Abstract
G-proteins mediate cellular function upon interaction with G-protein coupled receptors. Of the 16 mammalian G-protein α subunits identified, G-protein subunit α-11 (GNA11) and -14 (GNA14) have been implicated in modulating hypertension and endothelial function. However, little is known about their expression and roles in human placentas. Here, we examined GNA11 and GNA14 protein expression in first trimester (FT), normal term (NT), and severe preeclamptic (sPE) human placentas as well as in NT human umbilical cords. We found that GNA11 and GNA14 were immunolocalized primarily in trophoblasts, villous stromal cells, and endothelial cells in placentas as well as in endothelial and/or smooth muscle cells of the umbilical cord artery and vein. Western blotting revealed that the GNA14, but not GNA11, protein levels were increased (2.5-2.9 fold; p<0.01) in sPE vs. NT placentas. GNA11 protein was detected only in NT, but not FT, placentas, whereas GNA14 protein levels were increased (7.7-10.6 fold; p<0.01) in NT vs. FT placentas. Thus, GNA11 and GNA14 may mediate the function of several cell types in placentas. Moreover, the high expression of GNA14 in sPE placentas may also imply its importance in sPE pregnancies as in the other hypertension-related disorders.
Keywords: G-protein, placenta, pregnancy, preeclampsia
Introduction
During pregnancy, normal placental growth and development, in association with remarkable vascular formation and growth, are required to provide effective materno-fetal exchange in support of the rapid growing fetus (Reynolds and Redmer 2001). Thus, impaired placental development, such as that seen with inadequate trophoblast invasion or dysfunction of the placental vasculature, could contribute to pregnancy complications, such as preeclampsia and intrauterine growth retardation (Cerdeira and Karumanchi 2012).
Preeclampsia, a leading cause of neonatal and maternal morbidity and mortality, occurs in approximately 5-10% of all pregnancies worldwide and is characterized by the onset of high blood pressure and proteinuria (Solomon and Seely 2004, 2006). To date, the exact etiology of preeclampsia remains elusive; however, defective placentation, placental ischemia, and endothelial dysfunction are thought to be three major insults associated with the increased risk of preeclampsia (Solomon and Seely 2004, 2006).
G-proteins mediate a diverse array of cellular functions upon interaction with G-protein coupled receptors (GPCRs), which represent by far the largest family of cell-surface molecules (Neves et al. 2002; Dorsam and Gutkind 2007). The heterotrimeric G-protein comprises α-, β- and γ-subunits, the latter two forming an indissociable complex (Wettschureck and Offermanns 2005; Hubbard and Hepler 2006). The mammalian Gα subunit, which binds to and hydrolyzes guanosine-5’-triphosphate (GTP) and thus defines the basic properties of a G-protein, can be grouped into four families: Gαs, Gαi, Gαq, and Gα12/13 (Wettschureck and Offermanns 2005; Hubbard and Hepler 2006). Each family further contains various members. For example, the Gαq family consists of Gαq, Gα11 (GNA11), Gα14 (GNA14), and Gα15/16. Upon activation, these G-proteins induce a number of downstream signal molecules (e.g., PLC, ERK1/2, and Ca++), thereby initiating cellular processes (Wettschureck and Offermanns 2005; Hubbard and Hepler 2006). The importance of the Gα subunit in the fetus has been recognized since the knockdown of Gα12/13, Gαq, and GNA11 in mice results in intrauterine fetal death, largely due to severe defects in fetal vasculature (Offermanns et al. 1997, 1998; Ruppel et al. 2005). Recent evidence has also shown that GNA11 is an essential mediator in the activation of vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) and is required for the VEGF-stimulated cell migration and proliferation as well as VEGF-induced ERK1/2 activation in human umbilical vein endothelial cells (HUVECs) (Zeng et al. 2002, 2003). Moreover, although GNA14 has received much less attention, it has been identified as a hypertension-susceptibility gene in humans (Kohara et al. 2008). Along the same lines, proteomic analysis also revealed that GNA14 protein expression was significantly increased in lung tissues from patients with pulmonary artery hypertension (Abdul-Salam et al. 2010), suggesting importance of GNA14 in hypertension-related diseases.
The expression of GNA11 and GNA14 has been detected in various mammalian tissues including brain, heart, lung, liver, kidney, thyroid, testis, and skeletal muscle tissues (Nakamura et al. 1991; Wilkie et al. 1991; Laugwitz et al. 1996). However, information on the cellular distribution and expression of GNA11 and GNA14 in human placentas is lacking. Thus, in this study, we examined GNA11 and GNA14 protein expression in human placentas obtained from the first trimester (FT), normal term (NT), and severe preeclamptic (sPE) pregnancies, as well as in human umbilical cord vessels from NT pregnancies using immunohistochemistry and/or Western blot analysis.
Materials & Methods
Collection of Placental Tissues
Two sets of placental tissue samples were collected from two different sites. The first set of samples were collected from NT (n=10; nine were from vaginal delivery and one from cesarean section delivery) and sPE (n=10; all were from cesarean section delivery) pregnancies in the Meriter Hospital, Madison, WI, as previously described (Chung et al. 2004; Jiang et al. 2010). Umbilical cords from NT pregnancies were also collected. The tissue collection protocol was approved by the Institutional Review Board of Meriter Hospital, and the Health Sciences Institutional Review Board of the University of Wisconsin-Madison, and followed the recommended guidelines for using human subjects.
The second set of samples were obtained from FT (n=10) and NT (n=10) pregnancies in Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, China. These FT placentas were collected from patients with induced abortion at the gestational age of 6-8 weeks, as described elsewhere (Hao et al. 2012). Collection of the placentas was approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital. Written informed consent to participate in the study was obtained from each patient.
Preeclampsia was defined according to the standard criteria (National High Blood Pressure Education Program 2000). Preeclampsia was considered severe if one or more of the following criteria were present: maternal blood pressure higher than or equal to 160/110 mmHg on two separate readings; proteinuria more than 2+ by dipstick or more than 2 g/24 hr. None of the study subjects had signs of infection. Smokers were excluded. Patient ages were similar (23 ± 0.9 years) between NT and sPE pregnancies. Gestational ages for NT pregnancies (39 ± 0.2 weeks) were significantly (p<0.05) higher than those for sPE pregnancies (34 ± 0.7 weeks). Fetal weights for NT pregnancies (3404 ± 90.9 g) were higher (p<0.05) than in sPE pregnancies (2198 ± 236.4 g). Each of these parameters was analyzed using the Student’s t-test.
Placental villi from beneath the chorionic and basal plates were quickly dissected (~10 g each), snap-frozen, and stored in liquid nitrogen for Western blot analysis. Additional placental tissues and umbilical cords were fixed overnight at 4C in 4% paraformaldehyde in 10 mM PBS and embedded in paraffin for immunohistochemistry.
HUVECs were also isolated from NT pregnancies and cultured as described previously (Wang et al. 2009; Dai et al. 2011). At passage 2, five cell preparations were pooled and expanded. These HUVECs express GNA11 and GNA14 (Zeng et al. 2002, 2003; Jiang et al. 2013), and were used as a standard in the Western blot analysis.
Validation of GNA11 and GNA14 Antibodies
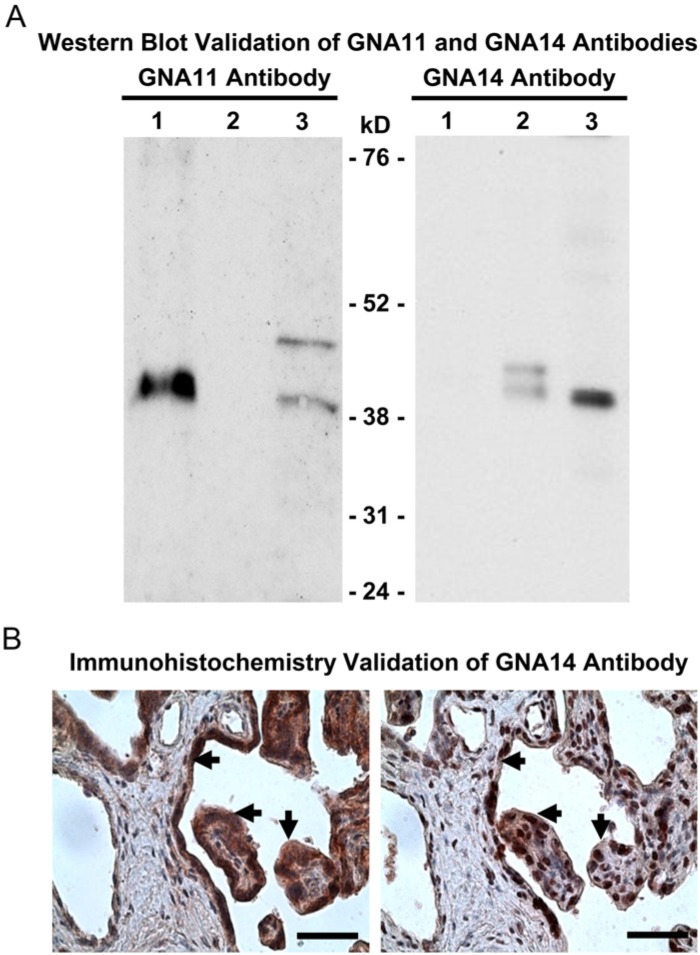
A rabbit antibody against human GNA11 antibody (cat # AP19441) was purchased from Abgent (San Diego, CA). The specificity of the GNA11 antibody was confirmed in cell lysates from GNA11-overexpressing 293T cells (10 µg; sc-120367, Santa Cruz Biotech, Santa Cruz, CA; a positive control), GNA14-overexpressing 293T cells (10 µg; sc-174410, Santa Cruz Biotech, a negative control), and HUVECs (20 µg; a positive control) using Western blotting (see below). As shown in Fig. 1A, this GNA11 antibody detected a single band at ~42 kDa in GNA11-overexpressing 293T cells and a predominant ~42 kDa band in HUVECs; however, no band was observed between 24-76 KDa in GNA14-overexpressing 293T cells.
Figure 1.
Validation of GNA11 and GNA14 antibodies. (A) Western blot validation of GNA11 and GNA14 antibodies. Lane 1: GNA11-overexpressing 293T cells (10 µg protein); Lane 2: GNA14-overexpressing 293T cells (10 µg protein); Lane 3: HUVECs (20 µg protein). (B) Immunohistochemistry validation of GNA14 antibody in human placentas (n=4) from severe preeclamptic (sPE) pregnancies. After counterstaining with hematoxylin, the adjacent tissue sections were probed with the GNA14 antibody alone (4 μg/ml, left panel) or the GNA14 antibody pre-immunoneutralized with its peptide immunogen (right panel). Representative images are shown. Arrows: syncytiotrophoblasts. Bar, 50 µm.
GNA14 antibody was generated by immunizing 2 rabbits with synthesized peptides based on the predicted sequence of human GNA14 (GenBank access # NM_004297) by GeneTel, Madison, WI. The antisera were pooled, affinity purified, and used for immunohistochemistry and Western blot analysis. The specificity of the GNA14 antibody was first verified in cell lysates from GNA11-overexpressing 293T cells (5 µg; a negative control), GNA14-overexpressing 293T cells (5 µg; a positive control), and HUVECs (20 µg; a positive control) using Western blotting. As shown in Fig. 1A, GNA14 antibody detected one predominant band at ~42 kDa in GNA14-overexpressing 293T cells and HUVECs, but not in GNA11-overexpressing 293T cells. The specificity of the GNA14 antibody was further confirmed in human placental tissue sections using immunohistochemistry (Fig. 1B). Placental tissue sections were probed with the GNA14 antibody (4 μg/ml) or with the GNA14 antibody which was preimmunoneutralized at 4C overnight with an excess amount (100-fold) of the synthesized GNA14 peptide immunogen. We observed that the synthesized GNA14 peptide immunogen greatly decreased the intensity of the GNA14 staining (Fig. 1B). In addition, in preliminary experiments, we also transfected HUVECs with specific GNA14 siRNA, and detected a significant decrease in the protein expression of GNA14, but not GNA11 (data not shown). All of these data attest to the specificity of the GNA14 antibody.
Immunohistochemistry
Immunolocalization of GNA11 and GNA14 was visualized by indirect detection via the avidin: biotinylated-peroxidase complex method (Vector Laboratories, Burlingame, CA), as previously described (Chung et al. 2004; Jiang et al. 2010). Paraffin-embedded tissue sections (n=4–5 for each experimental group) were cut at 5-μm thickness. Antigen retrieval was performed in sodium citrate buffer solution (10 mM, pH 6.5) before staining. After endogenous peroxidase quenching with 3% H2O2, tissue sections were counterstained lightly with hematoxylin. Nonspecific binding was blocked with 1% horse serum albumin. Tissue sections were then probed with a rabbit antibody against human GNA11 (4 μg/ml; Abgent) or GNA14 (4 μg/ml; GeneTel).
The controls consisted of replacing the primary antibody with preimmune rabbit IgG at the same concentration as the primary antibody. The secondary antibody used was a biotinylated universal antibody. The specific immunoreactivity was visualized by 3-amino-9-ethylcarbazole (Vector Laboratories).
Western Blot Analysis
Western blot analysis was conducted as described (Chung et al. 2004; Jiang et al. 2010). Placental tissues were homogenized and lysed by sonication in buffer (50 mM HEPES, 0.1 M NaCl, 10 mM EDTA, 4 mM sodium pyrophosphate, 10 mM sodium fluoride, 2 mM sodium orthovanadate [pH 7.5], 1 mM phenylmethylsulfonylfluoride, 1% Triton X-100, 5 µg/ml leupeptin, 5 µg/ml aprotinin). After centrifugation, protein concentrations of the supernatant were determined with BSA (fraction V; Sigma, St. Louis, MO) as a standard. Protein samples of placental tissue supernatants (50 or 100 μg/sample) were separated on 10% SDS-PAGE gels, and electrically transferred to polyvinylidene difluoride membranes. In parallel, supernatants of HUVECs (20 µg protein/sample) were included in each gel as standards.
The membranes were first probed with GNA11 (1:1000) or GNA14 antibody (1:500), followed by reprobing with glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:10,000; Novus, Littleton, CO) and β-actin (1:10,000; Life Technologies, Grand Island, NY) as loading controls. Proteins were visualized using enhanced chemiluminescence (ECL) reagents from Amersham Biosciences (Piscataway, NJ), followed by exposure to chemiluminescence films. The immunoreactive signals were analyzed by densitometry using NIH Image-J imaging analysis software (Bethesda, MD).
Statistical Procedures
Data were analyzed using t-test (SigmaStat, Jandel Co., San Rafael, CA). Differences were considered significant at p<0.05.
Results
Immunolocalization
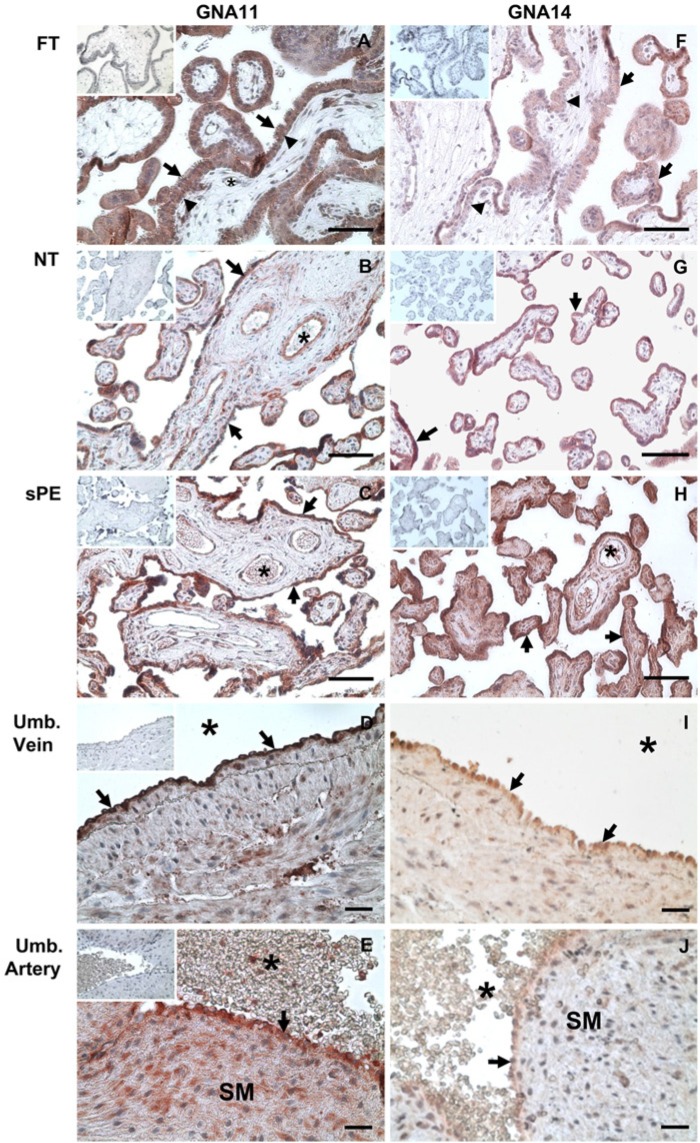
To determine the cellular distribution of GNA11 and GNA14 in human placentas and umbilical cord vessels, immunohistochemistry was conducted. We observed that GNA11 and GNA14 were localized in the FT, NT and sPE placentas as well as in umbilical cord vessels (Figs. 1 and 2). In placental villi, GNA11 and GNA14 were present primarily in syncytiotrophoblasts and cytotrophoblasts in FT, and syncytiotrophoblasts in NT and sPE placentas (Fig. 2A-2C and 2F-2H). GNA11 and GNA14 were also present in villous stromal cells and vascular endothelial cells (Fig. 2A-2C and 2F-2H). In addition, GNA14 was also detected in erythrocytes in NT and sPE placentas (Fig. 2H). In umbilical cords, GNA11 and GNA14 was immunolocalized in endothelial cells of the umbilical cord artery and vein (Fig. 2D, 2E, 2I, and 2J), whereas only GNA11 was present in the smooth muscle cells of the umbilical artery (Fig. 2E). No positive GNA11 and GNA14 staining was observed in the IgG control in all tissues studied. These data indicate that GNA11 and GNA14 are expressed in multiple cell types in human placentas and umbilical cords.
Figure 2.
Immunolocalization of GNA11 and GNA14 in human placentas from first trimester (FT), normal term (NT), and severe preeclamptic (sPE) pregnancies as well as in the umbilical (Umb.) vein and artery from NT pregnancies. The tissue sections (n=4–5 placentas for each group) were probed with GNA11 or GNA14 antibodies (4 μg/ml) after light counterstaining with hematoxylin. The preimmune IgG controls (4 μg/ml) are shown in the inset images. SM: smooth muscle layer. *: lumen of blood vessels; arrows: syncytiotrophoblasts in placental tissues and endothelial cells in umbilical vessels; arrow heads: trophoblasts. Bar, 50 µm.
Western Blot Analysis
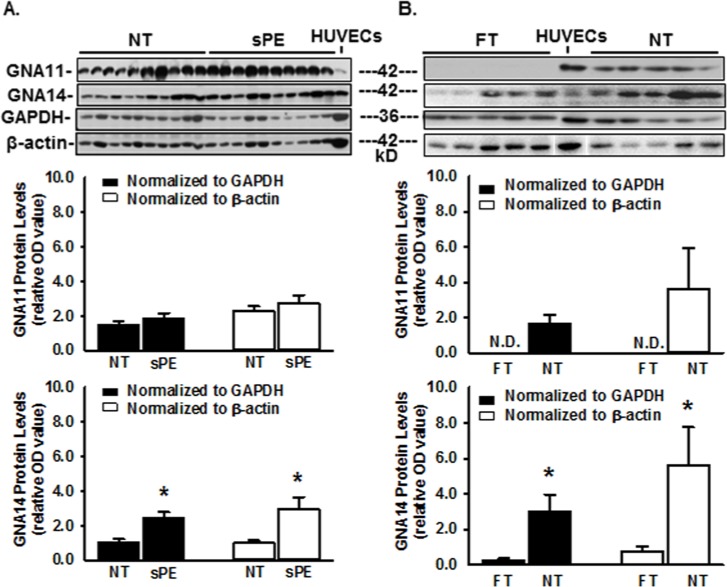
To quantify changes in GNA11 and GNA14 proteins in human placentas, Western blot analysis was performed. Both GNA11 and GNA14 were detected at ~42 kDa in human placental tissues and in HUVECs (positive control) (Figs. 1 and 3), corresponding to the reported molecular masses of GNA11 (UniProtKB # P29992; Nakamura et al., 1991; Wilkie et al., 1991; Laugwitz et al., 1996 ) and GNA14 (UniProtKB # O95837; Wilkie et al., 1991). When normalized to GAPDH and β-actin, the levels of GNA14, but not GNA11 protein were increased (p<0.01) by ~2.5- and 2.9-fold, respectively, in sPE vs. NT placentas(Fig. 3A). GNA11 was undetectable in FT but was abundantly present in NT placentas (Fig. 3B). When normalized to GAPDH and β-actin, the protein levels of GNA14 were increased (p<0.01) by ~10.7- and 7.7-fold, respectively, in NT vs. FT placentas (Fig. 3B). These data demonstrate that, whereas the protein levels of both GNA11 and GNA14 in placentas are increased from FT to NT, only the GNA14, and not GNA11, protein levels are elevated in sPE vs. NT placentas.
Figure 3.
Western blot analysis for GNA11 and GNA14 in human placentas and in HUVECs. (A) Placentas from normal term (NT) and severe preeclamptic (sPE) pregnancies (n=10/group) and HUVECs from NT pregnancies. (B) Placentas from FT and NT pregnancies (n=10/group). A representative image is shown for Panel B. Proteins (100 μg in A and 50 μg in B for each placental sample, as well as 20 μg for HUVECs) were subjected to Western blotting. Data are expressed as mean ± SEM of OD values normalized to GAPDH or β-actin. N.D.: non-detectable. *p<0.01.
Discussion
In the current study, we have demonstrated the cellular distribution and expression of GNA11 and GNA14 proteins in human placentas from FT, NT and sPE pregnancies. We found that GNA11 and GNA14 were present in several cell types in placentas and umbilical cord vessels (e.g., trophoblast cells, endothelial cells, vascular smooth muscle cells, and villous stromal cell). More importantly, we observed that, as the protein levels of both GNA11 and GNA14 in placentas exhibited similar increases from in FT to NT, only GNA14 was further elevated in sPE as compared to NT. Thus, together with the evidence resulting from the knockout mice (Offermanns et al. 1997,1998), a systemic multiple gene approach (Kohara et al. 2008), and in vitro studies (Zeng et al. 2002, 2003), our current data suggest that although both GNA11 and GNA14 may affect the function of multiple human placental cells, GNA14 may have a unique role in sPE placentas as in other hypertension-related diseases, such as hypertension and pulmonary artery hypertension (Kohara et al. 2008; Abdul-Salam et al. 2010).
It is not surprising that GNA11 and GNA14 are expressed in syncytiotrophoblasts, trophoblasts, stromal cells and endothelial cells in all placentas from FT, NT and sPE pregnancies because GNA11 and GNA14 are known to be expressed in many cell types of various mammalian tissues (Nakamura et al. 1991; Wilkie et al. 1991; Laugwitz et al. 1996). Thus, given that GNA11 and GNA14 are critically involved in mediating fetal vascular development (Offermanns et al. 1998; Kohara et al. 2008) and endothelial function (Zeng et al. 2002, 2003), these data suggest that both GNA11 and GNA14 may also mediate functions of these placental cells.
The current finding that only the GNA14, and not GNA11, protein levels were elevated in sPE over NT placentas implies that GNA14 may be a key mediator in placentas from sPE pregnancies, in which hypertension is one of the hallmarks (Solomon and Seely 2004, 2006). This observation is extremely interesting, as other investigators have reported that GNA14 expression is also high in lung tissues from patients with pulmonary artery hypertension (Abdul-Salam et al. 2010). Thus, our current data support the notion that GNA14 is a hypertension-susceptibility gene in humans (Kohara et al. 2008) and suggest that GNA14 overexpression might be used as an index for predicting hypertension-related diseases, especially when in conjunction with other clinical diagnoses. To date, it is unclear what are the underlying mechanisms elevating GNA14 expression. However, we have recently shown that chronic low oxygen significantly increases expression of GNA14 mRNA in HUVECs (Jiang et al. 2013). Thus, chronic low oxygen and/or hypoxia within the tissues may upregulate GNA14 expression in the placenta tissues. Moreover, the exact role of GNA14 in hypertension also remains elusive. Nonetheless, because many hypertension-related diseases are associated with endothelial dysfunction (Ross 1999; Berk et al. 2000; Granger et al. 2001) and endothelium is one of major cell types expressing GNA14 (Fig. 2), it is possible that GNA14 overexpression in endothelial cells may cause endothelial dysfunction (e.g., decreased vasodilator production and release or increased vasoconstrictor production and release), leading to hypertension-related diseases.
One may consider that the different expression of GNA14 between NT and sPE placentas is due to the different gestational ages of sPE and NT pregnancies, as observed in the current study. However, the protein levels of both GNA11 and GNA14 were increased in placentas from FT to NT pregnancies (Fig. 3B), suggesting an increasing trend in the expression of placental GNA11 and GNA14 proteins from early pregnancy to full term. Thus, together with the observation that only GNA14 protein levels were elevated in sPE placentas (Fig. 3A), it is unlikely that the shorter gestational age in PE pregnancies would be a major factor contributing to high GNA14 expression in sPE placentas, unless GNA14 expression uniquely (relative to GNA11) varies in a biphasic fashion (e.g., low in FT, high in ~33 weeks, and low again in NT).
In conclusion, the current data suggest that GNA11 and GNA14 may play important roles in mediating normal cellular function in human placentas; however, GNA14 overexpression in placentas may contribute to placental cellular dysfunction during sPE pregnancies, a hypertension-related disease. Further studies are warranted and are currently underway to explore the actions and signaling mechanisms of GNA11 and GNA14 in placental cells.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is partially supported by the National Institutes of Health Grants HD38843 (RRM/JZ), and the R & D grant from Department of Ob/Gyn, University of Wisconsin-Madison (JZ), by the National Science Foundation of China No. 81100429 (KW), and Shanghai Natural Science Foundation No.11 ZR1428700 (KW).
References
- Abdul-Salam VB, Wharton J, Cupitt J, Berryman M, Edwards RJ, Wilkins MR. (2010). Proteomic analysis of lung tissues from patients with pulmonary arterial hypertension. Circulation 122:2058-2067 [DOI] [PubMed] [Google Scholar]
- Berk BC, Haendeler J, Sottile J. (2000) Angiotensin II, atherosclerosis, and aortic aneurysms. J Clin Invest 105:1525-1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdeira AS, Karumanchi SA. (2012). Angiogenic factors in preeclampsia and related disorders. Cold Spring Harb Perspect Med 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Song Y, Wang Y, Magness RR, Zheng J. (2004). Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab 89:2484-2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CF, Jiang YZ, Li Y, Wang K, Liu PS, Patankar MS, Zheng J. (2011). Expression and roles of Slit/Robo in human ovarian cancer. Histochem Cell Biol 135:475-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. (2007). G-protein-coupled receptors and cancer. Nat Rev Cancer 7:79-94 [DOI] [PubMed] [Google Scholar]
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. (2001). Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension 38:718-722 [DOI] [PubMed] [Google Scholar]
- Hao KH, Zhou Q, He QZ, Zheng J, Wang K. (2012) Protein expression of aryl hydrocarbon receptors in human placentas from mild preeclamptic and early pregnancies. In: Recent Advances in Research on the Human Placenta. Ed Zheng J, InTech-Open Access, New York, NY, pp. 119-126 [Google Scholar]
- Hubbard KB, Hepler JR. (2006). Cell signalling diversity of the Gqα family of heterotrimeric G proteins. Cell Signal 18:135-150 [DOI] [PubMed] [Google Scholar]
- Jiang YZ, Wang K, Fang R, Zheng J. (2010). Expression of aryl hydrocarbon receptor in human placentas and fetal tissues. J Histochem Cytochem 58:679-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YZ, Wang K, Li Y, Dai CF, Wang P, Kendziorski C, Chen DB, Zheng J. (2013). Transcriptional and functional adaptations of endothelial cells to physiological chronic low oxygen. Biol Reprod 88: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Tabara Y, Nakura J, Imai Y, Ohkubo T, Hata A, Soma M, Nakayama T, Umemura S, Hirawa N, Ueshima H, Kita Y, Ogihara T, Katsuya T, Takahashi N, Tokunaga K, Miki T. (2008). Identification of hypertension-susceptibility genes and pathways by a systemic multiple candidate gene approach: the millennium genome project for hypertension. Hypertens Res 31:203-212 [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G. (1996). The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci U S A 93:116-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Ogata K, Shiozaki K, Kameyama K, Ohara K, Haga T, Nukada T. (1991). Identification of two novel GTP-binding protein α-subunits that lack apparent ADP-ribosylation sites for pertussis toxin. J Biol Chem 266:12676-12681 [PubMed] [Google Scholar]
- National High Blood Pressure Education Program. Working group report on high blood pressure in pregnancy. (2000). NHLBI (NIH) Publication No. 00-3029. [Google Scholar]
- Neves SR, Ram PT, Iyengar R. (2002). G protein pathways. Science 296:1636-1639 [DOI] [PubMed] [Google Scholar]
- Offermanns S, Mancino V, Revel JP, Simon MI. (1997). Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science 275:533-536 [DOI] [PubMed] [Google Scholar]
- Offermanns S, Zhao LP, Gohla A, Sarosi I, Simon MI, Wilkie TM. (1998). Embryonic cardiomyocyte hypoplasia and craniofacial defects in Gαq/Gα11-mutant mice. EMBO J 17:4304-4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. (2001). Angiogenesis in the placenta. Biol Reprod 64:1033-1040 [DOI] [PubMed] [Google Scholar]
- Ross R. (1999). Atherosclerosis-an inflammatory disease. N Engl J Med 340:115-126 [DOI] [PubMed] [Google Scholar]
- Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, Yin L, Xu SM, Coughlin SR. (2005). Essential role for Gα13 in endothelial cells during embryonic development. Proc Natl Acad Sci U S A 102:8281-8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon CG, Seely EW. (2004). Preeclampsia-searching for the cause. N Engl J Med 350:641-642 [DOI] [PubMed] [Google Scholar]
- Solomon CG, Seely EW. (2006). Hypertension in Pregnancy. Endocrinol Metab Clin N A. 35: 157-171 [DOI] [PubMed] [Google Scholar]
- Wang K, Jiang YZ, Chen DB, Zheng J. (2009). Hypoxia enhances FGF2- and VEGF-stimulated human placental artery endothelial cell proliferation: roles of MEK1/2/ERK1/2 and PI3K/AKT1 pathways. Placenta 30:1045-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. (2005). Mammalian G proteins and their cell type specific functions. Physiol Rev 85:1159-1204 [DOI] [PubMed] [Google Scholar]
- Wilkie TM, Scherle PA, Strathmann MP, Slepak VZ, Simon MI. (1991). Characterization of G-protein α subunits in the Gq class: expression in murine tissues and in stromal and hematopoietic cell lines. Proc Natl Acad Sci U S A 88:10049-10053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Zhao D, Mukhopadhyay D. (2002). KDR stimulates endothelial cell migration through heterotrimeric G protein Gq/11-mediated activation of a small GTPase RhoA. J Biol Chem 277:46791-46798 [DOI] [PubMed] [Google Scholar]
- Zeng H, Zhao D, Yang S, Datta K, Mukhopadhyay D. (2003). Heterotrimeric Gαq/Gα11 proteins function upstream of vascular endothelial growth factor (VEGF) receptor-2 (KDR) phosphorylation in vascular permeability factor/VEGF signaling. J Biol Chem 278:20738-20745 [DOI] [PubMed] [Google Scholar]