Abstract
The typical life cycle of filamentous fungi commonly involves asexual sporulation after vegetative growth in response to environmental factors. The production of asexual spores is critical in the life cycle of most filamentous fungi. Normally, conidia are produced from vegetative hyphae (termed mycelia). However, fungal species subjected to stress conditions exhibit an extremely simplified asexual life cycle, in which the conidia that germinate directly generate further conidia, without forming mycelia. This phenomenon has been termed as microcycle conidiation, and to date has been reported in more than 100 fungal species. In this review, first, we present the morphological properties of fungi during microcycle conidiation, and divide microcycle conidiation into four simple categories, even though fungal species exhibit a wide variety of morphological differences during microcycle conidiogenesis. Second, we describe the factors that influence microcycle conidiation in various fungal species, and present recent genetic studies that have identified the genes responsible for this process. Finally, we discuss the biological meaning and application of microcycle conidiation.
Keywords: Conidiation, Conidium, Filamentous fungi, Germination, Microcycle
The typical life cycle of filamentous fungi involves conidiation after a period of vegetative growth [1, 2]. Filamentous fungi produce several types of asexual spores, such as macroconidia, microconidia, and chlamydospores. The production of asexual spores is critical in the life cycle of most filamentous fungi, because spores contribute to survival under unfavorable environmental conditions, as a means of dispersal from one site to many potentially viable sites. In any case, spores remain dormant until the environmental conditions become favorable. Dormant spores tend to swell in water that contains ideal nutrients, and produce germ tube(s) following vegetative hyphal growth. Vegetative hyphae (or mycelia) produce asexual reproductive apparatus called conidiophores, which generate conidia when environmental factors limit the vegetative phase [1, 2]. However, several fungal species exhibit an extremely simplified asexual life cycle when subjected to stress conditions, in which the conidia germinate and directly generate further conidia, without forming mycelia [1, 2, 3, 4, 5, 6]. This phenomenon has been defined as microcycle conidiation (MC) [1].
In this review, first, we present the morphological properties of fungi during MC, and divide MC into four basic categories based on the morphological characteristics of conidiogenesis, even though there are a considerable number of morphological differences during microcycle conidiogenesis among fungal species. Second, we describe the factors that influence MC in various fungal species, and present recent genetic studies that have identified the genes responsible for this process. Finally, we discuss the biological meaning and application of MC.
MORPHOLOGICAL CHARACTERISTICS OF MC IN DIFFERENT FUNGAL SPECIES
MC was first reported in 1890 [7], but the term used to describe this phenomenon was first coined in 1971 by Anderson and Smith [1]. MC has been reported in more than 100 fungal species, and has a considerable number of morphological differences [3, 8]. To understand the morphogenesis of MC, we separated MC morphogenesis into four distinct categories.
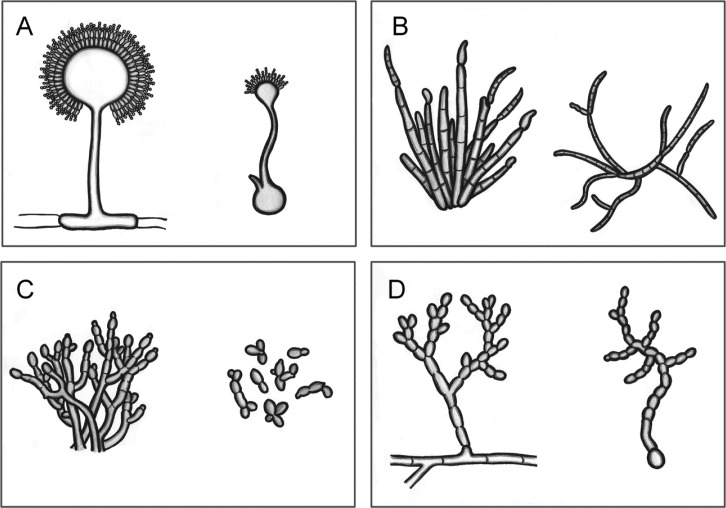
First, the majority of dormant conidia (3.5 µm, mean diameter) in Aspergillus niger swell to 7.0 µm before germtube outgrowth, and produce one to two germ tubes at 30℃ in nutrient-rich deionized water. The proportion of conidia that produce germ tubes gradually decreases from a temperature of 38℃ to 43℃, with the complete inhibition of germ tube formation on conidia at 44℃. At higher temperatures, conidia become large spherical cells (20 µm, mean diameter), and directly produce conidiophores following vesicle formation when the temperature drops to 30℃, without first forming mycelia [1]. When conidia are formed directly, the conidiophores are broader with thicker walls compared to the vegetative mycelium, and bear vesicles with phialides, while the metulae observed in the normal conidiation of this fungus are absent (Fig. 1A). A. flavus and Penicillium cyclopium exhibit similar MC morphological characteristics to A. niger [3, 9].
Fig. 1.
Morphological properties of typical conidiation (left) and microcycle conidiation (right) reported for Aspergillus niger (A), Cercospora zeae-maydis (B), Metarhizium anisopliae (C), and Neurospora crassa (D) [1, 4, 5, 15]. The figures were drawn by Boknam Jung.
The second type of MC is much simpler compared to the first type, and has been reported in the plant pathogenic fungi, Fusarium solani and Cercospora zeae-maydis [4, 10]. The conidia of these fungi also swell, but the level of swelling under conditions that induce MC is similar to the conditions for normal conidia germination. When the conidia are incubated in submerged culture or on water agar, conidiophores arise from the conidial cells or germ tubes. Most conidiophores produce conidia without the specialized phialides that are observed in normal conidiophores, with conidia also being frequently produced from intercalary phialides (Fig. 1B) [4, 10]. The MC of mutants generated from F. verticillioides and F. graminearum belongs to this category [11, 12].
The third type of MC is the simplest form, in which even the development of conidiophores is arrested. The entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana undergo a dimorphic transition in the host integument and hemocoel [13]. Under typical conditions for vegetative growth, fungal conidia produce germ tubes that elongate into hyphae, with new conidia being produced at the tip or sides of the hyphae, without any specialized asexual reproductive apparatus being formed, such as vesicles and metulae [13, 14, 15]. Under MC conditions, while the conidia germinate, the elongation of germ tubes is quickly arrested, after which they directly differentiate into conidia [14, 15]. This rapid differentiation of germ tubes into conidia results in the daughter conidia adjoining with the original conidia (Fig. 1C), and has the appearance of budding yeast.
The fourth type of MC is possibly unique to Neurospora classa. The microcycle conidia produced by the first three types described here are not significantly different to the conidia produced under normal conditions, although the pigmentation and size of the conidia are slightly different. However, the conidia produced by the fourth type of MC are very different to those produced during typical conidiation. In this type, the germ tubes multiply septated, with each cell rapidly enlarging and thickening. Eventually, fragmentation occurs to form a chain of conidia (Fig. 1D).
FACTORS INFLUENCING MC
MC has been proposed as a survival mechanism of fungi when unfavorable environmental conditions are encountered [4, 8]. Therefore, most MC is induced under stress conditions. Higher temperature inhibits the normal germination of conidia, and induces the formation of conidiophores that generate conidia in A. niger [1, 16]. In addition, glutamate is present at higher temperatures, which accelerates the initiation of conidiophore formation in this fungus [1]. Conidiophores that are produced from conidia have shorter stalk lengths and contain fewer pigments compared to normal conidiophores, but the conidia produced through MC are successfully germinated [1]. Glucose is required for the conidia to swell. Consequently, the absence of glucose from the medium decreases MC in A. niger and P. cyclopium [1, 9]. Another important factor that triggers MC is the change in pH during the first cultivation, which is probably caused by the faster incorporation of NH4+ or glutamate amino group. The buffering of the medium inhibits microcycle development [9]. The manipulation of glutamate and glucose levels in the medium efficiently inhibits the apical growth of P. cyclopium to induce MC. However, this process is independent of heat stress [9].
In the plant pathogenic fungus C. zeae-maydis, MC does not occur on the leaf surface, which might contain carbohydrates and amino compounds. Ammonium salts and hydrogen peroxide also suppress MC, although other nutrients do not affect MC, including amino acids, nitrate salts, and simple sugars [4]. Unlike C. zeae-maydis, the obligate biotrophic plant pathogens powdery mildew fungi conduct MC on the surface of host plants, where only 0.5% to 4% of germinated conidia conduct MC [6, 17]. This observation indicates that MC induction is not conserved among plant pathogens.
Fungal species belonging to the genus Colletotrichum also conduct MC on solid media, with the process possibly arising from a high-density conidial inoculum, regardless of heat stress [18, 19]. In this group of species, the MC process is characterized by accelerated development of short conidiophores across the inoculation site that support the production of a gelatinous mass of slime spores [18, 19, 20]. The high nutrient concentration promotes vegetative growth, and overrides the induction of MC metabolites, while nutrient-limited conditions sufficiently reduce vegetative growth and induce MC [19]. Secondary conidia in plant pathogenic fungi are efficiently produced in a water droplet, and are easily detached and dispersed. This observation indicates that MC may represent a viable strategy for producing new inoculums on nontarget plants or on plant surfaces [4, 20]. In addition, in plant pathogenic fungi, MC is closely related to the defense response of hosts, although secondary conidia produced through MC are viable and penetrate plant tissues [21].
In the entomopathogenic fungus B. bassiana, MC is efficiently induced when nitrogen sources reach the limitation level, which restricts vegetative growth. This observation indicates that fungal conidia contain endogenous nitrogen reserves for the synthesis of proteins required for MC [2]. In this fungus, carbon sources are not necessary for the induction of MC, because fungal strains produce MC, even in the complete absence of carbon sources [2]. However, carbon sources (such as glucose) are required for the conidia to swell, as shown in A. niger. When glucose concentrations are high, the conidia swell, with the germ tubes becoming longer and more vacuolated compared to those in the absence of glucose. Under glucose-poor conditions, the germination and microcycle processes of the swelling conidia are slower [2]. In the entomophathogenic fungi B. bassiana and M. acridum, heat stress does not induce MC, whereas the presence of glutamate induces vegetative growth, indicating that it is used as a nitrogen source [2, 14, 15].
MC might be a common phenomenon in filamentous fungi. However, MC has only been confirmed in a limited number of fungal species to date. This discrepancy exists because there is no unique set of environmental conditions that induce MC in most fungi. Consequently, MC might not have been observed in many other fungal species yet.
GENETIC STUDIES ON MC
Even though MC was first observed in 1890 [7] and has been reported in more than 100 fungal species, the molecular mechanisms underlying this process remain limited. The DNA of normal germinating conidia and MC is synthesized differently, whereby there is a sharp and premature resumption of DNA synthesis during MC [22]. The synthesis of new mRNA is also required for the initiation of MC. An inhibitor of mRNA synthesis, α-amanitin, which does not interfere with germination or germ tube formation, suppresses MC in C. zeae-maydis [4].
Some wild isolates of N. crassa produce three types of spores, blastoconidia, arthroconidia, and microconidia through MC. Two genes, mcb and mcm, that control the specific patterns of MC were first identified in N. crassa using a conventional genetic approach. The single gene mcb, which is epistatic to mcm, controls blastoconidiation, while mcm controls both microconidiation and arthroconidiation, depending on the temperature of the culture [5].
To identify genes related to MC, suppression subtractive hybridization has been performed during MC vs. the normal conidiation of the hyphae of M. anisopliae [14]. This study identified 221 uniquely expressed sequence tags in MC, which belonged to various cellular processes, including protein synthesis, energy production, the cell cycle, general metabolism, DNA processing, cellular transport, transcription, signal transduction, and the stress response. This observation indicates that MC requires new differentiation from normal conidial germination. Of the genes involved in this process, one gene, mmc, showed specifically higher expression during MC in M. anisopliae. This gene is a homolog of the circadian clock controlled gene ccg-6 protein identified in N. crassa [23] and of a regulator of a G protein signaling gene, cag8, which regulates normal conidiation in M. anisopliae [24]. The down regulation of mmc through RNAi blocks MC and delays conidiation, but does not influence normal conidial germination. This observation indicates that this gene specifically controls MC and positively influences the speed of conidiation in this fungus [25].
Previous studies have indicated that the wild isolates of several species belonging to the genus Fusarium, including F. verticillioides and F. graminearum, do not conduct MC. However, MC may be induced in these fungi by the deletion of a certain gene, indicating that MC in these fungi is suppressed under natural conditions. The velvet gene, veA, is an important global regulator for asexual and sexual development in filamentous fungi [11, 26, 27, 28]. In F. verticillioides, the deletion of veA suppresses the formation of aerial hyphae and reduces the hydrophobicity of the hyphal surface. In addition, the mutant strains of this fungus alter the production ratio of macroconidia and microconidia, and significantly induce MC [11]. veA-mediated MC has only been reported for F. verticillioides, even though veA influences filamentous growth and conidiation is similar among F. verticillioides and Aspergillus spp. [11, 26, 27, 28]. This observation indicates that the mechanisms of MC differ among fungal species.
Fusarium graminearum presents another example of MC induction through genetic manipulation. In A. nidulans, the wetA gene activates spore-specific gene expression, and is required for spore maturation [29]. The deletion of the wetA homolog in F. graminearum results in pleiotrophic phenotypic changes [12]. The deletion mutants produce fewer conidia, which are longer with fewer septa, compared to the wild-type strain. These conidia are sensitive to both oxidative and heat stress. The most distinct phenotypic change in F. graminearum wetA mutants compared to those reported for other fungal species is the induction of MC that is dependent on autophagy [12].
Even though MC has attracted the interest of many researchers over the last century, few genes related to MC have been characterized. To date, most research on MC has been focused on the characterization of morphological and physiological properties, and the optimization for MC induction. Detailed molecular mechanisms of normal conidiation have been elucidated for many filamentous fungi, including those belonging to the genera Aspergillus, Penicillium, Neurospora, and Fusarium. Genes related to normal conidiogenesis are also closely related to MC. Therefore, the reexamination of known genes related to conidiogenesis might provide important clues for elucidating the mechanisms involved in MC.
BIOLOGICAL SIGNIFICANCE AND APPLICATION OF MC
It is obvious that MC provides significant advantages to some fungal species that are able to induce MC in nature. First, the process contributes to the survival strategy of spores that encounter unfavorable environmental conditions. The spores of many filamentous fungal species simply germinate when the minimum requirements are met, such as a certain level of humidity over a broad spectrum of temperatures. Microcycle conidia produced by M. acridum are more thermo tolerant, because more trehaloase is accumulated in the cells compared to normal aerial conidia, even though there are no significant differences against UV tolerance [15], supporting the advantage of MC in nature.
Another advantage of MC occurs in plant pathogens that only infect plants under a limited range of conditions. Many plant pathogenic fungi have a narrow spectrum of hosts, and must infect the correct hosts at germination. When the conidia germinate on the surface of non-hosts or on non-edible places, they must quickly move to the correct places for survival. Consequently, quick sporulation through MC using minimal nutrient requirements helps the fungi to quickly escape such unfavorable conditions. MC is also a good strategy for disease dispersal, because a large amount of primary propagules is produced. In pathogens, such as C. zeae-maydis, C. beticola, B. bassiana, and M. acridum, microcycle conidia penetrate hosts as efficiently as the original primary conidia. This observation indicates that microcycle conidia may serve as an important source of inoculums [2, 4, 15, 21].
It is interesting that MC is normally suppressed in many fungi, such as F. verticillioides and F. graminearum [11, 12]. In these fungi, only mutant strains that carry a certain gene deletion induce MC, indicating that these fungi retain the ability of MC, even though it has not been recorded under laboratory or natural conditions. The retention of MC mechanisms by these fungi indicates that MC continues to have biological meaning in these fungi in nature. Therefore, the evolutionary context and fitness costs of this process in nature need to be studied.
The genera Aspergillus and Penicillium have been widely used for food fermentation processes and the production of secondary metabolites. The entomopathogenic fungi B. bassiana and M. acridum have been developed as control agents against insects [13, 14, 15, 30]. In addition, several Trichoderma spp. exhibit strong antagonistic effects against plant pathogens, which has led to the development of biological control agents. The mass production of uniformed conidia is prerequisite for industrial fermentation or biological control agents. In this context, MC represents one of the best strategies for produce large quantities of uniformed conidia. However many more studies are required to elucidate the molecular mechanisms of the MC process, with the genetic manipulation of specific genes related to MC being essential for the utilization of MC.
ACKNOWLEDGEMENTS
This work was supported by the Dong-A University research fund.
References
- 1.Anderson JG, Smith JE. The production of conidiophores and conidia by newly germinated conidia of Aspergillus niger (microcycle conidiation) J Gen Microbiol. 1971;69:185–197. doi: 10.1099/00221287-69-2-185. [DOI] [PubMed] [Google Scholar]
- 2.Bosch A, Yantorno O. Microcycle conidiation in the entomopathogenic fungus Beauveria bassiana bals. (vuill.) Process Biochem. 1999;34:707–716. [Google Scholar]
- 3.Ahearm DG, Price D, Simmons RB, Mayo A, Zhang ST, Crow SA., Jr Microcycle conidiation and medusa head conidiophores of aspergilli on indoor construction materials and air filters from hospitals. Mycologia. 2007;99:1–6. doi: 10.3852/mycologia.99.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Lapaire CL, Dunkle LD. Microcycle conidiation in Cercospora zeae-maydis. Phytopathology. 2003;93:193–199. doi: 10.1094/PHYTO.2003.93.2.193. [DOI] [PubMed] [Google Scholar]
- 5.Maheshwari R. Microcycle conidiation and its genetic basis in Neurospora crassa. J Gen Microbiol. 1991;137:2103–2115. doi: 10.1099/00221287-137-9-2103. [DOI] [PubMed] [Google Scholar]
- 6.Pintye A, Legler SE, Kiss L. New records of microcyclic conidiogenesis in some powdery mildew fungi. Mycoscience. 2011;52:213–216. [Google Scholar]
- 7.Barclay A. On the life history of a Himalayan Gymnosporangium (G. cunninghamianum n. sp.) Sci Mem Med Off Army India. 1890;5:71–78. [Google Scholar]
- 8.Hanlin RT. Microcycle conidiation: a review. Mycoscience. 1994;35:113–123. [Google Scholar]
- 9.Pazout J, Schröder P. Microcycle conidiation in submerged cultures of Penicillium cyclopium attained without temperature changes. J Gen Microbiol. 1988;134:2685–2692. doi: 10.1099/00221287-134-10-2685. [DOI] [PubMed] [Google Scholar]
- 10.Kølmark HG. Mutants with continuous microcycle conidiation in the filamentous fungus Fusarium solani f. sp. pisi. Mol Gen Genet. 1984;198:12–18. [Google Scholar]
- 11.Li S, Myung K, Guse D, Donkin B, Proctor RH, Grayburn WS, Calvo AM. FvVE1 regulates filamentous growth, the ratio of microconidia to macroconidia and cell wall formation in Fusarium verticillioides. Mol Microbiol. 2006;62:1418–1432. doi: 10.1111/j.1365-2958.2006.05447.x. [DOI] [PubMed] [Google Scholar]
- 12.Son H, Kim MG, Min K, Lim JY, Choi GJ, Kim JC, Chae SK, Lee YW. WetA is required for conidiogenesis and conidium maturation in the ascomycete fungus Fusarium graminearum. Eukaryot Cell. 2014;13:87–98. doi: 10.1128/EC.00220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendland JC, Hung SY, Boucias DG. Evasion of host defense by in vivo-produced protoplast-like cells of the insect mycopathogen Beauveria bassiana. J Bacteriol. 1993;175:5962–5969. doi: 10.1128/jb.175.18.5962-5969.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Xia Y. Identification of genes preferentially expressed during microcycle conidiation of Metarhizium anisopliae using suppression subtractive hybridization. FEMS Microbiol Lett. 2008;286:71–77. doi: 10.1111/j.1574-6968.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Peng G, Xia Y. Microcycle conidiation and the conidial properties in the entomopathogenic fungus Metarhizium acridum on agar medium. Biocontrol Sci Technol. 2010;20:809–819. [Google Scholar]
- 16.Sekiguchi J, Gaucher GM, Costerton JW. Microcycle conidiation in Penicillium urticae: an ultrastructural investigation of conidiogenesis. Can J Microbiol. 1975;21:2069–2083. doi: 10.1139/m75-296. [DOI] [PubMed] [Google Scholar]
- 17.Kiss L, Pintye A, Zséli G, Jankovics T, Szentiványi O, Hafez YM, Cook RT. Microcyclic conidiogenesis in powdery mildews and its association with intracellular parasitism by Ampelomyces. Eur J Plant Pathol. 2010;126:445–451. [Google Scholar]
- 18.Lingappa BT, Lingappa Y. Role of auto-inhibitors in mycelia growth and dimorphism of Glomerella cingulata. J Gen Microbiol. 1969;56:35–45. doi: 10.1099/00221287-56-1-35. [DOI] [PubMed] [Google Scholar]
- 19.Slade SJ, Harris RF, Smith CS, Andrews JH. Microcycle conidiation and spore-carrying capacity of Colletotrichum gloeosporioides on solid media. Appl Environ Microbiol. 1987;53:2106–2110. doi: 10.1128/aem.53.9.2106-2110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boosalis MG. Precocious sporulation and longevity of conidia of Helminthosporium sativum in soil. Phytopathology. 1962;52:1172–1177. [Google Scholar]
- 21.Rathaiah Y. Stomatal tropism of Cercospora beticola in sugarbeet. Phytopathology. 1977;67:358–362. [Google Scholar]
- 22.Grange F, Turian G. Differential deoxyribonucleic acid synthesis during microcycle conidiation in Neurospora crassa. Arch Microbiol. 1978;119:257–261. doi: 10.1007/BF00405404. [DOI] [PubMed] [Google Scholar]
- 23.Bell-Pedersen D, Shinohara ML, Loros JJ, Dunlap JC. Circadian clock-controlled genes isolated from Neurospora crassa are late night- to early morning-specific. Proc Natl Acad Sci U S A. 1996;93:13096–13101. doi: 10.1073/pnas.93.23.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang W, Pei Y, Bidochka MJ. A regulator of a G protein signaling (RGS) gene, cag8, from the insect-pathogenic fungus Metarhizium anisopliae is involved in conidiation, virulence and hydrophobin synthesis. Microbiology. 2007;153:1017–1025. doi: 10.1099/mic.0.2006/002105-0. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Cao Y, Xia Y. Mmc, a gene involved in microcycle conidiation of the entomopathogenic fungus Metarhizium anisopliae. J Invertebr Pathol. 2010;105:132–138. doi: 10.1016/j.jip.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Calvo AM, Bok J, Brooks W, Keller NP. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl Environ Microbiol. 2004;70:4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato N, Brooks W, Calvo AM. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot Cell. 2003;2:1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HS, Han KY, Kim KJ, Han DM, Jahng KY, Chae KS. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet Biol. 2002;37:72–80. doi: 10.1016/s1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 29.Marshall MA, Timberlake WE. Aspergillus nidulans wetA activates spore-specific gene expression. Mol Cell Biol. 1991;11:55–62. doi: 10.1128/mcb.11.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khurana N, Saxena RK, Gupta R, Kuhad RC. Light-independent conidiation in Trichoderma spp.: a novel approach to microcycle conidiation. World J Microbiol Biotechnol. 1993;9:353–356. doi: 10.1007/BF00383079. [DOI] [PubMed] [Google Scholar]