Abstract
The cardiac extracellular matrix (ECM) is a dynamic structure, adapting to physiological and pathological stresses placed on the myocardium. Deposition and organization of the matrix falls under the purview of cardiac fibroblasts. While often overlooked compared to myocytes, fibroblasts play a critical role in maintaining ECM homeostasis under normal conditions and in response to pathological stimuli assume an activated, myofibroblast phenotype associated with excessive collagen accumulation contributing to impaired cardiac function. Complete appreciation of fibroblast function is hampered by the lack of fibroblast-specific reagents and the heterogeneity of fibroblast precursors. This is further complicated by our ability to dissect the role of myofibroblasts versus fibroblasts in myocardial in remodeling. This review highlights critical points in the regulation of collagen deposition by fibroblasts, the current panel of molecular tools used to identify fibroblasts and the role of fibroblast-matrix interactions in fibroblast function and differentiation into the myofibroblast phenotype. The clinical potential of exploiting differences between fibroblasts and myofibroblasts and using them to target specific fibroblast populations is also discussed.
Keywords: extracellular matrix, fibroblast, myofibroblast, integrin, Discoidin domain receptor, fibroblast specific protein 1, fibroblast activation protein, transformation
1. Introduction
The cardiac extracellular matrix (ECM) is comprised of two basic structural organizations: the basal lamina, which surrounds individual myocytes and blood vessels, and the interstitial matrix which provides structural support for higher order cardiac myocyte organization as well as for larger blood vessels in the myocardium [1]. While the molecules within the ECM are diverse, structural ECM proteins include the fibrillar collagens which play a role in maintaining myocyte alignment, mechanically coupling cells within the myocardium and in preserving the structural integrity of the myocardial walls [2]. The organization of the cardiac collagen network is established shortly after birth and this ECM architecture persists in the normal adult heart. However, changes in the organization and composition of the ECM, particularly fibrillar collagen, are a structural milestone in the development and progression of heart failure, irrespective of etiology.
Fibrillar collagen expression, synthesis and post-translational modification is a fundamental role of the fibroblast. Within the myocardium, fibroblasts represent a rather poorly defined cell population compared to the other resident cell types (myocytes, endothelial and smooth muscle cells). Fibroblasts are most commonly defined as cells of mesenchymal origin, having an elongated morphology with multiple cell processes extending from their surface, and cells which lack a basement membrane [3]. For decades the main function assigned to cardiac fibroblasts was to maintain ECM homeostasis. Fibroblasts have an extensive Golgi and endoplasmic reticulum network and can produce virtually all of the cardiac ECM components, including a multiple collagens, glycoproteins such as fibronectin and laminin, and a variety of proteoglycans [4]. While the fibroblast plays a significant role in normal ECM homeostasis, little is known regarding the function of “normal” fibroblasts, as most studies focus upon fibroblast form and function during development or in specific cardiac disease states such as pressure overload or ischemia/infarction. The purpose of this review is to examine the definition of the myocardial fibroblast in terms phenotype and function, with a particular focus on fibrillar collagen synthesis, examine specific receptors that mediate fibroblast-ECM interactions and finally to place these profiles and processes in a translatable, clinical context.
2. The Cardiac Extracellular Matrix
The primary types of fibrillar collagens in the heart are collagen types I and III [5]. Collagen V, is also expressed in the heart and evidence supports a role for this collagen in providing a seed for fibrillar collagen assembly [6]. Production and secretion of fibrillar collagens requires a number of intracellular proteins that modify and assemble the trimeric collagen molecules prior to secretion [7]. HSP47 and prolyl hydroxylase are two examples of proteins that are required for assembly and subsequent secretion of triple helical collagen [7]. Fibrillar collagens are secreted in the form of soluble procollagens with N and C-terminal propeptides that prevent insoluble deposition of collagen. The propeptides are removed by specific proteases in the pericellular milieu [8]. ADAMTS 2 has been shown to cleave the N-terminal propeptide whereas BMP-1 is the protease primarily responsible for cleaving the C-propeptide [9, 10]. PCPE 1 and 2, enhancers of BMP-1 activity, facilitate the cleavage of the C-propeptide [11, 12]. Lack of PCPE-2 expression has been shown to reduce collagen accumulation in response to pressure-overload in the TAC model, demonstrating the importance of procollagen propeptide processing in cardiac collagen deposition [13]. Hence, strategies to limit procollagen processing might be worthwhile avenues of pursuit in attempts to limit tissue fibrosis.
Fibronectin expression is also a critical factor in cardiac ECM, particularly in developing myocardium and in response to injury [14, 15]. Fibronectin levels have been found to be higher in neonatal hearts when compared to adult [15, 16]. In addition, neonatal cardiac fibroblasts expressed increased amounts of fibronectin versus adult fibroblasts [16]. Recently, expression of fibronectin was shown to influence cardiac progenitor cell response after myocardial infarction in adult mice [17]. The splice variant, EDA-fibronectin is expressed in association with myofibroblast differentiation. In fact, expression of EDA-fibronectin has been proposed to be requisite for myofibroblast conversion [18]. Mice that lack this ED-A splice variant of fibronectin were found to deposit significantly less collagen in response to myocardial infarction [19]. A significant loss of myofibroblasts in the infarcted myocardium as well as a reduction in inflammatory cell recruitment was associated with the reduced collagen content in mice that do not express EDA-fibronectin [19].
In addition to structural ECM components, matricellular proteins are also expressed in the heart, particularly in response to injury and collagen deposition [20]. A lack of thrombospondin-1, for example, was shown to result in significant decreases in collagen accumulation [21]. Interestingly, an increase in the number of α-smooth muscle actin (α-SMA) positive myofibroblasts was associated with the absence of thrombospondin 1; however, these myofibroblasts were deemed dysfunctional due to a lack of fibrosis in the hearts of these mice. Two other matricellular proteins, tenascin C and SPARC are also associated with myofibroblasts. Whereas tenascin C is implicated in fibroblast migration and promotes the recruitment of cardiac myofibroblasts, SPARC is a primary contributor to collagen accumulation in the heart [22, 23, 24]. A lack of SPARC expression results in less collagen in response to pressure overload, myocardial infarction, and aging [25]. Hence, targeting of matricellular proteins might provide additional prospective avenues into alleviating cardiac fibrosis.
3. Cardiac Fibroblasts – A Dynamic Cell Population
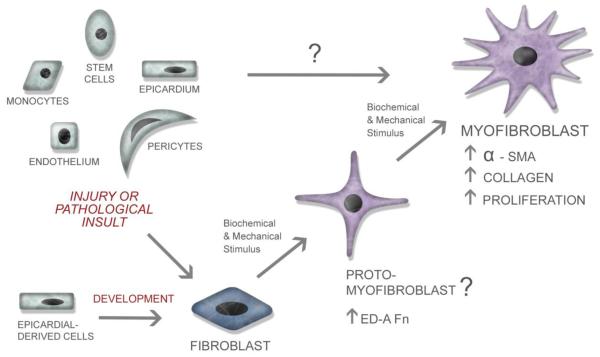
While once thought of as a homogenous population, it is becoming clear that cardiac fibroblasts can arise from multiple sources (Figure 1). During development, the vast majority of cardiac fibroblasts arise from epicardial derived cells (EPDCs). Cells within the proepicardium undergo epithelial to mesenchymal transformation (EMT) and migrate onto the surface of the heart giving rise to the epicardium [26, 27, 28]. These epicardial cells then undergo EMT producing EPDCs which can migrate into the myocardium, giving rise to interstitial fibroblasts [26, 27, 28]. Using expression of Discoidin Domain Receptor 2 to track the appearance of fibroblasts in the developing heart, DDR2 positive cells were first observed on the epicardial surface at ED11 and progressively migrated across the ventricular free wall, appearing throughout the myocardium by postnatal day four [29]. The distribution of fibroblasts within the neonatal myocardium is virtually indistinguishable from that in the adult, where fibroblasts are associated with the endomysial collagen network surrounding myocytes and are interconnected to one another and myocytes through gap junctions [30]. Overall, myocardial cell populations have been shown to be dynamic during adaptive, load dependent remodeling in development [31] and, in the adult rat heart, fibroblasts constitute almost two thirds of the myocardial cell population [31, 32, 33].
Figure 1. The Cardiac Fibroblast.
As shown in this figure, cardiac fibroblasts arise from divergent sources during development and in response to pathological insult. In response to various biochemical and mechanical stimuli, fibroblasts undergo a differentiation/transformation into a myofibroblastic phenotype. An intermediate proto-myofibroblast stage has been proposed for some fibroblasts [86], but it is unclear whether cardiac fibroblasts adopt this intermediary cell phenotype. In pathological conditions, when numerous cell types infiltrate the myocardium it is unknown if any of these cells transform directly into myofibroblasts or must they first differentiate into a fibroblast.
In response to a number of pathological insults, including myocardial infarction, pressure overload or ischemia/reperfusion, ventricular remodeling occurs accompanied by altered deposition of collagen and an increase in fibroblasts. Characterization of fibroblast proliferation during infarct healing [34] and in pressure overload models [35, 36, 37] indicate that fibroblasts undergo proliferation in response to pathological cues. In contrast to development, pathological stimuli results in the recruitment of fibroblasts from a number of sources including proliferation of resident fibroblasts, fibroblasts derived from endothelial cells (Endo EMT), stem cells (including bone marrow derived and mesenchymal), pericytes and myeloid-derived fibroblasts (Figure 1) [38, 39, 40, 3]. Given that fibroblasts are recruited from multiple sources, it is tempting to speculate that their initial source of origin may contribute to their ultimate function within the heart. Indeed, recent studies characterizing the origin of fibroblasts found in infarcts and ischemia/reperfusion injury suggest that two distinct cell populations, endogenous mesenchymal stem cells [39, 41] and myeloid fibroblasts [39, 42] respectively, are responsible for collagen deposition after these insults. Identification specific precursor cells which contribute to distinct injury-related fibroblast populations could provide a novel avenue for intervention to mediate fibrosis or adverse remodeling.
4. Cardiac Fibroblast Markers
The ability to identify myocardial fibroblasts has been a problematic issue and as such can yield difficulties in the interpretation of fibroblast form and function in the context of LV remodeling and failure. This is particularly true if changes in fibroblast phenotype occur in a cardiac disease state, which in turn can cause differential protein expression from ambient normal conditions. Nevertheless, there are several types and classes of proteins which have been utilized to identify fibroblasts in a general sense, yet none to date have yielded a “myocardial specific” protein signature. Some of these markers which have been utilized to identify fibroblasts in the context of LV remodeling are summarized in Table 1.
Table 1.
Cardiac Fibroblast Markers. This table lists proteins which have been used or could be used to identify fibroblasts. Fsp-1 – fibroblast specific protein 1; DDR2 – Discoidin domain receptor 2; Col I – collagen type I; Vim – vimentin; FAP – fibroblast activation protein; MyoFb – myofibroblasts; PO – pressure overload hypertrophy; MI – myocardial infarction
| Marker | Protein Type/Function | Expression in | Observed in Cardiac Diseases | Reference | ||
|---|---|---|---|---|---|---|
| Fibroblasts | MyoFb | Non-fibroblasts | ||||
| Fsp-1 | Ca2+ binding protein | Yes | Yes | Macrophages, endothelial, smooth muscle, myocyted | PO, MI | 45,46,48,49 |
| DDR2 | Receptor tyrosine kinase | Yes | Yes | Smooth muscle, activated epithelial cells | MI | 60 – 62 |
| Col I | ECM structural protein | Yes | Yes | Osteoblasts, odontoblasts, smooth muscle cells, mesenchymal cells | PO, MI | |
| Vim | Intermediate filament protein (cytoskeleton) | Yes | Yes | Endothelial, immune system cells, mesenchymal-derived cells | PO, MI | 72, 74 |
| FAP | Membrane bound serine protease | No (?) | Yes | ? | Atheroscl erosis | 80 |
4.1 Fibroblast Specific Protein
Fibroblast specific protein (FSP1), also known as SA100A4, is a member of the S100 family of intracellular calcium binding proteins which was originally identified in a comparative transcript analysis between fibroblasts and epithelial cells [43]. FSP1 expression was only observed in fibroblasts and not other cell lines, including adipocytes, endothelial cells, lymphocytes, hepatocytes or osteoblasts, which were screened. Whole organ expression analysis demonstrated weak expression in heart and further experiments in this study and others suggested that FSP1 is important in epithelial-mesenchymal transformation [43, 44]. FSP1 has been shown to be up-regulated in rodent models of pressure overload due to aortic banding and myocardial infarction as well as human patients with ischemic cardiomyopathy [45]. In sham animals, FSP1 co-localized with markers for fibroblasts, myofibroblasts, macrophages, endothelial and smooth muscles cells but not cardiac myocytes. Similar localization was observed in both injury models; however, in one study myocytes in the infarct border zone were also positive for FSP1 [45]. While Schneider et al. indicate that FSP1 staining of cardiac myocytes is due to myocyte uptake of released FSP1 and not de novo synthesis, in an isoproterenol hypertrophy model FSP1 was localized throughout cardiac myocytes while weak FSP1 staining was observed in the intercalated disks of normal myocytes [46]. To modulate gene expression in fibroblasts, an FSP1-Cre reporter mouse was created [47] and has been used to examine virus mediated reprogramming of non-cardiomyocytes into myocytes post-MI [48]. Recently, Kong et al. reported lack of specificity of FSP1 expression following infarction and pressure overload in mice. These authors found significant numbers of hematopoietic cells, endothelial cells and vascular smooth cells that expressed FSP1 in these models [49]. The lack of fibroblast specificity of FSP1 is not unique to the heart with a recent liver injury study noting that FSP1 is expressed by macrophages but not by collagen expressing cells, either fibroblasts or myofibroblasts [50]. Taken together these studies indicate that using FSP1 as a fibroblast marker may need to be considered in a model specific context.
4.2 Discoidin Domain Receptor 2
Discoidin domain receptor 2 (DDR2) belongs to the receptor tyrosine kinase protein family and has as its ligand various types of collagens [51, 52]. DDR2 expression has been detected within heart and other organs [30, 53, 54] as well as in a number of cancers [55, 56]. DDR2 has been shown to be expressed throughout heart development and within the heart was only detected on fibroblasts [30] and activated epithelial and mesenchymal cells during epithelial-mesenchymal transformation in atrioventricular valve development [57]. However, DDR2 expression has also been detected on normal vascular smooth muscle cells [58] and in smooth muscle cells associated with atherosclerotic vessels [59]. DDR2 has been used as a marker to identify cardiac fibroblasts in a number of studies [60, 61, 62], but the lack of a cloned DDR2 promoter has prevented its use to drive gene expression in fibroblasts or as a fibroblast lineage tracer.
4.3 Collagen Type I
Collagen type I is the major fibrillar component of the cardiac ECM and one of many ECM proteins produced by fibroblasts. The promoter region for the pro-α1(I) chain of type I collagen has been well characterized and it has been shown that different regions of this promoter can be used to drive expression of genes in specific collagen producing cell types including fibroblasts, osteoblasts, odontoblasts and some mesenchymal cells [63, 64]. A number of commercially available reporter mice (Cre, fluorescent protein expressing, inducible) have been generated using the type I collagen α1 chain promoter. Type I collagen is also produced by smooth muscle cells in response to TGFβ, EGF and angiotensin II stimulation [65, 66], therefore use of a fibroblast-specific regulatory region from the pro-α1(I) chain promoter could provide a valuable tool for marking fibroblasts within the heart. The promoter region of the α2 chain of type I collagen has also been characterized and a fibroblast-specific regulatory sequence used to develop a tamoxifen-inducible Cre reporter mouse [67]. Crossing this mouse with the ROSA26 reporter and induction with tamoxifen demonstrated strong staining in the pericardium [67] and crossing with a Tbx18-floxed reporter mouse showed that epicardial derived cells contribute to the myocardial fibroblast population [68]. However, this mouse has also been used as a tool to regulate gene expression in osteoblasts, indicating that this region of the α2 promoter used is not purely fibroblast specific [69].
4.4 Vimentin
Vimentin, an intermediate filament protein composing part of the cell cytoskeleton, has also been used extensively to identify fibroblasts. Vimentin is expressed by cells of mesenchymal origin and is found in a number of different cell types including fibroblasts, endothelial cells and some immune system cells [70]. Although the vimentin promoter has been cloned, no vimentin promoter driven reporter mice are commercially available likely due to the heterogeneous population of cells which would be labeled. Within the heart, however, vimentin has been has been used to identify fibroblasts in a variety of studies including during development [71], to localize fibroblasts derived from mesenchymal stem cells injected after myocardial infarction [72], to compare myofibroblast versus fibroblast populations of mitral valve fibroblasts after mechanical loading [73], and in fibrosis associated with pressure overload hypertrophy [74].
4.5 Fibroblast Activation Protein
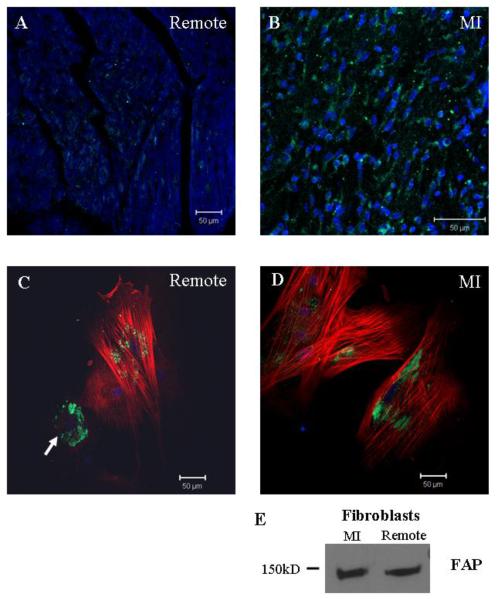
Fibroblast activation protein (FAP) is a membrane bound proline-specific serine protease, capable of cleaving gelatin and collagen type I, which was originally characterized on stromal fibroblasts associated with epithelial cancers but is not expressed on normal fibroblasts or in normal, healthy tissues [75, 76]. FAP expression was detected on fibroblasts of embryonic an adult origin from a variety of tissues and was also detected on scar fibroblasts [77]. More recent studies in models of liver cirrhosis and pulmonary fibrosis indicate that FAP staining does not precisely overlap α-SMA positive staining for activated fibroblasts (myofibroblasts), suggesting that this marker may identify a subset of activated fibroblasts involved in fibrosis and wound repair [78, 79]. Understanding the role of FAP in activated fibroblasts/myofibroblasts in cardiovascular disease has been very limited, with one study demonstrating TNFα induced expression of FAP in aortic smooth muscle cells in atherosclerotic plaques [80]. FAP has been detected on fibroblasts and α-SMA positive myofibroblasts isolated from MI and remote regions in a porcine myocardial infarct model (Figure 2), suggesting FAP may be useful in identifying myocardial fibroblasts/myofibroblasts. Given its limited expression, collagenolytic activity, proposed role in wound healing and the existence of FAP-specific inhibitors, this protein may be valuable marker in examining fibroblast activation during LV remodeling in cardiovascular disease.
Figure 2. New Tools for Identifying Fibroblasts.
Fibroblast activation protein (FAP) may provide a new marker for identifying fibroblasts. A) and B) confocal micrographs of tissue sections from remote myocardium and MI stained for FAP (green) and DAPI (blue). Very little FAP staining was detected in the remote region compared to the infarct. C) and D) confocal micrographs of fibroblasts isolated from remote and MI regions stained for FAP (green), α-SMA (red), and DAPI (blue). In the MI cells, FAP was detected on myofibroblasts whereas on cells from remote myocardium FAP was detected on fibroblasts (arrow; α-SMA - cell) and myofibroblasts (α-SMA + cells). E) Western blot of protein extracts from fibroblasts isolated from MI and remote myocardium. Scale bars = 50 μm.
5. Fibroblast Activation – Evolution of the Myofibroblast
Under normal physiological conditions, fibroblasts maintain ECM homeostasis and myofibroblasts are not detectable at any significant levels in the heart [81, 82]. In response to various insults, such as myocardial infarction, pressure overload or ischemic injury, these previously quiescent fibroblasts assume an activated, myofibroblast phenotype. A number of factors have been shown to modulate the transformation fibroblasts to myofibroblasts [83]. The defining feature of myofibroblasts, and what is used to distinguish them from fibroblasts, is their de novo expression of α-SMA and its assembly into stress fibers [83, 84]. In vitro, myofibroblasts develop what have been termed “supramature” focal adhesions or a fibronexus which are larger than focal adhesions found in fibroblasts and enriched in vinculin, paxillin and tensin along with certain integrins [85]. While often considered a one-step transformation, an intermediate stage, the proto-myofibroblast, has been proposed along this differentiation pathway (Figure 1) [86].
In general, tissue fibroblasts in vivo do not develop stress fibers; however, cells expressing stress fibers but not α-smooth muscle actin have been observed [86]. These cells, termed proto-myofibroblasts, express stress fibers composed of β- and γ-actin, as well as the ED-A fibronectin splice variant, and are found in areas of mechanical tension. Exposure of proto-myofibroblasts to TGFβ and continued interaction with ED-A fibronectin can stimulate the transformation of these cells into a fully differentiated, α-SMA expressing myofibroblast. Studies subjecting ED-A−/− mice to myocardial infarction revealed reduced fibrosis within the remote myocardium due to a decrease in myofibroblasts and less collagen within the infarct region as well [19]. Currently, studies of the proto-myofibroblast have been limited largely to in vitro models and nothing is known regarding their role in ECM remodeling in the heart. If proto-myofibroblasts represent an intermediate step in fibroblast to myofibroblast differentiation, then understanding what regulates their formation and maturation into myofibroblasts could produce exciting new therapeutic targets for regulating fibrosis and matrix remodeling.
6. Fibroblast-ECM Interactions
The cardiac ECM provides biochemical and mechanical cues for fibroblasts stimulating multiple cellular responses including differentiation into myofibroblasts, migration, and proliferation. Cardiac fibroblasts possess two classes of cell surface receptors which mediate fibroblast interactions with the ECM – integrins and Discoidin Domain Receptors (DDRs).
6.1 Integrins
Integrins are heterodimeric transmembrane proteins comprised of an α and β subunit which provide a bridge between the extracellular environment and the actin cytoskeleton. Integrins have large, extracellular ligand binding domains but relatively short cytoplasmic tails, necessitating the interaction with intracellular signaling molecules to mediate multiple cell functions including proliferation, migration, adhesion, differentiation and apoptosis [87]. Cardiac fibroblasts express a wide variety of integrin subunits, including β1 and β3 as well as multiple α chains (1, 2, 3, 5, 8, 9, 10 11, v) [87, 88, 89] allowing these cells to interact with a variety of matrix components. The α1β1 and α2β1 integrins have both been shown to be important in cardiac fibroblast-mediate remodeling of 3D collagen gels with α1β1 also playing a role in migration [90]. Compared to adult fibroblasts, neonatal cardiac fibroblasts demonstrate increased expression of β1 integrin and α-SMA which correlated with an improved ability to contract collagen gels [91], suggesting that fibroblast-ECM interactions are dynamic and change with age. Cardiac fibroblasts from β3 integrin−/− mice exhibit reduced proliferation, ECM attachment and migration [35]. Recent studies have shown that α11 integrin on diabetic cardiac fibroblasts is necessary for increased α-SMA expression associated with transformation to the myofibroblast phenotype [89]. Furthermore, it has been shown that cultured myofibroblasts can directly activate latent TGFβ within the ECM via multiple integrins (β1, β3 and αvβ5) depending upon ECM stiffness [92]. While this has not been demonstrated for cardiac fibroblasts/myofibroblasts, such autocrine control of local TGFβ may offer an explanation for the persistence of myofibroblasts within MI scars and a target for regulating adverse LV remodeling in the post-MI heart.
6.2 Discoidin Domain Receptors
The Discoidin domain receptors (DDR1 and DDR2) belong to the receptor tyrosine kinase protein family and bind various types of collagen as their ligands [51, 52]. DDR1 and DDR2 are both expressed on cardiac fibroblasts [91, 30] and have been shown to bind collagen at sites distinct from those used by integrins [93, 94, 95]. Other than the use of DDR2 as a marker to identify cardiac fibroblasts within the myocardium, little is known regarding the functional role of these receptors in the heart. No studies to date have demonstrated a cardiac phenotype for either DDR1 or DDR2 under normal conditions or in response to pathological stress such as pressure overload or MI. Examination of age-dependent cardiac fibroblast remodeling behavior revealed significant correlations between α-SMA and DDR1 or DDR2 expression in neonatal versus adult fibroblasts cultured in 3D collagen gels [91]. DDR1 has been implicated in the migration of smooth muscle cells and intimal thickening in response to vascular injury [96, 97]. A proteomics study of patients with chronic atrial fibrillation revealed elevated expression of DDR2 [98], with the authors postulating that DDR2 may play a role in monitoring the state of the cardiac ECM and directing either collagen synthesis or turnover. Given the ability of DDRs to differentially respond to multiple types of collagen by either increasing collagen production or up-regulating expression of matrix metalloproteinases, these receptors may play a role in fibroblast maintenance of ECM homeostasis in the myocardium and warrant further investigation.
7. Diagnostic and Therapeutic Potential and the Fibroblast
The changes in ECM structure and composition are a dynamic process and are influenced by a number of biological and mechanical signaling pathways, and thus assessment of ECM biology through quantitation of fibrillar collagen accumulation is unlikely to provide a direction for novel diagnostic and therapeutic applications. Indeed, the term “fibrosis”, which is often associated with excessive ECM accumulation, particularly that of fibrillar collagens does not hold a precise definition, and is often associated with an “end-stage” process. Thus, it will be critical to identify early changes in critical signaling and cellular pathways which may be harbingers of eventual malignant ECM accumulation. In terms of diagnosis, plasma profiling of determinants of ECM remodeling has been shown to be feasible in patients and abnormalities and can be detected prior to significant myocardial remodeling and progression to heart failure [99]. Since specific phenotype changes in fibroblasts occur early in the ECM remodeling process, integrating some of the protein signatures which may be reflective of fibroblast transdifferention, transformation and proliferation would hold diagnostic potential. For example, detection of the ED-A fibronectin variant may provide a means to identify when changes in fibroblast phenotype and function may be occurring within the myocardium. In terms of therapeutics, the conventional strategies have been to utilize antagonists of neurohormonal pathways and assess the effects on myocardial collagen accumulation. However, again this approach and response variable does not directly address critical cellular events within the ECM which promulgate pathological ECM remodeling. Through identification of specific fibroblast subtypes and unique fibroblast surface markers, it may be possible to develop therapies which are specific to those fibroblast subpopulations which contribute to adverse ECM remodeling. Since fibroblast proliferation and changes in phenotype are a ubiquitous event in maladaptive myocardial remodeling, targeting the source of these transformed fibroblasts as well as interrupting specific biological stimuli which promote expansion of this fibroblast population would afford a unique therapeutic intervention.
8. Conclusions
Fibroblasts and myofibroblasts represent the yin and yang of myocardial remodeling. While one cell, the fibroblast, is assumed to regulate homeostatic ECM remodeling, myofibroblasts are the culprits in adverse myocardial remodeling during cardiovascular disease. As noted, deposition and maturation of collagen is regulated post-translationally at many levels and while assumed, it is unclear if fibroblasts and myofibroblasts are identical when it comes to collagen deposition and processing as production of other ECM components can be associated with one cell type or the other. Myofibroblasts may represent a malignant, transformed phenotype with a unique cellular/remodeling signature that could be exploited therapeutically. Arising from diverse cellular precursors, it is unclear if certain precursors only contribute to the fibroblast populations in specific cardiac pathologies (i.e. pressure overload, myocardial infarction, ischemia/reperfusion) or what influence cellular origin may have on fibroblast remodeling behavior. Continued examination of fibroblast populations in multiple disease models is essential to address the issue. Characterization of fibroblast populations would be aided by the identification of novel, fibroblast-specific biomolecules. While there are a number of markers currently used for this purpose, many lack specificity and none appear to be capable of distinguishing fibroblasts from myofibroblasts, an important distinction for targeting adverse myocardial remodeling. Fibroblasts perceive biochemical and mechanical changes in the ECM through cell surface receptors which interrogate the matrix and can regulate fibroblast phenotype and function. These fibroblast-ECM interactions may represent the frontline in an adaptive process as fibroblasts transform into myofibroblasts, setting in motion a cascade of events which lead to ECM accumulation and myocardial dysfunction.
Highlights
Collagen processing, assembly and incorporation into the ECM is highly regulated
Different cell populations contribute to cardiac fibroblasts during development and disease
Identification of cardiac fibroblasts is challenging due to lack of specific markers
Cell-ECM interactions may perpetuate the myofibroblast phenotype
Elucidation of a unique fibroblast/myofibroblast signature may provide novel therapeutic targets
Table 2.
Unanswered Questions about Cardiac Fibroblasts.
| Do fibroblasts and myofibroblasts function similarly during collagen remodeling? |
| Are there cardiovascular pathological specific fibroblast precursors? |
| Does fibroblast origin influence fibroblast function? |
| Is there truly a “fibroblast specific” marker? Can it distinguish between fibroblasts, protomyofibroblasts and myofibroblasts? |
Acknowledgements
This work was supported by an American Heart Association Grant-In-Aid (ECG), by National Institutes of Health grant HL095608 (FGS), and Merit Award 1I01BX001385-01A1 to ADB from the Veterans' Affairs Health Administration. Drs. Francis Spinale and Michael Zile are supported by the Research Service of the Department of Veterans Affairs. The authors would like to thank Shaun Riffle for graphics assistance and Charity Fix and Lorain Junor for staining and microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures The authors have no conflicts of interest to disclose.
References
- [1].Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- [2].Caulfield JB, Borg TK. The collagen network of the heart. Lab Invest. 1979;40:364–72. [PubMed] [Google Scholar]
- [3].Souders CA, Bowers SLK, Baudino TA. Cardiac Fibroblast: The Renaissance Cell. Circ Res. 2009;105:1164–76. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem. 1990;96:1–14. doi: 10.1007/BF00228448. [DOI] [PubMed] [Google Scholar]
- [6].Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–37. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- [7].Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- [8].Prockop DJ, Hulmes DJS. “Assembly of collagen fibrils de novo from soluble precursors: polymerization and copolymerization of procollagen, pN-collagen, and mutated collagens” in Collagen: Primer in structure, processing and assembly. Academic Press; San Diego, CA: 1994. pp. 47–90. [Google Scholar]
- [9].Colige A, Vandenberghe I, Thiry M, Lambert CA, Van Beeumen J, Li SW, et al. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem. 2002;277:5756–66. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- [10].Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360–62. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- [11].Steiglitz BM, Keene DR, Greenspan DS. PCOLCE2 encodes a functional procollagen C-proteinase enhancer (PCPE2) that is a collagen-binding protein differing in distribution of expression and post-translational modification from the previously described PCPE1. J Biol Chem. 2002;277:49820–30. doi: 10.1074/jbc.M209891200. [DOI] [PubMed] [Google Scholar]
- [12].Takahara K, Kessler E, Biniaminov L, Brusel M, Eddy RL, Jani-Sait S, et al. Type I procollagen COOH-terminal proteinase enhancer protein: identification, primary structure, and chromosomal localization of the cognate human gene (PCOLCE) J Biol Chem. 1994;269:26280–85. [PubMed] [Google Scholar]
- [13].Baicu CF, Zhang Y, Van Laer AO, Renaud L, Zile MR, Bradshaw AD. Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am J Physiol Heart Circ Physiol. 2012;03:H234–40. doi: 10.1152/ajpheart.00227.2012. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boluyt MO, O'Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH, et al. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res. 1994;75:23–32. doi: 10.1161/01.res.75.1.23. [DOI] [PubMed] [Google Scholar]
- [15].Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984;104:86–96. doi: 10.1016/0012-1606(84)90038-1. [DOI] [PubMed] [Google Scholar]
- [16].Trombetta-Esilva J, Eadie EP, Zhang Y, Norris RA, Borg TK, Bradshaw AD. The Effects of Age and the Expression of SPARC on Extracellular Matrix Production by Cardiac Fibroblasts in 3-D Cultures. PLoS One. 2013;8:e79715. doi: 10.1371/journal.pone.0079715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Konstandin MH, Toko H, Gastelum GM, Quijada P, De La Torre A, Quintana M, et al. Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ Res. 2013;113:115–25. doi: 10.1161/CIRCRESAHA.113.301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, et al. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–92. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- [20].Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, et al. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–11. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, et al. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–80. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, et al. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC) J Mol Cell Cardiol. 2010;48:544–49. doi: 10.1016/j.yjmcc.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dettman RW, Denetclaw W, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–81. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- [27].Gittenberger-De Groot AC, Vrancken Peeters MPFM, Mentine MMT, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute to a novel population to the myocardial wall and the atrioventiruclar cushions. Circ Res. 1998;82:1043–52. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- [28].Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105:934–47. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morales MO, Price RL, Goldsmith EC. Expression of Discoidin Domain Receptor 2 (DDR2) in the developing heart. Microsc Microanal. 2005;11:260–67. doi: 10.1017/S1431927605050518. [DOI] [PubMed] [Google Scholar]
- [30].Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, et al. Organization of Fibroblasts in the Heart. Devel. Dyn. 2004;230:787–94. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- [31].Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–91. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- [32].Nag A. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- [33].Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;32:17–26. [PubMed] [Google Scholar]
- [34].Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–39. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- [35].Balasubramanian S, Quinones L, Kasiganesan H, Zhang Y, Pleasant DL, Sundararai KP, et al. β3 integrin in cardiac fibroblast is critical for extracellular matrix accumulation during pressure overload hypertrophy in mouse. PloS One. 2012;7:e45076. doi: 10.1371/journal.pone.0045076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overload rats. Circulation. 2002;106:130–35. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- [37].Stewart JA, Massey EP, Fix C, Zhu J, Goldsmith EC, Carver W. Temporal alterations in cardiac fibroblast function following induction of pressure overload. Cell Tissue Res. 2010;340:117–26. doi: 10.1007/s00441-010-0943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goldsmith EC, Bradshaw AD, Spinale FG. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol. 2013;304:C393–402. doi: 10.1152/ajpcell.00347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Crawford JR, Haudek SB, Cieslik KA, Trial J, Entman ML. Origin of developmental precursors dictates the pathophysiologic role of cardiac fibroblasts. J Cardiovasc Trans Res. 2012;5:749–59. doi: 10.1007/s12265-012-9402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Von Gise A, Pu WT. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res. 2012;110:1628–45. doi: 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Carlson S, Trial J, Soeller C, Entman ML. Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovasc Res. 2011;91:99–107. doi: 10.1093/cvr/cvr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci. 2006;103:18284–89. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al. Identification and characterization of fibroblast marker: FSP1. J Cell Biol. 1995;130(2):393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of FSP1 in epithelial-mesenchymal transformation. Am J Physiol Renal Physiol. 1997;273:F563–74. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- [45].Schneider M, Kostin S, Strom CC, Aplin M, Lyngbaek S, Theilade J, et al. S100A4 is upregulated in injured myocardium and promotes growth and survival of cardiac myocytes. Cardiovasc Res. 2007;75:40–50. doi: 10.1016/j.cardiores.2007.03.027. [DOI] [PubMed] [Google Scholar]
- [46].Inamoto S, Murao S, Yokoyama M, Kitazawa S, Maeda S. Isoproterenol-induced myocardial injury resulting altered S100A4 and S100A11 protein expression in the rat. Pathol Int. 2000;50:480–85. doi: 10.1046/j.1440-1827.2000.01069.x. [DOI] [PubMed] [Google Scholar]
- [47].Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-b signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- [48].Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305:H1363–72. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci. 2001;108:308–13. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- [52].Vogel W, Gish GD, Alves F, Pawson T. The Discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- [53].Lai C, Lemke G. Structure and expression of the Tyro 10 receptor tyrosine kinase. Oncogene. 1994;9:877–83. [PubMed] [Google Scholar]
- [54].Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- [55].Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–18. [PubMed] [Google Scholar]
- [56].Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc. Natl. Acad. Sci. 1993;90:5677–81. doi: 10.1073/pnas.90.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Goldsmith EC, Zhang X, Watson J, Hastings J, Potts JD. The collagen receptor DDR2 is expressed during early cardiac development. Anat Rec. 2010;293:762–69. doi: 10.1002/ar.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bhadriraju K, Chung KH, Spurlin TA, Haynes RJ, Elliott JT, Plant AL. The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the down-regulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials. 2009;30:6687–94. doi: 10.1016/j.biomaterials.2009.08.036. [DOI] [PubMed] [Google Scholar]
- [59].Ferri N, Carragher NO, Raines EW. Role of Discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164:1575–85. doi: 10.1016/S0002-9440(10)63716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Talasila A, Germack R, Dickenson JM. Characterization of P2Y receptor subtypes functionally expressed on neonatal rat cardiac myofibroblasts. Br J Pharmacol. 2009;158:339–53. doi: 10.1111/j.1476-5381.2009.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sun J, Li SH, Liu SM, Wu J, Weisel RD, Zhuo YF, et al. Improvement in cardiac function after bone marrow cell therapy is associated with an increase in myocardial inflammation. Am J Phyiol Heart Circ Physiol. 2009;296:H43–H50. doi: 10.1152/ajpheart.00613.2008. [DOI] [PubMed] [Google Scholar]
- [62].Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, et al. Altered fibroblast function following myocardial infarction. J. Mol Cell Cardiol. 2005;39:699–707. doi: 10.1016/j.yjmcc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- [63].Rossert J, Terraz C, Dupont S. Regulation of type I collagen gene expression. Nephrol Dial Transplant. 2000;15:66–8. doi: 10.1093/ndt/15.suppl_6.66. [DOI] [PubMed] [Google Scholar]
- [64].Rossert J, Eberspaecher H, de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-α1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995;129:1421–32. doi: 10.1083/jcb.129.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ford CM, Li S, Pickering JG. Angiotensin II stimulates collagen synthesis in human vascular smooth muscle cells: involvement of the AT1 receptor, transforming growth factor β and tyrosine phosphorylation. Arterioscler Thromb Vasc Biol. 1999;19:1843–51. doi: 10.1161/01.atv.19.8.1843. [DOI] [PubMed] [Google Scholar]
- [66].Schlumberger W, Thie M, Rauterberg J, Robenek H. Collagen synthesis in cultured aortic smooth muscle cells: modulation by collagen lattice culture, transforming growth factor-beta1 and epidermal growth factor. Arterioscler Thromb. 1991;11:1660–66. doi: 10.1161/01.atv.11.6.1660. [DOI] [PubMed] [Google Scholar]
- [67].Zheng B, Zhang Z, Black CM, de Brombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibrobalsts: a potentially powerful technique for investigating gene function in fibrosis. Am J Pathol. 2002;160:1609–17. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cai CL, Martin JC, Sun Y, Cui L, Want L, Ouyang K, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–9. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Duan J, Lee Y, Jania C, Gong J, Rojas M, Burk L, et al. Rib fractures and death from deletion of osteoblast βcatenin in adult mice is rescued by corticosteroids. PLoS One. 2013;8(2):e55757. doi: 10.1371/journal.pone.0055757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Evans RM. Vimentin: the conundrum of the intermediate filament gene family. BioEssays. 1998;20:79–86. doi: 10.1002/(SICI)1521-1878(199801)20:1<79::AID-BIES11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [71].Grigore A, Arsene D, Filipoiu F, Cionca F, Enache S, Ceausu M, et al. Cellular immunophenotypes in human embryonic, fetal and adult heart. Rom J Morphol Embryol. 2012;53:299–311. [PubMed] [Google Scholar]
- [72].Boopathy AV, Pendergrass KD, Che PL, Yoon YS, Davis ME. Oxidative stress-induced notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells. Stem Cell Res Ther. 2013;4:43. doi: 10.1186/scrt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Waxman AS, Kornreich BG, Gould RA, Noise NS, Butcher JT. Interactions between TGFβ1 and cyclic strain in modulation of myofibroblastic differentiation of canine mitral valve interstitial cells in 3D culture. J Vet Cardiol. 2012;14:211–21. doi: 10.1016/j.jvc.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Endo J, Sano M, Fujita J, Hayashida K, Yuasa S, Aoyama N, et al. Bone marrow-derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation. 2007;116:1176–84. doi: 10.1161/CIRCULATIONAHA.106.650903. [DOI] [PubMed] [Google Scholar]
- [75].Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci. 1990;87:7235–39. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274:36505–12. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- [77].Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci. 1988;85:3110–14. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Muller E, et al. Fibroblast activation protein: A cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodeling interface in human cirrhosis. Hepatology. 1999;29:1768–78. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- [79].Acharya PS, Zukas A, Zukas A, Chandan V, Katzenstein ALA, Pure E. Fibroblast activation protein: a serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis. Hum Path. 2006;37:352–60. doi: 10.1016/j.humpath.2005.11.020. [DOI] [PubMed] [Google Scholar]
- [80].Brokopp CE, Schoenauer R, Richards P, Bauer S, Lohmann C, Emmert MY, et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur Heart J. 2001;32:2713–22. doi: 10.1093/eurheartj/ehq519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Baum J, Duffy HS. Fibroblasts and myofibroblasts: What are we talking about? J Cardiovasc Pharmacol. 2011;57(4):376–79. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–78. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [83].Hinz B, Phan SH, Thannickai VJ, Prunotto M, Desmouliere A, Varga J, et al. Recent developments in myofibroblast biology. Amer J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- [85].Hinz B. Masters and servants of the force: The role of matrix adhesion in myofibroblast force perception and transmission. Eu J Cell Biol. 2006;85:175–81. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [86].Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Molec Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- [87].Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–19. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- [88].Hudon-David F, Bouzeghrane F, Couture P, Thibault G. Thy-1 expression by cardiac fibroblasts: lack of associate with myofibroblast contractile markers. J Mol Cell Cardiol. 2007;42:991–1000. doi: 10.1016/j.yjmcc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- [89].Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA. α11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res. 2012;96:265–75. doi: 10.1093/cvr/cvs259. [DOI] [PubMed] [Google Scholar]
- [90].Carver W, Molano I, Reaves TA, Borg TK, Terracio L. Role of the alpha 1 beta 1 integrin complex in collagen gel contraction in vitro by fibroblasts. J Cell Physiol. 1995;165:425–37. doi: 10.1002/jcp.1041650224. [DOI] [PubMed] [Google Scholar]
- [91].Wilson CG, Stone JW, Fowlkes V, Morales MO, Murphy CJ, Baxter SC, et al. Age-dependent expression of collagen receptors and deformation of type I collagen substrates by rat cardiac fibroblasts. Microsc Microanal. 2011;17:555–62. doi: 10.1017/S1431927611000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-b1 from the extracellular matrix. J Cell Biol. 2007;179:1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xu H, Bihan D, Chang F, Huang PH, Farndale RW, Leitinger B. Discoidin domain receptors promote α1β1- and α2β1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PLoS ONE. 2012;7:e52209. doi: 10.1371/journal.pone.0052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B. Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 2011;30:16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the Discoidin domain receptor DDR2 in collagen. J Biol Chem. 2008;283:6861–68. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- [96].Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of Discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90:1147–49. doi: 10.1161/01.res.0000022166.74073.f8. 2002. [DOI] [PubMed] [Google Scholar]
- [97].Hou G, Vogel W, Bendeck MP. The Discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107:727–35. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhang P, Wang W, Wang X, Wang X, Song Y, Han Y, et al. Protein analysis of atrial fibrosis via label-free proteomics in chronic atrial fibrillation patients with mitral valve disease. PLoS One. 2013;8:e60210. doi: 10.1371/journal.pone.0060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Spinale FG, Zile MR. Integrating the myocardial matrix into heart failure recognition and management. Circ Res. 2013;113:725–38. doi: 10.1161/CIRCRESAHA.113.300309. [DOI] [PMC free article] [PubMed] [Google Scholar]