Abstract
From April 2009 to October 2011, we surveyed the higher fungi in the Byeonsanbando National Park, Korea. In total, we identified 2 kingdoms, 3 divisions, 7 classes, 22 orders, 63 families, 149 genera, and 313 species (including 6 undocumented taxa: 2 families, 2 genera, and 2 species). Seventeen 17 orders, 49 families, 128 genera, and 286 species belonged to Basidiomycota; 7 orders, 9 families, 15 genera, and 21 species were of Ascomycota; and 4 orders, 5 families, 6 genera, and 6 species of primordial fungi. Among the Basidiomycota, Agaricomycetes were represented by 47 families, 126 genera, and 282 species. The most common fungi were Boletaceae (33 species), followed by Russulaceae (30), Agaricaceae (27), and Amanitaceae (24). Various species of most of the higher fungi occurred during periods with average temperatures of 23~24.9℃, maximum temperatures of 28~31.9℃, minimum temperatures of 22~23.9℃, > 82% relative humidity, and > 200 mm precipitation.
Keywords: Boletaceae, Byeonsanbando National Park, Ectomycorrhizal fungi, Higher fungi, Russulaceae
Byeonsanbando National Park spans 4 myeons (townships) in Buan-gun, Jeollabuk-do, Korea. The park is located in a relatively low mountainous area at <500 m altitude, where pine trees are widely distributed throughout the area. Pinus thunbergii, P. rigida, Quercus variabilis, Q. serrata, and Carpinus laxiflora are dominant, providing various habitats for higher fungi.
Most of the higher fungi belong to Basidiomycota and Ascomycota, although their ecotypes and habitats are different. Some of these fungi are parasitic pathogens that damage trees, but they also play a role as decomposers of plants, including leaves and timber, which is a crucial ecosystem function in breaking down organic matter and supplying various nutrients to trees while maintaining symbiotic relationships with higher plants [1].
For ectotrophic mycorrhizal fungi that live together with trees, species organization and numbers are greatly affected by anthropogenic factors, including logging [2, 3] and pollution [4], by natural factors, including drought [5] and soil temperature change [6], and by location factors such as microclimate, forest cover rate, stand density index, and the number of dead trees [7, 8, 9, 10, 11].
Here, we surveyed the distribution of higher fungi in Byeonsanbando National Park by altitude and time of year to provide basic data for biodiversity conservation and to clarify the effects of climate factors on the distribution of higher fungi.
Materials and Methods
Study period
We conducted 52 surveys between April 2009 and October 2011 (20 in 2009, 14 in 2010, and 18 in 2011). The surveyed area (126° 37'40" to 126° 44'20" E, 34° 21'40" to 34° 47'20" N) followed 2 main hiking paths: 1) Naebyeonsan hiking center to Jaebaeki gogae, Gwaneumbong fork, Naesosa, Gulbawui, Gamaso fork, Sebong fork, and Naesosa, and 2) Namyeochi park center to Ssangsunbong, Tower of environment protection, and Naebyeonsan hiking center. These hiking trails were assumed to be favorable grounds to support the growth of higher fungi. By using the road transect method, we surveyed 10 m on each side of the trails (Fig. 1).
Fig. 1.
Map of surveyed area. A indicated Byensanbando National Park.
Collection
Because all fungi changed their shapes ontogenetically during the survey, we collected both young and mature fruit bodies. The collected mushrooms were carefully sealed in envelopes and transferred to the lab after noting the location and date of collection and their habitats. For species that were difficult to categorize, we used Melzer fluid, assessed the chemical reaction to KOH or Guaiacol, and visualized them under a microscope to observe the basidium, spores, and cystidia. Fungi were initially categorized into Aphyllophorales [12] and Agaricales [13], and the final categorization followed CABI's Index Fungorum (http://www.indexfungorum.org/).
Climate and data analyses
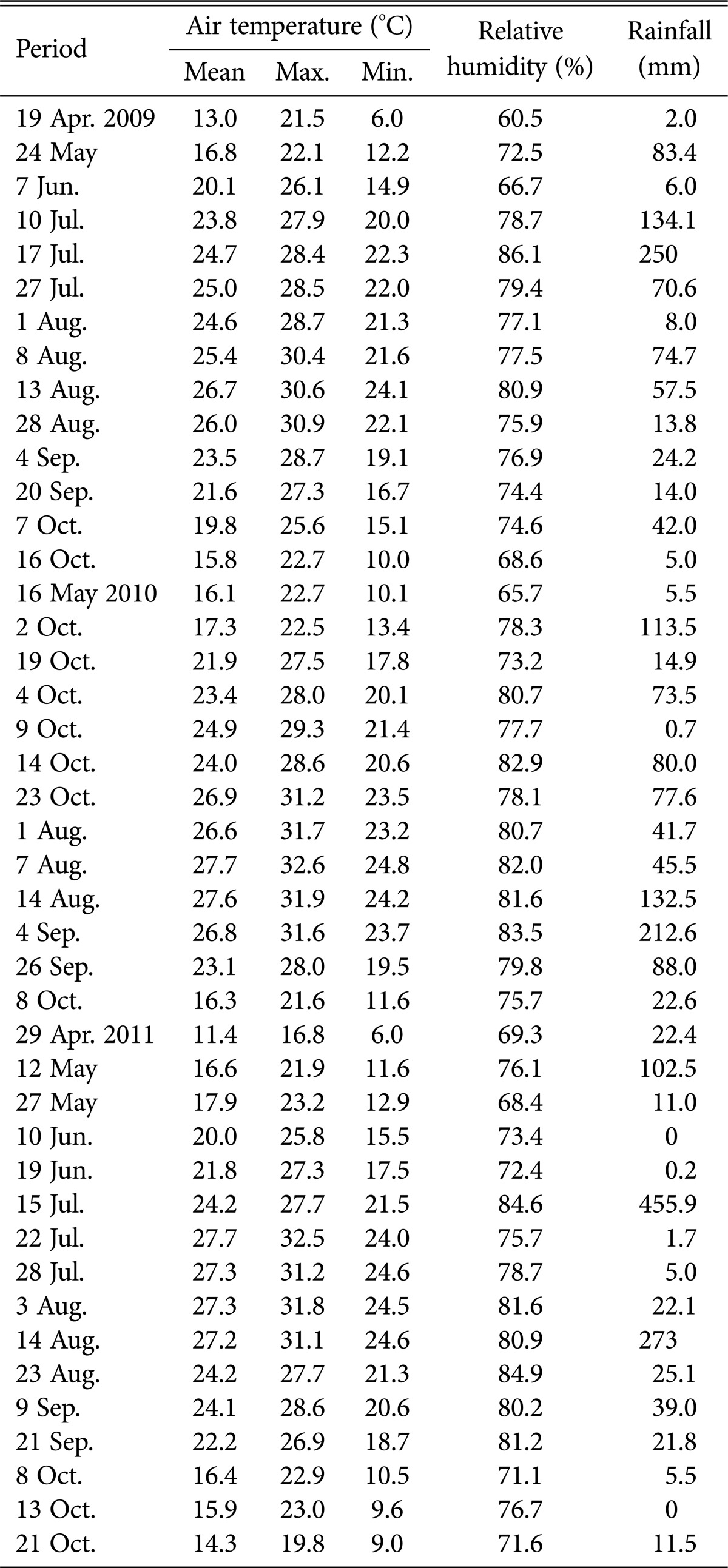
Climatic data from the Buan Weather Station were used, and the average climatic data during the research period (from the day after the survey to the day of survey) were used (Table 1). Fungi that occurred during the survey period were categorized into 5 phases of climatic factors (temperature [mean, maximum, and minimum], humidity, and precipitation). ANOVA was conducted to identify differences between species occurrence by environment, which were then compared using Duncan's test ver. 12.0K (SPSS Inc., Chicago, IL, USA).
Table 1.
Climatic data in Buan city from April 2009 to October 2011
RESULTS AND DISCUSSION
Diversity of higher fungi
In total, we identified 2 kingdoms, 3 divisions, 7 classes, 22 orders, 63 families, 149 genera, and 313 species (including 6 undocumented taxa: 2 families, 2 genera, and 2 species). Details are provided in Table 2.
Table 2.
List of higher fungi collected from 2004 to 2006 in Byeonsanbando National Park
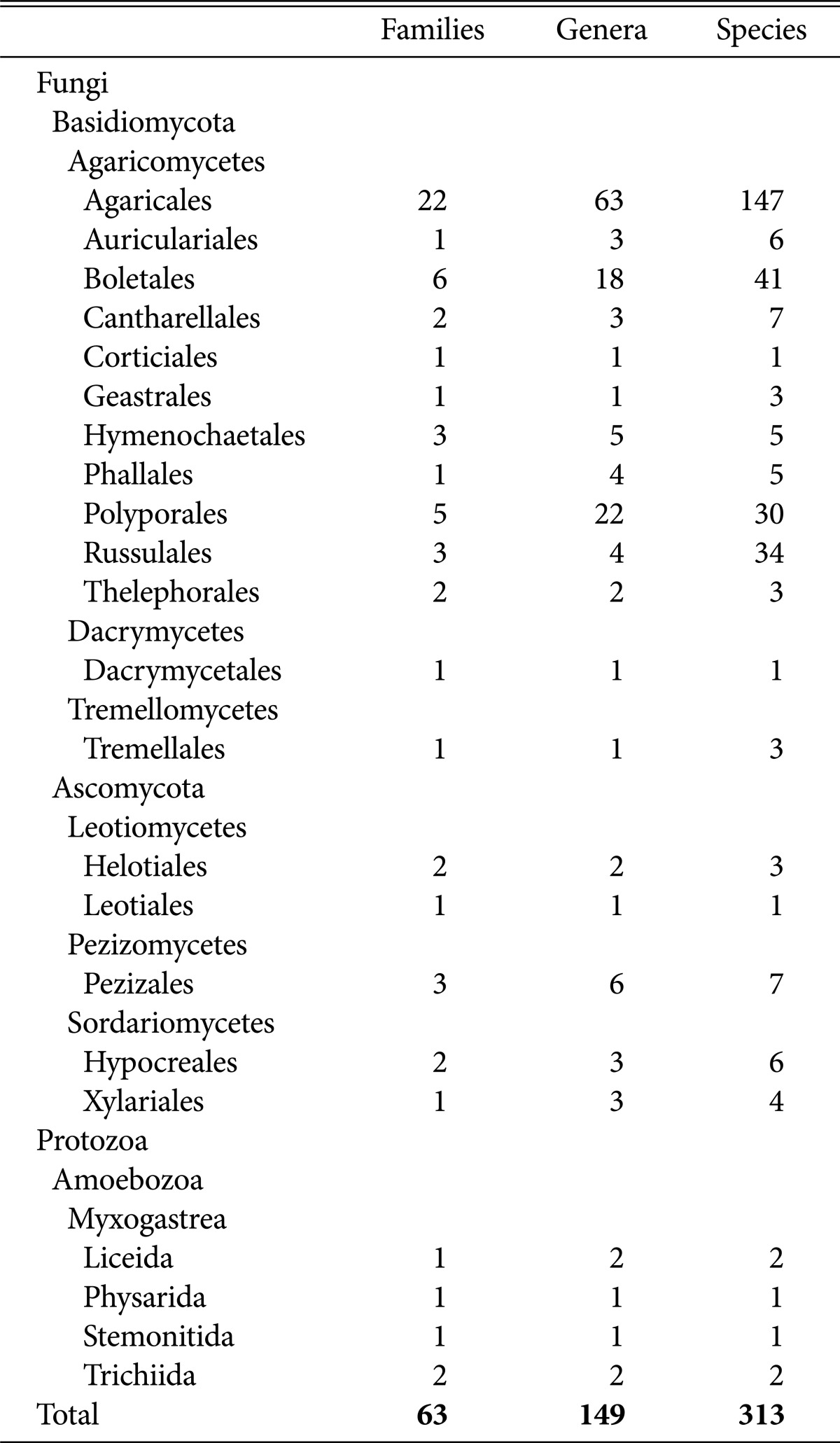
We found that 17 orders, 49 families, 128 genera, 286 species belonged to Basidiomycota; 7 orders, 9 families, 15 genera, and 21 species were of Ascomycota; and 4 orders, 5 families, 6 genera, and 6 species of primordial fungi. Among the Basidiomycota, 11 orders, 47 families, 126 genera, and 282 species belonged to Agaricomycetes; 1 order, 1 family, 1 genus, and 1 species belonged to Dacrymycetes; and 1 order, 1 family, 1 genus, and 3 species were Tremellomycetes. Among the Ascomycota, 2 orders, 3 families, 3 genera, and 4 species were identified as Leotiomycetes; 1 order, 3 families, 6 genera, and 7 species were Pezizomycetes; and 2 orders, 3 families, 6 genera, and 10 species belonged to Sordariomycetes, which implies that most of the higher fungi belonged either to Agaricomycetes of Basidiomycota.
Agaricales included 22 families, 63 genera, and 147 species; Boletales included 6 families, 18 genera, and 41 species; Russullales included 3 families, 4 genera, and 34 species; and Polyporales included 5 families, 22 genera, and 30 species. Together, the 4 orders included 252 species, accounting for 80.5% of the total species surveyed. We found 33 species of Boletaceae, 30 Russulaceae, 27 Agaricaceae, and 24 Amanitaceae.
Among the higher fungi, ectomycorrhizal mushrooms included 13 families, 26 genera, and 117 species (37.4%); leaf and wood-rotting fungi included 36 families, 78 genera, and 122 species (39.0%); ground fungi included 20 families, 42 genera, and 67 species (21.4%); and others included 3 families, 4 genera, and 7 species (2.2%). A previous study reported that out of 131 genera and 274 species of higher fungi found on Mt. Chiak, ectomycorrhizal mushrooms represented 32.7%, 63% were saprophyte parasites, and 4% were parasitic fungi [14]. The occurrence of ectomycorrhizal mushrooms was higher in our survey, but lower than that in a study on higher fungi on Mt. Odae, which showed that out of 281 species, 49.1% were ectomycorrhizal, 49.5% were saprophyte parasites, and 1.4% were parasitic fungi [15].
Distribution of higher fungi by survey period
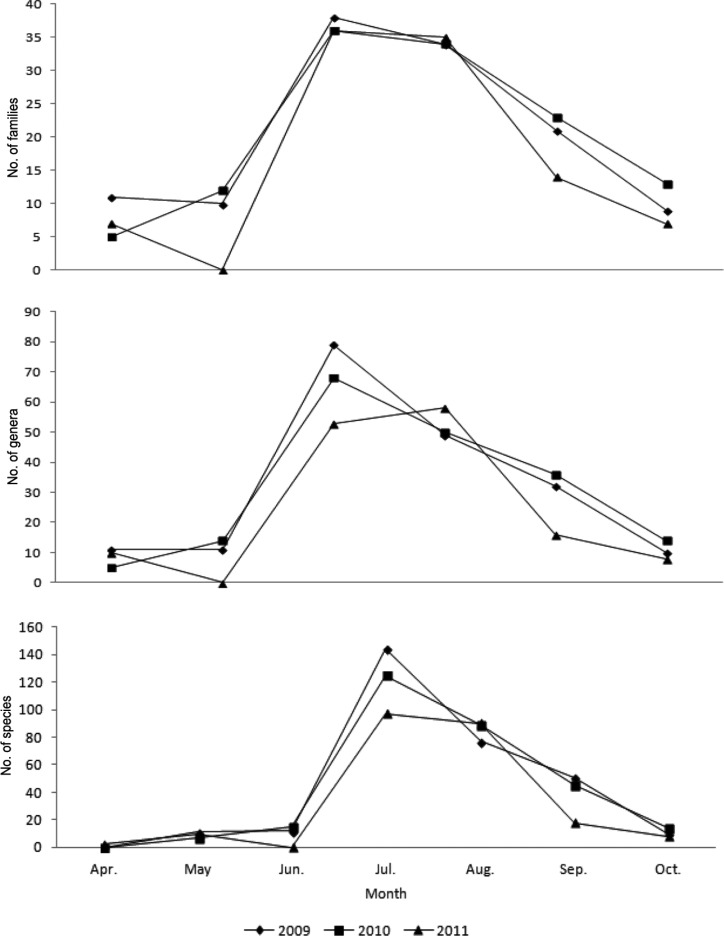
Annual occurrences of higher fungi (Figs. 2 and 3) were highest in 2009, with 53 families, 120 genera, and 223 species, followed by 2011 (51 families, 103 genera, 184 species) and 2010 (46 families, 90 genera, 176 species).
Fig. 2.
The number of higher fungi during the surveying periods in Byeonsanbando National Park.
Fig. 3.
The number of species of higher fungi according to the month in Byeonsanbando National Park.
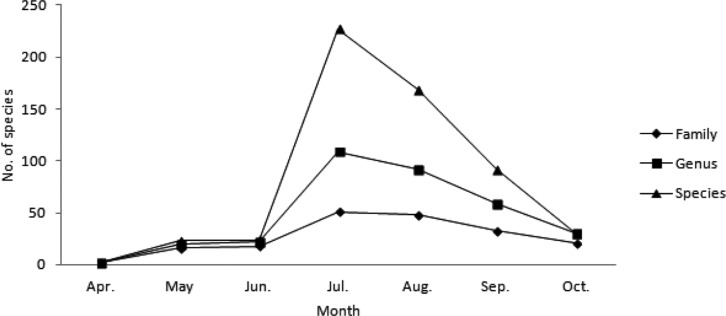
Among the survey periods, the highest number of fungi was recorded in July 2009, with 38 families, 79 genera, and 144 species, followed by July 2010 (36 families, 68 genera, 125 species) and July 2011 (36 families, 53 genera, 97 species); no fungi were observed in April 2009 and 2010 and June 2011. When the data were categorized by month (Fig. 3), the highest numbers were recorded in July (51 families, 109 genera, 227 species), followed by August (48 families, 92 genera, 169 species), September (33 families, 59 genera, 92 species), and April (2 families, 2 genera, 2 species). These results suggest that climate factors, including temperature, humidity, and precipitation, affected the occurrence of higher fungi, similar to reports on changes in ectomycorrhizal fungi group structure being affected by climate change [16, 17, 18].
Comparing dominant fungi by months (Table 3), 5 families (Agaricaceae, Amanitaceae, Boletaceae, Polyporaceae, Russulaceae) occurred from July to September; in particular, Boletaceae and Russulaceae were represented by over 30 species, making these families more dominant than other fungi. This was similar to a report that among higher fungi found on Mt. Naejang, Boletaceae, Russulaceae, and Polyporaceae were represented by many different species [19].
Table 3.
Distribution of species of higher fungi during the surveying periods in Byeonsanbando National Park
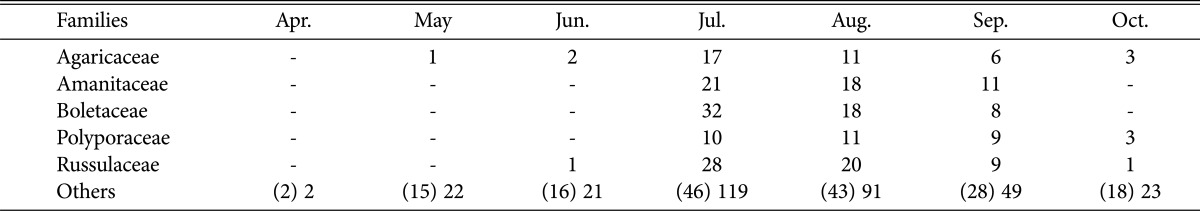
Numbers in parentheses are presented as numbers of families.
Categorization of higher fungi by altitude
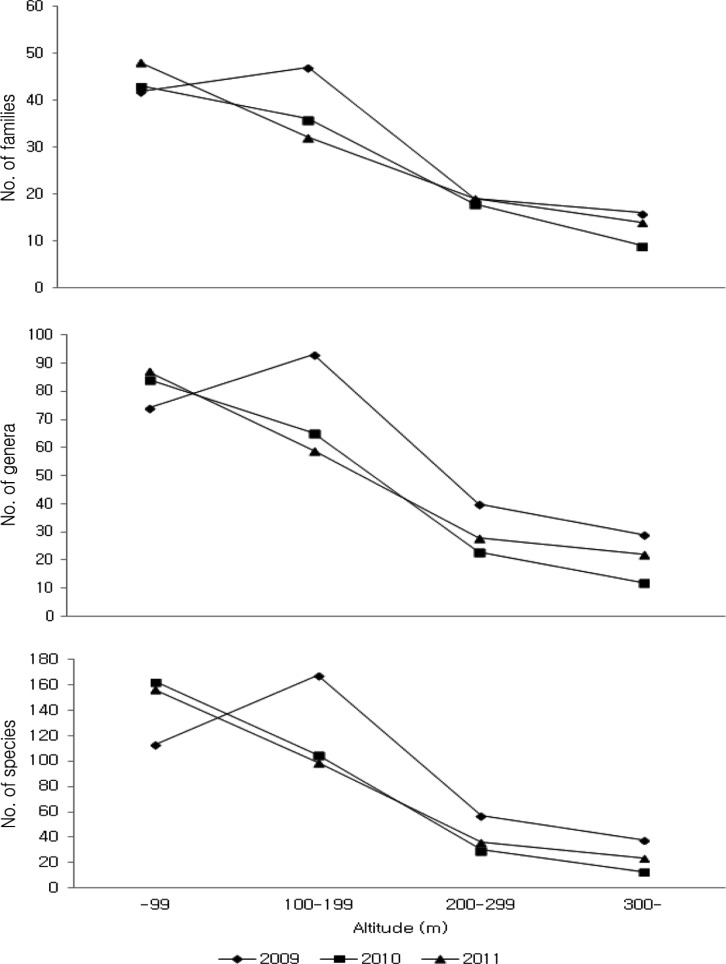
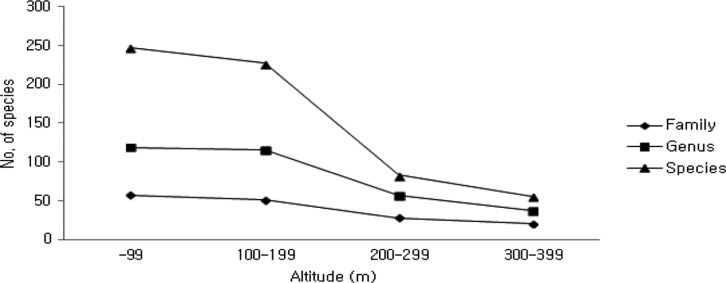
Comparing the distribution of higher fungi by altitude (Fig. 4), we recorded the highest number (47 families, 93 genera, 167 species) at 100~199 m altitude in 2009; the smallest number (9 families, 12 genera, 13 species) was recorded at > 300 m altitude in 2010. Combining all results (Fig. 5), most occurrences were recorded at 0~99 m (57 families, 119 genera, 247 species), followed by 100~199 m (51 families, 116 genera, 227 species), 200~299m (28 families, 57 genera, 83 species), and > 300 m (20 families, 37 genera, 56 species), implying that with increasing altitude, the number of species decreases. These results corroborate a previous report on declining richness and diversity of species with increased altitude [20].
Fig. 4.
The number of higher fungi according to the altitude during the surveying periods in Byeonsanbando National Park.
Fig. 5.
The number of species of higher fungi according to the altitude in Byeonsanbando National Park.
When examining the distribution of dominant fungi by altitude (Table 4), we found that the occurrences of Agaricaceae, Amanitaceae, Boletaceae, Polyporaceae, and Russulaceae were higher than those of the other taxa in most regions. In particular, the occurrence of Polyporaceae was relatively higher in areas > 300 m in altitude, whereas that of the other dominant fungi decreased sharply. This result suggests that the Polyporaceae are less affected by changes in climate and soil nutrition related to higher altitude than other fungi are. Previous research has demonstrated that the group structure of ectomycorrhizal fungi is affected by factors such as climate condition [21] and soil nutrition [22].
Table 4.
Distribution of species of higher fungi according to the altitude in Byeonsanbando National Park
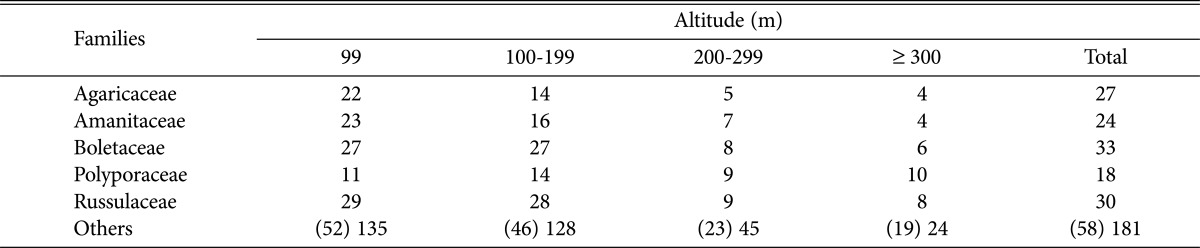
Numbers in parentheses are presented as numbers of families.
Distribution of climatic factors
The distribution of fungal habitats by climatic factors is presented in Tables 5, 6, 7, 8, 9.
Table 5.
Duncan's multiple range test between mean air temperature and species of higher fungi according to habitat environmental characteristics
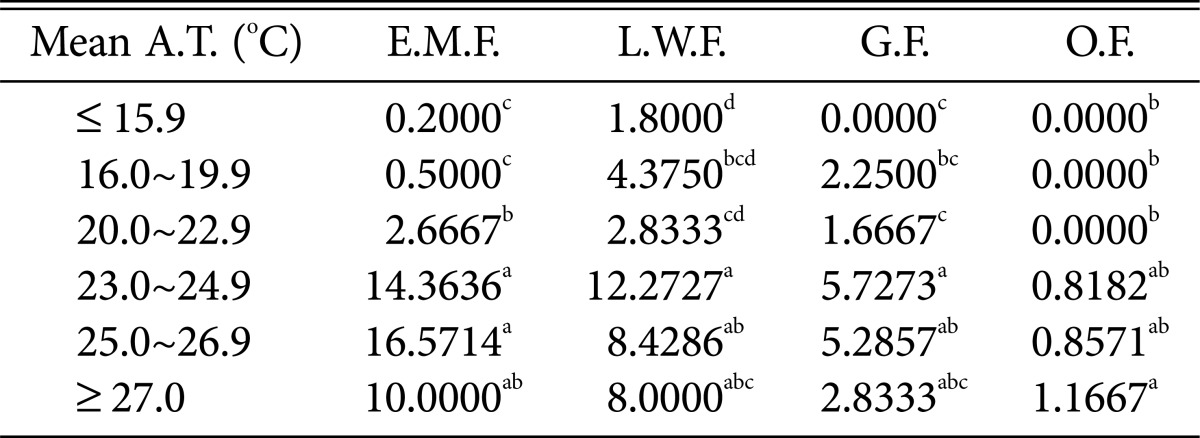
Mean A.T., mean air temperature; E.M.F., ectomycorrhizal fungi; L.W.F., litter decomposing and wood rotting fungi; G.F., grounding fungi; O.F., others fungi.
a-dThe mean difference is significant at the 0.05 level.
Table 6.
Duncan's multiple range test between maximum air temperature and species of higher fungi according to habitat environmental characteristics
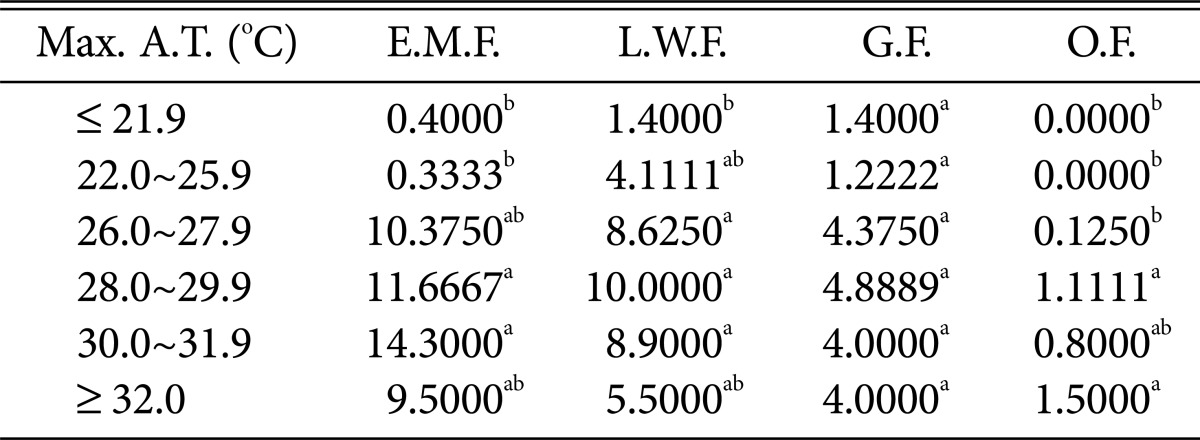
Max. A.T., maximum air temperature; E.M.F., ectomycorrhizal fungi; L.W.F., litter decomposing and wood rotting fungi; G.F., grounding fungi; O.F., others fungi.
a,bThe mean difference is significant at the 0.05 level.
Table 7.
Duncan's multiple range test between minimum air temperature and species of higher fungi according to habitat environmental characteristics
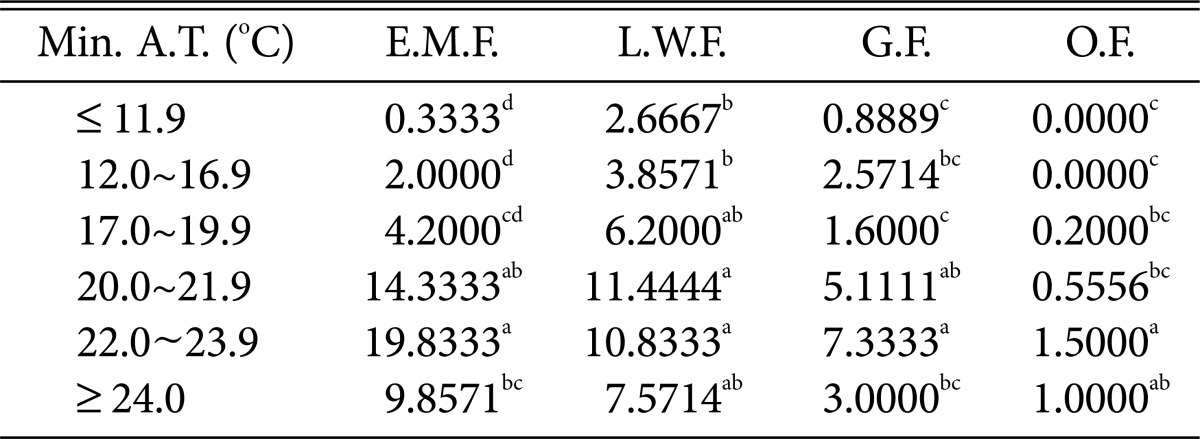
Min. A.T., minimum air temperature; E.M.F., ectomycorrhizal fungi; L.W.F., litter decomposing and wood rotting fungi; G.F., grounding fungi; O.F., others fungi.
a-dThe mean difference is significant at the 0.05 level.
Table 8.
Duncan's multiple range test between relative humidity and species of higher fungi according to habitat environmental characteristics
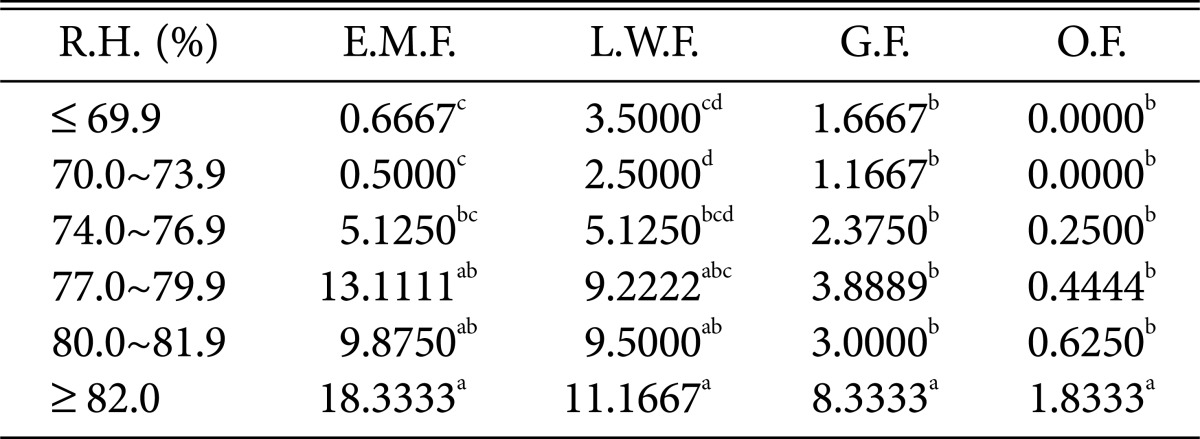
R.H., relative humidity; E.M.F., ectomycorrhizal fungi; L.W.F., litter decomposing and wood rotting fungi; G.F., grounding fungi; O.F., others fungi.
a-dThe mean difference is significant at the 0.05 level.
Table 9.
Duncan's multiple range test between Rainfall and species of higher fungi according to habitat environmental characteristics
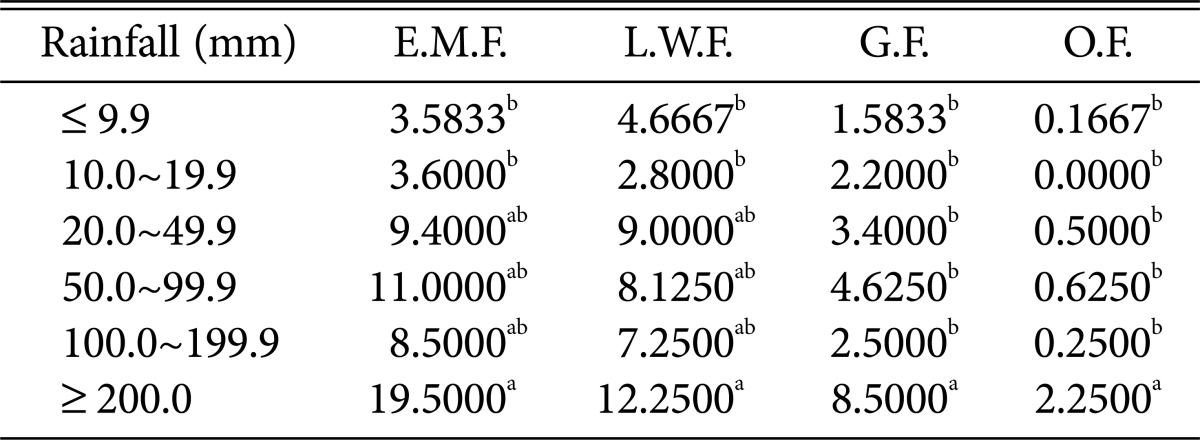
E.M.F., ectomycorrhizal fungi; L.W.F., litter decomposing and wood rotting fungi; G.F., grounding fungi; O.F., others fungi.
a,bThe mean difference is significant at the 0.05 level.
Most fungi, including ectomycorrhizal mushrooms, wood-rotting fungi, and ground fungi, were prevalent at mean temperatures of 23.0~24.9℃ (Table 5); in particular, ectomycorrhizal mushrooms were the most prevalent at 25.0~26.9℃. The occurrence of most fungi decreased above 27.0℃.
Regarding the maximum temperature (Table 6), all fungi, including ectomycorrhizal mushrooms, wood-rotting fungi, and ground fungi, were the most prevalent at 28~31.9℃. Ground fungi in particular were common above 32.0℃, whereas ectomycorrhizal mushrooms and wood-rotting fungi showed decreased numbers at higher temperatures.
Regarding the minimum temperature (Table 7), most fungi were found at 22~23.9℃. Wood-rotting fungi showed high prevalence at 20.0~21.9℃, and the numbers of most fungi decreased above 24.0℃.
Regarding relative humidity and precipitation (Tables 8 and 9), all fungi were the most prevalent at > 82% relative humidity and > 200 mm precipitation.
Most of the higher fungi occurred during periods with average temperatures between 23℃ and 24.9℃; maximum and minimum temperatures of 28~31.9℃ and 22~23.9℃, respectively; relative humidity > 82%; and precipitation > 200 mm. The results are similar to those in previous studies, indicating that the occurrence of ectomycorrhizal fruit bodies is affected by precipitation and temperature [23] and also by relative humidity and precipitation [24]. Further, the results are also similar to those of another study, indicating that several ectomycorrhizal fungal species occur during periods with average temperatures > 23℃, maximum temperatures > 29℃, and precipitation above 200 mm [25]. We propose that abrupt climate change in forests will likely accelerate the rapid decrease in populations of higher fungi that play diverse roles in forest ecosystems, which may in turn affect forest ecosystem health.
ACKNOWLEDGEMENTS
This work received financial support for natural resource surveys and resource monitoring from the Byeonsanbando National Park (2009~2011).
References
- 1.Taylor AF, Martin F, Read DJ. Fungal diversity in ectomycorrhizal communities of Norway spruce [Picea abies (L.) Karst] and beech (Fagus sylvatica L.) along north-south transects in Europe. In: Schulze ED, editor. Carbon and nitrogen cycling in European forest ecosystems-ecological studies. Berlin: Springer-Verlag; 2000. pp. 343–365. [Google Scholar]
- 2.Jones MD, Durall DM, Cairney JW. Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol. 2003;157:399–422. doi: 10.1046/j.1469-8137.2003.00698.x. [DOI] [PubMed] [Google Scholar]
- 3.Heinonsalo J, Koskiahde I, Sen R. Scots pine bait seedling performance and root colonizing ectomycorrhizal fungal community dynamics before and during the 4 years after forest clear-cut logging. Can J For Res. 2007;37:415–429. [Google Scholar]
- 4.Cairney JW, Meharg AA. Influences of anthropogenic pollution on mycorrhizal fungal communities. Environ Pollut. 1999;106:169–182. doi: 10.1016/s0269-7491(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 5.Shi LB, Guttenberger M, Kottke I, Hampp R. The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza. 2002;12:303–311. doi: 10.1007/s00572-002-0197-2. [DOI] [PubMed] [Google Scholar]
- 6.Buée M, Vairelles D, Garbaye J. Year-round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech (Fagus sylvatica) forest subjected to two thinning regimes. Mycorrhiza. 2005;15:235–245. doi: 10.1007/s00572-004-0313-6. [DOI] [PubMed] [Google Scholar]
- 7.Söderström L. The occurrence of epixylic bryophyte and lichen species in an old natural and a managed forest stand in northeast Sweden. Biol Conserv. 1988;45:169–178. [Google Scholar]
- 8.Gustafsson L, Fiskesjö A, Hallingbäck T, Ingelög T. Semi-natural deciduous broadleaved woods in southern Sweden: habitat factors of importance to some bryophyte species. Biol Conserv. 1992;59:175–181. [Google Scholar]
- 9.Hyvärinen M, Halonen P, Kauppi M. Influence of stand age and structure on the epiphytic lichen vegetation in the middle-boreal forests of Finland. Lichenologist. 1992;24:165–180. [Google Scholar]
- 10.Selva SB. Lichen diversity and stand continuity in the northern hardwoods and spruce-fir forests of northern New England and western New Brunswick. Bryologist. 1994;97:424–429. [Google Scholar]
- 11.Crites S, Dale MR. Diversity and abundance of bryophytes, lichens, and fungi in relation to woody substrate and successional stage in aspen mixedwood boreal forests. Can J Bot. 1998;76:641–651. [Google Scholar]
- 12.Donk MA. A conspectus of the families of Aphyllophorales. Persoonia. 1964;3:199–324. [Google Scholar]
- 13.Singer R. The Agaricales in modern taxonomy. 4th ed. Koenigstein: Koeltz Scientific Books; 1986. [Google Scholar]
- 14.Park YJ. Studies on the monitoring of fungal flora in Chiaksan National Park. Chuncheon: Kangwon National University; 2003. [Google Scholar]
- 15.Kim NK. Studies on the flora of soil microorganisms and higher fungi by forest types in the Odaesan National Park. Chuncheon: Kangwon National University; 2006. [Google Scholar]
- 16.Watling R. Dawyck Botanic Garden: the Heron Wood Cryptogamic Project. Bot J Scotl. 2004;56:109–118. [Google Scholar]
- 17.Gange AC, Gange EG, Sparks TH, Boddy L. Rapid and recent changes in fungal fruiting patterns. Science. 2007;316:71. doi: 10.1126/science.1137489. [DOI] [PubMed] [Google Scholar]
- 18.Kauserud H, Stige LC, Vik JO, Økland RH, Høiland K, Stenseth NC. Mushroom fruiting and climate change. Proc Natl Acad Sci U S A. 2008;105:3811–3814. doi: 10.1073/pnas.0709037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang SK. Distribution of higher fungi in NaeJangSan National Park. Korean J Mycol. 2007;35:11–27. [Google Scholar]
- 20.Kernaghan G, Harper KA. Community structure of ectomycorrhizal fungi across an alpine/subalpine ecotone. Ecography. 2001;24:181–188. [Google Scholar]
- 21.Ohenoja E. Effect of weather conditions on the larger fungi at different forest sites in northern Finland in 1976-1988. Acta Univ Ouluensis Ser A Sci Rerum Nat. 1993;243:1–69. [Google Scholar]
- 22.Avis PG, McLaughlin DJ, Dentinger BC, Reich PB. Long-term increase in nitrogen supply alters above- and below-ground ectomycorrhizal communities and increases the dominance of Russula spp. in a temperate oak savanna. New Phytol. 2003;160:239–253. doi: 10.1046/j.1469-8137.2003.00865.x. [DOI] [PubMed] [Google Scholar]
- 23.Park YW, Koo CD, Lee HY, Ryu SR, Kim TH, Cho YG. Relationship between macrofungi fruiting and environmental factors in Songnisan National Park. Korean J Environ Ecol. 2010;24:657–679. [Google Scholar]
- 24.Shim KM, Kim YS, Kim GY, Lee DB, Kang KK, So KH, Lee KH. Relationships between wild mushroom appearance and meteorological elements in Chiak National Park, Korea. Korean J Agric For Meteorol. 2012;14:170–178. [Google Scholar]
- 25.Jang SK, Kim SW. Relationship between higher fungi distribution and climatic factors in Naejangsan National Park. Korean J Mycol. 2012;40:19–38. [Google Scholar]