Abstract
The efficacy of magnesium sulphate in chronic obstructive pulmonary disease (COPD) was assessed by conducting a systematic review of published randomized clinical trials through extensive searches in MEDLINE and SCOPUS with no date limits, as well as manual review of journals. Outcome measures varied depending on route(s) of administration of magnesium sulphate and medications co-administered. Risk of bias was evaluated and quality of evidence was graded. Four (4) randomized trials were included. All trials had a moderate risk of bias and were of average methodological quality. Magnesium sulphate given intravenously did not seem to have an immediate bronchodilatory effect; however it appears to potentiate the bronchodilatory effect of inhaled beta-2 agonists. Increase in peak expiratory flow rate (PEFR) at 30 and 45 min was greater in those who received magnesium sulphate compared to placebo (P = 0.03), although the mean percentage change in PEFR was just 24%, without significant differences in dyspnoea scores, hospital admission rates, or emergency department readmission rates compared to placebo. Nebulized magnesium sulphate with salbutamol versus nebulized salbutamol with saline placebo showed no significant differences is forced expiratory volume in 1 s (FEV1) measured at 90 min after adjustment for baseline FEV1 (P = 0.34) or differences in the need for hospital admission. Combined inhalational and intravenous magnesium sulphate versus intravenous saline placebo and nebulized ipratropium bromide were comparable in terms of hospital admission, intubation and death, but the ipratropium bromide group showed better bronchodilator effect and improvement in arterial blood gas parameters. Overall, trial evidence for trial evidence for magnesium sulphate in acute exacerbation of COPD is poor, and further well-designed trials are needed.
Keywords: Airway disease, chronic obstructive, chronic obstructive pulmonary disease, emphysema, magnesium sulphate, pulmonary disease, review, treatment
Chronic obstructive pulmonary disease (COPD) is characterised by airflow limitation due to infiltration of small airways with inflammatory cells causing narrowing, and by destruction of the elastic recoil of lung parenchyma resulting in hyperinflation. The inflammation that occurs is a result of a protective response against inhaled toxins that becomes indolent and amplified, leading to persistent inflammatory and structural change that continues even after cessation of exposure, resulting in chronic airways obstruction.[1] Chronic stable COPD is best managed with long acting bronchodilators.[2]
Exacerbations occur after infection, irritation and ambient temperature changes. Exacerbations are often associated with increased neutrophilic or eosinophilic infiltration depending on severity. In mild exacerbations, there is minimal increase in airflow limitation, while severe exacerbations affect pulmonary gas exchange due with ventilation perfusion mismatch and respiratory muscle fatigue. Airway inflammation, oedema, mucous hypersecretion, and bronchoconstriction are the hallmark features of a severe exacerbation. The resultant hypoxic vasoconstriction of pulmonary arterioles impairs perfusion.[1]
Treatment of acute exacerbation of COPD is directed at arresting the pathogenesis and reversing potential precipitating factors, especially infections. Despite the lack of controlled trial evidence, inhaled short acting beta agonists, with or without anticholinergics, relieve dyspnea and improve respiratory parameters in patients with COPD. Steroids and antibiotics play a significant role in reducing inflammation and suppressing infection respectively, facilitating resolution of the acute exacerbation. Respiratory support and intensive care unit (ICU) admission may be needed depending on the clinical condition.[2]
Intravenous magnesium sulphate, in addition to bronchodilators, is beneficial in acute severe asthma attacks, or when the response to bronchodilators is inadequate.[3] This bronchodilatory effect is thought to occur through bronchial smooth muscle relaxation.[4] Trial evidence for the use of inhaled magnesium sulphate in acute exacerbations of asthma, either alone or in addition to bronchodilators, is lacking and further trials are needed to establish whether it is useful.[5] Intravenous and/or inhaled magnesium sulphate has also been used in acute severe exacerbation of COPD with mixed results.
Magnesium sulphate is not currently recommended as treatment for acute exacerbation of COPD in standard guidelines, nonetheless is used by clinicians in practice. Thus, the purpose of this review was to determine the role of magnesium sulphate in the treatment of acute exacerbation of COPD, based on current best evidence from randomized controlled clinical trials.
Methods
We searched MEDLINE and SCOPUS for publications, using the keywords ‘magnesium’ AND ‘COPD’ OR ‘Chronic obstructive pulmonary disease’ OR ‘chronic obstructive airways disease’ OR ‘Emphysema’ OR ‘COAD’ in title, abstract, and keywords with no date limits. The search was performed in May 2013. The reference lists were imported into ENDNOTE X5®, and duplicates were removed. There were 54 hits in the combined search of MEDLINE and SCOPUS. The two investigators (SR and MCS) independently went through the abstracts to identify relevant papers. We selected papers detailing the results of randomized trials only, comparing magnesium sulphate alone or in combination with other drugs compared with placebo or other drug or combination of drugs given for treatment of acute exacerbation of COPD. We also searched related publications and papers referenced in review articles. Non-randomised trials, case-control studies, and retrospective studies were excluded, as they would not provide high quality evidence. Full papers of relevant studies were read through by the two investigators, who extracted relevant data. Duplicate publications of the same data were excluded. Risk of bias analysis and grading of methodological quality was performed by two the authors independently. Inter-rater agreement was 100%.
Results
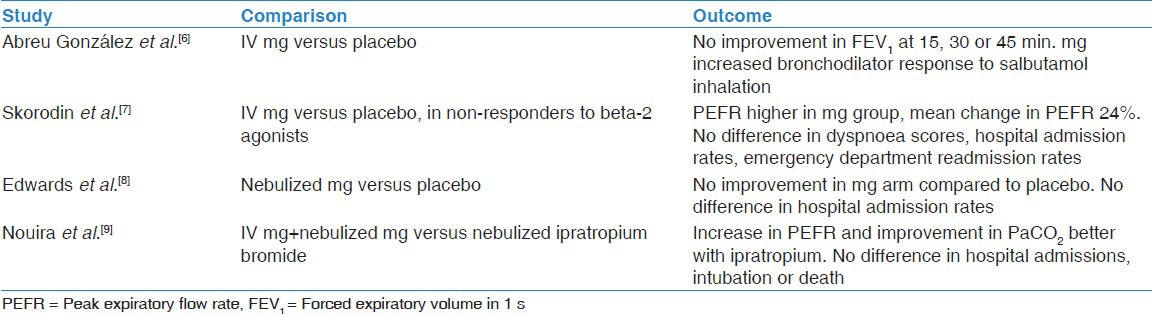
Four randomised controlled trials (RCTs) were eligible for inclusion. There were no trials with comparable outcomes, thus it was not possible to perform meta-analysis of data. The benefits of magnesium sulphate administered intravenously alone when compared with placebo were evaluated in two trials.[6,7] A third trial compared the efficacy of nebulized magnesium sulphate with placebo.[8] The fourth trial evaluated the combined effect of nebulized plus intravenous magnesium sulphate compared to intravenous salbutamol and nebulized ipratropium.[9] Our results are summarised in Table 1.
Table 1.
Summary of key outcomes of studies
Magnesium sulphate administered by intravenous route
Abreu González et al. conducted a randomized double-blind crossover trial consisting of two groups of twelve patients, with improvement in spirometry values as the outcome measure.[6] All participants received methylprednisolone, inhaled bronchodilators, antibiotics and intravenous fluids. Participants were given standard therapy 3 h prior to intravenous administration of either magnesium sulphate 1.5 g in the treatment arm or normal saline in the placebo arm. Administration of intravenous magnesium sulphate did not have an effect on forced expiratory volume in 1 s (FEV1) at 15, 30, or 45 min (P > 0.05). However when salbutamol 400 ug by aerosol inhaler was administered at 45 min, the resultant absolute increase in FEV1 (P < 0.004) and percentage increase in FEV1 (P < 0.008) were significantly higher in those who received magnesium. Thus although magnesium sulphate given intravenously did not seem to have an immediate direct effect, it appears to potentiate the bronchodilatory effect of inhaled beta-2 agonists.
Skorodin et al. demonstrated that intravenous magnesium sulphate use after administration of beta-2 agonist was safe with modest efficacy, based on a randomized double-blind controlled trial in COPD patients (n = 72) with a initial peak expiratory flow rate (PEFR) below 250 L/min which failed to rise at least two-fold after treatment with albuterol inhalation therapy.[7] The increase in PEFR at 30 and 45 min was greater in those who received magnesium sulphate compared to placebo (P = 0.03), although the mean percentage change in PEFR was just 24%. However were no significant differences in dyspnoea scores, hospital admission rates, or emergency department (ED) readmission rates within 2 weeks between the two groups. The groups were adequately matched; the placebo group was slightly older than the treatment group, though this did not reach significance (P = 0.06).
Magnesium sulphate administered by nebulized route alone
Edwards et al. studied 109 patients presenting to the ED with acute exacerbation of COPD in a randomised double-blind placebo-controlled trial.[8] All subjects enrolled were pre-dosed with salbutamol and ipratropium bromide nebulisations, and had FEV1 <50% predicted post bronchodilator. The treatment arm received 2.5 ml of isotonic magnesium sulphate mixed with 2.5 mg of salbutamol and the placebo arm received 2.5 ml of isotonic saline with 2.5 mg of salbutamol administered by a nebulizer. The authors excluded five cases of inadvertent re-enrolment and two cases where the FEV1 measurements were inaccurate. On intention-to-treat analysis, the primary outcome variable of FEV1 measured at 90 min showed no statistically significant difference between the placebo group (n = 61) and the magnesium sulphate group (n = 48) after adjustment for baseline FEV1 (P = 0.34). Differences in the need for hospital admission did not show significance either (relative risk, 0.98, P = 0.69).
Magnesium sulphate administered by combined nebulized and intravenous route
Nouira et al. carried out a randomized double-blind trial to assess multiple outcomes in 124 patients with acute exacerbation of COPD.[9] Their baseline characteristics were well matched. All were treated with standard therapy on admission to the ED. The participants were randomised to receive either intravenous magnesium sulphate with nebulized magnesium sulphate (n = 62) or intravenous normal saline and nebulized ipratropium bromide. There was no statistical difference in the likelihood of hospital admission (P = 0.26), intubation (P = 0.44), hospital mortality (P = 0.56), combined events (P = 0.26) or length of hospital stay (P = 0.28). The ipratropium bromide group showed significant increase in PEFR from baseline compared to the magnesium sulphate group (58 ± 19 L/min vs. 26 ± 13 L/min, mean difference 32, 95% confidence interval, 19-43 L). There was improvement in dyspnoea score and 180 min PaCO2 from admission values in both groups (P < 0.01), however 180 min PaCO2 was significantly lower in the ipratropium bromide group compared to the magnesium group. There was no difference in adverse events. Overall, magnesium sulphate and ipratropium bromide were comparable in terms of hospital admission, intubation and death, but the ipratropium bromide group showed better bronchodilator effect and improvement in arterial blood gas parameters.
Risk of bias, heterogeneity, and quality of trials
The risk of bias for most studies was low, with effective blinding of participants and evaluators, good allocation concealment, addressing of incomplete outcomes data and without selective reporting of outcomes. The evidence was of moderate quality, and studies were without overt methodological flaws.
Discussion
The place of intravenous magnesium sulphate in the treatment of acute exacerbation of COPD currently remains unclear; there are few RCTs, and while these are of moderate methodological quality, the studies are small, and the lack of comparable outcomes prevents meta-analysis of data. Despite some evidence of improved airflow dynamics with intravenous magnesium sulphate compared to placebo, inhalational ipratropium demonstrates better objective improvement in airflow and arterial blood gas parameters. The clinical benefit of the demonstrated improvement in airflow is questionable, as none of the hard clinical outcomes, such as the need for non-invasive or invasive ventilation, ICU admission, duration of hospital stay, or readmission were improved by the use of magnesium.
Nonetheless, magnesium sulphate could potentially play a useful role in augmenting the bronchodilatory effect of beta-2 agonists; this clearly warrants further evaluation.
There is no trial evidence to support the use of inhalational magnesium sulphate, with the two trials evaluating its benefit showing negative results. As a nebulised bronchodilator, ipratropium bromide is clearly effective and superior to magnesium; furthermore, it is difficult to determine whether the increase in PEFR noted with magnesium was due to intravenous or nebulized administration of the drug.[9]
However, given the satisfactory safety profile of magnesium, either when given intravenously or by inhalation, we recommend further trials to evaluate its benefit. The apparent improvement in airflow, and the augmenting effect of intravenous magnesium on nebulisation with beta-2 agonists cannot be ignored. Several important considerations should be made when designing such trials. Firstly, it would be unethical to conduct placebo controlled trials for intravenous, inhaled or combined magnesium sulphate therapy without any other form of treatment in acute exacerbation of COPD, given the volume of non-trial evidence and clinical experience supporting the beneficial effect of proven therapy. Thus, future trials should evaluate the efficacy of intravenous, inhalational and combined use of magnesium sulphate as add-on therapy on the background of maximum treatment with proven agents. Secondly, COPD patients are a heterogeneous group, with some patients having greater degrees of airway reversibility than others. Given that magnesium does appear to improve airflow, future studies should evaluate whether magnesium would play a useful role in augmenting this airflow reversibility by sensitizing the airways to conventional bronchodilators. Thirdly, and most importantly, trials must be adequately powered, with careful control of confounding factors, to demonstrate benefit in terms of hard outcomes, viz., improvement in blood gas parameters, need for hospital admission, need for ICU admission, need for non-invasive and invasive ventilation, and ICU and hospital stay. There is also currently no evidence regarding the long term use of magnesium by inhalation in stable COPD, and this is potentially another area for study.
Limitations
We searched the bibliographies of selected studies to identify any unpublished studies or published studies that might not have been included in the databases; none were found. Nonetheless, we acknowledge that non-indexed studies and unpublished studies may have been missed.
Conclusions
The routine use of magnesium sulphate for the treatment of acute exacerbation of COPD cannot be recommended. However, trials are relatively small. Given the possible bronchodilatory effects demonstrated by magnesium, further well designed RCTs are warranted, in particular to establish the role of magnesium sulphate as add-on therapy in exacerbations of COPD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mac Nee W. Pathology, pathogenesis, and pathophysiology. ABC of chronic obstructive pulmonary disease. BMJ. 2006;352:1202–4. [Google Scholar]
- 2.Global Strategy for Diagnosis M, and Prevention of COPD. Global Strategy for Diagnosis, Management, and Prevention of COPD. 2013. [Last accessed on 2013 Aug 7]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf .
- 3.Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA., Jr Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;2:CD001490. doi: 10.1002/14651858.CD001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spivey WH, Skobeloff EM, Levin RM. Effect of magnesium chloride on rabbit bronchial smooth muscle. Ann Emerg Med. 1990;19:1107–12. doi: 10.1016/s0196-0644(05)81513-6. [DOI] [PubMed] [Google Scholar]
- 5.Powell C, Dwan K, Milan SJ, Beasley R, Hughes R, Knopp-Sihota JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898. doi: 10.1002/14651858.CD003898.pub5. [DOI] [PubMed] [Google Scholar]
- 6.Abreu González J, Hernández García C, Abreu González P, Martín García C, Jiménez A. Effect of intravenous magnesium sulfate on chronic obstructive pulmonary disease exacerbations requiring hospitalization: A randomized placebo-controlled trial. Arch Bronconeumol. 2006;42:384–7. doi: 10.1016/s1579-2129(06)60551-x. [DOI] [PubMed] [Google Scholar]
- 7.Skorodin MS, Tenholder MF, Yetter B, Owen KA, Waller RF, Khandelwahl S, et al. Magnesium sulfate in exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 1995;155:496–500. [PubMed] [Google Scholar]
- 8.Edwards L, Shirtcliffe P, Wadsworth K, Healy B, Jefferies S, Weatherall M, et al. Use of nebulised magnesium sulphate as an adjuvant in the treatment of acute exacerbations of COPD in adults: A randomised double-blind placebo-controlled trial. Thorax. 2013;68:338–43. doi: 10.1136/thoraxjnl-2012-202225. [DOI] [PubMed] [Google Scholar]
- 9.Nouira S, Bouida W, Grissa MH, Beltaief K, Trimech MN, Boubaker H, et al. Magnesium sulfate versus ipratropium bromide in chronic obstructive pulmonary disease exacerbation: A randomized trial. Am J Ther. 2012. [Last accessed on 2014 Feb 26]. Epub ahead of print Available at http://journals.lww.com/americantherapeutics/Abstract/publishahead/Magnesium_Sulfate_Versus_Ipratropium_Bromide_in99541aspx . [DOI] [PubMed]


