Abstract
Introduction
Isoflavones are hypothesized to protect against breast cancer, but it is not clear whether they act as oestrogens or anti-oestrogens in breast tissue. Our aim was to determine the effects of taking a red clover-derived isoflavone supplement daily for 1 year on mammographic breast density. Effects on oestradiol, follicle-stimulating hormone (FSH), luteinizing hormone (LH), lymphocyte tyrosine kinase activity and menopausal symptoms were also assessed.
Methods
A total of 205 women (age range 49–65 years) with Wolfe P2 or DY mammographic breast patterns were randomly assigned to receive either a red clover-derived isoflavone tablet (26 mg biochanin A, 16 mg formononetin, 1 mg genistein and 0.5 mg daidzein) or placebo. Change in mammographic breast density, serum oestradiol, FSH, LH, menopausal symptoms and lymphocyte tyrosine kinase activity from baseline to 12 months were assessed.
Results
A total of 177 women completed the trial. Mammographic breast density decreased in both groups but the difference between the treatment and placebo was not statistically significant. There was a significant interaction between treatment group and oestrogen receptor (ESR1) PvuII polymorphism for the change in estimated percentage breast density (mean ± standard deviation): TT isoflavone 1.4 ± 12.3% and TT placebo -9.6 ± 14.2%; CT isoflavone -5.2 ± 12.0% and CT placebo -2.8 ± 10.3%; and CC isoflavone -3.4 ± 9.7% and CC placebo -1.1 ± 9.5%. There were no statistically significant treatment effects on oestradiol, FSH, or LH (assessed only in postmenopausal women), or on lymphocyte tyrosine kinase activity. Baseline levels of menopausal symptoms were low, and there were no statistically significant treatment effects on frequency of hot flushes or other menopausal symptoms.
Conclusion
In contrast to studies showing that conventional hormone replacement therapies increase mammographic breast density, the isoflavone supplement did not increase mammographic breast density in this population of women. Furthermore, there were no effects on oestradiol, gonadotrophins, lymphocyte tyrosine kinase activity, or menopausal symptoms.
Keywords: breast density, CYP17, CYP19, ESR1, isoflavone, oestradiol, polymorphism, tyrosine kinase
Introduction
Isoflavones are biologically active compounds that are naturally present in foods of plant origin. They have received considerable attention because of their potential cancer preventive, cardioprotective and bone sparing effects, and because of their potential to provide relief from menopausal symptoms [1,2]. Soybeans are particularly rich sources of isoflavones such as daidzein and genistein [3], and intakes of isoflavones are markedly higher among Asian populations than in Western ones, largely resulting from soy consumption patterns [4,5]. It is widely perceived that exposure to isoflavones is beneficial because rates of breast cancer and other hormone-dependent conditions are lower in Asian than in Western countries [6-8]. However, isoflavones can act as weak oestrogens, and two intervention studies with soy have provided evidence for stimulatory effects of isoflavones on breast tissue [9,10].
Mammographic breast density, characterized by the relative proportions of radiolucent fat and radiodense connective and epithelial tissue within the breast, has consistently been associated with risk for breast cancer. In a review of case–control studies [11], odds ratios for breast cancer among women with the highest versus the lowest extents of density ranged from 2.1 to 6.0. The mechanism underlying this relationship has not been fully explained, but it has been suggested that breast density provides an index of current and past hormonal and reproductive events that modulate risk for breast cancer [12]. In support of this, studies have shown that breast density increases when a woman begins hormone replacement therapy (HRT) and decreases when she discontinues [13-18], and anti-oestrogens such as tamoxifen reduce breast density [19-21]. As a result of such findings, we suggested that breast density can act as a biomarker of oestrogenic or anti-oestrogenic effects of a given treatment on breast tissue [22].
Diet has been shown to influence breast density, potentially through effects on endogenous hormone levels. For example, a low-fat, high-carbohydrate intervention significantly reduced breast density [23]; however, an inverse association has also been reported between breast density and saturated fat intake [24]. In a cross-sectional study of diet and mammographic density, total protein and carbohydrate intake was significantly and positively associated with breast density, and among postmenopausal women there was a significant positive association between total meat intake and breast density [25]. More recently, data from a cross-sectional study of soy food intakes and mammographic densities [26] indicated the presence of a significant trend toward higher percentage density through increasing quartiles of soy intake, but a small study of a soy isoflavone supplement [27] did not significantly alter breast density after 1 year of treatment.
Here, we report the results of a randomized, placebo-controlled trial that was conducted to determine the effects of a red clover-derived isoflavone supplement, taken daily for 1 year, on mammographic breast density. In addition, effects on circulating levels of oestradiol, gonadotrophins, lymphocyte tyrosine kinase activity, and menopausal symptoms are reported, and interactions between treatment group and polymorphisms in CYP17, CYP19, and oestrogen receptor (ESR1) genes.
Methods
Recruitment
Women were recruited from the Breast Screening Unit, Addenbrooke's Hospital, Cambridge, UK. Between November 1997 and May 1999, mammograms from 1908 healthy women aged 49–65 years were classified according to Wolfe pattern [28] and recruitment letters were sent to women with Wolfe P2 or DY breast patterns (n = 1149; Fig. 1). The letter contained a short description of the study and a reply slip. Women with a history of breast cancer and/or major breast surgery were not approached. Women who expressed an interest in taking part, and who were not taking HRT, were visited in their own home. During this initial home visit the study was explained in detail, and all women who wished to participate were asked for written informed consent. A total of 205 women were randomized (Fig. 1). Additional home visits were made after approximately 5.5 and 11 months on the study. All study procedures were approved by the Dunn Human Nutrition Unit Ethics Committee, and the Cambridge Local Research Ethics Committee.
Figure 1.
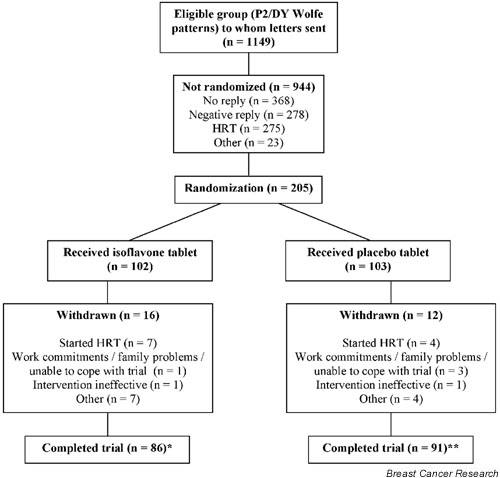
Flow chart describing progress of participants through trial. *Includes two women who completed the trial but were excluded from all analyses because they had taken an oral contraceptive (OC) or were being treated for alcoholism. **Includes one woman who was excluded from all analyses because she had taken an OC, two women who had mammograms taken before the end of the study, and one woman who did not attend her follow-up mammogram. HRT, hormone replacement therapy.
Intervention and randomization
Participants were randomly assigned to receive either an isoflavone tablet providing 26 mg biochanin A, 16 mg formononetin, 1 mg genistein and 0.5 mg daidzein derived from red clover (Promensil; Novogen Ltd, Sydney, Australia) or a placebo of identical appearance. Participants were asked to take one tablet each day for 1 year. An independent study has shown that Promensil tablets contain the quantity and type of isoflavones stated by the manufacturer [29]. Randomization was performed by the Outpatient Pharmacy, Addenbrooke's Hospital, Cambridge, UK, using random number generation in Microsoft Excel. Researchers and study participants remained blinded to tablet allocation throughout the study.
Mammographic density
Recruitment mammograms that were taken as part of the National Health Service Breast Screening Programme were used as the baseline measure, and women were scheduled to undergo a follow-up mammogram after approximately 12 months on the study. Independently of each other, and blinded to intervention or placebo status, two radiologists (RMLW and ES) assigned Wolfe patterns [28] and visually estimated the percentage density on each set (left and right mediolateral oblique views) of mammograms. Estimated percentage densities were assigned by drawing a cross on a 100 mm line (representing 0–100% density), and the distance from the start of the line and the cross was measured. Mammograms from the left and right breasts were presented to the radiologists, and an average of both breasts was used for assigning Wolfe pattern and estimated percentage density. One radiologist (RMLW) read baseline mammograms at the time of recruitment, and follow up mammograms at the end of the study, and another (ES) read all mammograms at the end of the study but was unaware of the sequence in which the mammograms had been taken (i.e. baseline or follow up). Correlations between estimated percentage densities assigned by both radiologists were highly significant for the baseline and 12-month mammograms (r = 0.79, P < 0.01 and r = 0.85, P < 0.01, respectively). Excellent (99%) observer agreement between the two radiologists has previously been reported for the P2 and DY categories [30]. In all statistical analyses, the average of the two radiologists' independent readings of percentage breast density was used. Two women in the placebo group had mammograms taken before the end of the study (because of suspicious palpable lumps), and one woman (also in the placebo group) did not attend her follow-up appointment.
Menopausal symptoms
All women were asked to complete a 28-day menopausal symptom diary at baseline and after approximately 12 months on the study [31]. They were asked to rate the severity of 21 symptoms associated with the menopause, including night sweats, heart beating quickly or strongly, feeling tense or nervous, difficulty in sleeping, irritability, and feeling unhappy or depressed. Severity ratings were as follows: 0 = absent, 1 = mild, 2 = moderate and 3 = severe. Women were also asked to note the number of hot flushes experienced on each of the 28 days. For each woman, a menopausal symptom score was calculated for the baseline and 12 month diaries (severity ratings for each symptom were averaged over the 28 days and a sum of the mean severity ratings was taken).
24-Hour urine samples
Participants were asked to provide 24-hour urine collections at baseline and after 12 months on the study. As a compliance check, women also were asked to make a 24-hour urine collection after 6 months. Collection bottles (containing 2 g boric acid/l as a preservative) and three para-amino benzoic acid (PABA) tablets (80 mg/tablet) were given to each participant at each of the three home visits [32]. Verbal and written instructions were given, and for each collection participants were asked to discard the first urine specimen of the day and to collect all subsequent samples up to and including the first specimen passed the following morning. Participants were asked to take one PABA tablet with each main meal on the day the collection began, and this was used to determine the completeness of urine collection (see below). Samples were brought to the laboratory in the morning on which the urine collection had been completed. Once received at the laboratory, samples were mixed thoroughly and the total volume of urine was measured. Three 25 ml aliquots were taken and stored at -20°C.
Urinary para-amino benzoic acid and isoflavone analysis
The PABA content of all 24-hour urine samples was measured using previously described methodology [32]. Samples containing 85–110% of the ingested PABA were designated satisfactory. For samples with PABA recoveries of between 70% and 85% (indicating that all tablets had been taken but that the urine collection was incomplete), urinary excretion of isoflavones was adjusted up to 93% PABA recovery [33]. Samples with less than 70% recovery were designated incomplete. Samples with greater than 110% PABA recovery were designated unsatisfactory, because additional sources of PABA may have been consumed (e.g. a multivitamin) and an accurate determination of sample completeness could therefore not be made.
Urinary excretions of genistein, daidzein, formononetin and biochanin A were measured by high-pressure liquid chromatography (HPLC) using a method modified from that of Setchell and coworkers [34] and Franke and coworkers [35]. Briefly, samples were incubated with β-glucuronidase for 20–72 hours at 37°C. Phenolic components were extracted into an ethyl-acetate (6:4) solvent mixture. Following 0.5-min vortex and subsequent 10-min centrifugation, the organic phase was transferred to a 2-ml vial and evaporated to dryness under vacuum at 43°C. Extraction residues were reconstituted in 100 μl 50% isopropanol solution and centrifuged for 10 min. A 5 μl aliquot was then injected directly onto the HPLC column; the HPLC column consisted of an Alltima 250 × 2.1 mm, 5 μmol/l, C-18 stationary phase (Alltech Associates, Sydney, New South Wales, Australia), and a mobile phase (acetonitrile/water) containing 0.05% trifluoroacetic acid with a gradient of acetonitrile from 25% to 100% was used for each run. Detection and quantification were via photo diode array detector. Flavone was used as the internal standard, and the limit of detection was 0.05 μg/ml and the limit of quantitation was 0.1 μg/ml. Baseline or 12-month urine samples were unavailable for two women in the isoflavone group and three women in the placebo group.
Blood samples and body mass index
Fasting blood samples were taken at baseline and at 12 months. Participants were asked to refrain from eating or drinking beverages (except water) from midnight until after the sample had been taken the following morning. A total of 35.5 ml blood was drawn at each visit, which included 9.0 ml blood that was drawn into a lithium heparin tube, 9.0 ml into a serum tube, 9 ml into an EDTA tube and 4 ml into an additional EDTA tube. The serum tube was left at room temperature for at least an hour before centrifugation to allow clotting. Following centrifugation, aliquots of plasma and serum were stored at -20°C. Lymphocytes were extracted from the EDTA tubes (total 13 ml whole blood) using Lymphoprep tubes (Nycomed Pharma AS, Oslo, Norway) and stored at -20°C. Approximately 1 ml whole blood was removed from the lithium heparin tube before centrifugation, and DNA was extracted using a QIAGEN kit (QIAGEN Ltd, Crawley, UK). Polymorphisms in the CYP17 gene (5' untranslated MspA1 polymorphism) and the CYP19 gene (G → T substitution on intron 6) were determined as described previously [36,37]. The PvuII polymorphism (generated by a C → T substitution on intron 1) in the ESR1 gene was determined via an automated method using TaqMan™ (Applied Biosystems, Cheshire, UK) [38]. The primers used were ESR1-F (TGTTGTCCATCAGTTCATCTGAGT) and ESR1-R (AACTCTAGACCACACTCAGGGTCTCT) and the probes were (AATGTCCCAGCCGTTTTATGCTTTGT) labelled with TET and (AAATGTCCCAGCTGTTTTATGCTTTGTCT) labelled with FAM (Applied Biosystems). Reactions were conducted according to manufacturer's instructions and detected using an ABI Prism 7700 Sequence Detector (Applied Biosystems).
Participants' height and weight were measured at baseline and 12 months, and body mass index (BMI) was calculated as follows: weight (kg)/height (m2).
Oestradiol and gonadotrophins, and menopausal status
Serum oestradiol was measured using previously described methodology [39]. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were measured using enzyme immunoassay on an Abbott Axsym automated analyzer (Abbott Diagnostics, Maidenhead, UK). Baseline and 12-month samples for each participant were assayed in the same batch. The limit of detection for oestradiol was 3.0 pmol/l. Menopausal status was determined using baseline levels of oestradiol and FSH as follows: women were classed as premenopausal if FSH < 30 IU/l and oestradiol > 100 pmol/l; postmenopausal if FSH > 30 IU/l and oestradiol < 100 pmol/l; and perimenopausal if FSH > 30 IU/l and oestradiol > 100 pmol/l, or if FSH < 30 IU/l and oestradiol < 100 pmol/l. However, if a woman had noted on her questionnaire at the initial home visit that she was currently menstruating, but her baseline hormone profile was that of a postmenopausal woman (i.e. FSH > 30 IU/l and oestradiol < 100 pmol/l), then she was classed as perimenopausal.
Tyrosine kinase
Lymphocytes were lysed according to methods reported elsewhere [40] and analyzed for tyrosine kinase activity using an enzyme-linked immunosorbent assay based kit and corrected for protein content (Tyrosine Kinase Assay Kit [product no. 29904] and BCA Protein Assay kit [product no. 23225]; Pierce, Rockford, IL, USA).
Data analysis
Based on the findings of our previous study on the effects of tamoxifen on breast density [19], we estimated that 20% of women in the isoflavone group and 5% of the women in the placebo group would change to a more lucent Wolfe pattern. Thus, to yield 80% power to detect a difference between treatment groups at the 5% significance level (two-tailed), a sample size of 76 women per treatment arm would be needed [41]. Statistical analyses were performed using the SAS statistical package version 6.12 (SAS Institute, Cary, NC, USA). Pearson correlation coefficients and Student's t-tests were used to assess inter-reader agreement in estimated percentage breast density. Changes in estimated percentage breast density, oestradiol, FSH, LH, tyrosine kinase activity, menopausal symptom score, and number of hot flushes from baseline to 12 months were expressed as absolute change (i.e. 12-month data – baseline data). Differences between treatment groups for changes in breast density and tyrosine kinase activity were tested using an unpaired t-test. Data for changes in oestradiol, FSH, LH, hot flushes, and menopausal symptom score were skewed, and therefore the nonparametric Wilcoxon rank sum test was used to test for differences between treatment groups. Changes in oestradiol, FSH, and LH were assessed only in postmenopausal women because of absence of data on phase of menstrual cycle in which blood samples were taken. A general linear model (using the SAS command PROC GLM) was used to determine interaction in effects of genotype on baseline measures and on change from baseline to 12 months. Three women who completed the study were excluded from all analyses either because they had taken an oral contraceptive regularly during the study (one in the isoflavone group, and one in the placebo group) or because they were being treated for alcoholism (one in the isoflavone group).
Results
Withdrawals and baseline data
Twenty-eight women withdrew from the study (Fig. 1); principal reasons for withdrawal were commencement of HRT and work commitments/family problems preventing completion of study activities. Other reasons included feeling no beneficial effects of the intervention or no interest in continuing on the trial, heavy menstrual bleeding, and illnesses preventing completion of study activities (e.g. severe hip pain, skin irritation and sores, sickness and diarrhoea). One woman in the isoflavone group was diagnosed with an interval cancer of the breast (i.e. a cancer detected in the interval after a negative mammographic result) 2 months after the start of the intervention and was withdrawn from the study. The difference between treatment groups in the number of withdrawals was not statistically significant (χ2 = 1.123, P = 0.29).
Age (mean ± standard deviation [SD]) was 55.1 ± 4.7 years for women in the isoflavone group and 55.2 ± 4.9 years for those in the placebo group (P = 0.65), and BMI (mean ± SD) was 25.3 ± 3.9 kg/m2 and 25.3 ± 3.5 kg/m2 for women in the isoflavone and placebo groups, respectively (P = 0.90). Percentages of women who were premenopausal, perimenopausal and postmenopausal were 16%, 14% and 67%, respectively, for women in the isoflavone group, and 17%, 16% and 68%, respectively, for women in the placebo group (χ2 = 0.04, P = 0.98). Time intervals (mean ± SD) between the screening mammogram and the start of the intervention, and between baseline and 12-month mammograms were 92 ± 32 and 452 ± 45 days, respectively, for women in the isoflavone group, and 87 ± 27 and 454 ± 43 days, respectively, for women in the placebo group. Differences between treatment groups were nonsignificant (P > 0.05). There were no significant effects of genotype (for genotype frequencies, see Table 1) on baseline breast density, oestradiol, FSH or LH (P > 0.05).
Table 1.
Genotype frequencies by treatment group
| Group | |||
| Gene/polymorphism | Isoflavone | Placebo | P |
| CYP17a | |||
| TT | 32 (40%) | 33 (39%) | |
| TC | 36 (44%) | 40 (47%) | 0.92 |
| CC | 13 (16%) | 12 (14%) | |
| CYP19b | |||
| GG | 21 (27%) | 28 (32%) | |
| GT | 40 (51%) | 45 (52%) | 0.49 |
| TT | 18 (23%) | 14 (16%) | |
| ESR1c | |||
| CC | 17 (22%) | 19 (22%) | |
| CT | 39 (51%) | 46 (53%) | 0.95 |
| TT | 21 (27%) | 22 (25%) | |
aCYP17 data not available for three and five women in the isoflavone and placebo groups, respectively. bCYP19 data not available for five and three women in the isoflavone and placebo groups, respectively. cESR1 data not available for seven and three women in the isoflavone and placebo groups, respectively. Percentages do not always add up to 100% because of rounding.
Compliance
According to the PABA check method, 58%, 72% and 77% of the women had complete urine collections at baseline, 6 and 12 months, respectively. A further 22%, 11% and 13% had PABA recoveries between 70% and 85% at baseline, 6 and 12 months, respectively. Differences between treatment groups in summed isoflavone excretion (sum of daidzein, genistein, formononetin and biochanin A) were nonsignificant at baseline, but differences were highly significant at 6 and 12 months (Fig. 2; data shown do not include women with < 70% or > 110% PABA recovery; inclusion of these women did not alter the results). Among women in the placebo group, isoflavone excretion did not change significantly from baseline to 6 months (P = 0.80) or from baseline to 12 months (P = 0.15; Fig. 2).
Figure 2.
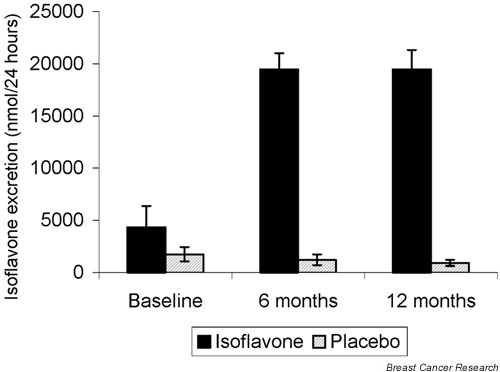
Urinary isoflavone (sum of daidzein, genistein, formononetin and biochanin A) excretion at baseline, midway, and at the end of the study, excluding samples with < 70% or > 110% para-amino benzoic acid recovery. Error bars represent ± standard error of the mean. Numbers at each evaluation were as follows: baseline, 66 and 72 in the isoflavone and placebo groups, respectively; 6 months, 70 and 73; and 12 months, 76 and 79. The difference in isoflavone excretion between treatment groups was not statistically significant at baseline (P = 0.23), but differences between treatment groups at 6 and 12 months were highly significant (P < 0.001 and P < 0.001).
Breast density
There were no differences between readers (RMLW and ES) in estimated percentage density at baseline or follow-up. Densities (mean ± SD) at baseline were 61.8 ± 14.4% and 61.5 ± 18.8% for RMLW and ES, respectively (P = 0.86), and at follow-up they were 58.0 ± 16.5% and 57.4 ± 21.6% for RMLW and ES, respectively (P = 0.81).
According to RMLW, among women in the isoflavone and placebo groups, respectively, 22% and 18% of women changed to a more lucent Wolfe pattern, 78% and 80% did not change, and 0% and 2% changed to a more dense Wolfe pattern. Differences between treatment groups were not statistically significant (χ2 = 3.57; P = 0.31). According to ES, among women in the isoflavone and placebo groups, respectively, 15% and 19% of women changed to a more lucent Wolfe pattern, 84% and 80% did not change, and 1% and 1% changed to a more dense Wolfe pattern. Again, differences between treatment groups were not statistically significant (χ2 = 0.43; P = 0.81).
Interactions between treatment group and menopausal status for changes in estimated percentage breast density were nonsignificant (P > 0.05), and so changes were assessed for the combined group. Percentage breast density (mean ± SD) decreased in both treatment groups to a similar extent: -3.2 ± 11.7% and -3.9 ± 11.7% among women in the isoflavone and placebo groups, respectively (P = 0.73; Fig. 3). When analyses were restricted to women with complete urine collections (i.e. samples containing 85–110% of ingested PABA) at all three time points, or when analyses were adjusted for baseline age and change in BMI, differences between treatment groups remained nonsignificant (P = 0.20 and P = 0.88, respectively).
Figure 3.
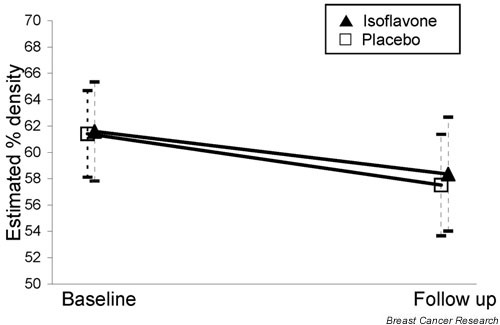
Estimated percentage density (and 95% confidence intervals) at baseline and follow up by treatment group (77 women in the isoflavone group and 85 in the placebo; P = 0.82).
The interaction between treatment group and the ESR1 polymorphism was significant (P = 0.009). Changes in estimated percentage breast density (mean ± SD) by treatment group and ESR1 genotype were as follows: TT isoflavone 1.4 ± 12.3% and TT placebo -9.6 ± 14.2%; CT isoflavone -5.2 ± 12.0% and CT placebo -2.8 ± 10.3%; and CC isoflavone -3.4 ± 9.7% and CC placebo -1.1 ± 9.5%. There were no interactions between treatment group and CYP17 or CYP19 genotypes.
Oestradiol, follicle-stimulating hormone, luteinizing hormone and tyrosine kinase activity
There were no significant effects of treatment group on change in oestradiol, FSH, or LH (Table 2). Restricting the analysis to women with complete urine collections at all three time points did not alter these results (data not shown). The interaction between CYP19 gene polymorphism and treatment group was of borderline statistical significance for the change in FSH (P = 0.05). Changes in FSH (IU/l) by treatment group and CYP19 genotype were as follows (mean ± SD): GG isoflavone 1.07 ± 12.74 and GG placebo -5.80 ± 18.46; GT isoflavone -2.93 ± 9.35 and GT placebo -2.69 ± 10.98; and TT isoflavone -10.63 ± 11.30 and TT placebo 1.00 ± 9.13.
Table 2.
Circulating levels of oestradiol, follicle-stimulating hormone and luteinizing hormone at baseline and after 12 months, and change from baseline to 12 months by treatment group
| Isoflavone group (n= 56) | Placebo (n = 61)a | ||||||
| Baseline | 12 months | Change | Baseline | 12 months | Change | P for changeb | |
| Oestradiol (pmol/l) | 22.6 ± 12.2 | 36.6 ± 65.0 | 14.0 ± 64.7 | 22.6 ± 13.3 | 21.6 ± 9.0 | -0.9 ± 11.5 | 0.49 |
| FSH (IU/l) | 76.2 ± 21.5 | 72.0 ± 23.5 | -4.2 ± 12.9 | 77.6 ± 25.1 | 74.5 ± 23.9 | -2.9 ± 13.0 | 0.83 |
| LH (IU/l) | 43.9 ± 15.4 | 39.9 ± 14.6 | -4.0 ± 11.7 | 45.5 ± 16.0 | 41.2 ± 12.0 | -4.2 ± 9.2 | 0.71 |
Values are expressed as means ± standard deviation. aFor change in FSH, n = 60. bP value for difference between treatment groups for change. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
For tyrosine kinase activity, data were available for 46 women in the isoflavone group and for 47 women in the placebo group. There was no significant difference in the mean change, from baseline to 12 months, in tyrosine kinase activity (activity/μg protein) between isoflavone and placebo groups (mean ± SD): 1.62 ± 2.18 for women in the isoflavone group and 0.90 ± SD 2.72 for women in the placebo group (P = 0.16).
Hot flushes and menopausal symptoms
Interactions between treatment group and menopausal status for changes in menopausal symptom score and number of hot flushes were not statistically significant (P > 0.05). Among women who experienced hot flushes at either assessment period, or among women whose menopausal symptom score was greater than zero at either assessment period, there were no significant effects of treatment group on hot flushes or menopausal symptoms (Table 3).
Table 3.
Daily number of hot flushes and menopausal symptom score at baseline and after 12 months, and change from baseline to 12 months by treatment group
| Isoflavone (n = 45, n = 70)a | Placebo (n = 54, n= 83)a | ||||||
| Baseline | 12 months | Change | Baseline | 12 months | Change | P for changeb | |
| Mean daily number of hot flushes | 2.1 ± 2.7 | 1.2 ± 2.3 | -0.8 ± 2.1 | 2.5 ± 3.0 | 1.5 ± 2.0 | -1.0 ± 1.8 | 0.41 |
| Menopausal symptom score | 4.3 ± 4.3 | 3.4 ± 4.1 | -0.9 ± 4.1 | 4.3 ± 4.3 | 3.5 ± 3.8 | -0.8 ± 2.8 | 0.88 |
Analyses based on women who reported hot flushes or menopausal symptoms in one or both diaries. Values are expressed as means ± standard deviation. a Shown in parentueses are the number of women with hot flushes and number of women with any menopausal symptoms, respectively. bP value for difference between treatment groups for change.
Discussion
Combined HRT is associated with an increased risk for breast cancer [42,43] and an increase in mammographic breast density [13,16,44]. Soy and phyto-oestrogens have been proposed as potential natural alternatives to HRT [45,46], but there are only limited data from small studies on the effect of isoflavones on breast density. In the largest and longest study conducted thus far, we did not observe a significant effect of the clover-derived isoflavone supplement, taken daily for 1 year, on mammographic breast density. This is in agreement with a small, exploratory study conducted in 30 premenopausal women [27], in which there were no significant effects of a soy isoflavone supplement, providing 100 mg/day isoflavones for 12 months, on mammographic breast density. In that study, there was a slight increase in percentage density among women taking the isoflavone supplement, but differences between the intervention and placebo groups were not statistically significant. In the present study, breast density decreased to a similar extent in both treatment groups. This was presumably due to natural changes over time because an inverse relationship between breast density and age exists (for review [11]). The decrease in percentage density was of a similar magnitude to that seen among control individuals in other studies [20,47] but was not as great as the decreases seen with anti-oestrogens such as tamoxifen [20].
An attractive hypothesis for the potential chemopreventive actions of isoflavones is via modulation of endocrine function. However, the literature is conflicting with regard to the effects of soy intakes on circulating levels of oestradiol in both premenopausal and postmenopausal women; some studies have reported decreases, increases, or little effect [9,48-53]. In a recent study of premenopausal women [54], consumption of soymilk from which the isoflavones had been removed resulted in a decrease in oestradiol, suggesting that soy protein rather than soy isoflavones may be responsible for the reduction in circulating levels of oestradiol seen in some studies.
Reported effects of soy intakes on gonadotrophins have also been inconsistent, with limited data showing either no effect [49,51,55] or decreases in FSH and LH with soy supplementation [53]. Despite the fact that this was one of the longest and largest studies to have investigated the effects of an isoflavone supplement on the hormonal status of postmenopausal women, we did not observe a significant effect of the isoflavone supplement on circulating levels of oestradiol, FSH or LH.
Phyto-oestrogens have been postulated to have several other anticancer effects and, because of the possible role of genistein as an inhibitor of protein tyrosine kinase activity [56], we investigated levels of lymphocyte tyrosine kinase activity. However, we were unable to show significant differences between treatment groups for the change in the levels of lymphocyte tyrosine kinase activity.
Soy and phyto-oestrogens have received considerable attention because of their potential role in relieving menopausal symptoms, but their efficacy in such a role remains to be fully established. Short-term intervention studies among women experiencing frequent hot flushes have shown reductions in the frequency and/or severity of hot flushes with soy consumption, but also among control individuals [57-59], whereas others have shown little or no effect [60-63]. However, in a recent study of women experiencing more than five hot flushes per day, all study participants were given a placebo tablet in a single-blind, 4-week run-in phase, and during the subsequent 12-week double-blind phase the frequency of hot flushes was reduced by 44% in the women taking two Promensil tablets per day; no further reduction occurred in the placebo group [64]. In our study, the occurrence of hot flushes and the severity of menopausal symptoms decreased to a similar extent in both treatment groups, suggesting natural improvement over time. It is possible that the lack of a significant treatment effect may, in part, have been due to our study population; women were not selected for the study on the basis of suffering from severe or frequent hot flushes and/or other menopausal symptoms. As a result, baseline levels of menopausal symptoms were relatively low, which presumably limited the room for improvement in the study.
Polymorphisms in genes involved in sex hormone metabolism and in genes that encode hormone receptors may have an effect on the production of and exposure to sex hormones, and on the subsequent activation of genes that are responsive to sex hormones. We investigated interactions between treatment and selected polymorphisms in the CYP17 and CYP19 genes, both of which are involved in the sex hormone biosynthesis pathway, and the oestrogen receptor (ESR1) gene. Polymorphisms in these genes have been associated with risk for breast cancer in some but not all studies [65,66]. We saw interesting potential gene–treatment effects for the changes in breast density and ESR1 polymorphism and FSH and CYP19 polymorphism, but the study was not powered to investigate these interactions and a larger study is needed to confirm these findings.
Using the PABA check method as an independent marker of compliance, and urinary excretion of isoflavones, compliance with study procedures appeared to be excellent. However, a potential limitation of this study is that, although the study size was based on a case–control study of tamoxifen and breast density [19], changes in breast density among control individuals in this study were greater than anticipated. In addition, our findings may not be generalizable to the population as a whole. We did not include women in this study who were taking HRT at the time of recruitment (approximately one-third of women in the UK between the ages of 50 and 64 years use HRT [67]), and we only selected women with Wolfe P2 or DY breast patterns (which comprised approximately 60% of all mammograms classified for recruitment purposes). A further limitation of the study is that several statistical tests were carried out, and some of the statistically significant findings may have been due to chance.
Conclusion
We found that a dietary supplement that provided 26 mg biochanin A, 16 mg formononetin, 1 mg genistein and 0.5 mg daidzein daily for 1 year did not increase mammographic breast density in women aged 49–65 years, unlike conventional oestrogen replacement therapies. Furthermore, there were no effects of the isoflavone supplement on oestradiol, FSH or LH in postmenopausal women, or on hot flushes or other menopausal symptoms. Taken together, our findings suggest that the isoflavone supplement at the dose given was not acting as an oestrogen or as an anti-oestrogen.
Competing interests
SA Bingham was in receipt of research support from Novogen Ltd (Australia), who also supplied the Promensil and placebo tablets and performed the urinary isoflavone analyses. All other authors have no competing interests to declare.
Abbreviations
BMI = body mass index; ESR1 = estrogen receptor; FSH = follicle-stimulating hormone; HPLC = high-pressure liquid chromatography; HRT = hormone replacement therapy; LH = luteinizing hormone; PABA = para-amino benzoic acid; SD = standard deviation.
Addendum
All authors participated fully in the manuscript preparation. In addition, individual authors were involved in the following aspects of the trial: Charlotte Atkinson, recruitment and day-to-day running of the trial, sample analyses, and statistical analyses; Ruth ML Warren, breast density; Evis Sala, breast density; Mitch Dowsett, hormone assays; Alison M Dunning, genotyping; Catherine S Healey, genotyping, Shirley Runswick, tyrosine kinase assay; Nicholas E Day, data analysis; Sheila A Bingham, experiment design, sample analysis, and significant advice and consultation regarding all aspects of the trial.
Acknowledgments
Acknowledgements
This study was supported by grants from the Food Standards Agency FS2034 and the Medical Research Council. The authors would thank Sue Gardner, Nicola Duffy, Nasima Siddiqui, Jane Bettany and Jayne Girvan for their assistance with the day-to-day running of the trial, Leanne West for mammogram retrieval, and Addenbrooke's Hospital Pharmacy for administering the tablets. SA Bingham was in receipt of research support from Novogen Ltd (Australia), who also supplied the Promensil and placebo tablets and performed the urinary isoflavone analyses.
See related Commentary: http://breast-cancer-research.com/content/6/3/140
References
- Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;Suppl:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- Reinli K, Block G. Phytoestrogen content of foods: a compendium of literature values. Nutr Cancer. 1996;26:123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zheng W, Custer LJ, Dai Q, Shu XO, Jin F, Franke AA. Usual dietary consumption of soy foods and its correlation with the excretion rate of isoflavonoids in overnight urine samples among Chinese women in Shanghai. Nutr Cancer. 1999;33:82–87. doi: 10.1080/01635589909514752. [DOI] [PubMed] [Google Scholar]
- de Kleijn MJ, van der Schouw YT, Wilson PW, Adlercreutz H, Mazur W, Grobbee DE, Jacques PF. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study(1–4) J Nutr. 2001;131:1826–1832. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594–606. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- Ling X, Lu A, Zhao X, Chen X, Cummings SR. Very low rates of hip fracture in Beijing, People's Republic of China the Beijing Osteoporosis Project. Am J Epidemiol. 1996;144:901–907. doi: 10.1093/oxfordjournals.aje.a009024. [DOI] [PubMed] [Google Scholar]
- Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, Lee MM, Miike R, Kirk M, Coward L. Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–794. [PubMed] [Google Scholar]
- Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, Howell A, Bundred NJ. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–4024. doi: 10.1210/jc.84.11.4017. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–1144. [PubMed] [Google Scholar]
- Boyd NF, Lockwood GA, Martin LJ, Byng JW, Yaffe MJ, Tritchler DL. Mammographic density as a marker of susceptibility to breast cancer: a hypothesis. IARC Sci Publ. 2001;154:163–169. [PubMed] [Google Scholar]
- Laya MB, Gallagher JC, Schreiman JS, Larson EB, Watson P, Weinstein L. Effect of postmenopausal hormonal replacement therapy on mammographic density and parenchymal pattern. Radiology. 1995;196:433–437. doi: 10.1148/radiology.196.2.7617857. [DOI] [PubMed] [Google Scholar]
- Leung W, Goldberg F, Zee B, Sterns E. Mammographic density in women on postmenopausal hormone replacement therapy. Surgery. 1997;122:669–673. doi: 10.1016/s0039-6060(97)90072-6. discussion 673-664. [DOI] [PubMed] [Google Scholar]
- Stomper PC, Van Voorhis BJ, Ravnikar VA, Meyer JE. Mammographic changes associated with postmenopausal hormone replacement therapy: a longitudinal study. Radiology. 1990;174:487–490. doi: 10.1148/radiology.174.2.2136958. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Sie A, Singh HR, Olson LK, Gatewood O, Bassett LW, Wasilauskas C, Bush T, Barrett-Connor E. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Postmenopausal Estrogen/ Progestin Interventions (PEPI) Investigators. Ann Intern Med. 1999;130:262–269. doi: 10.7326/0003-4819-130-4_part_1-199902160-00003. [DOI] [PubMed] [Google Scholar]
- Lundstrom E, Wilczek B, von Palffy Z, Soderqvist G, von Schoultz B. Mammographic breast density during hormone replacement therapy: differences according to treatment. Am J Obstet Gynecol. 1999;181:348–352. doi: 10.1016/s0002-9378(99)70560-0. [DOI] [PubMed] [Google Scholar]
- Rutter CM, Mandelson MT, Laya MB, Seger DJ, Taplin S. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285:171–176. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol Biomarkers Prev. 1999;8:863–866. [PubMed] [Google Scholar]
- Brisson J, Brisson B, Cote G, Maunsell E, Berube S, Robert J. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9:911–915. [PubMed] [Google Scholar]
- Chow CK, Venzon D, Jones EC, Premkumar A, O'Shaughnessy J, Zujewski J. Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomarkers Prev. 2000;9:917–921. [PubMed] [Google Scholar]
- Atkinson C, Bingham SA. Mammographic breast density as a biomarker of effects of isoflavones on the female breast. Br Cancer Res. 2002;4:1–4. doi: 10.1186/bcr410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NF, Greenberg C, Lockwood G, Little L, Martin L, Byng J, Yaffe M, Tritchler D. Effects at two years of a low-fat, high-carbohydrate diet on radiologic features of the breast: results from a randomized trial. Canadian Diet and Breast Cancer Prevention Study Group. J Natl Cancer Inst. 1997;89:488–496. doi: 10.1093/jnci/89.7.488. [DOI] [PubMed] [Google Scholar]
- Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA. Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev. 2000;9:151–160. [PubMed] [Google Scholar]
- Sala E, Warren R, Duffy S, Welch A, Luben R, Day N. High risk mammographic parenchymal patterns and diet: a case-control study. Br J Cancer. 2000;83:121–126. doi: 10.1054/bjoc.2000.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskarinec G, Meng L. An investigation of soy intake and mammographic characteristics in Hawaii. Br Can Res. 2001;3:134–141. doi: 10.1186/bcr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskarinec G, Williams AE, Carlin L. Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev. 2003;12:165–169. doi: 10.1097/00008469-200304000-00011. [DOI] [PubMed] [Google Scholar]
- Wolfe JN. Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol. 1976;126:1130–1137. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;Suppl:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- Sala E, Warren R, McCann J, Duffy S, Day N, Luben R. Mammographic parenchymal patterns and mode of detection: implications for the breast screening programme. J Med Screen. 1998;5:207–212. doi: 10.1136/jms.5.4.207. [DOI] [PubMed] [Google Scholar]
- Greene JG. A factor analytic study of climacteric symptoms. J Psychosom Res. 1976;20:425–430. doi: 10.1016/0022-3999(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Bingham S, Cummings JH. The use of 4-aminobenzoic acid as a marker to validate the completeness of 24 h urine collections in man. Clin Sci. 1983;64:629–635. doi: 10.1042/cs0640629. [DOI] [PubMed] [Google Scholar]
- Johansson G, Bingham S, Vahter M. A method to compensate for incomplete 24-hour urine collections in nutritional epidemiology studies. Pub Health Nutr. 1999;2:587–591. doi: 10.1017/s1368980099000786. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Welsh MB, Lim CK. High-performance liquid chromatographic analysis of phytoestrogens in soy protein preparations with ultraviolet, electrochemical and thermospray mass spectrometric detection. J Chromatogr. 1987;386:315–323. doi: 10.1016/S0021-9673(01)94608-4. [DOI] [PubMed] [Google Scholar]
- Franke AA, Custer LJ, Cerna CM, Narala K. Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Proc Soc Exp Biol Med. 1995;208:18–26. doi: 10.3181/00379727-208-43826. [DOI] [PubMed] [Google Scholar]
- Dunning AM, Healey CS, Pharoah PD, Foster NA, Lipscombe JM, Redman KL, Easton DF, Day NE, Ponder BA. No association between a polymorphism in the steroid metabolism gene CYP17 and risk of breast cancer. Br J Cancer. 1998;77:2045–2047. doi: 10.1038/bjc.1998.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey CS, Dunning AM, Durocher F, Teare D, Pharoah PD, Luben RN, Easton DF, Ponder BA. Polymorphisms in the human aromatase cytochrome P450 gene (CYP19) and breast cancer risk. Carcinogenesis. 2000;21:189–193. doi: 10.1093/carcin/21.2.189. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Marmaro J, Todd JA. Towards fully automated genome-wide polymorphism screening. Nat Genet. 1995;9:341–342. doi: 10.1038/ng0495-341. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Goss PE, Powles TJ, Hutchinson G, Brodie AM, Jeffcoate SL, Coombes RC. Use of the aromatase inhibitor 4-hydroxyandrostenedione in postmenopausal breast cancer: optimization of therapeutic dose and route. Cancer Res. 1987;47:1957–1961. [PubMed] [Google Scholar]
- Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, Baker J, Kerr DJ. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2:659–668. [PubMed] [Google Scholar]
- Campbell MJ, Julious SA, Altman DG. Estimating sample sizes for binary, ordered categorical, and continuous outcomes in two group comparisons. BMJ. 1995;311:1145–1148. doi: 10.1136/bmj.311.7013.1145. (Published erratum appears in BMJ 1996, 312:96.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/S0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Lundstrom E, Christow A, Kersemaekers W, Svane G, Azavedo E, Soderqvist G, Mol-Arts M, Barkfeldt J, von Schoultz B. Effects of tibolone and continuous combined hormone replacement therapy on mammographic breast density. Am J Obstet Gynecol. 2002;186:717–722. doi: 10.1067/mob.2002.121896. [DOI] [PubMed] [Google Scholar]
- Messina MJ. Soy foods and soybean isoflavones and menopausal health. Nutr Clin Care. 2002;5:272–282. doi: 10.1046/j.1523-5408.2002.05602.x. [DOI] [PubMed] [Google Scholar]
- Carusi D. Phytoestrogens as hormone replacement therapy: an evidence-based approach. Prim Care Update Ob Gyns. 2000;7:253–259. doi: 10.1016/S1068-607X(00)00055-X. [DOI] [PubMed] [Google Scholar]
- Freedman M, San Martin J, O'Gorman J, Eckert S, Lippman ME, Lo SC, Walls EL, Zeng J. Digitized mammography: a clinical trial of postmenopausal women randomly assigned to receive raloxifene, estrogen, or placebo. J Natl Cancer Inst. 2001;93:51–56. doi: 10.1093/jnci/93.1.51. [DOI] [PubMed] [Google Scholar]
- Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H. Effect of soymilk consumption on serum estrogen concentrations in premenopausal Japanese women. J Natl Cancer Inst. 1998;90:1830–1835. doi: 10.1093/jnci/90.23.1830. [DOI] [PubMed] [Google Scholar]
- Lu L-JW, Anderson KE, Grady JJ, Kohen F, Nagamani M. Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer Res. 2000;60:4112–4121. [PubMed] [Google Scholar]
- Lu LJ, Anderson KE, Grady JJ, Nagamani M. Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev. 1996;5:63–70. [PubMed] [Google Scholar]
- Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84:3479–3484. doi: 10.1210/jc.84.10.3479. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham S, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994;60:333–340. doi: 10.1093/ajcn/60.3.333. [DOI] [PubMed] [Google Scholar]
- Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR, Kurzer MS. Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab. 1999;84:192–197. doi: 10.1210/jc.84.1.192. [DOI] [PubMed] [Google Scholar]
- Lu LJ, Anderson KE, Grady JJ, Nagamani M. Effects of an isoflavone-free soy diet on ovarian hormones in premenopausal women. J Clin Endocrinol Metab. 2001;86:3045–3052. doi: 10.1210/jc.86.7.3045. [DOI] [PubMed] [Google Scholar]
- Baird DD, Umbach DM, Lansdell L, Hughes CL, Setchell KD, Weinberg CR, Haney AF, Wilcox AJ, McLachlan JA. Dietary intervention study to assess estrogenicity of dietary soy among postmenopausal women. J Clin Endocrinol Metab. 1995;80:1685–1690. doi: 10.1210/jc.80.5.1685. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Murkies AL, Lombard C, Strauss BJ, Wilcox G, Burger HG, Morton MS. Dietary flour supplementation decreases postmenopausal hot flushes: effect of soy and wheat. Maturitas. 1995;21:189–195. doi: 10.1016/0378-5122(95)00899-V. [DOI] [PubMed] [Google Scholar]
- Brzezinski A, Adlercreutz H, Shaoul R, Rösler A, Shmueli A, Tanos V, Schenker JG. Short-term effects of phytoestrogen-rich diet on postmenopausal women. Menopause. 1997;4:89–94. [Google Scholar]
- Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, De Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol. 1998;91:6–11. doi: 10.1016/S0029-7844(97)00597-8. [DOI] [PubMed] [Google Scholar]
- St Germain A, Peterson CT, Robinson JG, Alekel DL. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause. 2001;8:17–26. doi: 10.1097/00042192-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Knight DC, Howes JB, Eden JA. The effect of Promensil, an isoflavone extract, on menopausal symptoms. Climacteric. 1999;2:79–84. doi: 10.3109/13697139909025570. [DOI] [PubMed] [Google Scholar]
- Knight DC, Howes JB, Eden JA, Howes LG. Effects on menopausal symptoms and acceptability of isoflavone-containing soy powder dietary supplementation. Climacteric. 2001;4:13–18. [PubMed] [Google Scholar]
- Baber RJ, Templeman C, Morton T, Kelly GE, West L. Randomized placebo-controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric. 1999;2:85–92. doi: 10.3109/13697139909025571. [DOI] [PubMed] [Google Scholar]
- Van de Weijer PHM, Barentsen R. Isoflavones from red clover (Promensil) significantly reduce menopausal hot flush symptoms compared with placebo. Maturitas. 2002;42:187–193. doi: 10.1016/S0378-5122(02)00080-4. [DOI] [PubMed] [Google Scholar]
- Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8:843–854. [PubMed] [Google Scholar]
- Ye Z, Parry JM. The CYP17 MspA1 polymorphism and breast cancer risk: a meta-analysis. Mutagenesis. 2002;17:119–126. doi: 10.1093/mutage/17.2.119. [DOI] [PubMed] [Google Scholar]
- Million Women Study Collaborators Patterns of use of hormone replacement therapy in one million women in Britain, 1996–2000. Br J Obstet Gynaecol. 2002;109:1319–1330. doi: 10.1016/s1470-0328(02)02914-2. [DOI] [PubMed] [Google Scholar]


