Abstract
The use of growth factors in tissue engineering offers an added benefit to cartilage regeneration. Growth factors, such as insulin-like growth factor I (IGF-I), increase cell proliferation and can therefore decrease the time it takes for cartilage tissue to regrow. In this study, IGF-I was released from poly(lactic-co-glycolic) acid (PLGA) scaffolds that were designed to have a decreased burst release often associated with tissue engineering scaffolds. The scaffolds were fabricated from IGF-I-loaded PLGA microspheres by a double emulsion (W1/O/W2) technique. The microspheres were then compressed, sintered at 49°C, and salt leached. The bioactivity of soluble IGF-I was verified after being heat treated at 37, 43, 45, 49, and 60°C. Additionally, the bioactivity of IGF-I was confirmed after being released from the sintered scaffolds. The triphasic release lasted 120 days resulting in 20%, 55% and 25% of the IGF-I being released during days 1-3, 4-58, and 59-120, respectively. Seeding bone marrow cells directly onto the IGF-I loaded scaffolds showed an increase in cell proliferation, based on DNA content, leading to an increased glycosaminoglycan (GAG) production. The present results demonstrated that IGF-I remains active after being incorporated into heat-treated scaffolds, further enhancing tissue regeneration possibilities.
Keywords: scaffolds, poly(lactic-co-glycolic acid), insulin-like growth factor I, bioactivity, cartilage
Introduction
Drug- or growth factor-encapsulating devices offer an alternative to localized injections or oral dosage forms and can help improve tissue repair at the site of implantation. Furthermore, fabricating a device (or scaffold) that is biodegradable and biocompatible eliminates the need for retrieval surgery or potential long-term effects, sometimes associated with metal-based implants [1-3]. While polymeric implants have certain advantages over metals, there are still some barriers in creating a polymeric drug delivery system. Some of the difficulties associated with encapsulating drugs or growth factors into a scaffold are controlling the drug loading and release rate while maintaining bioactivity [3-7]. By changing the material properties and scaffold fabrication technique, a balance between each of these difficulties can be reached. Fabricating the scaffold from polymeric microspheres allows for control over the drug loading by adjusting the amount of drug added and type of polymer used. Additionally, choosing a polymer that has a tunable degradation rate will allow control over the drug release rate when the drug or protein incorporated has a degradation-controlled release (e.g., macromolecules such as proteins). Finally, using a fabrication method that does not require extreme temperatures or harsh solvents can prevent loss of drug bioactivity.
Poly(lactic-co-glycolic) acid (PLGA) is a material that possesses all the necessary properties to develop a drug delivery device. It has been used extensively to encapsulate hydrophilic and lipophilic drugs [8] and proteins [9] . PLGA systems are currently approved for drug delivery in the form of microspheres for the treatment of prostate cancer (Lupron Depot®) and endometriosis (Zoladex®). To alter the drug release rate and degradation time of the microspheres, the properties of PLGA can be changed. PLGA comprises polymeric chains made up of lactic and glycolic acid. Changing the ratio of lactic acid to glycolic acid, the end group terminating the macromolecules, or the overall length of the chain (e.g., molecular weight) will affect the degradation time.
Besides the already FDA-approved drug delivery systems, a wide range of research currently exists on encapsulating growth factors into scaffolds. Growth factors can be quite effective for tissue regrowth and can promote cell proliferation and differentiation. Insulin-like growth factor I (IGF-I) is a commonly used growth factor because it has the ability to increase proliferation of various cell types, such as mesenchymal stem cells, osteoblasts, and chondroblasts, that form new tissue. Studies showed that IGF-I increased bone growth and increased aggrecan and collagen-II content in vivo [10-14]. Also, IGF-I soaked into cartilage disks caused an increase in matrix deposition and glycosaminoglycan (GAG) production [15]. For IGF-I to be effective, however, the bioactivity must not be comprised when the growth factor is being incorporated into an implant or scaffold. This area of research has been often overlooked, and the IGF-I bioactivity must be verified. Some scaffold fabrication methods require elevated temperatures, mechanical forces, and harsh solvents that could potentially denature proteins and compromise the bioactivity of an incorporated growth factor [16-18]. Currently, IGF-I denaturation is mostly studied in agricultural research involving the proteins in cow milk and the effects of milk processing at temperatures exceeding 100°C, and these findings are not necessarily translatable to scaffold fabrication techniques [12, 19].
The objective of the present studies was to investigate whether incorporation of IGF-I into PLGA scaffolds formed by a microsphere sintering method compromised bioactivity. Specifically, activity of IGF-I was measured after being heat-treated in solution and then again after it had been released from PLGA scaffolds. The scaffolds used in this study have been previously characterized [20], and their mechanical properties can be suitable for soft or hard tissue applications. Addition of IGF-I to the scaffolds would provide the potential to enhance tissue regrowth and reduce recovery time.
Materials and Methods
Microsphere Fabrication
Poly(lactic-co-glycolic acid) (50:50, acid-terminated; Durect Corporation, Pelham, AL) with an inherent viscosity of 0.55–0.75 dL/g (molecular weight approximately 40 kDa) was used. PLGA microspheres were fabricated using a water/oil/water (W1/O/W2) emulsion technique. Two types of PLGA microspheres were fabricated, blank and IGF-I-loaded, which differed only in the W1 phase. The W1 phase was phosphate-buffered saline (PBS), pH 7.4, for blank microspheres, whereas the W1 phase for the IGF-I loaded microspheres contained 1.1 mg/mL IGF-I (PeproTech, Rocky Hill, NJ) in PBS, targeting a release of 2-20 ng/mL. For both types of microspheres, the oil phase (O) consisted of PLGA dissolved in dichloromethane (DCM) at 13wt/v%, and the W2 phase was made by dissolving 1% poly(vinyl alcohol) (PVA; Sigma-Aldrich, St. Louis, MO) into deionized water. W1 was emulsified into the O phase by sonication at 25 W for 10 seconds. The W1/O was homogenized into W2 at 3500 rpm for 3 minutes. The resulting microspheres were stirred overnight, washed six times in deionized water, and lyophilized. Only microspheres <250 μm were used for scaffold fabrication.
Scaffold Preparation
Scaffolds were fabricated using a salt-leaching method at a weight ratio of 40:60 (microspheres:salt) [20]. The salt size was <150 μm, controlled through grinding and sieving. Each scaffold was weighed out individually into a 0.6 mL microcentrifuge tube and mixed by hand for 45 seconds. The mixture was then compressed for 2 minutes at 1.5 ton using a 6 mm diameter die in a Carver press. The scaffolds were then incubated for two days at 49°C, the glass transition (Tg) temperature of the PLGA used, which allowed for the microspheres to fuse around the NaCl particles. Lastly, the scaffolds were stirred in deionized water overnight to leach out the NaCl particles and dried the following day. The final mass of the scaffolds was approximately 41 mg, with scaffold dimensions of 6.0 mm in diameter and a thickness of 2.4 mm, with an overall porosity of approximately 70%. Scaffolds were disinfected by washing in 70% ethanol and twice in cold PBS.
Encapsulation Efficiency and Loading
Encapsulation efficiency was measured by dissolving microspheres in DCM and then adding acetone at a volume ratio of 3:7 (DCM:acetone) followed by centrifugation at 14,000 g for 5 minutes and discarding the supernatant. The sample was washed and centrifuged three times using the DCM/acetone mixture and then left overnight for solvent evaporation. The dried samples were rehydrated in PBS, and the protein concentration was measured using MicroBCA assay (Thermo Fisher Scientific, NJ) according to the manufacturer's instructions.
Release Study
Scaffolds were placed in 12-well plates with 4 mL PBS and incubated at 37°C while on an orbital shaker. The supernatant was collected every 3-5 days and stored at −20°C until analyzed. Before being assayed, the supernatant was concentrated using centrifugal filters having a molecular weight cutoff of 3,000 Da (Amicon Ultra; Millipore, MA) following the manufacturer's instructions. The MicroBCA assay was then used to quantify the IGF-I concentration at each time point.
Bioactivity Studies
Two experiments were conducted to verify the bioactivity of IGF-I. First, the IGF-I activity was measured after incubated growth factor solutions at elevated temperatures for two days, and in the second, activity was determined as the IGF-I was released from the scaffolds during degradation. Before starting these experiments, effects of known concentrations of IGF-I (0-100 ng/mL) were examined to ensure samples were within the linear range of cell responsiveness to the growth factor. Both of the experiments used the same initial conditions, where SaOS-2 human osteosarcoma cells (ATCC HTB-85) were seeded in McCoy's 5A medium supplemented with 10% fetal bovine serum (FBS; HyClone, ThermoScientific) at a density of 2.5×104 cells/mL and allowed to attach overnight.
To test the bioactivity of IGF-I after being incubated at elevated temperatures for two days (which is experienced during scaffold fabrication, as described in the Scaffold Preparation section), an IGF-I solution with a concentration of 12 μg/mL in PBS was incubated at 37, 43, 45, 49, or 60°C for two days. A day after cell seeding, heat-treated IGF-I solution was diluted into McCoy's 5A medium containing 0.1% charcoal and dextran-treated fetal bovine serum (CDTFBS), which has reduced serum levels of growth factors, and added to the cells. The overall IGF-I concentration in medium was adjusted to 12 ng/mL, which pilot studies demonstrated was within the range of SaOS-2 responsiveness. The positive control was an IGF-I solution with no heat treatment, and the negative control was incubated at 100°C.
To measure the bioactivity of IGF-I released from the protein-loaded scaffolds, release supernatant was diluted in McCoy's medium containing 0.1% CDTFBS to be within the linear range of the IGF-I dose response. The released supernatant made up 15% of the total well volume. The same study was performed using unprocessed IGF-I to create a standard curve of expected cell proliferation based on a given IGF-I concentration.
At the end of both experiments, cell proliferation was assessed by measuring DNA contents with a Hoechst assay adapted from Labarca et. al [21]. Briefly, each well was washed twice with PBS before salt solution (50 mM NaH2PO4, 2 M NaCl, 2 mM EDTA) was added and sonicated for 10 seconds at 25 W. Hoechst 33258 (Sigma-Aldrich) was added to a final concentration of 1.25 μg/mL, shaken, and left in the dark. Ten minutes later, fluorescence readings (λex= 356 nm, λem=458 nm) were taken. Bioactivity, reflected by cell proliferation, was expressed as a percentage of the effect of control treatments.
Bone Marrow Stromal Cell Responses
Bone marrow stromal cells were harvested from 40-45 days old Sprague-Dawley rats shortly after euthanization. The femurs were dissected and the epiphyses removed. The diaphyses were flushed with MEM, Alpha modification medium (αMEM; HyClone, ThermoScientific) containing 10% FBS and antibiotic-antimycotic solution (100 U/mL penicillin G, 100 μg/mL streptomycin, 250 ng/mL amphotericin B; HyClone, ThermoScientific). The cells were transferred to a flask and cultured. Once confluent, the cells were seeded onto either blank or IGF-I-loaded scaffolds at a density of 150,000 cells per scaffold with 4 mL of αMEM containing 0.5% transferrin and 0.05% sodium selenite. The wells were coated with 2% agarose to prevent cell adhesion on the polystyrene, which enabled measurement of cell activity on only the porous PLGA scaffolds. The medium was changed every 2-3 days. After three and six weeks of culture, the scaffolds were washed twice with PBS and transferred to a 2 mL microcentrifuge tube and incubated with 300 μL Cell Lytic-M (Sigma-Aldrich, St. Louis, MO) for 15 minutes on an orbital shaker. Afterwards, the samples were centrifuged for 15 minutes at 12,000 g. The cell lysate was assayed according to the manufacturer's instructions for DNA and GAG content using a Quant-iT© PicoGreen™ Assay (Thermo Scientific, NJ) and Blyscan™ Glycosaminoglycan Assay, respectively. In addition, alkaline phosphatase activity was measured to assess pre-osteoblastic response. Briefly, the substrate consisted of 10 mM p-nitrophenyl phosphate in 0.6 M 2-amino-2-methyl-1-propanol buffer solution (pH 10) and just before use 4 mM magnesium chloride was added. In a 96-well plate the sample was mixed with the substrate at a 1:3 ratio (sample:substrate) and read at 400 nm [22].
Statistics
Analysis was carried out using GraphPad InStat software running ANOVA followed by a Tukey–Kramer Multiple Comparisons Test. Results were considered significant if p < 0.05.
Results
Encapsulation Efficiency and Loading
The encapsulation efficiency of IGF-I in PLGA microspheres was found to be 38±3% for a total loading of approximately 0.5 μg of IGF-I per mg of microspheres. Each scaffold contained 18-23 μg IGF-I. As reported previously [20], initial porosity of the scaffolds was 63% with an average pore size of 49 μm.
IGF-I Release
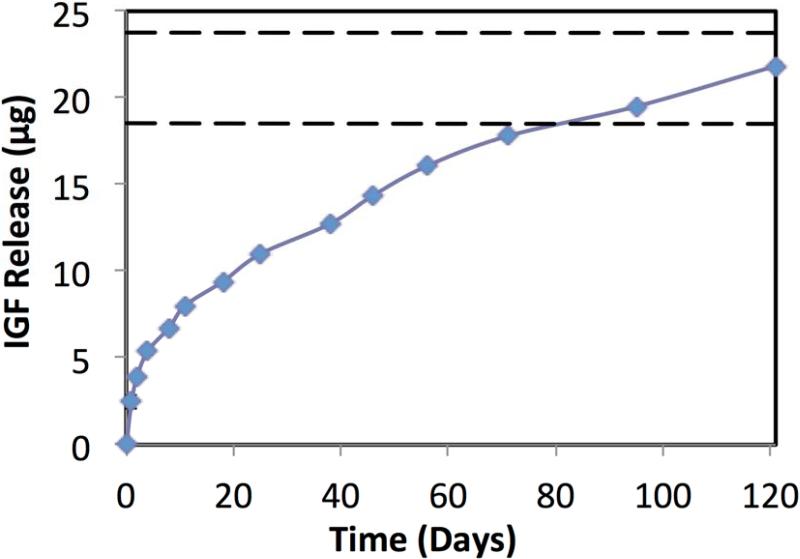
Figure 1 shows the release of IGF-I from the scaffolds over time. In the first five days, there was an initial release of 1.28 μg IGF-I per day, followed by a decreased rate of 0.24 μg/day through day 10, after which it became 0.11 μg/day throughout the rest of the release. The scaffolds degradation was done previous where full degraded was reached at 120 days [20].
Figure 1.
Cumulative release of IGF-I release from PLGA scaffolds. The dashed lines represent the range of expected drug release based on the loaded amount. Data are mean ± standard deviation (n=3). Standard deviation is shown, ranging from 0.06-0.12 μg.
IGF-I Dose-Response
To determine the concentration-dependent effect of IGF-I on cell proliferation, DNA contents were measured for SaOS-2 cells cultured with IGF-I at increasing concentrations. Figure 2 shows that IGF-I began to have an effect at 2 ng/mL and then reached the maximal effect around 20 ng/mL.
Figure 2.
Concentration-dependent effect of IGF-I on cell proliferation measured by DNA content, showing a plateau after approximately 20 ng/mL. The dashed line represents the control where no IGF-I was added. Data are mean ± standard deviation (n=3).
Bioactivity of IGF-I
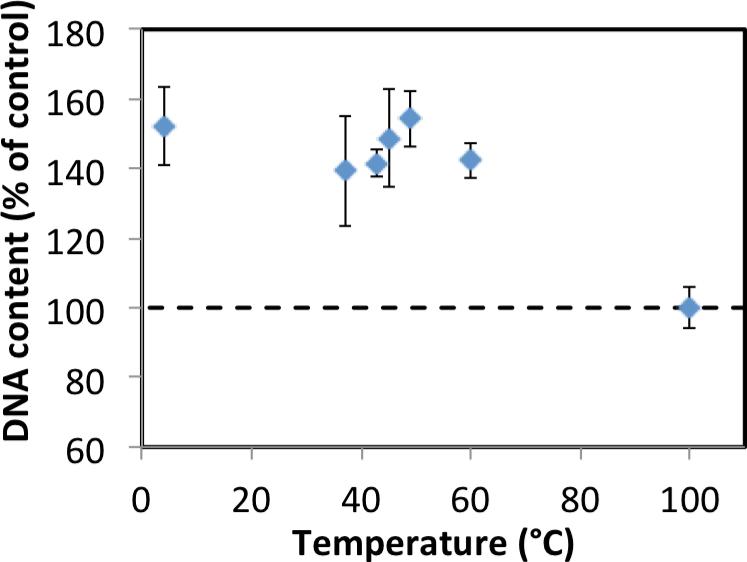
The bioactivity of IGF-I solutions was tested after incubation for two days at 37, 43, 45, 49, 60 or 100°C. The solutions incubated at 37, 43, 45, and 60°C caused a statistically significant increase (p<0.001) of about a 140% in cell proliferation compared to cultures without growth factor (Figure 3). Proliferation in response to solutions that were not incubated (preserved at 4°C) or incubated at 37, 43, 45, 49, and 60°C was not significantly different. The negative control (100°C) was not statistically different than cells cultured without growth factor, indicating that heat treatment to 100°C eliminated IGF-I activity.
Figure 3.
Effect of heat-treated IGF-I solution on cell proliferation as measured by DNA content. The dashed line represents the DNA content in cells cultured without growth factor. IGF-I solution that had been incubated at increasing temperatures for 2 days. Data are mean ± standard deviation (n=3).
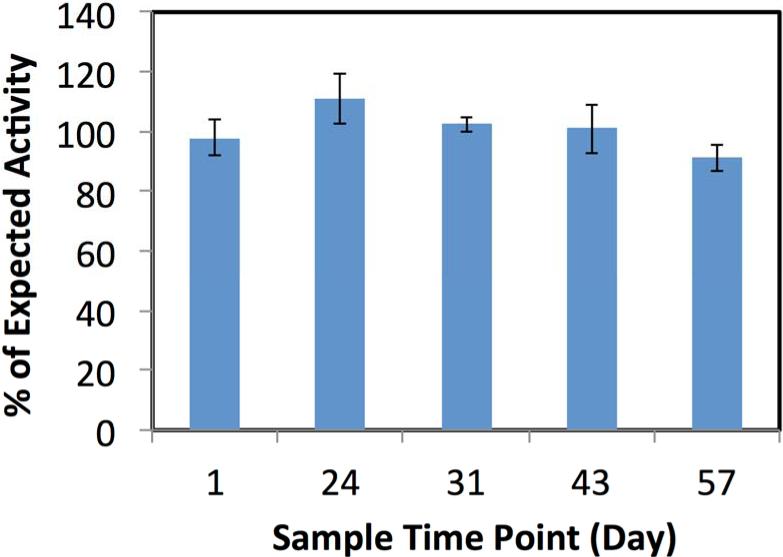
Bioactivity of the supernatants collected throughout the period of degradation of IGF-I-loaded scaffolds was measured at the time points 1, 24, 31, 43, 57 days. The actual activity was compared to the expected activity based on mitogenicity of unprocessed IGF-I at the same concentration. The results are shown in Figure 4, where 100% activity indicates no loss of bioactivity. The percentages of expected activity (±SEM) were 97±6, 110±8, 102±2, 100±8 and 91±4% at 1, 24, 31, 43 and 57 days, respectively, and were not statistically different from 100%.
Figure 4.
Bioactivity of IGF-I in the release supernatant at increasing times scaffold degradation. Each time point was compared to the expected level of activity for the amount of IGF-I released at the respective time point. Data are mean ± standard deviation (n=3).
Bone Marrow Stromal Cell Responses
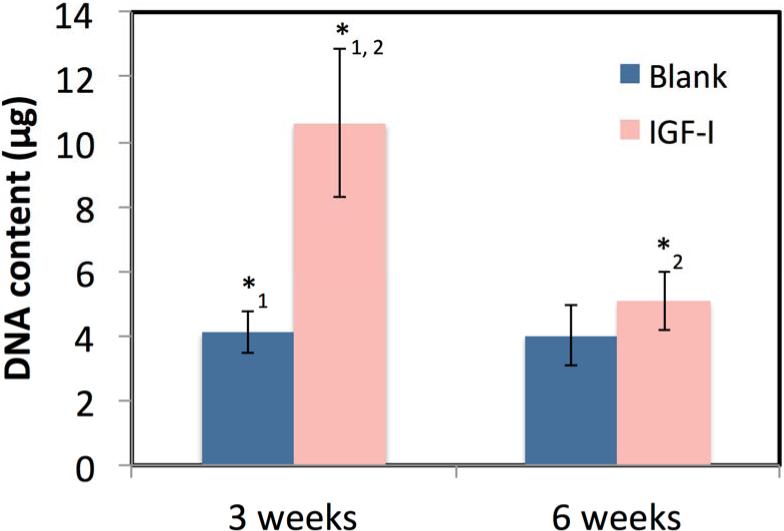
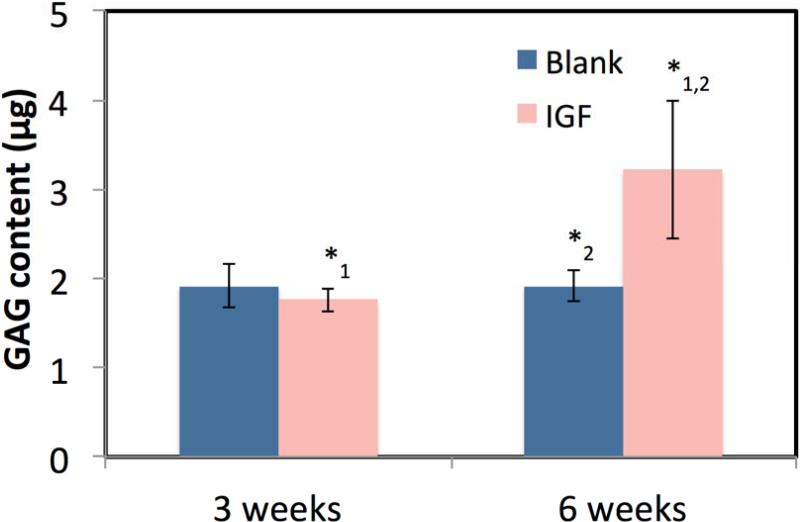
Figure 5 shows that the encapsulation of IGF-I in microspheres caused an increase in DNA production at 3 and 6 weeks compared to the blank scaffolds. A statistically significant difference (p<0.01) was seen between the blank and IGF-I-loaded scaffolds at 3 weeks with 4.1±0.7 and 10.6±2.3 μg of DNA and at 6 weeks with 4.0±1.0 and 5.08±0.9 μg of DNA, respectively. There was an overall significant decrease in DNA between 3 and 6 weeks for the IGF-I scaffolds (p<0.01) but not for the blank scaffolds. The GAG content is shown in Figure 6, where no statistical difference was seen at 3 weeks between the blank and IGF-I-loaded scaffolds with 1.9±0.3 and 1.8±0.1 μg of GAG, respectively. After 6 weeks, the blank scaffolds showed no change in GAG content at 1.9±0.2 μg, while the IGF-I-loaded scaffold saw a significant increase to 3.2±0.8 μg of GAG (p<0.01). AP activity did not increase between 3 and 6 weeks, and no significant difference was seen between scaffold types (results not shown).
Figure 5.
DNA content after culturing BMCs on blank and IGF-I-loaded scaffolds for 3 and 6 weeks. Data are mean ± standard deviation (n=3). *1,2 p<0.001
Figure 6.
GAG content after culturing BMCs on blank and IGF-I-loaded scaffolds for 3 and 6 weeks. Data are mean ± standard deviation (n=3). *1,2 p<0.001
Discussion
IGF-I Release
PLGA has been widely used for drug delivery options in the form of microspheres and scaffolds [23-26]. PLGA microspheres can be advantageous for injectable delivery due to their size, but they present more of a problem for extended release due to drug diffusion and the large surface area of microspheres. The release profile of PLGA microspheres commonly starts with an initial burst followed by a second phase of sustained release and then a third phase of a slower sustained released [27, 28]. The initial burst release is caused by a combination of drug dissolution from microsphere surfaces and initial diffusion. The second phase is attributed to diffusion from deeper in the microspheres and initial matrix degradation, whereas the third phase is primarily due to matrix degradation.
The amount of IGF-I released from microspheres during the initial burst usually accounts for a large portion of the overall loaded protein and can be an excessive amount, not necessary to see an effect and can possibly cause adverse effects such as swelling and joint aches [29]. Approximately 20% of the IGF-I loaded in PLGA microspheres, using the same type of PLGA (50:50) polymer as in the present study, was released within the first 24 hours of a 13-18 day degradation time, releasing 1.1 to 2.2 μg of IGF-1 [30, 31]. IGF-I burst release also occurs in other scaffold types, such as tricalcium phosphate, which released 10-78% of the protein within 24 hours when degraded in water and serum, respectively [32]. The present results, however, showed that sintering the microspheres into a scaffold reduced the 24 hour burst, with 20% release not reached until three days. The decreased burst release can also be attributed to the microsphere wash steps to remove surfactant and the salt leaching process, both of which reduced the amount of surface protein. Altering the burst release will help prevent drug loss during the initial increase in blood flow around the implant site as a result of the inflammatory response [33].
Other methods for decreasing the burst release from microspheres involve changing the polymer properties (molecular weight, end group, and lactic to glycolic ratio), polymer concentration, PVA concentration, the water phase volume, and homogenization speed during microsphere fabrication [34]. Mao et al. evaluated each step of the W/O/W process and found that increasing the PVA concentration, homogenization speed, and drug loading or decreasing the water phase (W2) volume and drug molecular weight all caused a decrease in the burst release. Another way to avoid the burst is pre-degrading/washing the microspheres, and start the drug release after the initial burst [35]. However, this could be costly to waste expensive drugs or growth factors, and it reduces the amount of drug delivered.
The present IGF-I loaded scaffolds released 50% of the IGF-I within the first 24 days, which is a crucial time frame to allow for the development of new tissue ingrowth throughout the scaffold. New bone tissue can take 4 weeks to fill a non-critical size wound [36], and cartilage formation can take 2 weeks before GAG and collagen I and II production become apparent based on histological staining for GAG chondrogenic markers [37]. Capito et al. saw an effect of scaffold fabrication on DNA and GAG content at 2 weeks. Their collagen scaffold with and without crosslinking IGF-I plasmid into the scaffold resulted in no significant difference without the crosslinking. Additionally, they seeded 4 million cells onto their scaffold compared to the 150,000 cells in this study, which could be another factor in the non-significant difference of GAG content with and without IGF-I [38].
Techniques used to decrease the burst release from scaffolds include varying the internal porosity and microstructure of the scaffold. To alter the internal porosity of the microspheres, it has been shown that increasing the temperature during the second emulsion to 38-42°C resulted in a lower burst release, which may be a problem for drug bioactivity [39]. For PLGA scaffolds, the burst release is also affected by the scaffold porosity, which increases surface area and allows for more drug diffusion. However, interconnected pores in a PLGA scaffold help prevent acid build up and allow for cell migration into the scaffold, improving tissue ingrowth [40]. Using a non-porous PLGA (50:50) system can have a negative effect on the implant site and inhibit new tissue formation [41]. The present scaffold fabrication method yielded an overall scaffold porosity of approximately 70%, yet eliminated the burst release [20].
Bioactivity of IGF-I
Some growth factors, such as vascular endothelial growth factor, transforming growth factor-beta and bone morphogenetic protein 2, have already been shown to maintain their bioactivity after heat treatment or solvent interaction [42-46]. Jabbarzadeh et al. incorporated vascular endothelial growth factor into sintered PLGA scaffolds and were able to show retained bioactivity [47]. However, little is known about the effects that scaffold fabrication will have on IGF-I protein and the ability to maintain bioactivity. IGF-I encapsulated in PLGA retained mitogenicity. The small amount loaded, which was appropriate for achieving physiologically relevant concentrations of the growth factor, was at least partially responsible for avoiding adverse effects because of solvent exposure. Regarding the influence of temperature during scaffold sintering, most of the research surrounding heat treatment of IGF-I is in the agricultural sciences studying the protein in bovine milk during pasteurization. Collier et al. examined IGF-I denaturation after heat treatment at 121°C for 5, 15, and 30 minutes and found that, after only 5 minutes, 93% of the IGF-I was unrecognizable and therefore considered denatured [19]. Even heat-treating IGF-I at 75 or 85°C for 15 minutes caused a decrease of 45 and 45.2%, respectively, in active IGF-I [48]. Furthermore, the same group found that heating IGF-I to 121°C, resulted in no active IGF-I.
The present study analyzed the temperature range of 37-60°C, which was used for scaffold fabrication. The temperatures were based on glass transition temperature of different types of PLGA used to fabricate the scaffold [20]. After 2 days of incubation at these elevated temperatures, IGF-I retained full bioactivity as shown by the analysis of cell proliferation compared to non-heat-treated growth factor. Additionally, there was no effect from scaffold fabrication, involving the use of solvents, sonication, homogenization, and compression, on IGF-I bioactivity. Other types of PLGA scaffolds containing IGF-I have been fabricated using solvents, but no heat treatment was used. Jaklenec et al. verified the bioactivity of IGF-I as it was released from scaffolds using a MCF-7 proliferation assay similar to the present methods [49]. The scaffolds were fabricated using dichloromethane vapor method, whereas the current method used temperature treatment to fuse the microspheres. Heat treatment (or sintering) allows for a 10-fold increase in mechanical properties compared to non-sintered scaffolds [20]. No mechanical properties were reported for scaffolds fabricated using solvent vapor [49].
Bone Marrow Stromal Cell Responses to Growth Factor
Seeding cells on tissue engineering scaffolds allows cell infiltration and subsequent tissue responses. For example, pore sizes of 20-120 μm have been shown to be appropriate for cell infiltration in scaffolds for hard and soft tissues regeneration [8, 29, 50]. Although the present study focused on bioactivity measurements, we have previously demonstrated infiltration of cells into our scaffolds [50]. In the present studies, encapsulation of IGF-I into PLGA scaffolds increased cell proliferation. Prolonged release of IGF-I continued to have an effect on bone marrow stromal cells since there was a 2.6 fold increase at 3 weeks and a 1.3 fold increase at 6 weeks over blank scaffolds. In other studies, Longobardi et al. showed that IGF-I had an effect on chondrogenesis through increased cell proliferation, decreased apoptosis, and increased collagen II expression [51]. Their findings on IGF-I dose-dependent cell proliferation were similar to the present data, which show that the IGF-I effect plateaued around 20 ng/mL.
The DNA production between 3 and 6 weeks did not change for the blank scaffolds but significantly decreased in the IGF-I-loaded scaffolds, which was likely due to a combined effect of scaffold degradation, cell maturation, and scaffold saturation. Between 3 and 6 weeks, the scaffolds lost approximately 30% mass, for a total of 12.6 mg, which accounts for a total of 57% of the total volume reported in previous studies [20]. Normalizing the DNA content to milligrams of scaffold remaining, the IGF-I scaffolds had 0.28±0.06 μg of DNA per mg of scaffold at 3 weeks, significantly (p<0.01) more than the blank scaffolds had with 0.11±0.02 μg. The same trend was seen at 6 weeks, though not statistically significant, where the IGF-I-loaded scaffolds had more DNA content per milligram of scaffold than the blank scaffolds with 0.20±0.04 μg and 0.16±0.04 μg, respectively. The combined effect of scaffold mass loss and scaffold saturation can account for the similarity in DNA contents seen between the IGF-I-loaded scaffolds and blank scaffolds at 6 weeks, and it may not be due to a lack of IGF-I activity but rather a lack of space or surface area for cell proliferation.
The increased cell content seen at 3 week did not cause an effect on GAG content, with no statistical difference in GAG content between the blank and IGF-I-loaded scaffolds. However, at 6 weeks the GAG content in IGF-I-loaded scaffolds was 1.7 times higher than that in the blank scaffolds. It has been reported that human marrow cells can take 2-4 weeks before showing chondrogenic markers or producing GAG, so it is possible that a difference in GAG content between the scaffold types may not be detectable at 3 weeks [52, 53]. Lastly, it is relevant to note that alkaline phosphatase, commonly used as an indicator of early osteoblastic activity [54-56], was not increased during the 6 weeks of culture of bone marrow stromal cells on either the blank or IGF-I-loaded scaffolds.
Conclusion
The present study demonstrated that sintering IGF-I-loaded, PLGA microspheres into three-dimensionally porous scaffolds allowed for the release of active IGF-I. Scaffold fabrication process and temperatures up to 49°C did not compromise the bioactivity of IGF-I. Cell proliferation was initially enhanced with the addition of IGF-I to the scaffold, which lead to an eventual increase in GAG production after 6 weeks. Additionally, the sintered scaffolds eliminated the typical initial burst release seen with PGLA microspheres. Preventing the burst release helps to reduce growth factor waste, avoid adverse effects possibly caused by a large localized dose of growth factor, and instead creates a long slow release (~120 days) that can aid in tissue repair and growth.
Acknowledgements
This research was supported in part by the Pediatric Orthopaedic Society of North America, the NIH (AR060964), and an NSF IGERT traineeship to AC (DGE-0653710).
REFERENCES
- 1.McGee MA, Howie DW, Costi K, Haynes DR, Wildenauer CI, Pearcy MJ, McLean JD. Implant retrieval studies of the wear and loosening of prosthetic joints: a review. Wear. 2000;241:158–165. [Google Scholar]
- 2.Black J. Systemic effects of biomaterials. Biomaterials. 1984;5:11–18. doi: 10.1016/0142-9612(84)90061-9. [DOI] [PubMed] [Google Scholar]
- 3.Steinemann S. Metal implants and surface reactions. Injury. 1996;27:16–22. doi: 10.1016/0020-1383(96)89027-9. [DOI] [PubMed] [Google Scholar]
- 4.Lu W, Park TG. Protein release from poly(lactic-co-glycolic acid) microspheres: protein stability problems. PDA J Pharm Sci Technol. 1995;49:13–19. [PubMed] [Google Scholar]
- 5.McCall JD, Anseth KS. Thiol-ene photopolymerizations provide a facile method to encapsulate proteins and maintain their bioactivity. Biomacromolecules. 2012;13:2410–2417. doi: 10.1021/bm300671s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabata Y, Takebayashi Y, Ueda T, Ikada Y. A formulation method using D, L-lactic acid oligomer for protein release with reduced initial burst. J Controlled Release. 1993;23:55–63. [Google Scholar]
- 7.Ghaderi R, Carlfors J. Biological activity of lysozyme after entrapment in poly(d,llactide-co-glycolide)-microspheres. Pharm Res. 1997;14:1556–1562. doi: 10.1023/a:1012122200381. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Lee IW, Lee YM, Lee HB, Khang G. Macroporous biodegradable natural/synthetic hybrid scaffolds as small intestine submucosa impregnated poly (D, L-lactide-co-glycolide) for tissue-engineered bone. J Biomater Sci Polym Ed. 2004;15:1003–1017. doi: 10.1163/1568562041526487. [DOI] [PubMed] [Google Scholar]
- 9.Benita S. Microencapsulation: Methods and Industrial Applications. CRC Press; 2006. pp. 99–122. [Google Scholar]
- 10.Hock JM, Centrella M, Canalis E. Insulin-like growth factor-I has independent effects on bone-matrix formation and cell replication. Endocrinology. 1988;122:254–260. doi: 10.1210/endo-122-1-254. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy TL, Centrella M, Canalis E. Regulatory effects of insulin-like growth factors I and II on bone collagen synthesis in rat calvarial cultures. Endocrinology. 1989;124:301–309. doi: 10.1210/endo-124-1-301. [DOI] [PubMed] [Google Scholar]
- 12.Yun Z, Zhang H, Cai X, Wang A, Zhang L. Kinetic and thermodynamic studies on the thermal denaturation of bovine milk insulin-like growth factor-I in model systems. Lait. 2007;87:139–148. [Google Scholar]
- 13.Morisset S, Frisbie DD, Robbins PD, Nixon AJ, McIlwraith CW. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462:221–228. doi: 10.1097/BLO.0b013e3180dca05f. [DOI] [PubMed] [Google Scholar]
- 14.Damien E, Hing K, Saeed S, Revell PA. A preliminary study on the enhancement of the osteointegration of a novel synthetic hydroxyapatite scaffold in vivo. J Biomed Mater Res A. 2003;66:241–246. doi: 10.1002/jbm.a.10564. [DOI] [PubMed] [Google Scholar]
- 15.Guenther HL, Guenther HE, Froesch ER, Fleisch H. Effect of insulin-like growth factor on collagen and glycosaminoglycan synthesis by rabbit articular chondrocytes in culture. Cell Mol Life Sci. 1982;38:979–981. doi: 10.1007/BF01953688. [DOI] [PubMed] [Google Scholar]
- 16.Coleman J, Lowman A. Biodegradable nanoparticles for protein delivery: analysis of preparation conditions on particle morphology and protein loading, activity and sustained release properties. J Biomater Sci Polym Ed. 2011 doi: 10.1163/092050611X576648. [DOI] [PubMed] [Google Scholar]
- 17.England JL, Haran G. Role of solvation effects in protein denaturation: from thermodynamics to single molecules and back. Annu Rev Phys Chem. 2011;62:257–277. doi: 10.1146/annurev-physchem-032210-103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo Y, Baek N, Park K. Microencapsulation methods for delivery of protein drugs. Biotechnol Bioprocess Eng. 2001;6:213–230. [Google Scholar]
- 19.Collier RJ, Miller MA, Hildebrandt JR, Torkelson AR, White TC, Madsen KS, Vicini JL, Eppard PJ, Lanza GM. Factors affecting insulin-like growth factor-I concentration in bovine milk. J Dairy Sci. 1991;74:2905–2911. doi: 10.3168/jds.S0022-0302(91)78473-7. [DOI] [PubMed] [Google Scholar]
- 20.Clark A, Milbrandt TA, Hilt JZ, Puleo DA. Tailoring properties of microsphere-based poly(lactic-co-glycolic acid) scaffolds. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34706. [DOI] [PubMed] [Google Scholar]
- 21.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 22.Vreven J, Lieberherr M, Vaes G. The acid and alkaline phosphatases, inorganic pyrophosphatases and phosphoprotein phosphatase of bone. II. Distribution in subcellular fractions of bone tissue homogenates and structure-linked latency. Biochim Biophys Acta. 1973;293:170–177. doi: 10.1016/0005-2744(73)90388-4. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Yoshioka T, Lucarelli M, Hwang L, Langer R. Controlled Delivery Systems for Proteins Based on Poly(Lactic/Glycolic Acid) Microspheres. Pharm Res. 1991;8:713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 24.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier Syst. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 26.Crotts G, Park TG. Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: Release kinetics and stability issues. J Microencapsul. 1998;15:699–713. doi: 10.3109/02652049809008253. [DOI] [PubMed] [Google Scholar]
- 27.Garcia JT, Farina JB, Munguia O, Llabres M. Comparative degradation study of biodegradable microspheres of poly(DL-lactide-co-glycolide) with poly(ethyleneglycol) derivates. J Microencapsul. 1999;16:83–94. doi: 10.1080/026520499289338. [DOI] [PubMed] [Google Scholar]
- 28.Raghuvanshi RS, Singh M, Talwar GP. Biodegradable delivery system for single step immunization with tetanus toxoid. Int J Pharm. 1993;93:R1–R5. [Google Scholar]
- 29.Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, Sledge CB, Yannas IV, Spector M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18:769–776. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 30.Meinel L, Illi OE, Zapf J, Malfanti M, Peter Merkle H, Gander B. Stabilizing insulin-like growth factor-I in poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2001;70:193–202. doi: 10.1016/s0168-3659(00)00352-7. [DOI] [PubMed] [Google Scholar]
- 31.Elisseeff J, McIntosh W, Fu K, Blunk BT, Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 32.Laffargue P, Fialdes P, Frayssinet P, Rtaimate M, Hildebrand HF, Marchandise X. Adsorption and release of insulin-like growth factor-I on porous tricalcium phosphate implant. J Biomed Mater Res. 2000;49:415–421. doi: 10.1002/(sici)1097-4636(20000305)49:3<415::aid-jbm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 33.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel T. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm. 2007;334:137–148. doi: 10.1016/j.ijpharm.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23:1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 36.Uematsu K, Hattori K, Ishimoto Y, Yamauchi J, Habata T, Takakura Y, Ohgushi H, Fukuchi T, Sato M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273–4279. doi: 10.1016/j.biomaterials.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Veilleux N, Spector M. Effects of FGF-2 and IGF-1 on adult canine articular chondrocytes in type II collagen–glycosaminoglycan scaffolds in vitro. Osteoarthr Cartilage. 2005;13:278–286. doi: 10.1016/j.joca.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Capito RM, Spector M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Ther. 2007;14:721–732. doi: 10.1038/sj.gt.3302918. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y-Y, Chia H-H, Chung T-S. Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. J Controlled Release. 2000;69:81–96. doi: 10.1016/s0168-3659(00)00291-1. [DOI] [PubMed] [Google Scholar]
- 40.Fu K, Pack D, Klibanov A, Langer R. Visual Evidence of Acidic Environment Within Degrading Poly(lactic-co-glycolic acid) (PLGA) Microspheres. Pharm Res. 2000;17:100–106. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 41.Rhee SH, Lee SJ. Effect of acidic degradation products of poly(lactic-coglycolic)acid on the apatite-forming ability of poly(lactic-co-glycolic)acid-siloxane nanohybrid material. J Biomed Mater Res A. 2007;83:799–805. doi: 10.1002/jbm.a.31405. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence DA, Pircher R, Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun. 1985;133:1026–1034. doi: 10.1016/0006-291x(85)91239-2. [DOI] [PubMed] [Google Scholar]
- 43.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gentry LE, Nash BW. The pro domain of pre-pro-transforming growth factor .beta.1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry. 1990;29:6851–6857. doi: 10.1021/bi00481a014. [DOI] [PubMed] [Google Scholar]
- 45.Winkler L, Müller R, Wiemann M. Heat-treated BMP-2 depots release BMP-2 in its bioactive form. Materialwiss Werkst. 2006;37:436–440. [Google Scholar]
- 46.Schwarz C, Wulsten D, Ellinghaus A, Lienau J, Willie BM, Duda GN. Mechanical load modulates the stimulatory effect of BMP2 in a rat nonunion model. Tissue Eng Part A. 2013;19:247–254. doi: 10.1089/ten.tea.2012.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jabbarzadeh E, Deng M, Lv Q, Jiang T, Khan YM, Nair LS, Laurencin CT. VEGF-incorporated biomimetic poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2012;100:2187–2196. doi: 10.1002/jbm.b.32787. [DOI] [PubMed] [Google Scholar]
- 48.Kang SH, Kim JU, Imm JY, Oh S, Kim SH. The effects of dairy processes and storage on insulin-like growth factor-I (IGF-I) content in milk and in model IGF-I-fortified dairy products. J Dairy Sci. 2006;89:402–409. doi: 10.3168/jds.S0022-0302(06)72104-X. [DOI] [PubMed] [Google Scholar]
- 49.Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. Sequential release of bioactive IGF-I and TGF-β1 from PLGA microsphere-based scaffolds. Biomaterials. 2008;29:1518–1525. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Sundararaj SK, Cieply RD, Gupta G, Milbrandt TA, Puleo DA. Treatment of growth plate injury using IGF-I-loaded PLGA scaffolds. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1670. [DOI] [PubMed] [Google Scholar]
- 51.Longobardi L, O'Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21:626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 52.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 53.Martin I, Padera RF, Vunjak-Novakovic G, Freed LE. In vitro differentiation of chick embryo bone marrow stromal cells into cartilaginous and bone-like tissues. J Orthop Res. 1998;16:181–189. doi: 10.1002/jor.1100160205. [DOI] [PubMed] [Google Scholar]
- 54.Weinreb M, Shinar D, Rodan GA. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J Bone Miner Res. 1990;5:831–842. doi: 10.1002/jbmr.5650050806. [DOI] [PubMed] [Google Scholar]
- 55.Van der Kraan P, Buma P, Van Kuppevelt T, Van den Berg W. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthr Cartilage. 2002;10:631–637. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 56.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik A, Bonow RO. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]