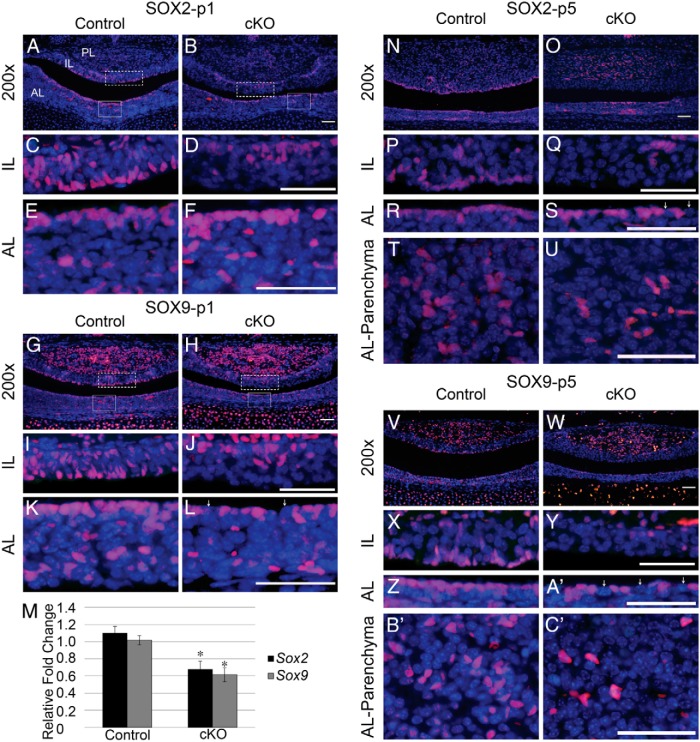
Our analysis shows that Notch2 is maintained in cleft cells after birth, indicating that it may have a role in progenitor maintenance during the postnatal wave of pituitary development. We, therefore, examined SOX2 expression at p1 in control and cKO mice. At p1, SOX2 is normally expressed in most cells lining the cleft of the pituitary on both the AL and IL sides. In addition, SOX2 is also found in cells scattered throughout the IL and the AL (Figure 3, A, C, and E). In cKO mice, none of the cells lining the pituitary cleft on the IL side are positive for SOX2. Instead, SOX2-immunoreactive cells are found in the dorsal IL, at the IL-posterior lobe (PL) border (Figure 3, B and D). Interestingly, cells in the AL appear to be largely unaffected in localization or number in the cKO mice (Figure 3, B and F). The expression pattern of SOX9, another progenitor marker, is similar to that of SOX2. In control mice at p1, SOX9-positive cells are found lining the pituitary cleft and scattered throughout the IL and AL, as well as in many cells in the PL (Figure 3, G, I, and K). In cKO mice, SOX9 is not found in cells lining the cleft in the IL, but, similar to SOX2 localization, is restricted to cells in the dorsal IL (Figure 3, H and J). In addition, in cKO mice, the number of SOX9-immunoreactive cells lining the cleft and in the parenchyma of the AL appears to be reduced (Figure 3, H and L; arrows indicate SOX9-negative cleft cells). In support of these observations, the mRNA levels of Sox2 and Sox9 are both significantly decreased in cKO mice compared with those in controls (Figure 3M). Taken together, these results show that Notch2 is necessary for the maintenance and localization of progenitor cells at p1.
Figure 3.
Loss of Notch2 results in misplacement of pituitary progenitors at p1 and decreased progenitor number at p5. SOX2 is expressed at p1 in cells lining the pituitary cleft and in scattered cells in the IL and AL of control mice (A, C, and E). In contrast, in cKO mice, SOX2 is only present in cells in the dorsal IL and is absent from IL cleft cells (B and D), whereas AL expression of SOX2 appears to be unaffected in cKO mice (B and F). SOX9 follows an expression pattern similar to that of SOX2 in control mice (G, I, and K). SOX9 is restricted to dorsal IL cells (H and J) and appears to be reduced in the AL (H and L) of cKO mice. mRNA levels of Sox2 and Sox9 are reduced in whole pituitaries of cKO mice at p1 compared with those in controls (M). SOX2 is expressed in cells lining the cleft in the AL and IL in control mice at p5 (N, P, and R). In cKO mice, SOX2-containing cells in the IL and the AL are reduced (O, Q, and S). In the AL parenchyma, SOX2 cells are seen in clusters in control mice (T) and appear reduced in cKO mice (U). SOX9 is also expressed in cells lining the cleft in the AL and IL in control mice (V, X, and Z) and shows reductions in cKO mice (W, Y, and A′) similar to as those seen with SOX2. In control mice, SOX9 is seen in clusters in the AL parenchyma (B′). These clusters are not observed in cKO mice (C′). Magnification, ×200 (A, B, G, H, N, O, V, and W) and ×400 (C–F, I–L, P–U, and X–C′). White boxes (A, B, G, and H) indicate where higher magnification photos were taken. Arrows (L, S, and A′) indicate SOX9- and SOX2-negative cleft cells. Bars, 25 μm (E, F, K, and L); 50 μm (A–D, G–J, and N–C′). *, P < .05. n = 6 to 8 (histology) and n = 4 to 9 (qRT-PCR).
Loss of Notch2 results in a progressive decrease in pituitary progenitor number
To assess whether progenitor cells are maintained in the Notch2 cKO throughout early postnatal expansion, we stained p5 control and cKO pituitaries for SOX2 and SOX9. In control mice, SOX2 expression in the IL is mostly limited to cells lining the cleft (Figure 3, N and P). In cKO mice, fewer SOX2-expressing cells remain in the IL than are observed at p1. The cells that do remain are still present at the IL-PL border (Figure 3, O and Q), rather than lining the cleft. On the AL side of the cleft, SOX2 is present in nearly every cell in control mice (Figure 3, N and R), and appears to be expressed in fewer cells in cKO mice (Figure 3, O and S, arrows). In addition to SOX2-positive progenitors lining the cleft, there are clusters of SOX2-expressing cells in the AL parenchyma (Figure 3T). Although these clusters are still present in cKO mice, they appear to be reduced (Figure 3U). In control mice, SOX9 expression follows a pattern in the IL similar to that of SOX2 (Figure 3, V and X). Likewise, SOX9 is expressed in only a few cells in the IL of cKO, and these cells are present only in the dorsal IL (Figure 3, W and Y). As with SOX2, SOX9 is expressed in nearly every cell lining the cleft in the AL in control mice (Figure 3, V and Z). However, SOX9 expression appears to be lost in many AL cleft cells in cKO mice (Figure 3, W and 3A′, arrows). SOX9 is expressed in clusters in the AL parenchyma in control mice (Figure 3B′). In contrast, very few, if any, clusters are observed in the AL parenchyma of cKO mice, and the SOX9-positive cells that are present appear to be morphologically different from their control counterparts (Figure 3C′). Taken together, these data suggest that Notch2 is necessary to maintain and expand the progenitor population during postnatal pituitary maturation.
Loss of Notch2 results in decreased pituitary proliferation at p1 and decreased AL volume at p6
Although proliferation is not affected at e12.5 when Notch signaling is reduced, we observe a disruption of progenitor cells at p1 and p5. Therefore, we examined whether these changes in progenitor cell number and localization correlate with changes in postnatal proliferation. In control mice, Ki67-immunoreactive cells are observed throughout the AL, IL, and PL (Figure 4A). In the IL, Ki67 expression is found throughout, especially in cells lining the cleft (Figure 4C). In cKO mice, there appears to be an overall reduction in the number of Ki67-expressing cells in the AL and a striking reduction of immunopositive cells in the IL (Figure 4, B and D). In addition, an apparent decrease in cells in mitosis is also observed in the cKO pituitaries as evidenced by a reduction in the number of cells expressing PH3 (Figure 4F) compared with those in the controls (Figure 4E). Furthermore, mKi67 and Ccne1 mRNA levels are reduced in cKO pituitaries. This finding is coincident with an increase in Cdknc1 (p57) mRNA (Figure 4G), a cell cycle inhibitor, shown to be necessary for pituitary progenitors to exit the cell cycle before differentiating (38). Taken together, these data indicate that Notch2 is an important regulator of pituitary proliferation at p1.
Figure 4.
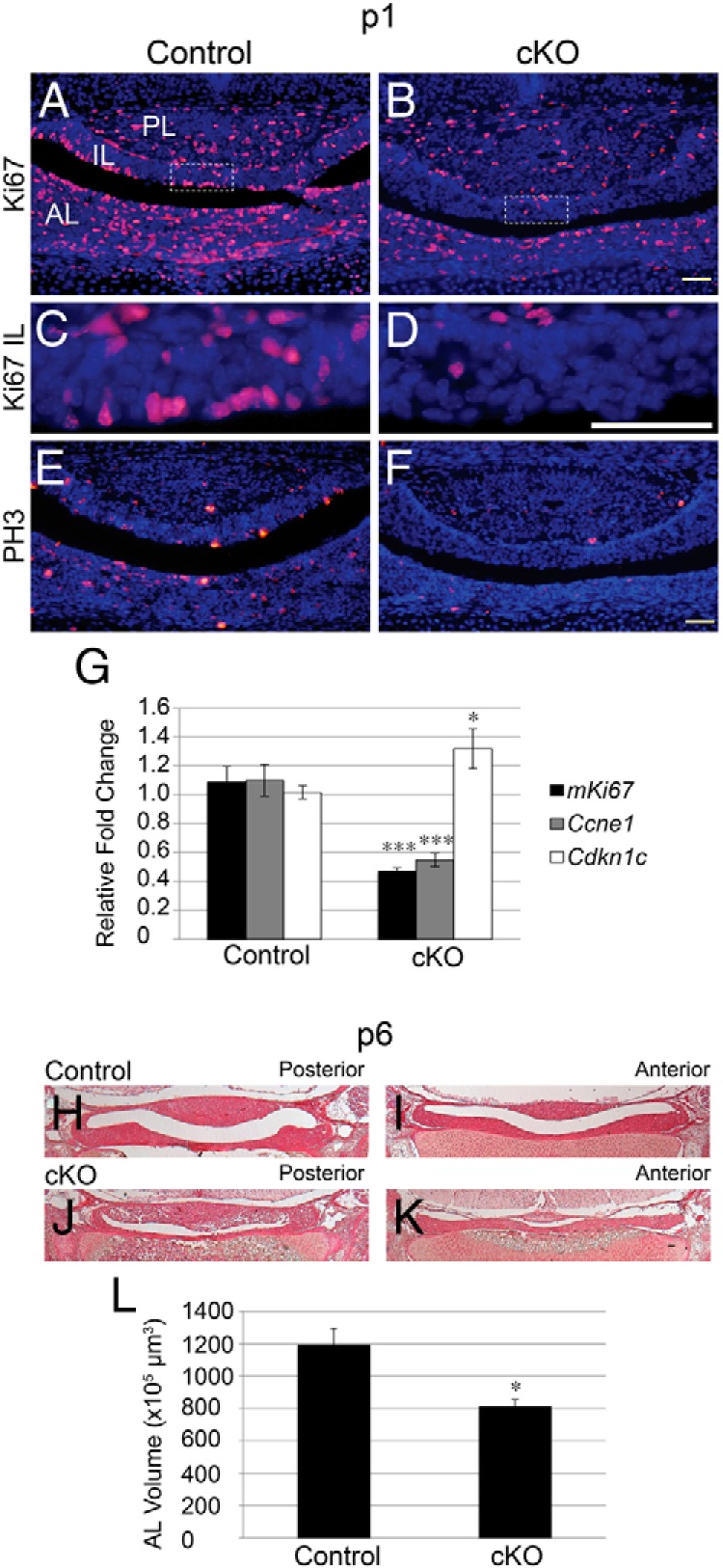
Decreased proliferation is observed in mice with conditional loss of Notch2, resulting in a hypoplastic pituitary by p6. Ki67-immunoreactive cells are found in the AL and IL of control (A and C) pituitaries at p1. The number of Ki67-immunopositive cells appears to be reduced in both lobes of cKO (B and D) mice. PH3 expression is scattered throughout the pituitary at p1 in control (E) mice and is markedly decreased in cKO (F) pituitaries. qRT-PCR in whole pituitaries at p1 reveals a significant decrease in the mRNA levels of mKi67 and Ccne1 and a significant increase in Cdkn1c mRNA in cKO mice compared with that in controls (G). Hematoxylin and eosin staining reveals a hypoplastic AL at p6 in cKO mice (J and K) compared with that in control mice (H and I). The volume of AL at p6 is decreased in cKO mice. Magnification, ×50 (H–K), ×200 (A, B, E, and F), and ×400 (C and D). White boxes (A and B) indicate where higher magnification photos were taken. Bars, 50 μm. *, P < .05; ***, P < .001. n = 6 to 7 (immunohistochemistry), n = 4 to 7 (qRT-PCR), and n = 3 to 4 (AL volume approximation).
Because we observed decreased proliferation at p1, we examined the approximate AL volume at p6 to determine how these changes in proliferation may result in changes in pituitary size. We found that at p6 the AL of cKO mice (Figure 4, J and K) appears hypoplastic compared with the AL of control mice (Figure 4, H and I). In addition, the approximate volume of the cKO AL is significantly reduced compared with that of the control (Figure 4L). These findings suggest that a reduction in Notch2 can lead to a hypoplastic pituitary as early as p6.
Chemical Notch inhibition results in decreased postnatal proliferation in vivo
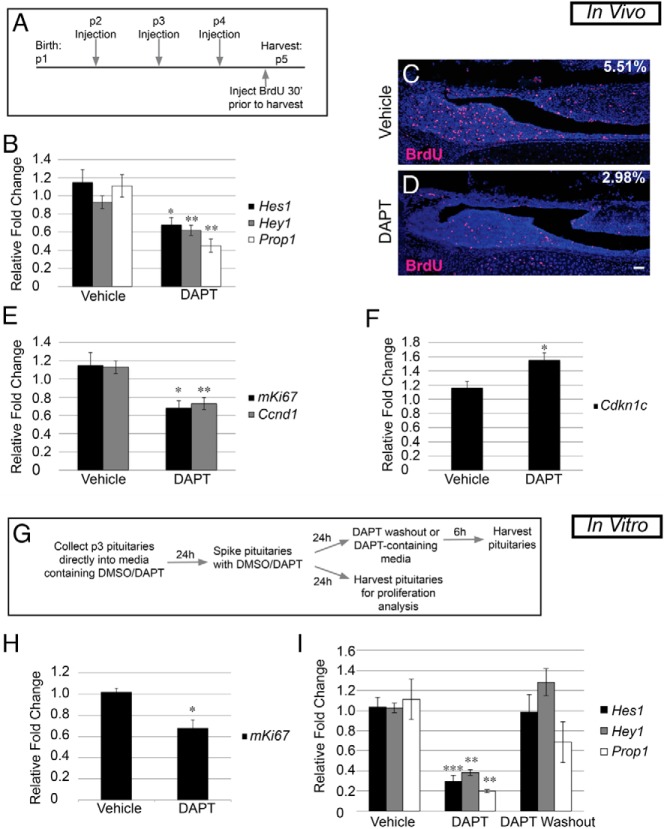
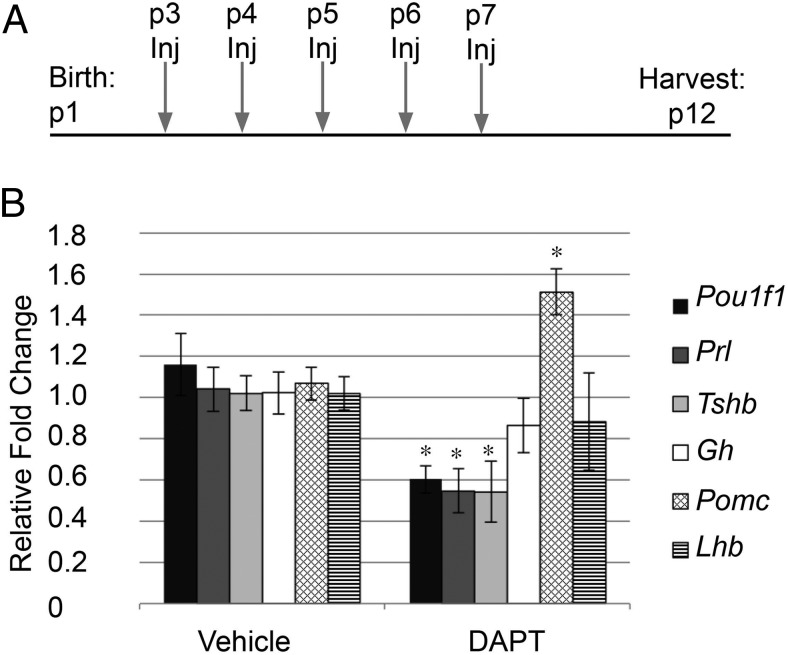
In Notch2 cKO mice, we did not observe proliferation defects until after birth, indicating that Notch signaling may be specifically important for proliferation that occurs postnatally. To confirm that the postnatal changes we observed in Notch2 cKO mice are due to direct actions postnatally, we injected mice during early postnatal development with the γ-secretase inhibitor DAPT. DAPT inhibits cleavage of the Notch intracellular domain, thus preventing activation of the downstream pathway (39–41). Mice were injected with 100 mg/kg DAPT or the vehicle control (DMSO) daily between p2 and p4, and pituitaries were harvested 24 hours after the last injection (Figure 5A). This time period was chosen because, based on expression analysis, many Notch signaling components appear to be most highly expressed between p1 and p5. To determine the efficacy of Notch inhibition in DAPT-injected mice, we examined the mRNA levels of the canonical Notch targets Hes1 and Hey1 and the putative pituitary-specific Notch target Prop1 in whole pituitaries. We saw a significant decrease in all targets examined, with Prop1 levels being most highly suppressed after DAPT treatment compared with those in vehicle-treated controls (Figure 5B).
Figure 5.
Postnatal Notch inhibition results in decreased proliferation and identifies Prop1 as a direct target. A, Experimental design for in vivo DAPT injection. B, Subcutaneous injection with DAPT results in a decrease in whole pituitary mRNA levels of Hes1, Hey1, and Prop1 compared with those in vehicle-injected mouse pituitaries. C and D, BrdU-positive cells (red) are present throughout the pituitaries of vehicle-treated mice (C, 5.51 ± 0.20%) and are significantly reduced (P = .0001) in pituitaries of DAPT-treated mice (D, 2.98 ± 0.26%). E, mRNA levels of mKi67 and Ccnd1 in whole pituitaries are decreased after DAPT injection compared with those after for vehicle injection. F, Conversely, mRNA levels of Cdkn1c are increased in the whole pituitary of DAPT-injected mice. G, Experimental design for in vitro DAPT treatment and DAPT washout assay. H, After 48 hours in culture, mRNA levels of mKi67 are significantly decreased compared with those of vehicle-treated controls. I, mRNA levels of Hes1, Hey1, and Prop1 are reduced after DAPT treatment and recover after 6 hours of DAPT washout. Magnification, ×100. Bars, 50 μm. *, P < .05; **, P < .01; ***, P < .001. n = 4 (immunohistochemistry), n = 5 to 8 (qRT-PCR in vivo), and n = 4 to 8 (qRT-PCR in vitro).
Because Notch signaling is known to be important for proliferation in many developing organs and because we observed decreases in proliferation at p1 in Notch2 cKO mice, we examined proliferation by quantifying the percentage of BrdU-positive cells in the AL and IL of pituitaries of DAPT-treated mice and compared these values with those for vehicle controls. In vehicle-injected mice, there were an average of 5.51% (±0.20%) of cells that were positive for BrdU (Figure 5C) compared with 2.98% (±0.26%) of cells in pituitaries of DAPT-treated mice (Figure 5D). In addition, we found a reduction in the mRNA levels of mKi67, which is present in cells during all phases of the cell cycle, and in Ccnd1, which is important for the transition from the G1 to the S phase, in mice treated with DAPT (Figure 5E). This result was coincident with an increase in Cdknc1 (p57) mRNA (Figure 5F). These observations show that Notch signaling contributes to postnatal pituitary proliferation in vivo and are similar to those seen in Notch2 cKO mice at p1.
In vitro chemical Notch inhibition reveals direct effects on the pituitary
To elucidate whether the effects observed in the in vivo DAPT injection study are consistent with direct effects on the pituitary, we used an in vitro organ culture system (Figure 5G). We examined whether proliferation in DAPT-cultured pituitaries is affected in a manner analogous to that seen in DAPT-injected mice. Similarly, we found a significant decrease in the mRNA levels of mKi67 in pituitaries treated with DAPT compared with those in vehicle-treated pituitaries after 48 hours in culture (Figure 5H). Taken together, the results of the Notch2 cKO and acute DAPT treatment studies implicate Notch signaling in maintaining at least a subset of progenitors and allowing their proliferation during postnatal development.
To identify potentially direct targets of Notch signaling, we performed a 6-hour washout assay, using the in vitro explant system. Targets that are altered after DAPT treatment, but recover within the 6-hour period, are considered likely to be the direct targets of the Notch signaling pathway (42–44). We observed significant reductions in the mRNA levels of the Notch targets Hes1, Hey1, and Prop1, with Prop1 levels again being the most decreased after culture in the presence of DAPT. Six hours after washout, the mRNA levels of the canonical Notch targets Hes1 and Hey1 have recovered to levels comparable to those in vehicle-treated pituitaries. Prop1 levels recover to levels that are statistically similar to those in vehicle-treated pituitaries, albeit not as complete a response as that for Hes1 or Hey1 (Figure 5I). This result further suggests that Prop1 is probably a direct Notch target and shows that Notch signaling can influence Prop1 expression after birth.
Loss of Notch2 results in decreased Pit1 cell number and increased POMC number at p1
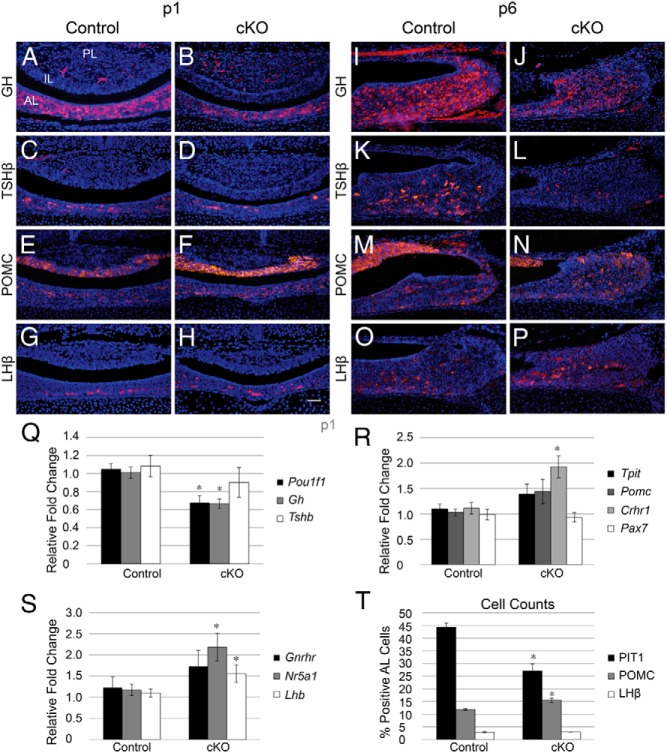
In addition to its role in progenitor maintenance and proliferation, Notch signaling has been shown to be a critical regulator of cell fate in many systems (45–48). To determine whether Notch signaling has a similar role in the pituitary, we examined the fate of Pit1 lineage cells, which contain GH, TSH, and PRL, as well as corticotropes and gonadotropes at p1 in control and Notch2 cKO mice. GH is normally expressed in the AL of control mice (Figure 6A) and appears to be reduced in cKO pituitaries (Figure 6B). TSHβ appears throughout the AL in control mice (Figure 6C) and appears to be largely unchanged in cKO pituitaries (Figure 6D). In parallel, we examined the mRNA levels of Gh, Tshb, and Pou1f11. We observed a significant reduction in cKO mRNA levels of Pou1f1 and Gh, with Tshb levels being statistically similar between control and cKO mice (Figure 6Q). To determine whether the observed changes in the mRNA levels correlate with changes in cell number, we quantified the percentage of Pit1-positive cells in the AL of control and cKO mice. In control mice, 44.35% (±1.59%) of AL cells are positive for Pit1, compared with only 26.97% (±2.85%) in cKO mice (Figure 6T), suggesting that Notch2 is, at least in part, necessary to obtain the proper number of Pit1-positive cells.
Figure 6.
Conditional loss of Notch2 results in decreased expression of Pit1 lineage hormones and increased expression of POMC. GH-immunopositive cells are found scattered throughout the AL of control mice (A) and appear to be reduced in pituitaries of cKO mice (B) at p1. TSHβ is localized to the AL of control (C) and cKO (D) mice. POMC-immunoreactive cells are found in the AL and the IL of control (E) pituitaries and appear slightly increased in the AL of cKO mice (F). LHβ expression is confined to the AL and appears similar between control (G) and cKO (H) mice. At p6, GH is present in the AL of control mice (I) and appears reduced in cKO mice (J). Similarly, TSHβ is strongly reduced in cKO mice (L) compared with those in control mice (K). POMC is present in the IL and AL of control mice (M) and appears slightly increased in the AL of cKO mice (N). LHβ appears similar between control (O) and cKO (P) pituitaries. qRT-PCR analysis at p1 reveals a significant decrease in Pou1f1 (Pit1) and Gh mRNA, but no change in Tshb mRNA between control and cKO mice (Q). In addition, p1 mRNA levels of Tpit and Pomc show a trend toward an increase in cKO mice, whereas Crhr1 is significantly increased and Pax7 is unchanged (R). mRNA levels of Nr5a1 and Lhb are significantly increased in pituitaries, whereas Gnrhr is trending toward an increase at p1 (S). The percentages of Pit1-, POMC-, and LHβ-positive cells in the AL were quantified in control and cKO mice, and the percentage of Pit1-positive cells was found to be deceased in cKO mice, whereas the percentage of POMC-immunoreactive cells is increased (T). *, P < .05. Magnification, ×200. Bars, 50 μm. n = 5 to 7 (histology), n = 4 to 7 (qRT-PCR), and n = 3 to 5 (cell counts).
POMC is expressed in melanotropes in the IL and corticotropes in the AL in both control (Figure 6E) and cKO (Figure 6F) mice and appears to be slightly increased in the AL of cKO mice compared with that in controls. In addition, we observe a trend toward an increase in the mRNA levels of Tpit and Pomc, which are present in both corticotropes and melanotropes, in cKO mice. To more specifically explore corticotrope and melanotrope lineages, we examined expression levels of genes that are specific to each cell type (49). We show an increase in the levels of Crhr1, which is expressed in corticotropes, in cKO pituitaries. Conversely, no changes in the mRNA levels of Pax7, a melanotrope-specific marker, are observed in cKO mice (Figure 6R). To determine whether these changes in mRNA are coincident with changes in cell number, we quantified the number of POMC-positive cells in the AL. Control mice have 11.87% (±0.40%) POMC-expressing cells in the AL. cKO mice have a significantly increased number of POMC-containing AL cells (15.49% ± 0.83%) (Figure 6T), suggesting that Notch2 is an important factor regulating corticotrope number.
LHβ is found in a few cells scattered throughout the ventral pituitary in control mice (Figure 6G), and the number of LHβ-positive cells does not appear to be altered in cKO pituitaries (Figure 6H). Although the number of LHβ-immunopositive cells does not appear to be changed in cKO mice, Lhb mRNA levels are significantly increased in cKO pituitaries compared with those in controls. In addition, Nr5a1, a transcription factor expressed only in gonadotropes in the pituitary, is significantly increased in cKO pituitaries at p1, whereas Gnrhr shows a trend toward an increase (Figure 6S). To determine whether these changes in mRNA levels correlate with changes in LHβ-positive cell numbers, we quantified the percentage of AL cells that express this hormone. We found no difference between control (2.95% ± 0.20%) and cKO mice (2.99% ± 0.08%) (Figure 6T), suggesting that regulation of the gonadotrope cell number is probably independent of Notch2.
Mice with conditional loss of Notch2 have reduced GH and TSHβ at p6
To examine how loss of Notch2 affects expansion of the pituitary hormone lineages during postnatal development, we immunohistochemically examined the expression of pituitary hormones at p6. Similar to what was observed at p1, GH appears to be reduced in cKO pituitaries (Figure 6J) compared with that in control pituitaries (Figure 6I). In contrast to p1, TSHβ appears to be strongly reduced in cKO mice at p6 (Figure 6L) compared with that in control mice (Figure 6K). POMC is present in the IL and AL of control mice (Figure 6M) and appears to be slightly increased in the AL of cKO mice (Figure 6N), mirroring the qRT-PCR results and cell counts observed at p1. LHβ is present in scattered AL cells of control mice (Figure 6O) and cKO mice (Figure 6P) and appears similar between the two groups. Taken together, these data show that Notch2 is necessary to control the number of Pit1-positive cells and POMC-positive cells during postnatal pituitary expansion.
To eliminate the possibility that the reduction in Gh is a result of the presence of the Cre transgene alone, we examined Gh mRNA in control mice with (Notch2+/+Foxg1+/cre or Notch2+/flFoxg1+/cre) and without (Notch2+/+Foxg1+/+, Notch2+/flFoxg1+/+, or Notch2fl/flFoxg1+/+) the Cre transgene. No differences were observed between the two groups in Gh levels (Supplemental Figure 2A) at p1. In addition, although Foxg1 Cre should not be expressed in the developing hypothalamus and thus Notch2 expression should not be altered there, we examined whether reduced GH expression could be due to unexpected alterations in Ghrh in the hypothalamus in the cKO mice. In control mice, in situ hybridization reveals that Ghrh mRNA is present in cells lateral to the third ventricle in the arcuate nucleus (Supplemental Figure 2B). A similar pattern of expression is observed for cKO mice (Supplemental Figure 2C). These data suggest that the phenotype of the Notch2 cKO mice is probably due to the pituitary intrinsic effects of loss of Notch2.
Postnatal chemical Notch inhibition results in decreased mRNA levels of Pit1 lineage hormones and increased Pomc mRNA levels
Because alterations in pituitary hormones were observed when Notch2 was lost from early embryogenesis, we investigated whether Notch signaling is also specifically important for postnatal pituitary cell fate choice. To examine this question, we injected mice with DAPT daily from p3 through p7 and harvested whole pituitaries on p12 to allow changes in differentiated cells to manifest (Figure 7A). In mice treated with DAPT, we observed a significant decrease in the mRNA levels of Pou1f1, Prl, and Tshb. Interestingly, although part of the Pit1 lineage, mRNA levels of Gh are unchanged after DAPT treatment. Pomc mRNA levels are increased after DAPT treatment, whereas Lhb mRNA levels appear unaffected (Figure 7B). These findings show that Notch signaling is necessary for proper mRNA levels of Pou1f1, Tshb, Prl, and Pomc and suggest a role for Notch in postnatal pituitary cell fate choice, similar to what was uncovered in the Notch2 cKO mouse.
Figure 7.
Postnatal chemical Notch inhibition results in decreased mRNA levels of Pou1f1 (Pit1), Prl, and Tshb and increased levels of Pomc. A, Experimental design for in vivo subcutaneous DAPT injection (Inj). mRNA levels of Pou1f1, Prl, and Tshb are significantly decreased and mRNA levels of Pomc are significantly increased in whole pituitaries after DAPT injection compared with those in vehicle-treated mice. B, mRNA levels of Gh and Lhb are unchanged between the two groups. *, P < .05. n = 3 to 5.
Discussion
Taken together, our studies have defined key roles for Notch signaling during postnatal development. We show that Notch signaling is required for pituitary proliferation and progenitor maintenance during postnatal pituitary expansion but appears to be less important for these processes during embryonic development. Furthermore, during both periods of development, we show that Notch signaling regulates the balance between the Pit1 and POMC lineages.
We have identified Notch2 as an important regulator of postnatal pituitary progenitor placement and number. Pituitary stem/progenitor cells contain the markers SOX2 and SOX9 and have been shown to contribute to postnatal pituitary homeostasis during both normal and stressed conditions (50, 51). Therefore, it is critical that an appropriate number of progenitor cells are maintained. We show that Notch2 cKO mice have progressive loss of this progenitor population after birth, suggesting that Notch signaling is necessary for maintenance of these cells. Loss of SOX2 in the embryonic pituitary results in impaired proliferation, pituitary hypoplasia, and a reduction in cells of the Pit1 lineage (52), mirroring what is observed in Notch2 cKO mice. In addition, overexpression of activated Notch in POMC-expressing cells results in increased SOX2 expression in the IL (20), suggesting that Notch may have the ability to induce SOX2 expression. Both Sox2 and Sox9 are direct Notch targets in neural progenitors in vivo (53), indicating the potential for Notch to directly control these factors in the pituitary.
Loss of Notch2 results in differential effects in AL and IL progenitors. IL progenitors are misplaced at p1, similar to what is observed with conditional loss of the Notch pathway components Numb and Numblike (54) and are nearly absent by p5. This is probably due to the fact that they lack the proper progenitor niche. In contrast, AL progenitors appear to be correctly localized but decreased in number by p5. These differences may be due to the heterogeneity of progenitor populations observed throughout the pituitary (37, 55). In addition, we show that Notch2 is not uniformly expressed in all progenitors, which may contribute to differences observed in different populations. These differential effects in progenitor populations may also be a factor contributing to the changes in hormone-producing cell numbers observed at p1, as some lineage-committed or intermediate progenitors may have an altered ability to expand.
We show that reductions in Notch2 have little effect on embryonic pituitary proliferation, but that Notch2 is required for proliferation during postnatal pituitary expansion. This result is in contrast to studies showing reduced proliferation in the embryonic pituitaries of Hes1 knockout mice (13, 17, 18, 56) and indicates that the Hes1-null phenotype may be due to factors extrinsic to the pituitary or that the reduced but detectable presence of Hes1 and other Notch signaling components, including Notch3, in Notch2 cKO mice is sufficient to maintain embryonic proliferation. Additional studies examining pituitary-specific loss of Hes1 will be required to further explore this question. Although we did not observe alterations in proliferation in cKO mice at e12.5, we saw a striking decrease in proliferation at p1. We saw a similar reduction in proliferation in mice treated postnatally with the chemical Notch inhibitor DAPT. This correlates with studies showing decreased proliferation in adult dissociated pituitary cells in vitro when treated with DAPT (21, 22). However, it remains unclear whether Notch signaling directly controls proliferation or alters the ability of stem cells to respond to proliferative signals.
We showed that loss of Notch2 resulted in alterations in cell fate, a phenomenon that has been described in multiple contexts, including pancreatic and central nervous system development (45–48). Specifically, after loss of Notch2, we detect a decrease in Pit1 cell number. This finding is similar to that observed in a conditional pituitary knockout of Rbpj, an essential Notch cofactor (13). In addition, we have extended these observations showing that alterations in hormone-producing cell numbers are maintained during postnatal expansion in Notch2 cKO mice and that the Pit1 lineage is similarly affected after postnatal DAPT treatment. It is likely that the changes observed in the Pit1 lineage in both models are due to reductions in Prop1 levels because Prop1 is necessary for differentiation of cells of the Pit1 lineage (10–12). However, it is also likely that the levels of Prop1 in Notch2 cKO and DAPT-injected mice are sufficient to allow for some expression of Pit1, because we did not observe a complete absence of this lineage. Taken together, our results show that reduced levels of Notch2 or of Notch signaling components in general result in specific and incomplete reduction in Pit1 and hormones of the Pit1 lineage. This finding suggests that other signaling pathways or molecules are also important in the development and function of Pit1 lineage hormones. Such factors may include GATA2, which helps to maintain Tshb expression (57), and Math3, which is required for somatotrope maturation (13).
In addition to the decrease observed in Pit1 lineage cells when Notch is reduced, we found that the POMC lineage is affected by reductions in Notch signaling. In Notch2 cKO mice, we observed an increase in the percentage of POMC-containing AL cells and an increase in mRNA levels of Crhr1, which is restricted to corticotropes, in Notch2 cKO mice. These changes are paralleled by an increase in Pomc mRNA in mice treated with the chemical Notch inhibitor, DAPT. A similar increase in POMC-expressing cells is observed in mice with loss of both Hes1 and Prop1 and in mice with pituitary-specific loss of Rbpj (13, 33). In addition, to obtain ACTH-producing cells from embryonic stem cells, these cells must first be incubated with DAPT (58). Taken together, these data all suggest that Notch signaling must be inhibited for corticotrope differentiation to proceed. This is also confirmed by persistent expression of activated Notch in corticotropes, which results in an inhibition in their differentiation (20). Interestingly, the IL POMC-expressing melanotropes appear to be less affected by alterations in Notch signaling in our model. This may be due to the fact that we observed no changes in Pax7, a pioneer factor that dictates melanotrope fate by altering chromatin accessibility (49). In addition, despite these changes in corticotrope and Pit1 lineage cell numbers, our data suggest that the gonadotrope lineage differentiates independently of Notch signaling. Taken together, these data strongly suggest that Notch2 may help to regulate the balance between the Pit1 and POMC lineages. This role may be direct or may be a result of the altered ability of different progenitor populations to expand.
Our studies have defined Notch2 as a critical regulator of Prop1 expression. We show that loss of Notch2 leads to decreased expression of Prop1 at both e12.5 and p1 and that in vivo and in vitro exposure to the chemical Notch inhibitor DAPT results in strong reductions in mRNA levels of Prop1. A recovery in Prop1 mRNA levels in vitro is observed 6 hours after DAPT washout, mirroring the recovery seen in the canonical Notch targets Hes1 and Hey1. Recent work has shown that RBPJ can bind to the first intron of the Prop1 gene, further solidifying Prop1 as a direct Notch target (13). In addition, the phenotype observed in Notch2 cKO mice is similar to, although less severe, than that observed in Prop1-deficient mice, with both showing a reduction in hormones of the Pit1 lineage and an inability of the pituitary to appropriately expand after birth (11, 12, 59, 60). Although some aspects of the phenotype observed when Notch2 is lost are similar to those of Prop1 mutants, we did not see increased adhesion and inability of cells to migrate out of the Rathke pouch in Notch2 cKO mice, as is seen in mice with loss of Prop1 (59). These differences indicate that the levels of Prop1 in Notch2 cKO mice may be sufficient to prevent cell migration defects or that other Notch targets are able to compensate for defects observed when Prop1 is lost.
Genetic causes of CPHD include mutations resulting in hypomorphic SOX2 expression and loss-of-function mutations in PROP1 and PIT1 (3, 5, 61). Our studies show that reductions in Notch signaling can result in alterations in expression of all of these factors during embryonic and postnatal development. Furthermore, we show that loss of Notch2 can lead to a hypoplastic pituitary as early as p6, indicating that the Notch signaling pathway is an important regulator of pituitary size and cell fate. Based on these studies, it may be advisable to consider whether Notch2 or other members of the Notch signaling pathway are altered in patients with CPHD who have no known genetic mutations.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We are grateful to Dr Ann Nardulli for use of equipment. Pituitary hormone antibodies were kindly provided by Dr A. F. Parlow and the National Hormone and Pituitary Program (University of California, Los Angeles).
This work was supported by the National Institutes of Health (Grant T32 GM007283 [to L.B.N.] and R01 DK076647 [to L.T.R.]).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AL
- anterior lobe
- BrdU
- bromodeoxyuridine
- cKO
- conditional knockout
- CPHD
- combined pituitary hormone deficiency
- DAPI
- 4′,6-diamidino-2-phenylindole
- DAPT
- N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester
- DMSO
- dimethylsulfoxide
- e
- embryonic day
- IL
- intermediate lobe
- p
- postnatal day
- PH3
- phospho-histone H3
- PL
- posterior lobe
- POMC
- proopiomelanocortin
- PRL
- prolactin
- qRT-PCR
- quantitative RT-PCR.
References
- 1. Regal M, Paramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf). 2001;55:735–740. [DOI] [PubMed] [Google Scholar]
- 2. Prince KL, Walvoord EC, Rhodes SJ. The role of homeodomain transcription factors in heritable pituitary disease. Nat Rev Endocrinol. 2011;7:727–737. [DOI] [PubMed] [Google Scholar]
- 3. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30:790–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cogan JD, Wu W, Phillips JA, et al. The PROP1 2-base pair deletion is a common cause of combined pituitary hormone deficiency. J Clin Endocrinol Metab. 1998;83:3346–3349. [DOI] [PubMed] [Google Scholar]
- 5. Wu W, Cogan JD, Pfäffle RW, et al. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet. 1998;18:147–149. [DOI] [PubMed] [Google Scholar]
- 6. Flück C, Deladoey J, Rutishauser K, et al. Phenotypic variability in familial combined pituitary hormone deficiency caused by a PROP1 gene mutation resulting in the substitution of Arg→Cys at codon 120 (R120C). J Clin Endocrinol Metab. 1998;83:3727–3734. [DOI] [PubMed] [Google Scholar]
- 7. Pernasetti F, Toledo SP, Vasilyev VV, et al. Impaired adrenocorticotropin-adrenal axis in combined pituitary hormone deficiency caused by a two-base pair deletion (301–302delAG) in the prophet of Pit-1 gene. J Clin Endocrinol Metab. 2000;85:390–397. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal G, Bhatia V, Cook S, Thomas PQ. Adrenocorticotropin deficiency in combined pituitary hormone deficiency patients homozygous for a novel PROP1 deletion. J Clin Endocrinol Metab. 2000;85:4556–4561. [DOI] [PubMed] [Google Scholar]
- 9. Böttner A, Keller E, Kratzsch J, et al. PROP1 mutations cause progressive deterioration of anterior pituitary function including adrenal insufficiency: a longitudinal analysis. J Clin Endocrinol Metab. 2004;89:5256–5265. [DOI] [PubMed] [Google Scholar]
- 10. Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. [DOI] [PubMed] [Google Scholar]
- 11. Nasonkin IO, Ward RD, Raetzman LT, et al. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum Mol Genet. 2004;13:2727–2735. [DOI] [PubMed] [Google Scholar]
- 12. Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Mol Endocrinol. 1996;10:1570–1581. [DOI] [PubMed] [Google Scholar]
- 13. Zhu X, Zhang J, Tollkuhn J, et al. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20:2739–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol. 2004;265:329–340. [DOI] [PubMed] [Google Scholar]
- 15. Mortensen AH, MacDonald JW, Ghosh D, Camper SA. Candidate genes for panhypopituitarism identified by gene expression profiling. Physiol Genomics. 2011;43:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monahan P, Rybak S, Raetzman LT. The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology. 2009;150:4386–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006;20:2898–2908. [DOI] [PubMed] [Google Scholar]
- 20. Goldberg LB, Aujla PK, Raetzman LT. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Dev Biol. 2011;358:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tando Y, Fujiwara K, Yashiro T, Kikuchi M. Localization of Notch signaling molecules and their effect on cellular proliferation in adult rat pituitary. Cell Tissue Res. 2013;351:511–519. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Crabbe A, Van Duppen V, Vankelecom H. The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol. 2006;20:3293–3307. [DOI] [PubMed] [Google Scholar]
- 23. Carbajo-Pérez E, Watanabe YG. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990;261:333–338. [DOI] [PubMed] [Google Scholar]
- 24. Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Mitoses of thyrotrophs contribute to the proliferation of the rat pituitary gland during the early postnatal period. Anat Embryol. 2002;206:67–72. [DOI] [PubMed] [Google Scholar]
- 25. Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of rat anterior pituitary cells. Anat Embryol. 2002;206:1–11. [DOI] [PubMed] [Google Scholar]
- 26. Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of pituitary somatotrophs and mammotrophs during late fetal and postnatal periods. Anat Embryol. 2001;204:469–475. [DOI] [PubMed] [Google Scholar]
- 27. Taniguchi Y, Kominami R, Yasutaka S, Kawarai Y. Proliferation and differentiation of pituitary corticotrophs during the fetal and postnatal period: a quantitative immunocytochemical study. Anat Embryol. 2000;201:229–234. [DOI] [PubMed] [Google Scholar]
- 28. Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci USA. 2011;108:11482–11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gremeaux L, Fu Q, Chen J, Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev. 2012;21:801–813. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Martin JF, Bai CB. Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Dev Biol. 2010;348:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. [DOI] [PubMed] [Google Scholar]
- 32. Hébert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. [DOI] [PubMed] [Google Scholar]
- 33. Himes AD, Raetzman LT. Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Dev Biol. 2009;325:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cushman LJ, Watkins-Chow DE, Brinkmeier ML, et al. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum Mol Genet. 2001;10:1141–1153. [DOI] [PubMed] [Google Scholar]
- 35. Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87:933–963. [DOI] [PubMed] [Google Scholar]
- 36. Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA. 2008;105:2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia-Lavandeira M, Quereda V, Flores I, et al. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One. 2009;4:e4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol. 2009;29:1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dovey HF, John V, Anderson JP, et al. Functional γ-secretase inhibitors reduce β-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. [DOI] [PubMed] [Google Scholar]
- 40. Sastre M, Steiner H, Fuchs K, et al. Presenilin-dependent γ-secretase processing of β-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep. 2001;2:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weng AP, Millholland JM, Yashiro-Ohtani Y, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang H, Zou J, Zhao B, et al. Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proc Natl Acad Sci USA. 2011;108:14908–14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chadwick N, Zeef L, Portillo V, et al. A Identification of novel Notch target genes in T cell leukaemia. Mol Cancer. 2009;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J Biol Chem. 2001;276:30467–30474. [DOI] [PubMed] [Google Scholar]
- 47. Siveke JT, Lubeseder-Martellato C, Lee M, et al. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. [DOI] [PubMed] [Google Scholar]
- 48. Patten BA, Peyrin JM, Weinmaster G, Corfas G. Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J Neurosci. 2003;23:6132–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Budry L, Balsalobre A, Gauthier Y, et al. The selector gene Pax7 dictates alternate pituitary cell fates through its pioneer action on chromatin remodeling. Genes Dev. 2012;26:2299–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rizzoti K, Akiyama H, Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell. 2013;13:419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andoniadou CL, Matsushima D, Mousavy Gharavy SN, et al. Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13:433–445. [DOI] [PubMed] [Google Scholar]
- 52. Jayakody SA, Andoniadou CL, Gaston-Massuet C, et al. SOX2 regulates the hypothalamic-pituitary axis at multiple levels. J Clin Invest. 2012;122:3635–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells. 2012;30:741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moran TB, Goldberg LB, Serviss SL, Raetzman LT. Numb deletion in POMC-expressing cells impairs pituitary intermediate lobe cell adhesion, progenitor cell localization, and neuro-intermediate lobe boundary formation. Mol Endocrinol. 2011;25:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rizzoti K. Adult pituitary progenitors/stem cells: from in vitro characterization to in vivo function. Eur J Neurosci. 2010;32:2053–2062. [DOI] [PubMed] [Google Scholar]
- 56. Kita A, Imayoshi I, Hojo M, et al. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol Endocrinol. 2007;21:1458–1466. [DOI] [PubMed] [Google Scholar]
- 57. Kashiwabara Y, Sasaki S, Matsushita A, et al. Functions of PIT1 in GATA2-dependent transactivation of the thyrotropin β promoter. J Mol Endocrinol. 2009;42:225–237. [DOI] [PubMed] [Google Scholar]
- 58. Suga H, Kadoshima T, Minaguchi M, et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–62. [DOI] [PubMed] [Google Scholar]
- 59. Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. [DOI] [PubMed] [Google Scholar]
- 60. Gage PJ, Roller ML, Saunders TL, Scarlett LM, Camper SA. Anterior pituitary cells defective in the cell-autonomous factor, df, undergo cell lineage specification but not expansion. Development. 1996;122:151–160. [DOI] [PubMed] [Google Scholar]
- 61. Castinetti F, Reynaud R, Saveanu A, Barlier A, Brue T. Genetic causes of combined pituitary hormone deficiencies in humans. Ann Endocrinol (Paris). 2012;73:53–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.