Abstract
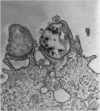
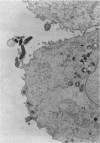
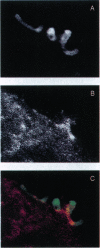
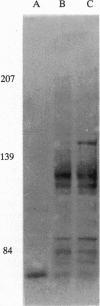
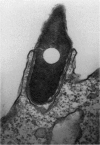
The consequences of Helicobacter pylori attachment to human gastric cells were examined by transmission electron microscopy and immunofluorescence microscopy. H. pylori attachment resulted in (i) effacement of microvilli at the site of attachment, (ii) cytoskeletal rearrangement directly beneath the bacterium, and (iii) cup/pedestal formation at the site of attachment. Double-immunofluorescence studies revealed that the cytoskeletal components actin, alpha-actinin, and talin are involved in the process. Immunoblot analysis showed that binding of H. pylori to AGS cells induced tyrosine phosphorylation of two host cell proteins of 145 and 105 kDa. These results indicate that attachment of H. pylori to gastric epithelial cells resembles that of enteropathogenic Escherichia coli. Coccoid H. pylori, which are thought to be terminally differentiated bacterial forms, are capable of binding and inducing cellular changes of the same sort as spiral H. pylori, including tyrosine phosphorylation of host proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen L. P., Blom J., Nielsen H. Survival and ultrastructural changes of Helicobacter pylori after phagocytosis by human polymorphonuclear leukocytes and monocytes. APMIS. 1993 Jan;101(1):61–72. [PubMed] [Google Scholar]
- Bliska J. B., Guan K. L., Dixon J. E., Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode G., Mauch F., Malfertheiner P. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol Infect. 1993 Dec;111(3):483–490. doi: 10.1017/s0950268800057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borén T., Falk P., Roth K. A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993 Dec 17;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Catrenich C. E., Makin K. M. Characterization of the morphologic conversion of Helicobacter pylori from bacillary to coccoid forms. Scand J Gastroenterol Suppl. 1991;181:58–64. [PubMed] [Google Scholar]
- Cellini L., Allocati N., Angelucci D., Iezzi T., Di Campli E., Marzio L., Dainelli B. Coccoid Helicobacter pylori not culturable in vitro reverts in mice. Microbiol Immunol. 1994;38(11):843–850. doi: 10.1111/j.1348-0421.1994.tb02136.x. [DOI] [PubMed] [Google Scholar]
- Chen M., Lee A., Hazell S. Immunisation against gastric helicobacter infection in a mouse/Helicobacter felis model. Lancet. 1992 May 2;339(8801):1120–1121. doi: 10.1016/0140-6736(92)90720-n. [DOI] [PubMed] [Google Scholar]
- Dytoc M., Gold B., Louie M., Huesca M., Fedorko L., Crowe S., Lingwood C., Brunton J., Sherman P. Comparison of Helicobacter pylori and attaching-effacing Escherichia coli adhesion to eukaryotic cells. Infect Immun. 1993 Feb;61(2):448–456. doi: 10.1128/iai.61.2.448-456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton K. A., Catrenich C. E., Makin K. M., Krakowka S. Virulence of coccoid and bacillary forms of Helicobacter pylori in gnotobiotic piglets. J Infect Dis. 1995 Feb;171(2):459–462. doi: 10.1093/infdis/171.2.459. [DOI] [PubMed] [Google Scholar]
- Eaton K. A., Morgan D. R., Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989 Apr;57(4):1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology. 1992 May;102(5):1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Moulds J. J., Graham D. Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988 Nov;56(11):2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Zobeley S., Rinnerthaler G., Small J. V. Fluorescent phallotoxins as probes for filamentous actin. J Muscle Res Cell Motil. 1988 Oct;9(5):370–383. doi: 10.1007/BF01774064. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Rosenshine I., Donnenberg M. S., Kaper J. B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992 Jun;60(6):2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Hessey S. J., Spencer J., Wyatt J. I., Sobala G., Rathbone B. J., Axon A. T., Dixon M. F. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990 Feb;31(2):134–138. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neman-Simha V., Mégraud F. In vitro model for Campylobacter pylori adherence properties. Infect Immun. 1988 Dec;56(12):3329–3333. doi: 10.1128/iai.56.12.3329-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius M., Bode G., Büchler M., Malfertheiner P. Adhesion of Helicobacter pylori and Escherichia coli to human and bovine surface mucus cells in vitro. Eur J Clin Invest. 1994 Jul;24(7):454–459. doi: 10.1111/j.1365-2362.1994.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Rosenshine I., Donnenberg M. S., Kaper J. B., Finlay B. B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992 Oct;11(10):3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Duronio V., Finlay B. B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992 Jun;60(6):2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudmann D. G., Eaton K. A., Krakowka S. Ultrastructural study of Helicobacter pylori adherence properties in gnotobiotic piglets. Infect Immun. 1992 May;60(5):2121–2124. doi: 10.1128/iai.60.5.2121-2124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E. D., Shon J., Tompkins L. S. Characterization of Helicobacter pylori urease mutants. Infect Immun. 1992 May;60(5):1883–1889. doi: 10.1128/iai.60.5.1883-1889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoot D. T., Resau J. H., Naab T., Desbordes B. C., Gilliam T., Bull-Henry K., Curry S. B., Nidiry J., Sewchand J., Mills-Robertson K. Adherence of Helicobacter pylori to cultured human gastric epithelial cells. Infect Immun. 1993 Jan;61(1):350–355. doi: 10.1128/iai.61.1.350-355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P., Rosenshine I., Finlay B. B. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol Biol Cell. 1994 Apr;5(4):455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkonen K. H., Wadström T., Moran A. P. Interaction of lipopolysaccharides of Helicobacter pylori with basement membrane protein laminin. Infect Immun. 1994 Sep;62(9):3640–3648. doi: 10.1128/iai.62.9.3640-3648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyle F. A., Tarnawski A., Dabros W., Gergely H. Campylobacter pylori interactions with gastric cell tissue culture. J Clin Gastroenterol. 1990;12 (Suppl 1):S99–103. doi: 10.1097/00004836-199001001-00017. [DOI] [PubMed] [Google Scholar]
- Young V. B., Falkow S., Schoolnik G. K. The invasin protein of Yersinia enterocolitica: internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992 Jan;116(1):197–207. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]