Abstract
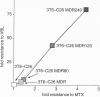
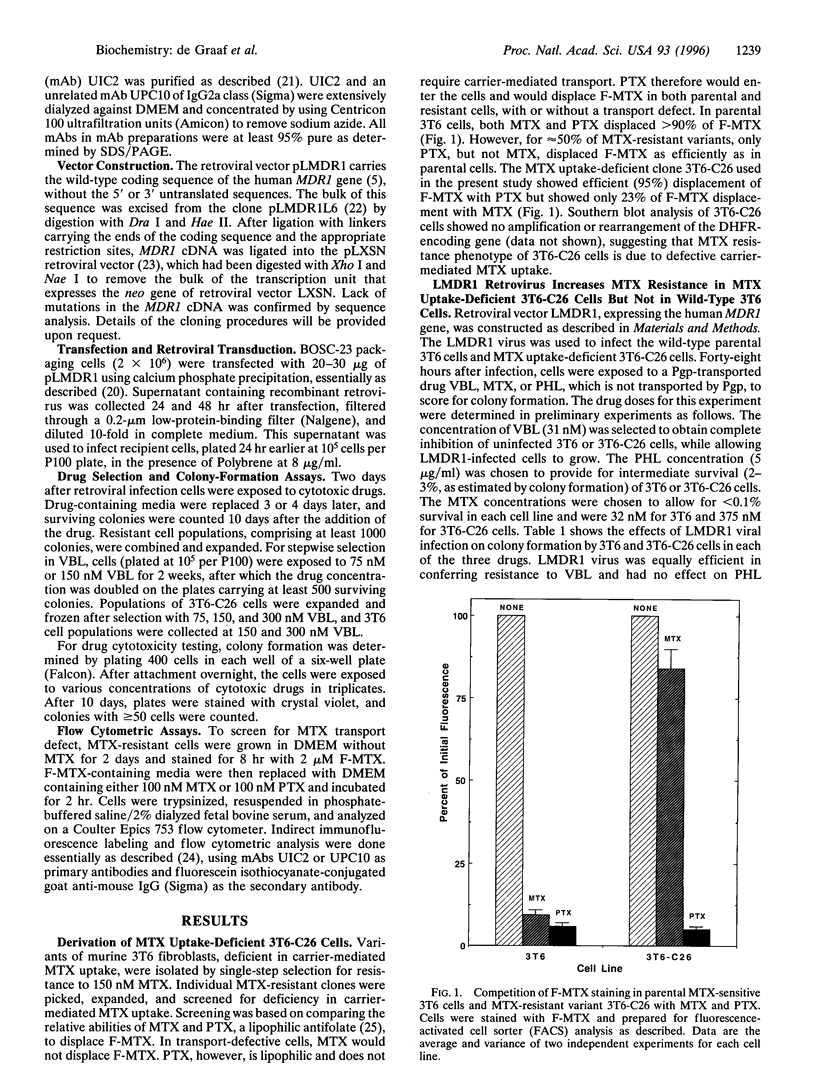
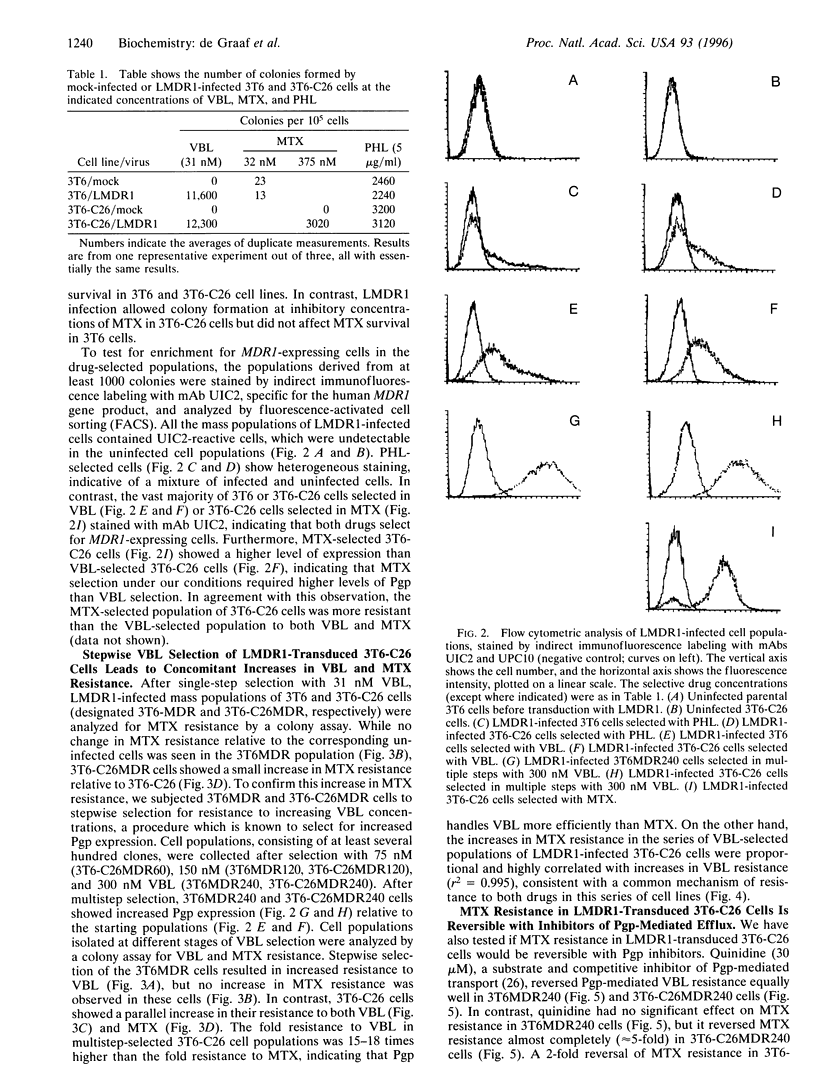
P-glycoprotein (Pgp), a transmembrane efflux pump encoded by the MDR1 gene, transports various lipophilic drugs that enter the cell by passive diffusion through the lipid bilayer. Pgp-expressing multidrug-resistant cell lines are not usually cross-resistant to a hydrophilic antifolate methotrexate (MTX). MTX enters cells primarily through a folate carrier, but passive diffusion becomes the primary mode of MTX uptake in carrier-deficient cells. To test if a deficiency in MTX carrier would allow Pgp to confer resistance to MTX, a MTX carrier-deficient cell line (3T6-C26) was infected with a recombinant retrovirus expressing the human MDR1 gene. The infected 3T6-C26 cells showed increased survival in MTX relative to uninfected cells. Multistep selection of the infected cells with vinblastine led to increased Pgp expression and a concomitant increase in resistance to MTX. MTX resistance of Pgp-expressing 3T6-C26 cells was reduced by Pgp inhibitors, including a Pgp-specific monoclonal antibody UTC2. In contrast, the expression and the inhibition of Pgp had no effect on MTX resistance in 3T6 cells with normal carrier-mediated MTX uptake. Thus, a deficiency in the MTX carrier enables Pgp to confer resistance to MTX, suggesting that hydrophilic compounds may become Pgp substrates when such compounds enter cells by passive diffusion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkin H., Ohnuma T., Kamen B. A., Holland J. F., Vallabhajosula S. Multidrug resistance in a human leukemic cell line selected for resistance to trimetrexate. Cancer Res. 1989 Dec 1;49(23):6556–6561. [PubMed] [Google Scholar]
- Assaraf Y. G., Molina A., Schimke R. T. Cross-resistance to the lipid-soluble antifolate trimetrexate in human carcinoma cells with the multidrug-resistant phenotype. J Natl Cancer Inst. 1989 Feb 15;81(4):290–294. doi: 10.1093/jnci/81.4.290. [DOI] [PubMed] [Google Scholar]
- Beck W. T. Cellular pharmacology of Vinca alkaloid resistance and its circumvention. Adv Enzyme Regul. 1984;22:207–227. doi: 10.1016/0065-2571(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Chaudhary P. M., Roninson I. B. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991 Jul 12;66(1):85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Choi K. H., Chen C. J., Kriegler M., Roninson I. B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell. 1988 May 20;53(4):519–529. doi: 10.1016/0092-8674(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Choi K., Frommel T. O., Stern R. K., Perez C. F., Kriegler M., Tsuruo T., Roninson I. B. Multidrug resistance after retroviral transfer of the human MDR1 gene correlates with P-glycoprotein density in the plasma membrane and is not affected by cytotoxic selection. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7386–7390. doi: 10.1073/pnas.88.16.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine S. E., Ling V., Melera P. W. Amino acid substitutions in the sixth transmembrane domain of P-glycoprotein alter multidrug resistance. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4564–4568. doi: 10.1073/pnas.89.10.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. W., Besserer J. A. Characterization of trimetrexate transport in human lymphoblastoid cells and development of impaired influx as a mechanism of resistance to lipophilic antifolates. Cancer Res. 1988 Dec 15;48(24 Pt 1):6986–6991. [PubMed] [Google Scholar]
- Goldman I. D., Matherly L. H. The cellular pharmacology of methotrexate. Pharmacol Ther. 1985;28(1):77–102. doi: 10.1016/0163-7258(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gros P., Dhir R., Croop J., Talbot F. A single amino acid substitution strongly modulates the activity and substrate specificity of the mouse mdr1 and mdr3 drug efflux pumps. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7289–7293. doi: 10.1073/pnas.88.16.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B. Folate-binding proteins. Annu Rev Nutr. 1990;10:319–335. doi: 10.1146/annurev.nu.10.070190.001535. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gottesman M. M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992 Jan;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Hill B. T., Dedhar S., Goldie J. H. Preliminary communications. Biochem Pharmacol. 1982 Jan 15;31(2):263–266. doi: 10.1016/0006-2952(82)90223-4. [DOI] [PubMed] [Google Scholar]
- Hoof T., Demmer A., Hadam M. R., Riordan J. R., Tümmler B. Cystic fibrosis-type mutational analysis in the ATP-binding cassette transporter signature of human P-glycoprotein MDR1. J Biol Chem. 1994 Aug 12;269(32):20575–20583. [PubMed] [Google Scholar]
- Horio M., Lovelace E., Pastan I., Gottesman M. M. Agents which reverse multidrug-resistance are inhibitors of [3H]vinblastine transport by isolated vesicles. Biochim Biophys Acta. 1991 Jan 9;1061(1):106–110. doi: 10.1016/0005-2736(91)90274-c. [DOI] [PubMed] [Google Scholar]
- Loo T. W., Clarke D. M. Functional consequences of glycine mutations in the predicted cytoplasmic loops of P-glycoprotein. J Biol Chem. 1994 Mar 11;269(10):7243–7248. [PubMed] [Google Scholar]
- Mechetner E. B., Roninson I. B. Efficient inhibition of P-glycoprotein-mediated multidrug resistance with a monoclonal antibody. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5824–5828. doi: 10.1073/pnas.89.13.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Pear W. S., Nolan G. P., Scott M. L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce H. L., Safa A. R., Bach N. J., Winter M. A., Cirtain M. C., Beck W. T. Essential features of the P-glycoprotein pharmacophore as defined by a series of reserpine analogs that modulate multidrug resistance. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5128–5132. doi: 10.1073/pnas.86.13.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. C., Assaraf Y. G., Schimke R. T. A phenotype conferring selective resistance to lipophilic antifolates in Chinese hamster ovary cells. Cancer Res. 1991 Jun 1;51(11):2949–2959. [PubMed] [Google Scholar]
- Stein W. D., Cardarelli C., Pastan I., Gottesman M. M. Kinetic evidence suggesting that the multidrug transporter differentially handles influx and efflux of its substrates. Mol Pharmacol. 1994 Apr;45(4):763–772. [PubMed] [Google Scholar]
- Su G. M., Davey M. W., Davey R. A., Kidman A. D. Development of extended multidrug resistance in HL60 promyelocytic leukaemia cells. Br J Haematol. 1994 Nov;88(3):566–574. doi: 10.1111/j.1365-2141.1994.tb05075.x. [DOI] [PubMed] [Google Scholar]
- Tang-Wai D. F., Kajiji S., DiCapua F., de Graaf D., Roninson I. B., Gros P. Human (MDR1) and mouse (mdr1, mdr3) P-glycoproteins can be distinguished by their respective drug resistance profiles and sensitivity to modulators. Biochemistry. 1995 Jan 10;34(1):32–39. doi: 10.1021/bi00001a005. [DOI] [PubMed] [Google Scholar]
- Volm M., Efferth T., Günther A., Lathan B. Detection of murine S180 cells expressing a multidrug resistance phenotype using different in vitro test systems and a monoclonal antibody. Arzneimittelforschung. 1987 Jul;37(7):862–867. [PubMed] [Google Scholar]