Abstract
Mirror neurons are theorized to serve as a neural substrate for spoken language in humans, but the existence and functions of auditory–vocal mirror neurons in the human brain remain largely matters of speculation. Songbirds resemble humans in their capacity for vocal learning and depend on their learned songs to facilitate courtship and individual recognition. Recent neurophysiological studies have detected putative auditory–vocal mirror neurons in a sensorimotor region of the songbird's brain that plays an important role in expressive and receptive aspects of vocal communication. This review discusses the auditory and motor-related properties of these cells, considers their potential role on song learning and communication in relation to classical studies of birdsong, and points to the circuit and developmental mechanisms that may give rise to auditory–vocal mirroring in the songbird's brain.
Keywords: auditory, vocal, mirror neuron, birdsong, learning, communication
1. Introduction
Since their discovery in the monkey frontal cortex almost three decades ago, ‘mirror neurons’ that are active both when an individual observes and executes a specific movement have been advanced as a substrate for imitative learning, including for skills that form the basis of communicative behaviours, such as spoken language [1–10]. Beyond a role in skill learning, their capacity to encode both sensory and motor aspects of complex sequential behaviours would appear to predispose mirror neurons to function as a critical cellular interface for switching rapidly and efficiently back and forth between receptive and expressive modes of communication. Indeed, both motor theories of speech perception and forward models of speech learning invoke a congruent sensory-motor interface that could be served by auditory–vocal mirror neurons [11–14].
Despite the postulated importance of mirror neurons to learned vocal communication, whether and how mirror neurons operate to facilitate vocal learning, perception and production in the human brain remains largely speculative. Moreover, a detailed understanding of neural mechanisms for learned vocal communication will depend not only on studies in humans, but also on integrated physiological, anatomical and behavioural studies that are only practical in suitable animal models. Few such models exist: although human speech undoubtedly evolved from the vocalizations of our animal ancestors, vocal learning is a uniquely human trait among extant primates and a rarely encountered trait in only a few other vertebrate taxa, including cetaceans, echolocating bats and songbirds. As discussed here, studies of auditory–vocal sensorimotor neurons in songbirds provide compelling evidence that mirror neurons are engaged in vocal learning, perception and production.
Birdsong shares numerous traits with human speech: both are complex sequential vocal behaviours learned by imitation; and both serve an essential communication function, facilitating individual recognition, courtship and group cohesion [15–20]. Furthermore, songbirds and humans are both specialized for low-frequency hearing and display similar auditory perceptual capabilities, including a capacity for categorical perception of learned vocalizations [21–23]. And despite the evolutionary distance separating songbirds and humans, birdsong and speech exhibit strong developmental and neural parallels, including juvenile critical periods for auditory and vocal motor learning, a developmental progression from fragmentary babbling vocalizations to more complex, sequential and stereotyped vocal patterns, and specialized sensorimotor circuits that play an essential role in vocal learning, production and perception [15,17,19,24].
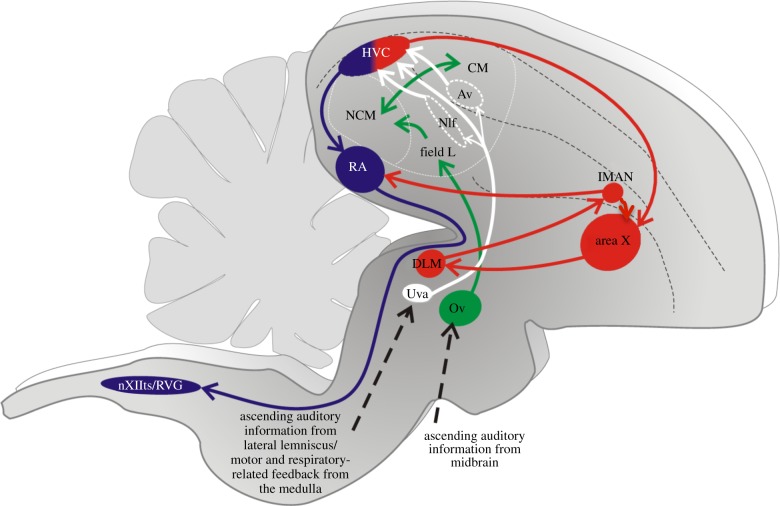
The song system (figure 1) refers to a distributed network of forebrain and brainstem nuclei that distinguishes the songbird's brain from the brains of other birds that only produce innate, unlearned vocalizations [19,20,25]. The song system is commonly divided into two functionally distinct pathways, a song motor pathway (SMP) that plays an essential role in singing and an anterior forebrain pathway (AFP) that shares strong similarities to mammalian cortical-basal ganglia loops. While AFP lesions do not prevent singing, lesions made in the AFP of juvenile zebra finches interfere with their ability to accurately copy a tutor song, whereas AFP lesions made in adults prevent audition-dependent vocal plasticity and the more subtle modulation of song variability that occurs when a female is nearby [26,27]. Thus, the SMP and the AFP have motor roles in adult singing, and it is speculated that coordination of activity in these two pathways plays an integral role in song learning [28].
Figure 1.
A schematic of the song system emphasizing HVC and its connections. This parasagittal view of the songbird brain shows the SMP (blue) and AFP (red), the ascending auditory pathways (green) and the auditory inputs to HVC (white). At the microscopic level, HVCX and HVCRA cells are randomly intermingled within HVC. Av, nucleus avalanche; CM, caudal mesopallium; DLM, medial part of the dorsolateral thalamic nucleus; HVC, abbreviation used as proper name; LMAN, lateral magnocellular nucleus of the anterior nidopallium; NCM, caudomedial nidopallium; NIf, nucleus interface; OV, nucleus ovoidalis; RA, robust nucleus of the arcopallium; Uva, nucleus uvaeformis; VRG, ventral respiratory group; nXIIts, tracheosyringeal division of the hypoglossal nucleus.
Notably, both of these pathways receive input from distinct pools of projection neurons that are intermingled in the nucleus HVC [29,30], which is located on the dorsal aspect of the caudal telencephalon and occupies a functional position at or near the apex of a sensorimotor hierarchy for song [25,31,32]. By rough analogy to the human brain, HVC can be likened to Broca's area (vocal premotor cortex in primates), containing one projection neuron type (HVCRA) that innervates the song motor nucleus RA (an analogue of the vocal motor representation in the primary motor cortex) in the SMP and another projection neuron type (HVCX) that provides input to a striatopallidal structure (area X) in the AFP. Beyond their similar anatomical locations in the vocal motor hierarchy, HVC and Broca's area share comparable functional roles in the generation of learned vocalizations: similar to Broca's aphasics, adult songbirds with HVC lesions cannot sing, although they continue to produce innate vocalizations, such as alarm calls, and also produce simpler vocalizations that resemble the babbling vocalizations produced by juvenile songbirds at the earliest stages of song learning (i.e. subsong) [25,33]. Furthermore, chronic recordings made in singing birds indicate that both HVCRA and HVCX cells fire in temporally precise bursts during singing [34–38], and both focal cooling and microstimulation experiments point to HVC as a core component of the neural machinery for the temporal patterning of song [32,39].
2. Sensorimotor roles for HVC and the phenomenon of auditory–vocal mirroring
Interestingly, songbirds with HVC lesions also show deficits in the ability to recognize the songs of other birds of their own species [40] or to learn new contingencies to these songs [41], suggesting that HVC serves auditory as well as premotor functions. Consistent with this idea, some of the earliest electrophysiological recordings made from HVC in awake songbirds found that certain HVC neurons could be excited by auditory presentation (i.e. playback) of the bird's own song (BOS) [42]. Subsequent recordings in a variety of anaesthetized songbird species, including sparrows and zebra finches, detected HVC auditory neurons that were highly tuned to temporal and spectral features in the BOS but that could also respond to other acoustically similar songs of conspecific birds, including the tutor song on which the BOS was modelled [38,43–51]. In fact, in anaesthetized zebra finches, BOS playback is sufficient to excite neurons in all nuclei in the song system downstream of HVC, including the motor neurons and nerves that innervate the vocal organ, and reversible inactivation and functional connectivity studies indicate that HVC is the source of this widespread auditory responsiveness [52–56].
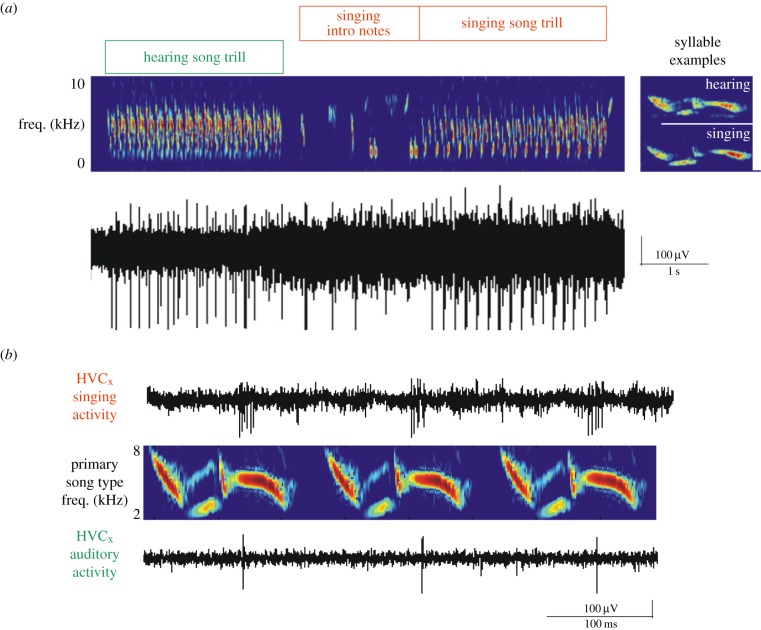
Although the detection of neural activity in HVC during singing and BOS playback are suggestive of auditory–vocal mirror neurons, the earliest studies of singing and auditory-related activity in the HVC of freely behaving birds used multiunit methods and thus could not resolve whether the same neurons were active during singing and song playback. Subsequent single unit studies in freely behaving swamp sparrows (Melospiza georgiana) and Bengalese finches (Lonchura striata var. domestica) that used antidromic stimulation methods to distinguish HVCX from HVCRA cells established that only HVCX cells exhibit auditory activity during quiet wakefulness and also revealed that about a third of HVCX cells are active both during singing and BOS playback (figure 2a) [35,38]. Remarkably, this subset of HVCX neurons discharge action potentials at exactly the same time in the song phrase, regardless of whether the bird is singing or listening to its song through a speaker (figures 2a,b and 3b). One explanation for this behaviour is that these cells respond to auditory feedback during singing and to similar auditory cues when the BOS is played through a speaker. However, the singing-related activity of these cells is unperturbed by acoustic manipulations that disrupt auditory feedback, suggesting that HVCX cells switch from auditory to purely motor-related activity as the bird transitions from listening to singing [38,57]. Therefore, HVCX cells display a temporally precise form of auditory–vocal mirroring that could be well suited to facilitate song learning and perception.
Figure 2.
Chronic extracellular recordings from freely singing swamp sparrows reveal auditory–vocal mirroring in HVCX cells. (a) Action potential activity (bottom trace) of an HVCX cell as it listens to its song, which consists of a trilled syllable (top inset at right) played through a nearby speaker (green box over sonogram) and as it transitions to singing the same trilled syllable (red box over sonogram and bottom inset at right). (b) An expanded view of the action potential activity of this cell during hearing (bottom trace) and singing (top trace) three repeated syllables in the song (sonogram shown in the middle). Adapted from Prather et al. [38].
Figure 3.
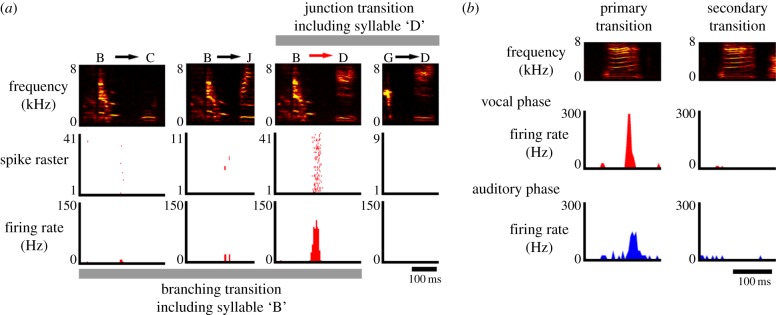
Auditory–vocal mirror neurons can display similar selectivity for specific syllable transitions during motor and auditory phases. (a) Extracellular recordings from an HVCX cell in the Bengalese finch reveals that singing-related action potential activity occurs during the transition from syllable B to D, but not during other syllable–syllable transitions involving either syllable B or D. (b) The selective firing for a primary syllable–syllable transition exhibited during singing (i.e. the vocal phase) is maintained during auditory playback of various syllable transitions (auditory phase). Adapted from Fujimoto et al. [35].
Before considering how HVC auditory–vocal mirror neurons might function to facilitate song learning and communication, it is notable that auditory responses can be detected in the HVC of female songbirds that typically do not sing [58,59], and HVC lesions in female songbirds impair their ability to discriminate appropriately between conspecific and heterospecific songs [40,60], indicating that HVC's auditory function, at least in females, may not be a simple consequence of singing. Furthermore, the auditory responses of HVC cells in adult male zebra finches are strongly state-dependent, only emerging during sleep or under anaesthesia [61–64]. Songbird neurobiologists use zebra finches for experimental subjects because of their suitable captive breeding habits, with the consequence that there has been some uncertainty as to whether auditory–vocal mirroring is a common feature of HVCX cells across species. Recent experiments from our own laboratory have used longitudinal recordings across the sleep–wake boundary to establish that precise auditory–vocal mirroring also is a trait of HVCX cells in male zebra finches [57]. Thus, although the auditory responses of HVCX cells are more tightly regulated by behavioural state in zebra finches than in swamp sparrows and Bengalese finches, auditory–vocal mirroring appears to be a common trait of this cell type in a wide variety of songbird species.
3. Role in production
A prevailing view is that HVC sits atop the song motor hierarchy and plays an integral role in encoding temporal aspects of song [65]. Although HVC lesions abolish singing, indicating a critical role in song production, and HVCRA cells are thought to form a synfire chain that generates a precise timing signal for song patterning [34,36,39], whether and how HVCX cells contribute to these processes is unclear. Notably, HVCX axons form synapses on other neurons in HVC and on striatopallidal neurons, but not on song premotor neurons in RA [30,66] (by analogy to mammalian cortical circuitry, HVCX cells resemble cortical pyramidal neurons that extend axons to the basal ganglia but not to the pyramidal tract). While lesions made downstream of HVC in the AFP of adult songbirds do not prevent singing, they can render the song less variable [67,68]. Therefore, while a prevailing view is that HVCX cells are not essential to song production, whether these cells may play a more nuanced role in singing has not been rigorously tested and remains plausible.
A more direct attempt to test whether HVCX cells are necessary to singing used retrogradely transported chlorin-e6-coated latex microspheres from area X to facilitate the targeted laser ablation of HVCX cells [69]. This manipulation not only had no effect on song, but also killed only slightly more than half of the HVCX cell population. Therefore, a subtler role for HVCX cells in song motor control cannot be excluded, mediated perhaps by their long-range projections to area X or by the excitatory synapses that their local collaterals make on HVCRA cells and HVC interneurons [66]. In the former scenario, the singing-related activity of HVCX cells could influence to what extent the AFP contributes to performance variability and action selection, two prominent functions that have been ascribed to mammalian cortico-basal ganglia pathways. In the latter case, HVCX cell activity could either directly or indirectly alter the timing or magnitude of HVCRA firing patterns during singing, which are thought to shape song's temporal features through a synfire mechanism local to HVC [34,36,39,65].
Analyses of the singing-related activity of HVCX activity reveal correlations with song features that are consistent with a premotor encoding role. In zebra finches, a single HVCX cell fires brief action potential bursts several times during a single song phrase, the timing of which is temporally precise and invariant from one song bout to the next [37]. Correlational analyses revealed that the multiple bursts of a single cell precede similar song syllable features (i.e. either periods of sound or silence) in the song phrase by approximately 40 ms, consistent with a premotor activity signature [37]. These observations support a model in which HVCX cells transmit precise timing information about song to the AFP and to other HVC cells during singing but cannot address whether this information is used for ongoing song motor control or serves other slower processes related to song learning and maintenance.
Zebra finches sing a single highly stereotyped song phrase comprising a linear sequence of syllables, with the result that a single syllable typically occurs in fixed context. This organization makes it difficult to determine whether the bursting activity of HVCX cells correlates with specific syllables or syllable sequences regardless of context. By contrast, other songbird species, including swamp sparrows and Bengalese finches, sing multiple songs that are distinguished by different syllables or by different syllable–syllable transitions. Electrophysiological recordings in singing swamp sparrows and Bengalese finches indicate that bursting activity of HVCX mirror neurons can correlate with specific syllables [35,38]. Furthermore, recordings made in Bengalese finches, which often sing variable sequences of syllables, indicate that some HVCX neurons can fire bursts in an all-or-none fashion for specific syllable–syllable transitions, or can display different levels of activity for different syllable–syllable transitions (figure 3a). Finally, HVCX cells in Bengalese finches also can encode information about the initiation, evolution and termination of strings of repeated syllables [35]. Taken together, these observations indicate that the singing-related activity of HVCX cells can encode syntactic information about song in a hierarchical fashion, spanning from the identity of individual syllables to the number of repeated syllables and the nature of inter-syllable transitions. Notably, these cells supply input to basal ganglia circuitry, which plays an important role in the initiation and termination of motor sequences, raising the possibility that HVCX cell activity plays an important role in controlling song's syntactic features. In this light, an important goal of future experiments will be to systematically and selectively manipulate the activity of HVCX cells, preferably through optogenetic methods, to determine whether their singing-related bursting activity can influence ongoing aspects of song production, including song syntax.
4. Role in perception and the effects of auditory experience
In monkeys and humans, the ‘observation-related’ activity of mirror neurons is speculated to facilitate understanding of another's actions by representing the corresponding sensations in the context of the observer's own action framework [8,10]. More broadly, motor circuits have been advanced as a substrate for perceptual processing of spoken language [13,14]. In this context, an important question is whether auditory–vocal mirror neurons in songbirds, which are embedded in a premotor nucleus critical for learned vocal control, are linked to song perception. Interestingly, lesions of HVC and in the AFP can interfere with the recognition of conspecific song [40,70,71], HVCX cells are thought to be the sole source of auditory input to the AFP [53,72], and HVCX cells can display highly selective auditory responses to specific features of the BOS, including individual notes and note sequences [38,44,73–75]. Taken together, these observations implicate HVCX cells in auditory processing important to song perception.
An analysis of HVCX mirror neurons in swamp sparrows suggests how their auditory properties could contribute to song perception [44]. Swamp sparrows sing several distinct song types each comprising a monosyllabic trill, with each syllable composed of two to five acoustically distinct notes drawn from a pool of note types common to this species. Playback experiments in awake but non-singing sparrows reveal that individual HVCX mirror neurons respond only to a single song type in the bird's repertoire, firing one or two action potentials very reliably at a precise time within the syllable [38,44]. Furthermore, reversing the order of the notes in the syllable of the effective song type is sufficient to attenuate or strongly abolish the cell's auditory response, indicating that they are selectively responsive to specific note combinations within an effective song type [38]. This sensitivity to specific note sequences, which also has been described for HVC neurons in other songbird species [46,76], requires a pronounced capacity for spectrotemporal integration [46,48,50,76]. Moreover, similar note sequences from the songs of other swamp sparrows also can evoke auditory responses from these cells, indicating that despite their remarkable selectivity, they are capable of encoding information about the songs of other birds and thus potentially suited to a role in song perception.
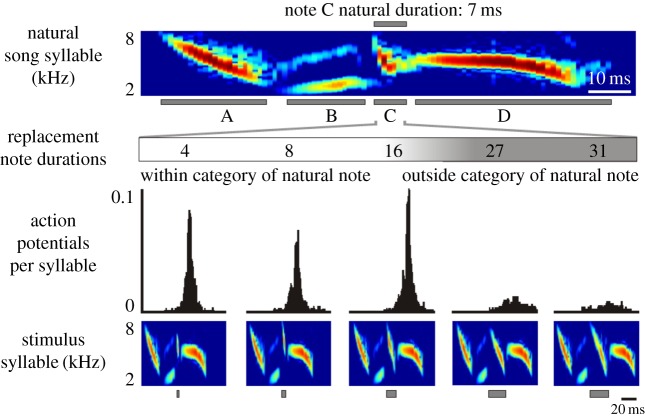
Swamp sparrows also provide a convenient platform for investigating neural correlates of song perception, as different breeding populations sing distinct dialects characterized by subtle differences in note morphology, including millisecond differences in the durations of specific note types [23,44]. Additionally, these subtle differences in note morphology, which are acquired through imitation, provide an acoustic basis by which individuals learn to recognize and distinguish birds from different breeding populations. Finally, behavioural studies have demonstrated that swamp sparrows perceptually group song notes that vary systematically in their absolute durations into distinct categories, similar to the manner in which humans group phonemes that vary continuously in voice onset times into two distinct perceptual categories [23,44]. A further similarity is that these categorical perceptual boundaries are not fixed in either humans or swamp sparrow populations but instead are determined by the individual's experience with a natal dialect [44]. Notably, swamp sparrow HVCX mirror neurons respond categorically to changes in note duration in the effective song type, and the neural response boundary closely aligns with the categorical perceptual boundary evinced by sparrows that sing the same dialect, but not with the perceptual boundary of sparrows that sing another dialect (figure 4) [44]. Thus, the auditory response properties of individual HVCX mirror neurons closely parallel the swamp sparrow's perceptual ability to distinguish subtle differences in vocal dialects, suggesting that these neurons could provide the neural substrate for categorical perception of learned vocalizations.
Figure 4.
Categorical responsiveness of swamp sparrow mirror neurons to changes in note duration. Systematically varying the duration of the third note (note ‘C’) in the four-note syllable of the effective song type reveals that mirror neurons are sharply tuned to changes in note duration. Behavioural experiments (not shown) reveal that the neural response boundary parallels the perceptual response boundary for note duration in this breeding population of sparrows (approx. 20 ms) but differs from the categorical perceptual boundary (approx. 14 ms) in a separate breeding population of sparrows that sing a distinct song dialect. Adapted from Prather et al. [39].
The auditory mirroring of motor-related activity may also extend beyond simpler phonological features, to the syntactical level. At least some auditory–vocal mirror neurons in the Bengalese finch that fire differentially at alternative syllable–syllable transitions during singing also respond differently to these alternative transitions, indicating that transition selectivity is maintained across motor and auditory states (figure 3b) [35]. Because songbirds use both phonological and syntactical information to distinguish different songs, the auditory properties of mirror neurons in HVC are well suited to extract features that are important to song perception at multiple levels of acoustical complexity.
A remaining question is the extent to which the auditory activity of songbird mirror neurons is required for song recognition, including the discrimination of the songs of other conspecifics, which to the human ear can sound remarkably similar to one another. Interestingly, while most HVCX neurons in anaesthetized songbirds display strong and selective auditory responses, less than half of HVCX cells that display singing-related activity also respond to song playback during periods of quiet wakefulness [35,38,73,74]. Furthermore, studies in adult zebra finches reveal that auditory activity can only be detected in HVCX and most other HVC cells when the animal is sedated, anaesthetized or asleep [57,62,63,77]. Indeed, auditory responses in the HVC of sedated zebra finches are strongly suppressed by arousing stimuli, such as a brief air puff delivered to the bird's chest [62]. These various findings point to the existence of a gating mechanism [78] that can control auditory drive to HVCX cells as a function of arousal and, presumably, in the service of attention. Thus, an important avenue for future research will be to determine whether the auditory activity of HVCX cells, and thus auditory–vocal mirroring, is more widespread when the animal is performing demanding song recognition tasks.
5. Circuit and synaptic mechanisms underlying motor and sensory activity of auditory–vocal mirror neurons
A fundamental question in mirror neuron research is the nature of the functional circuitry and synaptic machinery that accounts for the complex sensory and motor properties of these cells. Are the sensorimotor properties of mirror neurons simply inherited from sensory and motor neurons in other brain regions, or do they instead arise through computations performed by the local circuit in which the mirror neuron is embedded? Such an understanding is important because it can shed light on the neural computations that facilitate imitation, perception and communication, and also because localizing where mirroring originates can inform the search for how experience shapes the underlying circuits to generate such a precise sensorimotor correspondence. Although this question is far from resolved in any system, the song system is highly amenable to a wide range of powerful methods, including intracellular and extracellular recordings in singing and anaesthetized birds, in vitro intracellular recordings, reversible inactivation, focal cooling, in vivo imaging, viral tracing and genetic manipulation of activity, that facilitate the detailed analysis of functional connectivity necessary to provide an answer. In fact, the product of several decades of research by a wide variety of groups has provided considerable insights into the circuit mechanisms that underlie the expression of singing-related and auditory-evoked activity of auditory–vocal mirror neurons.
Similar to the manner in which mammalian motor cortex is recurrently connected to brainstem and basal ganglia structures, the songbird HVC is recurrently connected with downstream elements in the SMP and the AFP, as well as with neurons in the auditory telencephalon [79–85]. Thus, the functional properties of HVC mirror neurons must in some manner arise through complex and potentially reciprocal interactions with other brain regions. Nonetheless, a variety of evidence supports the idea that HVC is a site where a precise timing signal necessary for song motor patterning originates. Although one of HVC's major telencephalic afferents, NIf, contains neurons that may fire in a temporally precise manner during singing [86], completely ablating NIf disrupts singing for only a few days, after which the bird continues to sing normally [77,87]. And although lesions in a thalamic afferent to HVC, Uva, can permanently disrupt the song pattern [88], presumably by interfering with the bilateral coordination of HVC activity mediated through recurrent circuitry in which Uva is embedded, Uva neurons do not display singing-related activity that is precisely time-locked to the song [89]. Thus, HVC and more precisely HVCRA cells are presumed to be the sites where an explicit, temporally precise code necessary for song motor control originates. The details of the synaptic machinery that generate this precise code are not well understood, but paired intracellular recordings reveal that HVCRA and HVCX cells form excitatory synapses on local interneurons, which in turn form inhibitory synapses on other HVCRA and also HVCX cells [66], reminiscent of half centre oscillatory circuitry known to drive rhythmical bursting activity in other pattern-generating circuits [90]. Furthermore, HVCRA cells make direct excitatory and feed-forward inhibitory connections onto HVCX cells [66], providing a circuit by which HVCRA cells could transmit a copy of the premotor timing command through HVCX cells to the AFP.
The remarkably selective auditory response properties of HVC neurons, including the sequence sensitivity of HVCX cells, have motivated extensive studies of where and how such selectivity originates. Several of HVC's afferents contain auditory-responsive cells, and reversible inactivation studies implicate two of these—NIf and CM/Av—as the sources of much or all of the auditory drive to HVC [77,87,91,92]. Comparisons of auditory-evoked activity in these two afferents and HVC indicate that HVCX cells are sites where highly phasic, BOS-selective responses arise. That is, although NIf and CM/Av contain neurons that are selective for temporal features of the BOS, these neurons fire in a more sustained fashion in response to BOS playback than do HVCX cells [91,92]. Moreover, single and dual in vivo intracellular recordings from interneurons and HVCX cells, as well as intracellular dialysis of HVCX cells with blockers of inhibitory currents, show that the highly phasic and temporally precise action potential output of HVCX cells arises through the sculpting effects of local inhibitory circuits acting on more sustained BOS-evoked excitatory drive originating from extrinsic sources [73,75,93]. Interactions between inhibitory and excitatory synapses are also implicated in the generation of note combination-sensitive responses of HVCX cells [76,94]. Thus, two features of auditory–vocal mirror neurons, namely their temporally precise action potential responses to BOS playback and their sensitivity to specific note combinations, are likely to depend on local circuit interactions involving inhibitory interneurons.
Taken together, these studies shine the spotlight on HVC as the site where temporally precise premotor and auditory representations originate, while also delineating local circuitry that could account for the convergence and registration of these motor and sensory representations on individual HVCX cells. Furthermore, because birdsong is learned through imitation, the synaptic and circuit mechanisms that result in this precise sensorimotor registration are likely to be influenced by auditory and motor experience acting on HVC during song development. The important role for inhibitory interneurons in transmitting signals from HVCRA to HVCX cells and for sculpting auditory responses in this latter cell type, as well as the key role assigned to inhibitory interneurons in regulating experience-dependent plasticity in other systems [95–98], suggest that inhibitory synapses onto HVCX cells play an especially important role in the development and ongoing expression of auditory–vocal mirroring in the songbird's HVC.
6. Developmental origins of auditory–vocal mirroring
While HVC is a likely site where temporally precise auditory and vocal representations are generated and merged at a single-cell level, it is less clear exactly how this correspondence develops. Modelling studies suggest that if motor-related signals emanating from HVCRA cells and auditory feedback signals from extrinsic auditory sources could be integrated by single HVCX cells during singing, synaptic plasticity mechanisms that depend on the precise timing of pre- and postsynaptic spiking activity (i.e. spike-timing-dependent plasticity; STDP) could result in precise auditory–vocal mirroring [99]. Support for an effect of feedback on the auditory properties of HVCX cells comes from the observation that BOS-selectivity in HVC and the AFP develops in parallel with the process of song imitation [43,100,101], and from the finding that exposing adult finches for prolonged periods to distorted auditory feedback can drive auditory retuning to the distorted BOS in both HVC and the AFP [53,87]. Perhaps most compellingly, in vivo multiphoton imaging reveals that deafening can destabilize and shrink dendritic spines on HVCX but not HVCRA cells, an effect that precedes and predicts the severity of subsequent song deterioration [102].
Despite evidence indicating that feedback shapes HVC auditory selectivity, a significant challenge to this STDP model is that the singing-related action potential activity of HVCX cells is entirely insensitive to feedback perturbations [37,38]. Moreover, studies from our group using intracellular recordings in singing birds also reveal that the singing-related synaptic activity of HVCX cells is unaltered by acute manipulations of auditory feedback [57]. Thus, how and when auditory feedback-related information impinges on HVC remains unclear. One possibility is that feedback signals are gated in a highly dynamic fashion as a function of behavioural and/or developmental state. In fact, HVC neurons in juvenile zebra finches appear to respond more readily to auditory stimulation during periods of quiet wakefulness than do HVC neurons in adults [61,103]. However, whether this developmental difference in responsiveness extends to feedback is unclear, and recordings made in the HVC of juvenile zebra finches have failed to detect changes in singing-related action potential activity in response to acute feedback perturbations [37]. Alternatively, processes of sensorimotor replay operating during sleep may play a role in organizing a mirror neuron network in HVC [104].
Another idea is that mirroring is a consequence of supplying excitatory auditory drive to a strongly interconnected and thus highly resonant premotor circuitry. In this view, temporally precise bursting activity in HVC initially emerges in the form of premotor activity, rather than through auditory experience. Subsequently, as the juvenile sings its song many thousands of times over the course of song learning, synaptic connectivity in the premotor network would become deeply ingrained. Supplying excitatory drive to the premotor network, which could be achieved by activating auditory afferents to HVC, would trigger the network to recapitulate a premotor-like pattern of activity, resulting in mirroring. Although this idea deserves important consideration, it is insufficient by itself to account for the existence of selective auditory responses in the HVC of female songbirds that do not sing [58], or for the highly selective auditory responses in the adult male swamp sparrow HVC to tutor songs that are not maintained as copies in the bird's adult repertoire [45]. In fact, in vivo multiphoton imaging, electrophysiological, optogenetic and reversible inactivation experiments in juveniles indicate that HVC plays an essential role in helping to encode auditory experience of the tutor song [105,106]. These studies reveal that a naive juvenile's first auditory experience of a tutor song rapidly stabilizes and strengthens excitatory synapses on HVCX and HVCRA cells, a process that is paralleled by the emergence of pronounced, spontaneous bursting activity in both HVC and RA [106,107]. Therefore, early auditory experience of the tutor song and not simply singing-related premotor activity could be critical to the development of a precise auditory–vocal correspondence in HVC.
7. Mirror neurons in vocal learning and communication
In the absence of techniques for systematically manipulating the activity of specific neuron types in the HVC of freely behaving songbirds, the specific function of auditory–vocal mirror neurons must remain a matter of conjecture. However, their remarkable sensorimotor properties and critical location in the sensorimotor hierarchy raise the possibility that they play important roles in song learning, perception and, in those species that sing multiple song types, the adaptive matching of songs to conspecific neighbours. An important near-term goal is the application of intersectional strategies to selectively express genetically encoded modulators of neural activity in HVCX cells to test their roles in song learning, perception and communication.
One major function of HVCX cells, including mirror neurons, appears to be to provide the AFP with a copy (i.e. corollary discharge) of the vocal motor commands issued by HVC. In fact, although the singing-related activity in the AFP persists in deafened birds [108], indicative of a motor origin, lesions placed in the AFP do not prevent singing [67], consistent with a corollary discharge signal. One idea is that HVCX cells transmit corollary discharge signals to the striatopallidal circuitry that provides precise timing information about song, which in an error correction framework could facilitate the temporal assignment of variability signals used to adaptively modify song [28]. However, the auditory–vocal correspondence exhibited by some HVCX cells also has the potential to function in a more sophisticated manner by providing a predictive estimate of the auditory feedback that will result from the corresponding premotor signal generated by HVC [38]. Indeed, it is speculated that such forward (i.e. motor to sensory) predictions are an integral ingredient for complex skill learning, including speech learning [11,12]. In both songbirds and humans, forward models could be compared with vocalization-related feedback to generate error signals that adaptively train the vocal motor network. This idea presumes that the comparison occurs downstream of HVCX cells, either in the AFP or possibly in CM/Av, an auditory region that also receives input from HVC [79]. The absence of feedback sensitivity in the AFP makes the CM/Av region of special interest in this regard.
Auditory–vocal mirror neurons in HVC are likely to contribute to song recognition and also could play a subtler role in singing in birds with multiple song types by helping guide the selection of appropriate song types as determined by communicative context. As previously discussed, the close parallel between acoustic selectivity of mirror neurons and the bird's perceptual boundaries advance these cells as likely candidates for facilitating song recognition in the framework of the individual's own repertoire. In birds with multiple song types, such as swamp sparrows, male–male rivalries can involve a process of matched countersinging, where a resident male sings a song from its own repertoire that is most like that of its rival. Auditory–vocal mirror neurons could facilitate this vocal matching process: listening to the rival's song would activate mirror neurons in the resident that encode a similar song in the resident's repertoire; this auditory activity could then lead to the selective recruitment of premotor neurons encoding for that song. One possibility is that the mirror neurons function as premotor neurons; another possibility is that they recruit HVCRA cells that encode the desired song type, either through local connections in HVC or long-range recurrent connections through the SMP or AFP.
8. Conclusion and future directions
Although still nascent, mirror neuron research in songbirds affords scientists the potential to understand how these fascinating cells contribute to the complex behavioural and perceptual skills necessary to learned vocal communication. Furthermore, it is likely that the synaptic and circuit basis for sensorimotor mirroring will be most readily explored in the songbird, an organism in which a variety of high-resolution recording and imaging methods are now practical, and in which the generation time is sufficiently rapid to facilitate ontological experiments that seek to determine how experience shapes the development of mirror neurons. Current evidence suggests that auditory and vocal experience act at the level of local circuitry in HVC to generate a precise form of auditory–vocal mirroring that is not evident in earlier stages of the sensorimotor hierarchy.
The recent application of viral genetic methods to label and manipulate the activity of HVC neurons holds the promise that the role of auditory–vocal mirror neurons in vocal learning and perception can be tested more directly. An important near-term goal will be to use intersectional viral strategies to limit expression of transgenes, such as channelrhodopsin or archaerhodopsin, to HVCX cells and employ song-triggered optogenetic methods to disrupt their activity in song learning, production and recognition assays. Another important goal will be to map in greater detail the organization of the functional circuitry in which auditory–vocal mirror neurons are embedded, a process that can provide greater insight into the structure and function of the complex networks which can account for such remarkable sensorimotor properties. A reasonable goal over the next decade will be to understand at a synaptic and circuit level how the properties of mirror neurons are generated and how they contribute to imitative vocal learning, perception and communication.
References
- 1.Arbib MA. 2005. From monkey-like action recognition to human language: an evolutionary framework for neurolinguistics. Behav. Brain Sci. 28, 105–124. [DOI] [PubMed] [Google Scholar]
- 2.Arbib MA, Liebal K, Pika S. 2008. Primate vocalization, gesture, and the evolution of human language. Curr. Anthropol. 49, 1053–1063. ( 10.1086/593015) [DOI] [PubMed] [Google Scholar]
- 3.Fadiga L, Craighero L, Buccino G, Rizzolatti G. 2002. Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur. J. Neurosci. 15, 399–402. ( 10.1046/j.0953-816x.2001.01874.x) [DOI] [PubMed] [Google Scholar]
- 4.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. 1996. Action recognition in the premotor cortex. Brain 119, 593–609. ( 10.1093/brain/119.2.593) [DOI] [PubMed] [Google Scholar]
- 5.Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. 1998. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc. Natl Acad. Sci. USA 95, 15 061–15 065. ( 10.1073/pnas.95.25.15061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. 2005. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 3, e79 ( 10.1371/journal.pbio.0030079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. 1999. Cortical mechanisms of human imitation. Science 286, 2526–2528. ( 10.1126/science.286.5449.2526) [DOI] [PubMed] [Google Scholar]
- 8.Rizzolatti G. 2005. The mirror neuron system and its function in humans. Anat. Embryol. 210, 419–421. ( 10.1007/s00429-005-0039-z) [DOI] [PubMed] [Google Scholar]
- 9.Rizzolatti G, Arbib MA. 1998. Language within our grasp. Trends Neurosci. 21, 188–194. ( 10.1016/S0166-2236(98)01260-0) [DOI] [PubMed] [Google Scholar]
- 10.Rizzolatti G, Craighero L. 2004. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192. ( 10.1146/annurev.neuro.27.070203.144230) [DOI] [PubMed] [Google Scholar]
- 11.Ventura MI, Nagarajan SS, Houde JF. 2009. Speech target modulates speaking induced suppression in auditory cortex. BMC Neurosci. 10, 58 ( 10.1186/1471-2202-10-58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickok G, Houde J, Rong F. 2011. Sensorimotor integration in speech processing: computational basis and neural organization. Neuron 69, 407–422. ( 10.1016/j.neuron.2011.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberman AM, Cooper FS, Shankweiller DS, Studdert-Kennedy M. 1967. Perception of the speech code. Psychol. Rev. 74, 431–461. ( 10.1037/h0020279) [DOI] [PubMed] [Google Scholar]
- 14.Liberman AM, Mattingly IG. 1985. The motor theory of speech perception revised. Cognition 21, 1–36. ( 10.1016/0010-0277(85)90021-6) [DOI] [PubMed] [Google Scholar]
- 15.Doupe A, Kuhl P. 1999. Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631. ( 10.1146/annurev.neuro.22.1.567) [DOI] [PubMed] [Google Scholar]
- 16.Konishi M. 1985. Birdsong: from behavior to neuron. Annu. Rev. Neurosci. 8, 125–170. ( 10.1146/annurev.ne.08.030185.001013) [DOI] [PubMed] [Google Scholar]
- 17.Marler P. 1970. Birdsong and speech development: could there be parallels? Am. Sci. 58, 669–673. [PubMed] [Google Scholar]
- 18.Marler P, Tamura M. 1964. Culturally transmitted patterns of vocal behaviour in sparrows. Science 146, 1483–1486. ( 10.1126/science.146.3650.1483) [DOI] [PubMed] [Google Scholar]
- 19.Mooney R. 2009. Neural mechanisms for learned birdsong. Learn. Mem. 16, 655–669. ( 10.1101/lm.1065209) [DOI] [PubMed] [Google Scholar]
- 20.Mooney R, Prather J, Roberts T. 2008. Neurophysiology of birdsong learning. In Memory systems. Learning and memory: a comprehensive reference, vol. 3 (ed. Eichenbaum H.), pp. 441–474. Oxford, UK: Elsevier. [Google Scholar]
- 21.Dooling RJ, Mulligan JA, Miller JD. 1971. Auditory sensitivity and song spectrum of the common canary (Serinus canarius). J. Acoust. Soc. Am. 50, 700–709. ( 10.1121/1.1912686) [DOI] [PubMed] [Google Scholar]
- 22.Okanoya K, Dooling RJ. 1990. Temporal integration in zebra finches (Poephila guttata). J. Acoust. Soc. Am. 87, 2782–2784. ( 10.1121/1.399069) [DOI] [PubMed] [Google Scholar]
- 23.Nelson DA, Marler P. 1989. Categorical perception of a natural stimulus continuum: birdsong. Science 244, 976–978. ( 10.1126/science.2727689) [DOI] [PubMed] [Google Scholar]
- 24.Dudek S, Friedlander M. 1996. Intracellular blockade of inhibitory synaptic responses in visual cortical layer IV neurons. J. Neurophysiol. 75, 2167–2173. [DOI] [PubMed] [Google Scholar]
- 25.Nottebohm F, Stokes TM, Leonard CM. 1976. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 165, 457–486. ( 10.1002/cne.901650405) [DOI] [PubMed] [Google Scholar]
- 26.Kao MH, Brainard MS. 2006. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J. Neurophysiol. 96, 1441–1455. ( 10.1152/jn.01138.2005) [DOI] [PubMed] [Google Scholar]
- 27.Thompson JA, Basista MJ, Wu W, Bertram R, Johnson F. 2011. Dual pre-motor contribution to songbird syllable variation. J. Neurosci. 31, 322–330. ( 10.1523/JNEUROSCI.5967-09.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fee MS, Goldberg JH. 2011. A hypothesis for basal ganglia-dependent reinforcement learning in the songbird. Neuroscience 198, 152–170. ( 10.1016/j.neuroscience.2011.09.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirn JR, Alvarez-Buylla A, Nottebohm F. 1991. Production and survival of projection neurons in a forebrain vocal center of adult male canaries. J. Neurosci. 11, 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wild JM, Williams MN, Howie GJ, Mooney R. 2005. Calcium-binding proteins define interneurons in HVC of the zebra finch (Taeniopygia guttata). J. Comp. Neurol. 483, 76–90. ( 10.1002/cne.20403) [DOI] [PubMed] [Google Scholar]
- 31.McCasland JS. 1987. Neuronal control of birdsong production. J. Neurosci. 7, 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vu ET, Mazurek ME, Kuo YC. 1994. Identification of a forebrain motor programming network for the learned song of zebra finches. J. Neurosci. 14, 6924–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronov D, Andalman AS, Fee MS. 2008. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 320, 630–634. ( 10.1126/science.1155140) [DOI] [PubMed] [Google Scholar]
- 34.Long MA, Jin DZ, Fee MS. 2010. Support for a synaptic chain model of neuronal sequence generation. Nature 468, 394–399. ( 10.1038/nature09514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto H, Hasegawa T, Watanabe D. 2011. Neural coding of syntactic structure in learned vocalizations in the songbird. J. Neurosci. 31, 10 023–10 033. ( 10.1523/JNEUROSCI.1606-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahnloser RH, Kozhevnikov AA, Fee MS. 2002. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419, 65–70. ( 10.1038/nature00974) [DOI] [PubMed] [Google Scholar]
- 37.Kozhevnikov AA, Fee MS. 2007. Singing-related activity of identified HVC neurons in the zebra finch. J. Neurophysiol. 97, 4271–4283. ( 10.1152/jn.00952.2006) [DOI] [PubMed] [Google Scholar]
- 38.Prather JF, Peters S, Nowicki S, Mooney R. 2008. Precise auditory–vocal mirroring in neurons for learned vocal communication. Nature 451, 305–310. ( 10.1038/nature06492) [DOI] [PubMed] [Google Scholar]
- 39.Long MA, Fee MS. 2008. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456, 189–194. ( 10.1038/nature07448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenowitz EA. 1991. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science 251, 303–305. ( 10.1126/science.1987645) [DOI] [PubMed] [Google Scholar]
- 41.Gentner TQ, Hulse SH, Bentley GE, Ball GF. 2000. Individual vocal recognition and the effect of partial lesions to HVC on discrimination, learning, and categorization of conspecific song in adult songbirds. J. Neurobiol. 42, 117–133. () [DOI] [PubMed] [Google Scholar]
- 42.McCasland JS, Konishi M. 1981. Interaction between auditory and motor activities in an avian song control nucleus. Proc. Natl Acad. Sci. USA 78, 7815–7819. ( 10.1073/pnas.78.12.7815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volman SF. 1993. Development of neural selectivity for birdsong during vocal learning. J. Neurosci. 13, 4737–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prather JF, Nowicki S, Anderson RC, Peters S, Mooney R. 2009. Neural correlates of categorical perception in learned vocal communication. Nat. Neurosci. 12, 221–228. ( 10.1038/nn.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prather JF, Peters S, Nowicki S, Mooney R. 2010. Persistent representation of juvenile experience in the adult songbird brain. J. Neurosci. 30, 10 586–10 598. ( 10.1523/JNEUROSCI.6042-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margoliash D. 1983. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J. Neurosci. 3, 1039–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margoliash D. 1986. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J. Neurosci. 6, 1643–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margoliash D, Fortune ES. 1992. Temporal and harmonic combination-sensitive neurons in the zebra finch's HVC. J. Neurosci. 12, 4309–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theunissen FE, Amin N, Shaevitz SS, Woolley SM, Fremouw T, Hauber ME. 2004. Song selectivity in the song system and in the auditory forebrain. Ann. N. Y. Acad. Sci. 1016, 222–245. ( 10.1196/annals.1298.023) [DOI] [PubMed] [Google Scholar]
- 50.Theunissen FE, Doupe AJ. 1998. Temporal and spectral sensitivity of complex auditory neurons in the nucleus HVC of male zebra finches. J. Neurosci. 18, 3786–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volman SF. 1996. Quantitative assessment of song-selectivity in the zebra finch ‘high vocal center’. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 178, 849–862. ( 10.1007/BF00225832) [DOI] [PubMed] [Google Scholar]
- 52.Doupe AJ, Konishi M. 1991. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc. Natl Acad. Sci. USA 88, 11 339–11 343. ( 10.1073/pnas.88.24.11339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy A, Mooney R. 2007. Auditory plasticity in a basal ganglia-forebrain pathway during decrystallization of adult birdsong. J. Neurosci. 27, 6374–6387. ( 10.1523/JNEUROSCI.0894-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sturdy CB, Wild JM, Mooney R. 2003. Respiratory and telencephalic modulation of vocal motor neurons in the zebra finch. J. Neurosci. 23, 1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams H. 1989. Multiple representations and auditory–motor interactions in the avian song system. Ann. N. Y. Acad. Sci. 563, 148–164. ( 10.1111/j.1749-6632.1989.tb42196.x) [DOI] [Google Scholar]
- 56.Williams H, Nottebohm F. 1985. Auditory responses in avian vocal motor neurons: a motor theory for song perception in birds. Science 229, 279–282. ( 10.1126/science.4012321) [DOI] [PubMed] [Google Scholar]
- 57.Hamaguchi K, Tschida K, Yoon I, Donald BR, Mooney R. 2014. Auditory synapses to song premotor neurons are gated off during vocalization in zebra finches. eLIFE 3, e01833 ( 10.7554/eLife.01833.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Del Negro C, Edeline JM. 2001. Differences in auditory and physiological properties of HVc neurons between reproductively active male and female canaries (Serinus canaria). Eur. J. Neurosci. 14, 1377–1389. ( 10.1046/j.0953-816x.2001.01758.x) [DOI] [PubMed] [Google Scholar]
- 59.Del Negro C, Edeline JM. 2002. Sex and season influence the proportion of thin spike cells in the canary HVc. Neuroreport 13, 2005–2009. ( 10.1097/00001756-200211150-00003) [DOI] [PubMed] [Google Scholar]
- 60.Del Negro C, Gahr M, Leboucher G, Kreutzer M. 1998. The selectivity of sexual responses to song displays: effects of partial chemical lesion of the HVC in female canaries. Behav. Brain Res. 96, 151–159. ( 10.1016/S0166-4328(98)00009-6) [DOI] [PubMed] [Google Scholar]
- 61.Rauske PL, Shea SD, Margoliash D. 2003. State and neuronal class-dependent reconfiguration in the avian song system. J. Neurophysiol. 89, 1688–1701. ( 10.1152/jn.00655.2002) [DOI] [PubMed] [Google Scholar]
- 62.Cardin JA, Schmidt MF. 2003. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. J. Neurophysiol. 90, 2884–2899. ( 10.1152/jn.00391.2003) [DOI] [PubMed] [Google Scholar]
- 63.Cardin JA, Schmidt MF. 2004. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J. Neurosci. 24, 7745–7753. ( 10.1523/JNEUROSCI.1951-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cardin JA, Schmidt MF. 2004. Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. J. Neurophysiol. 91, 2148–2163. ( 10.1152/jn.00918.2003) [DOI] [PubMed] [Google Scholar]
- 65.Fee MS, Kozhevnikov AA, Hahnloser RH. 2004. Neural mechanisms of vocal sequence generation in the songbird. Ann. N. Y. Acad. Sci. 1016, 153–170. ( 10.1196/annals.1298.022) [DOI] [PubMed] [Google Scholar]
- 66.Mooney R, Prather JF. 2005. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J. Neurosci. 25, 1952–1964. ( 10.1523/JNEUROSCI.3726-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bottjer SW, Miesner EA, Arnold AP. 1984. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224, 901–903. ( 10.1126/science.6719123) [DOI] [PubMed] [Google Scholar]
- 68.Sohrabji F, Nordeen EJ, Nordeen KW. 1990. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav. Neural Biol. 53, 51–63. ( 10.1016/0163-1047(90)90797-A) [DOI] [PubMed] [Google Scholar]
- 69.Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. 2000. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds (see comments). Neuron 25, 481–492. ( 10.1016/S0896-6273(00)80910-1) [DOI] [PubMed] [Google Scholar]
- 70.Burt JM, Lent KL, Beecher MD, Brenowitz EA. 2000. Lesions of the anterior forebrain song control pathway in female canaries affect song perception in an operant task. J. Neurobiol. 42, 1–13. () [DOI] [PubMed] [Google Scholar]
- 71.Scharff C, Nottebohm F, Cynx J. 1998. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J. Neurobiol. 36, 81–90. () [DOI] [PubMed] [Google Scholar]
- 72.Gale SD, Perkel DJ. 2010. A basal ganglia pathway drives selective auditory responses in songbird dopaminergic neurons via disinhibition. J. Neurosci. 30, 1027–1037. ( 10.1523/JNEUROSCI.3585-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mooney R. 2000. Different subthreshold mechanisms underlie song-selectivity in identified HVc neurons of the zebra finch. J. Neruosci. 20, 5420–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mooney R, Hoese W, Nowicki S. 2001. Auditory representation of the vocal repertoire in a songbird with multiple song types. Proc. Natl Acad. Sci. USA 98, 12 778–12 783. ( 10.1073/pnas.221453298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosen MJ, Mooney R. 2003. Inhibitory and excitatory mechanisms underlying auditory responses to learned vocalizations in the songbird nucleus HVC. Neuron 39, 177–194. ( 10.1016/S0896-6273(03)00357-X) [DOI] [PubMed] [Google Scholar]
- 76.Lewicki MS, Konishi M. 1995. Mechanisms underlying the sensitivity of songbird forebrain neurons to temporal order. Proc. Natl Acad. Sci. USA 92, 5582–5586. ( 10.1073/pnas.92.12.5582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cardin JA, Raksin JN, Schmidt MF. 2005. Sensorimotor nucleus NIf is necessary for auditory processing but not vocal motor output in the avian song system. J. Neurophysiol. 93, 2157–2166. ( 10.1152/jn.01001.2004) [DOI] [PubMed] [Google Scholar]
- 78.Schmidt MF, Konishi M. 1998. Gating of auditory responses in the vocal control system of awake songbirds. Nat. Neurosci. 1, 513–518. ( 10.1038/2232) [DOI] [PubMed] [Google Scholar]
- 79.Akutagawa E, Konishi M. 2010. New brain pathways found in the vocal control system of a songbird. J. Comp. Neurol. 518, 3086–3100. ( 10.1002/cne.22383) [DOI] [PubMed] [Google Scholar]
- 80.Ashmore RC, Wild JM, Schmidt MF. 2005. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. J. Neurosci. 25, 8543–8554. ( 10.1523/JNEUROSCI.1668-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamaguchi K, Mooney R. 2012. Recurrent interactions between the input and output of a songbird cortico-basal ganglia pathway are implicated in vocal sequence variability. J. Neurosci. 32, 11 671–11 687. ( 10.1523/JNEUROSCI.1666-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Striedter GF, Vu ET. 1998. Bilateral feedback projections to the forebrain in the premotor network for singing in zebra finches. J. Neurobiol. 34, 27–40. () [DOI] [PubMed] [Google Scholar]
- 83.Wild JM. 2004. Functional neuroanatomy of the sensorimotor control of singing. Ann. N. Y. Acad. Sci. 1016, 438–462. ( 10.1196/annals.1298.016) [DOI] [PubMed] [Google Scholar]
- 84.Wild JM, Kubke MF, Mooney R. 2009. Avian nucleus retroambigualis: cell types and projections to other respiratory-vocal nuclei in the brain of the zebra finch (Taeniopygia guttata). J. Comp. Neurol. 512, 768–783. ( 10.1002/cne.21932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wild JM, Williams MN, Suthers RA. 2000. Neural pathways for bilateral vocal control in songbirds. J. Comp. Neurol. 423, 413–426. () [DOI] [PubMed] [Google Scholar]
- 86.Lewandowski B, Vyssotski A, Hahnloser RH, Schmidt M. 2013. At the interface of the auditory and vocal motor systems: NIf and its role in vocal processing, production and learning. J. Physiol. Paris 107, 178–192. ( 10.1016/j.jphysparis.2013.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roy A, Mooney R. 2009. Song decrystallization in adult zebra finches does not require the song nucleus NIf. J. Neurophysiol. 102, 979–991. ( 10.1152/jn.00293.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coleman MJ, Vu ET. 2005. Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J. Neurobiol. 63, 70–89. ( 10.1002/neu.20122) [DOI] [PubMed] [Google Scholar]
- 89.Williams H, Vicario DS. 1993. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. J. Neurobiol. 24, 903–912. ( 10.1002/neu.480240704) [DOI] [PubMed] [Google Scholar]
- 90.Selverston AI, Moulins M. 1985. Oscillatory neural networks. Annu. Rev. Physiol. 47, 29–48. ( 10.1146/annurev.ph.47.030185.000333) [DOI] [PubMed] [Google Scholar]
- 91.Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. 2008. A synaptic basis for auditory–vocal integration in the songbird. J. Neurosci. 28, 1509–1522. ( 10.1523/JNEUROSCI.3838-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coleman MJ, Mooney R. 2004. Synaptic transformations underlying highly selective auditory representations of learned birdsong. J. Neurosci. 24, 7251–7265. ( 10.1523/JNEUROSCI.0947-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosen MJ, Mooney R. 2006. Synaptic interactions underlying song-selectivity in the avian nucleus HVC revealed by dual intracellular recordings. J. Neurophysiol. 95, 1158–1175. ( 10.1152/jn.00100.2005) [DOI] [PubMed] [Google Scholar]
- 94.Lewicki MS. 1996. Intracellular characterization of song-specific neurons in the zebra finch auditory forebrain. J. Neurosci. 16, 5855–5863. [PubMed] [Google Scholar]
- 95.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. 1999. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755. ( 10.1016/S0092-8674(00)81509-3) [DOI] [PubMed] [Google Scholar]
- 96.Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. 2010. Cortical plasticity induced by inhibitory neuron transplantation. Science 327, 1145–1148. ( 10.1126/science.1183962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hensch TK. 2005. Critical period mechanisms in developing visual cortex. Curr. Top. Dev. Biol. 69, 215–237. ( 10.1016/S0070-2153(05)69008-4) [DOI] [PubMed] [Google Scholar]
- 98.Sugiyama S, Prochiantz A, Hensch TK. 2009. From brain formation to plasticity: insights on Otx2 homeoprotein. Dev. Growth Differ. 51, 369–377. ( 10.1111/j.1440-169X.2009.01093.x) [DOI] [PubMed] [Google Scholar]
- 99.Hanuschkin A, Ganguli S, Hahnloser RH. 2013. A Hebbian learning rule gives rise to mirror neurons and links them to control theoretic inverse models. Front. Neural Circuits 7, 106 ( 10.3389/fncir.2013.00106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nick TA, Konishi M. 2005. Neural auditory selectivity develops in parallel with song. J. Neurobiol. 62, 469–481. ( 10.1002/neu.20115) [DOI] [PubMed] [Google Scholar]
- 101.Doupe AJ. 1997. Song- and order-selective neurons in the songbird anterior forebrain and their emergence during vocal development. J. Neurosci. 17, 1147–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tschida KA, Mooney R. 2012. Deafening drives cell-type-specific changes to dendritic spines in a sensorimotor nucleus important to learned vocalizations. Neuron 73, 1028–1039. ( 10.1016/j.neuron.2011.12.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nick TA, Konishi M. 2005. Neural song preference during vocal learning in the zebra finch depends on age and state. J. Neurobiol. 62, 231–242. ( 10.1002/neu.20087) [DOI] [PubMed] [Google Scholar]
- 104.Dave A, Margoliash D. 2000. Song replay during sleep and computational rules for sensorimotor vocal learning. Science 290, 812–816. ( 10.1126/science.290.5492.812) [DOI] [PubMed] [Google Scholar]
- 105.Roberts TF, Gobes SM, Murugan M, Olveczky BP, Mooney R. 2012. Motor circuits are required to encode a sensory model for imitative learning. Nat. Neurosci. 15, 1454–1459. ( 10.1038/nn.3206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts TF, Tschida KA, Klein ME, Mooney R. 2010. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature 463, 948–952. ( 10.1038/nature08759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shank SS, Margoliash D. 2009. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature 458, 73–77. ( 10.1038/nature07615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hessler NA, Doupe AJ. 1999. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J. Neurosci. 19, 10 461–10 481. [DOI] [PMC free article] [PubMed] [Google Scholar]